Abstract
Introduction:
Many women continue tobacco use during pregnancy despite known adverse consequences on neonatal growth and development. Testing meconium, the first neonatal feces, for tobacco biomarkers offers objective evidence of prenatal tobacco exposure. However, relationships between the amount, frequency, and timing of cigarette smoking during gestation and tobacco biomarker meconium concentrations and neonatal outcomes are unclear.
Methods:
Eighty-seven pregnant women provided detailed self-reports of daily tobacco consumption throughout pregnancy. Nicotine, cotinine, and trans-3′-hydroxycotinine were quantified in neonatal meconium by liquid chromatography–tandem mass spectrometry.
Results:
Among nonsmokers, all meconium specimens were negative, whereas nearly all meconium specimens were positive if the mother self-reported tobacco use into the third trimester. Tobacco biomarker concentrations were significantly albeit weakly correlated with mean cigarettes per day in the third trimester. Reduced birth weight, gestational age, or head circumference were observed if meconium contained one or more tobacco biomarkers, but deficits did not correlate with biomarker concentrations.
Conclusion:
While previously thought to reflect second and third trimester drug exposure, meconium appears to reliably identify only third trimester drug use. While a 10 ng/g nicotine, cotinine, or trans-3′-hydroxycotinine cutoff in meconium was previously proposed to differentiate tobacco-exposed from nonexposed or passively exposed neonates, improved maternal self-reporting techniques in this cohort suggest that a lower cutoff, equivalent to the analytic limits of quantification, is more appropriate.
Introduction
Tobacco use is a significant health concern in the United States. According to the 2004 Surgeon General Report, smoking-attributable health care costs exceed $150 billion annually, including nearly $500 million on neonatal care (U.S. Department of Health and Human Services, 2004b). Despite long-established health consequences and federally mandated warnings, 16.4% of American women continue to smoke while pregnant (Substance Abuse and Mental Health Services Administration, 2009). Identifying affected infants is critical to establishing social, behavioral, and educational interventions.
Biological monitoring of maternal and/or neonatal specimens can identify affected children and offers a more objective measurement than maternal self-report (Boyd, Windsor, Perkins, & Lowe, 1998; Britton, Brinthaupt, Stehle, & James, 2004; Markovic et al., 2000; Owen & McNeill, 2001; Webb, Boyd, Messina, & Windsor, 2003). Multiple matrices are available for testing, with maternal urine, oral fluid, and hair and neonatal urine, hair, and meconium among the most popular (Florescu et al., 2009).
Meconium, the first neonatal feces, is an important matrix offering several advantages over other neonatal matrices, including a longer window of drug detection, easy collection, and larger specimen volume (Gray & Huestis, 2007). While meconium analysis is well established for other drugs of abuse, including opiates and cocaine, testing for nicotine and metabolites is less prevalent, and many questions remain regarding the disposition of tobacco biomarkers in meconium and the utility of quantitative concentration data. It is not yet clear if meconium biomarker presence or a specific concentration can unequivocally differentiate active exposure (through smoking), passive exposure (through secondhand smoke), or nonexposure. Our previous research proposed a 10 ng/g nicotine, cotinine, or trans-3′-hydroxycotinine (OHCOT) concentration cutoff (Gray, Magri, Shakleya, & Huestis, 2008), but this has not yet been validated in a second independent cohort.
In addition to identifying exposed children, quantitative biomarker determinations may directly reflect the magnitude of maternal cigarette consumption or, more importantly, predict neonatal outcomes, such as weight, height, head circumference, or developmental deficits later in childhood. The existing literature correlating tobacco biomarkers in meconium, particularly with nicotine and OHCOT, and neonatal parameters is limited.
Meconium is currently thought to reflect maternal substance use during the second and third trimester; however, prospective clinical data monitoring maternal opioid and cocaine use by thrice weekly urine specimens suggest that the detection window is shorter, predominantly the last 3 months of pregnancy (Kacinko, Jones, Johnson, Choo, & Huestis, 2008). Few data are available to define the window of tobacco detection in meconium and whether it is similar to detection windows of other drugs. Since individual tobacco use patterns tend to be relatively stable, chronic daily exposure may extend tobacco’s detection in meconium as compared with a more varied pattern of illicit drug use.
The aims of this research were to (a) evaluate how the timing and magnitude of maternal cigarette consumption influences tobacco biomarker disposition in meconium; (b) compare maternal self-reported tobacco use and meconium analysis results, including validating our 10 ng/g concentration cutoff proposed previously; and (c) determine if meconium concentrations predict neonatal growth deficits.
Methods
Pregnant women between 12- and 20-week gestation were approached to participate in this University at Buffalo Institutional Review Board–approved research protocol. Exclusion criteria included maternal age <18, illicit drug use (other than cannabis), heavy alcohol, or cannabis consumption (more than one drink or five joints per day or more than four drinks or four joints on a single occasion after pregnancy recognition) based on the initial screening and multiple-birth pregnancy. After providing written informed consent, participants completed four assessments (three prenatal and one postpartum). The prenatal interviews were conducted near the end of each trimester. The postpartum interview was conducted at approximately 2 months of infant age corrected for prematurity. At each appointment, a timeline followback interview (Sobell & Sobell, 1992; Sobell, Sobell, Leo, & Cancilla, 1988) was used to gather retrospective daily tobacco, alcohol, and cannabis use data for the previous 3 months; thus, self reported data spanned 3 months prior to conception through delivery. Participants also provided information regarding their partner and/or household member’s smoking status, number of cigarettes smoked daily, and if smoking occurred within the home. An oral fluid specimen was collected for toxicological evaluation to provide objective evidence of recent exposure.
After birth, meconium specimens were collected from soiled diapers twice daily until the appearance of milk stool, transferred to storage containers, and frozen until transport to the National Institute on Drug Abuse for analysis. Neonatal birth parameters, including gender, gestational age, birth weight, length, head circumference, and 1- and 5-min Apgar scores, were extracted from medical records.
Oral fluid specimens were analyzed by a commercial laboratory for cotinine, the primary nicotine biomarker, with enzyme-linked immunosorbent assay (ELISA) or liquid chromatography–tandem mass spectrometry (LC-MSMS) at a 10 ng/ml cutoff.
Meconium specimens were assayed with a validated LS-MSMS method for nicotine, cotinine, and OHCOT (Gray, Shakleya, & Huestis, 2009). Quantification limits were 2.5 ng/g nicotine, 1 ng/g cotinine, and 5 ng/g OHCOT. The same assay also permitted quantification of opiate, cocaine, and amphetamine biomarkers, including morphine, codeine, 6-acetylmorphine, hydrocodone, hydromorphone, oxycodone, methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenyl-pyrrolidine, buprenorphine, norbuprenorphine, cocaine, benzoylecgonine, cocaethylene, m-hydroxybenzoylecgonine, methamphetamine, amphetamine, and p-hydroxyamphetamine (Gray, Shakleya, & Huestis, 2009).
Statistical evaluations were completed with SPSS for Windows (Chicago, IL). Kolmorgov–Smirnov tests assessed normality. For normally distributed variables, t tests normal variables were evaluated with Mann–Whitney U and Spearman’s rho tests. Mean differences between three or more groups were compared with analysis of variance. Chi-square tests examined variable independence.
Results
Recruitment
In total, 129 women met initial eligibility criteria at screening; 120 returned for the first prenatal interview. Of the nine who did not return, one woman, a smoker, had an abortion and eight miscarried (six smokers and two nonsmokers). Following the first prenatal interview, 16 women did not return for their second or third prenatal interview or elected to dropout, 1 miscarried, and 1 relocated. Thus, 102 women proceeded to delivery while in the study. Meconium was not collected from 14 neonates; in three cases, only milk stool was available, six specimens were discarded due to nursing error, and five women did not deliver at the recruitment hospital. An additional woman moved soon after delivery before the final interview; thus, complete maternal self-report data were not available. Only mother/infant dyads with complete self-report data and meconium results (n = 87) were included in subsequent evaluations.
Sociodemographic characteristics
Of 87 participants, 24 (27.6%) denied smoking during pregnancy (“nonsmokers”), 8 (9.2%) reportedly quit in the first or second trimester (“quitters”), and 55 (63.2%) continued smoking into the third trimester (“smokers”). Maternal demographics for each group are presented in Table 1; no statistical differences were observed between groups, except for the partner’s smoking status. Smokers were more likely to live with a partner or spouse who also smoked (χ2 = 6.98, p = .03) than nonsmokers or quitters (Table 1).
Table 1.
Maternal demographics for 87 pregnant women classified by self-reported tobacco smoking status
| Nonsmoker (n = 24) | Quitter (n = 8) | Smoker (n = 55) | Total (n = 87) | |
| Age in years (M ± SD, range) | 25.0 ± 5.5, 19–37 | 23.1 ± 3.9, 20–31 | 23.2 ± 4.4, 18–39 | 24.3 ± 5.1, 18–39 |
| Gravida (M ± SD, range) | 2.3 ± 2.4, 0–8 | 0.9 ± 1.5, 0–4 | 2.5 ± 2.5, 0–10 | 2.3 ± 2.4, 0–10 |
| Para (M ± SD, range) | 1.5 ± 1.2, 0–4 | 0 | 1.9 ± 1.8, 0–8 | 1.7 ± 1.6, 0–8 |
| Race | ||||
| Caucasian (%) | 16.7 | 50.0 | 25.5 | 25.3 |
| Black (%) | 50.0 | 37.5 | 41.8 | 43.7 |
| Hispanic (%) | 33.3 | 12.5 | 10.9 | 17.2 |
| Multiracial/other (%) | 0.0 | 0.0 | 21.8 | 13.8 |
| Employed (%) | 43.6 | 37.5 | 37.5 | 41.4 |
| Martial status | ||||
| Single (%) | 25.0 | 25.0 | 16.4 | 19.5 |
| In a relationship but does not live with partner (%) | 25.0 | 25.0 | 41.8 | 35.6 |
| Married or lives with partner (%) | 50.0 | 50.0 | 41.8 | 44.8 |
| Spouse/live-in partner also smokes (%) | 33.3 | 50.0 | 78.3 | 61.5 |
Prevalence of biomarkers in meconium based on self-reported smoking status
For the 24 nonsmokers, all maternal oral fluid specimens and neonatal meconium specimens were negative, corroborating maternal self-report. Even among women (n = 5) whose partner or housemate smoked, meconium results were negative.
Among eight quitters, five meconium specimens were negative, whereas three contained nicotine, cotinine, and/or OHCOT. In two of the three, third trimester oral fluid specimens were positive for cotinine, questioning the veracity of maternal abstinence. The third neonate, whose mother reportedly ceased smoking in the first trimester, contained a low concentration (4.2 ng/g) of nicotine.
Most participants (n = 55) continued cigarette use into the third trimester, and cotinine was found in 81.5%, 86.0%, and 92.0% of first, second, and third trimester oral fluid specimens. Nearly all (90.9%) neonatal meconium specimens contained one or more nicotine biomarkers; the prevalence and concentrations of nicotine, cotinine, and OHCOT are presented in Table 2. Of the 50 specimens who contained one or more tobacco biomarker, all three were present in most (n = 45, 90%). In the five remaining specimens, two contained nicotine and cotinine, one cotinine and OHCOT, and two cotinine only; in all but one of these five specimens, concentrations were <10 ng/g.
Table 2.
Tobacco biomarker prevalence and concentrations in meconium from 55 neonates born to women who smoked into the third trimester
| n | Median (ng/g) | Interquartile interval (ng/g) | Range (ng/g) | |
| Nicotine | 47 | 51.5 | 21.5–95.6 | 3.6–295 |
| Cotinine | 50 | 62.0 | 24.2–95.2 | 1.8–316 |
| trans-3′-Hydroxycotinine | 46 | 76.2 | 34.6–107 | 5.7–275 |
Factors influencing the presence of tobacco biomarkers in meconium
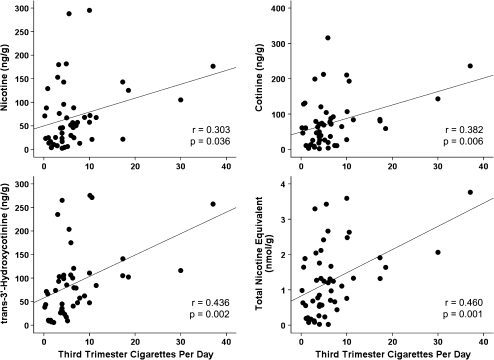
The presence of meconium tobacco biomarkers was influenced by the timing and magnitude of maternal cigarette consumption within the last trimester. Of 44 women who continued smoking within 2 weeks of delivery (median [range] cigarettes per day, 5.3 [0.4–37.1]), all neonatal meconium specimens were positive. Five women reportedly stopped cigarette consumption (3.1 [1.5–5.2]) 2–4 weeks before birth, and tobacco biomarkers were found in four meconium specimens. The remaining six women ceased smoking (0.3 [0.1–1.7]) at least 1 month (range 30–73 days) before delivery, and only 33% of meconium specimens were tobacco positive. Furthermore, the mean number of cigarettes consumed daily in the third trimester was significantly related to meconium nicotine, cotinine, OHCOT, and total tobacco biomarker concentrations (Figure 1). The effect of environmental tobacco smoke in addition to maternal smoking was investigated; among women smoking similar numbers of cigarettes, mean meconium concentrations were not significantly increased when mothers lived with another smoker.
Figure 1.
Tobacco biomarkers concentrations are correlated to number of cigarettes consumed per day during the third trimester.
Meconium concentration as a predictor of maternal smoking status and neonatal growth deficits
Applying our previously proposed 10 ng/g cutoff concentration of nicotine, cotinine, or OHCOT to differentiate active from passive or nonsmokers, 24 were identified as true negatives, 47 as true positives, 16 as false negatives, and none as false positives. For this evaluation, active smokers were women who self-reported cigarette use or had a positive oral fluid cotinine test at the time of the first interview, including those who reportedly quit smoking during pregnancy. The 10 ng/g cutoff achieved 74.6% sensitivity and 100% specificity. Lowering the cutoff to the method’s limits of quantification (2.5 ng/g nicotine, 1 ng/g cotinine, or 5 ng/g OHCOT), six more true positives were identified, increasing the sensitivity to 84.1% while maintaining 100% specificity.
The presence and concentration of tobacco biomarkers were evaluated as predictors of neonatal growth deficits. If meconium was positive for one or more tobacco biomarkers, significant reductions in gestational age, birth weight, and head circumference were observed (Table 3), whereas no difference in birth length or 1- and 5-min Apgar scores was found. No significant correlations between nicotine, cotinine, OHCOT, or total tobacco biomarkers and neonatal growth parameters were observed. We also evaluated concentration–response relationships by grouping total biomarker concentrations into no, low, medium, and high exposure groups. The no exposure group consisted of neonates with negative meconium results, whereas low, medium, and high exposure groups corresponded to the first, second, and third tertiles of total biomarker concentration, respectively. No significant differences in birth weight, length, or gestational age were observed; however, there were differences in head circumference. Specifically, no exposure (34.9 ± 1.6 cm) was greater than low exposure (33.2 ± 2.7 cm, p = .021) but not medium (33.6 ± 1.6 cm) or high exposure (33.9 ± 1.6 cm). While a significant statistical difference was observed, the difference was not clinically significant, as these head circumferences are within normal range. As with linear correlations, thus, there were no concentration–response relationships after grouping individuals by concentration levels.
Table 3.
Neonatal growth parameters and the presence of tobacco biomarkers in meconium
| Negative meconium |
Positive meconium |
||
| M ± SD | M ± SD | p Value | |
| Estimated gestational age at delivery (weeks) | 39.6 ± 1.2 | 38.6 ± 2.0 | .017 |
| Birth weight (g) | 3466 ± 453 | 3193 ± 672 | .041 |
| Head circumference (cm) | 34.9 ± 1.6 | 33.6 ± 2.0 | .002 |
| Length (cm) | 50.7 ± 2.0 | 50.1 ± 3.6 | .334 |
| 1-min Apgar score | 8.3 ± 1.3 | 8.6 ± 1.1 | .348 |
| 5-min Apgar score | 9.0 ± 0.3 | 9.0 ± 0.2 | .714 |
Polydrug exposure determined by biological testing
Polydrug exposure was evident only among smokers. Cocaine biomarkers were present in one woman’s oral fluid and two other neonates’ meconium. Seventeen smokers provided cannabis positive oral fluid specimens; to date, meconium specimens have not been analyzed for cannabinoids to corroborate or refute maternal self-report. Three additional meconium specimens contained hydrocodone and/or hydromorphone; these neonates’ mothers received analgesic narcotics for pain during extended labor.
Discussion
Tobacco consumption during pregnancy is associated with adverse obstetrical and neonatal outcomes, including premature rupture of membranes, placenta previa, placenta abruption, preterm delivery, shortened gestation, fetal growth restriction, and low birth weight (U.S. Department of Health and Human Services, 2004a). Furthermore, increased risks of behavioral problems, externalizing behaviors, attention deficit hyperactivity disorder, cognitive impairments, and lower academic achievement are observed in prenatally exposed children (Herrmann, King, & Weitzman, 2008). Thus, identifying affected infants is critical. Meconium analysis for identifying prenatal tobacco exposure has gained in popularity in recent years. The aims of this research were to investigate factors contributing to tobacco biomarker disposition in meconium, evaluate a proposed concentration for differentiating active and nonsmoking mothers, and determine if biomarkers’ presence or concentrations could predict infant outcomes.
As observed for opiates and cocaine (Kacinko et al., 2008), the timing and magnitude of tobacco exposure during pregnancy impacts the presence of nicotine and metabolites in meconium. Higher daily cigarette consumption and fewer days between last reported cigarette and birth influenced whether drug biomarkers were found in neonatal meconium. Meconium is currently thought to reflect second and third trimester drug and tobacco exposure; however, our data suggest that only third trimester tobacco exposure can be reliably documented in meconium. Previous studies investigating cigarette consumption have not reported specific relationships between number of cigarettes smoked per day and time elapsed between last cigarette and birth. Kohler, Avenarius, Rabsilber, Gerloff, and Jorch (2007) evaluated meconium results, maternal urine concentrations, and self-reported gestational age at smoking cessation among nine maternal/fetal dyads, yet no clear relationship between the time interval between smoking cessation and birth, presence of nicotine biomarkers in maternal urine at delivery, and positive neonatal meconium were noted.
Additional research is needed to confirm tobacco detection windows observed in this cohort, particularly among women who stop smoking in pregnancy. Of 87 participants, 8 women reportedly ceased tobacco consumption in the first or second trimester and another 6 quit early in the third trimester, more than 1 month before delivery. A larger population of women who successfully stopped smoking during gestation and who were closely monitored with toxicological testing could refine our detection window estimates.
Recently, researchers employed more sensitive and selective procedures allowing quantification of nicotine and its major metabolites, cotinine, and OHCOT in meconium (Gray, Shakleya, & Huestis, 2008, 2009; Kohler et al., 2007). While others failed to observe nicotine or OHCOT in meconium (Baranowski, Pochopien, & Baranowska, 1998; Ostrea, Knapp, Romero, Montes, & Ostrea, 1994), previous data from our laboratory and others demonstrated that nicotine and OHCOT are as prevalent and abundant as cotinine (Gray, Magri, Shakleya, & Huestis, 2008; Kohler et al.); moreover, an additional 25% of neonates were identified as tobacco exposed by including both nicotine and OHCOT in testing procedures (Gray, Magri, et al.). However, in this cohort, the majority of meconium specimens contained all three tobacco biomarkers. Differences in analyte distribution between studies may be due to maternal cigarette consumption; the women in the current study reported smoking more cigarettes per day than the previous cohort.
Differentiation of active and nonsmoking women by meconium nicotine and/or metabolites’ concentrations also was investigated. Earlier reports, based on cotinine immunoassay, found concentration differences between nonsmokers, passive smokers, and active smokers (Ostrea et al., 1994; Sherif et al., 2004), but passive and women smoking <20 cigarettes/day could not be differentiated based on meconium concentrations (Ostrea et al., 1994). However, more recent research employing more sensitive analytic methodology suggest that active smokers can be differentiated from nonsmokers and environmentally exposed women, but nonsmokers and environmental-exposed women are indistinguishable based on meconium concentrations. Kohler et al. (2007) clearly identified active smokers from environmentally exposed or nonsmokers, as all meconium specimens from the active smoking group were positive at a 20 pmol/g (∼3 ng/g) analytic limit, whereas meconium specimens from environmentally exposed and nonsmoking groups were all negative. Similarly, our laboratory previously proposed a 10 ng/g nicotine, cotinine or OHCOT cutoff to differentiate active smokers from passive or nonsmokers (Gray, Magri, et al., 2008). In a cohort of Uruguayan women of predominately low economic status, the 10 ng/g cutoff achieved 82.4% sensitivity and 97.0% specificity (Gray, Magri, et al.). Applying the 10 ng/g cutoff in the current investigation yielded 74.6% sensitivity and 100% specificity; lowering the cutoff to the analytic limits of quantification increased sensitivity to 84.1% without changing specificity. We reevaluated the Uruguayan cohort with the 1 ng/g cotinine, 2.5 ng/g nicotine, or 5 ng/g OHCOT concentration cutoff and observed a larger number of false positives and decreasing specificity (78.1%) at the lower cutoffs and no change in sensitivity as compared with maternal self-reported tobacco use. Different maternal interviewing techniques may have contributed to the specificity discrepancies observed in the two populations; the timeline followback interview administered several times during pregnancy in the current study likely generated more accurate responses than a single multiple-choice style postpartum interview employed in the Uruguayan cohort. For future research endeavors, we suggest the lower 2.5 ng/g nicotine, 1 ng/g cotinine, or 5 ng/g OHCOT cutoff for differentiating maternal smoking status. Furthermore, a larger population of environmentally exposed nonsmoking women is needed to confirm the appropriateness of these cutoffs.
The reliance on maternal self-report is a limitation of most prenatal substance use research. The timeline followback is a well-established interview technique effective for several drugs of abuse and various populations (Sobell et al., 1988), including obstetric patients (Magnusson, Gransson, & Heilig, 2005; Sarkar et al., 2009); however, it is not infallible. To facilitate maternal truthfulness, oral fluid specimens were collected for cotinine testing during each interview session. In some instances, cotinine oral fluid concentrations indicated smoking when mothers reported abstinence. It is well established that self-reported tobacco use often is misrepresented by pregnant women (England et al., 2007; Gray, LaGasse, et al., 2009). The 10 ng/ml oral fluid cutoff to differentiate smokers from nonsmokers is slightly lower than previous reports by Hegaard, Kjaergaard, Moller, Wachmann, and Ottesen (2007), 13 ng/ml; Owen and McNeill (2001),14 ng/ml; and Boyd et al. (1998), 30 ng/ml but correspond to levels probable for regular nicotine use, as suggested by Etzel (1990) and Parazzini et al. (1996). Furthermore, the 10 ng/ml cutoff was recommended by the Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification (Benowitz et al., 2002).
Linear relationships between maternal cigarettes smoked in the third trimester and meconium nicotine, cotinine, OHCOT, and total biomarker concentrations were found. To our knowledge, only one other report has evaluated the relationship between the number of maternal cigarettes consumed daily and meconium concentrations, finding a moderate correlation (r = .492–.678) for nicotine, cotinine, OHCOT, and the summed meconium concentrations (Kohler et al., 2007). Despite finding statistically significant correlations in our data and those of Kohler, predicting the number of cigarettes with meconium concentrations would be difficult given the low correlation coefficients. Furthermore, observing a relationship between maternal consumption and meconium concentrations is in contrast to other studies evaluating how maternal dose is reflected in meconium concentrations; a monitored buprenorphine administration study observed no dose–concentration relationships based on dose per day, cumulative pregnancy dose, or third trimester dose (Kacinko et al., 2008).
To date, the relationship between meconium tobacco biomarker concentrations and neonatal outcomes is poorly defined. Only birth weight was shown to negatively correlate with cotinine concentrations (Sherif et al., 2004), but this finding was not reproduced in earlier research by our laboratory (Gray, Magri, et al., 2008) and in the current data. Likewise, binomial tobacco biomarker meconium results (positive/negative) have not consistently proven to be effective predictors of growth deficits. Ostrea et al. (2008) observed no differences in gestational age at birth, birth weight, length, and head circumference based on meconium positivity for tobacco biomarkers, whereas our laboratory observed decreased head circumference when one or more nicotine biomarkers was present (Gray, Magri, et al.). In the present cohort, significantly reduced gestational age, birth weight, and head circumference were noted when meconium specimens contained nicotine, cotinine, or OHCOT.
Other drug classes were assayed in meconium to identify additional drug exposure that could contribute to negative growth outcomes. Three neonates had opioid-positive meconium that was attributable to pain medication given to the mother during extended labor; this peripartum opioid exposure would not be expected to adversely affect development and growth. Only two other neonates had cocaine-positive meconium. The few cocaine-exposed neonates would not meaningfully confound the effects of nicotine. Future research will evaluate the effect of maternal cannabis use on fetal growth with and without concurrent tobacco exposure.
In conclusion, the detection window for tobacco biomarkers in meconium appears to be shorter than currently thought, reliably reflecting only third trimester tobacco exposure. The presence of tobacco biomarkers in meconium predicts reduced gestational age, birth weight, and/or head circumference, but higher concentrations did not imply more severe deficits.
Funding
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (Intramural Research Program and grant number R01 DA 013190).
Declaration of Interests
None declared.
References
- Baranowski J, Pochopien G, Baranowska I. Determination of nicotine, cotinine and caffeine in meconium using high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications. 1998;707:317–321. doi: 10.1016/s0378-4347(97)00619-1. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III., Ahjevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Boyd NR, Windsor RA, Perkins LL, Lowe JB. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Maternal and Child Health Journal. 1998;2:77–83. doi: 10.1023/a:1022936705438. [DOI] [PubMed] [Google Scholar]
- Britton GRA, Brinthaupt J, Stehle J, James G. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2004;33:306–311. doi: 10.1177/0884217504264866. [DOI] [PubMed] [Google Scholar]
- England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine & Tobacco Research. 2007;9:1005–1013. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Preventive Medicine. 1990;19:190–197. doi: 10.1016/0091-7435(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Therapeutic Drug Monitoring. 2009;31:14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Analytical and Bioanalytical Chemistry. 2007;388:1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, LaGasse LL, Smith LM, Derauf C, Grant P, Shah R, et al. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology in the Infant Development, Environment and Lifestyle (IDEAL) Study. Therapeutic Drug Monitoring. 2009;31:769–775. doi: 10.1097/FTD.0b013e3181bb438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Magri R, Shakleya DM, Huestis MA. Meconium nicotine and metabolites by liquid chromatography-tandem mass spectrometry: differentiation of passive and nonexposure and correlation with neonatal outcome measures. Clinical Chemistry. 2008;54:2018–2027. doi: 10.1373/clinchem.2008.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Shakleya DM, Huestis MA. Quantification of nicotine, cotinine, trans-3’-hydroxycotinine, nornicotine and norcotinine in human meconium by liquid chromatography tandem mass spectrometry. Journal of Chromatography B. 2008;863:107–114. doi: 10.1016/j.jchromb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Shakleya DM, Huestis MA. A liquid chromatography tandem mass spectrometry method for the simultaneous quantification of 20 drugs of abuse and metabolites in human meconium. Analytical and Bioanalytical Chemistry. 2009;393:1977–1990. doi: 10.1007/s00216-009-2680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnant non-smokers. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:401–406. doi: 10.1080/00016340601147517. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Current Opinion in Pediatrics. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clinical Pharmacology and Therapeutics. 2008;84:604–612. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E, Avenarius S, Rabsilber A, Gerloff C, Jorch G. Assessment of prenatal tobacco smoke exposure by determining nicotine and its metabolites in meconium. Human and Experimental Toxicology. 2007;26:535–544. doi: 10.1177/0960327107072391. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Gransson M, Heilig M. Unexpectedly high prevalence of alcohol use among pregnant Swedish women: Failed detection by antenatal care and simple tools that improve detection. Journal of Studies on Alcohol. 2005;66:157–164. doi: 10.15288/jsa.2005.66.157. [DOI] [PubMed] [Google Scholar]
- Markovic N, Ness RB, Cefilli D, Grisso JA, Stahmer S, Shaw LM. Substance use measures among women in early pregnancy. American Journal of Obstetrics and Gynecology. 2000;183:627–632. doi: 10.1067/mob.2000.106450. [DOI] [PubMed] [Google Scholar]
- Ostrea E, Villanueva-Uy E, Ngerncham S, Punnakanta L, Batilando MJP, Agarwal P, et al. An epidemiologic study comparing fetal exposure to tobacco smoke in three Southeast Asian countries. International Journal of Occupational and Environmental Health. 2008;14:257–262. doi: 10.1179/oeh.2008.14.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Knapp DK, Romero AI, Montes M, Ostrea AR. Meconium analysis to assess fetal exposure to nicotine by active and passive maternal smoking. Journal of Pediatrics. 1994;124:471–476. doi: 10.1016/s0022-3476(94)70378-7. [DOI] [PubMed] [Google Scholar]
- Owen L, McNeill A. Saliva cotinine as indicator of cigarette smoking in pregnant women. Addiction. 2001;96:1001–1006. doi: 10.1046/j.1360-0443.2001.96710019.x. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Davoli E, Rabaiotti M, Restelli S, Stramare L, Dindelli M, et al. Validity of self-reported smoking habits in pregnancy: A saliva cotinine analysis. Acta Obstetricia et Gynecologica Scandinavica. 1996;75:352–354. doi: 10.3109/00016349609033330. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Burnett M, Carrire S, Cox L, Dell C, Gammon H, et al. Screening and recording of alcohol use among women of child-bearing age and pregnant women. Canadian Journal of Clinical Pharmacology. 2009;16:e242–e263. [PubMed] [Google Scholar]
- Sherif NA, Kamel SM, Al-Ashkar OS, Sharaki OA, Seif EA, Hegazy EA. Detection of cotinine in neonate meconium as a marker for nicotine exposure in utero. Eastern Mediterranean Health Journal. 2004;10:96–105. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back. A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–73. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National findings. 2009. No. DHHS Publication No. SMA 09-4434). Rockville, MD: Department of Health and Human Services. [Google Scholar]
- U.S. Department of Health and Human Services. Chapter 5. Reproductive effects. In: Samet JM, Norman LA, Wilbanks C, editors. The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: U.S. Government Printing Office; 2004a. pp. 550–601. [Google Scholar]
- U.S. Department of Health and Human Services. Chapter 7: The impact of smoking on disease and the benefits on smoking reduction. In: Samet JM, Norman LA, Wilbanks C, editors. The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: U.S. Government Printing Office; 2004b. pp. 853–93. [Google Scholar]
- Webb D, Boyd N, Messina D, Windsor R. The discrepancy between self-reported smoking status and urine cotinine levels among women enrolled in prenatal care at four publicly funded clinical sites. Journal of Public Health Management and Practice. 2003;9:322–325. doi: 10.1097/00124784-200307000-00011. [DOI] [PubMed] [Google Scholar]