Abstract
Preweanling rats are highly sensitive to the locomotor stimulation induced by relatively high ethanol doses. In adult mice this ethanol effect is modulated by stress. The goal of the present study was to analyze the role of stress and corticosterone in the stimulating effect of ethanol in preweanling rats. In Experiment 1 15-day-old rats were separated from the mother during a period of 4 hours in which subjects remained isolated or paired with a littermate. In a third condition pups remained in the home-cage with the dam. After this isolation period pups were given ethanol (0 or 2.5 g/kg) and were tested in a novel environment. Previous data have shown that a similar period of isolation is enough to increase corticosterone levels in preweanling rats. Experiment 2 evaluated the effect of exogenous administration of corticosterone (0, 3 or 6 mg/kg) along with ethanol, and Experiment 3 tested ethanol-mediated locomotor activation in adrenalectomized preweanling rats. The last Experiment aimed to test the role of corticotropic releasing factor 1 (CRF1) receptors in locomotion induced by ethanol in isolated pups. According to our results there is a synergism between stress or corticosterone and ethanol in preweanling rats. The interaction between stress (induced by social isolation) and ethanol seems to be mediated by CRF, since blockade of CRF1 receptors cancelled the effect of ethanol in isolated pups. This study highlights the importance of considering stress as a possible intervening variable in studies evaluating ethanol effects in developing animals when maternal separation is used in the experimental procedure.
Keywords: Preweanling rat, ethanol, locomotor activity, stress, maternal separation, corticosterone, corticotropic releasing factor
Over the past few years a growing number of studies have shown that infant rats are highly sensitive to a variety of ethanol effects. By the end of the second postnatal week of life rats are more sensitive to the acute tolerance induced by ethanol than adults (see Arias, Molina, Mlewski, Pautassi, & Spear, 2008; Silveri & Spear, 2001). Ethanol-mediated negative reinforcement (Pautassi, Sanders, Miller, Spear, & Molina, 2006), aversion conditioning (Arias & Chotro, 2006a, 2006b) or positive appetitive reinforcement (Arias & Chotro, 2006a; Chotro & Arias, 2007; Nizhnikov, Pautassi, Molina, & Spear, 2009) can easily be observed in preweanling rats. Recently we also found a particular sensitivity in infant rats to the locomotor stimulating effect of ethanol (Arias, Mlewski, Hansen, Molina, Paglini and Spear, 2009; Arias, Mlewski, Miller, Molina, & Spear, 2009; Arias, Mlewski, Molina, & Spear, 2009a, 2009b). This effect was consistently observed when preweanling rats were tested in a novel environment during the second postnatal week of life in response to relatively high ethanol doses intragastrically (i.g.) delivered (1.25 or 2.5 g/kg).
The study of the mechanisms mediating ethanol-mediated stimulation is relevant because of its possible mechanistic association with the reinforcing effects of drugs of abuse (Wise & Bozarth, 1987). Recent evidence suggests that this association may be present in infant rats in response to ethanol. Relatively neutral stimuli paired with the early stage of intoxication induced by a high ethanol dose (2 g/kg, blood ethanol levels (BECs): 157 mg%) became positive reinforcers (Molina, Pautassi, Truxell, & Spear, 2007). Molina and colleagues (2007) also reported that when stimuli were presented when blood ethanol reached peak values infant rats learned conditioned aversion (BECs: 200 mg%). The occurrence of these biphasic motivational effects of ethanol followed a time-course similar to that of the biphasic locomotor effects of the drug at the same age (Arias et al., 2008). A relatively high ethanol dose (2.5 g/kg) induced stimulation during the rising phase of the blood ethanol curve (BECs: 187 mg%) while sedation was promoted when BECs reached peak values (228 mg%). The association between ethanol-mediated positive reinforcing and the stimulating effect of the drug is also supported by recent pharmacological studies from our laboratory showing that mu-opioid antagonists can reduce both of these effects of ethanol (Arias, Molina and Spear, 2010; Nizhnikov, Pautassi, Molina, & Spear, 2009). Additionally, blocking other neurochemical systems involved in ethanol-mediated conditioned place preference in adult rodents, such as the dopaminergic (Matsuzawa, Suzuki, Misawa & Nagase, 1999) or GABA B (Bechtholt & Cunningham, 2005) systems, also suppress ethanol-mediated locomotor stimulation in preweanling rats (Arias et al., 2009b; Arias, Mlewski, Hansen et al., 2009). Considering these antecedents, the analysis of the mechanisms and variables that modulate the locomotor effects of ethanol may represent a valuable tool for understanding the neural basis of affective states induced by ethanol early in ontogeny. This is important since exposure to ethanol and other drugs of abuse early in life is considered a risk factor for later use and abuse of these substances (Chotro et al., 2007; Spear, 2000; Spear & Molina, 2005).
We recently reported that the testing environment must be novel for ethanol to induce stimulation in preweanling rats. When infant rats are pre-exposed to the testing environment before testing, the stimulating effect of ethanol is attenuated (Arias, Mlewski, Miller et al., 2009). This result does not clarify whether it is the novelty itself or the stress induced by the novel environment that is critical for these activating effects of ethanol. Additionally it is a common procedure in studies with infant rats to separate subjects from the homecage before testing, a treatment well known to induce stress in infants (Levine, 2005). In fact, in those studies showing stimulation induced by ethanol in preweanling rats, subjects were separated from the homecage for at least one hour (Arias, Mlewski, Hansen et al., 2009; Arias, Mlewski, Miller et al., 2009; Arias, Mlewski, Molina et al., 2009a, 2009b). In adult rats novelty and stress modulate the locomotor stimulating effects of a variety of drugs (Marinelli & Piazza, 2002). Exposure to a novel environment or to an acute stressor induces activation of the hypothalamus-pituitary-adrenal (HPA) axis (Ader & Friedman, 1968; Friedman, Ader, Grota, & Larson, 1967). The administration of exogenous corticosterone enhances the acute or chronic locomotor effects of a variety of drugs such as ethanol (Marinelli & Piazza, 2002; Roberts, Lessov, & Phillips, 1995), while adrenalectomy reduces the psychomotor activation induced by psychostimulants (Marinelli & Piazza, 2002).
The goal of the present study was to analyze the role of stress and corticosterone in the stimulating effect of ethanol in preweanling rats. We hypothesized that ethanol-mediated locomotor stimulation would be enhanced by stress. Additionally, we also hypothesized that corticosterone would mediate the stress-induced potentiation of the stimulating effect of ethanol. The present study aimed to test these hypotheses. The procedure selected to induce stress was maternal separation. The long-term effects of social isolation during infancy have been well studied. For example, rearing rats in isolation increased their sensitivity to the locomotor stimulating effects of psychostimulant drugs later in life (Brake, Zhang, Diorio, Meaney, & Gratton, 2004). Although the first two postnatal weeks of life is a period characterized by a low corticosterone response to most stressful stimuli, during this period maternal separation can nevertheless increase the production of corticotropic releasing factor (CRF) adrenocorticotropic hormone (ACTH) and corticosterone (Levine, 2001, 2005; Faturi et al., 2009; Stanton et al., 1988), while maternal stimuli reduce corticosterone release produced by stressful and painful stimuli (Stanton, Gutierrez, & Levine, 1988; Suchecki, Mozaffarian, Gross, Rosenfeld, & Levine, 1993).
In Experiment 1 15-day-old rats were separated from the mother during a period of 4 hours in which subjects remained isolated or paired with a littermate. In a third condition pups remained in the home-cage with the dam. Unpublished data from our laboratory indicate that these isolation treatments induce a significant increase in plasma corticosterone levels [home cage (n=14): 9.64 ± 3.08 ng/ml; isolated in pairs (n=14): 37.50 ± 8.10 ng/ml; isolated alone (n=12): 38.58 ± 7.65 ng/ml; values represent means ± SEM]. After this isolation period pups were given ethanol (0 or 2.5 g/kg) and were tested in a novel environment. This relatively high ethanol dose consistently has been found to increase locomotion in preweanling rats soon after administration (Arias, Mlewski, Molina et al., 2009a, 2009b). Our hypothesis is that isolated pups will show more stimulation after ethanol administration than subjects whcih were not separated from the homecage. Experiment 2 evaluated the effect of exogenous administration of corticosterone (0, 3 or 6 mg/kg) along with ethanol, and Experiment 3 tested ethanol- mediated locomotor activation in adrenalectomized preweanling rats. Our prediction is that exogenous corticosterone will potentiate the effect of ethanol, while adrenalectomy should reduce the stimulating effect of ethanol. The last Experiment (4) aimed to test the role of CRF1 receptors in locomotion induced by ethanol in isolated pups. Additional Experiments were performed to analyze BECs as a function of the different treatments employed in the study.
Material and Methods
Subjects
Forty-seven (26 females and 21 males) and forty-three (24 females and 19 males) Wistar WKAH/HOK rats, representative of 8 litters, were utilized for each of Experiments 1a and 1b, respectively. Fifty-five (29 females and 26 males) and forty-one (22 females and 19 males) Wistar WKAH/HOK rats were utilized for Experiment 2a and 2b, respectively. Those subjects were representative of 10 and 8 litters respectively. Fifty-seven (26 females and 31 males) and forty-seven (25 females and 22 males) Wistar WKAH/HOK rats were utilized for the Experiment 3a and 3b, respectively. Nine litters were used for each of these Experiments. Finally, for Experiments 4a and 4b we employed forty-seven (27 males and 20 females) and thirty-two (17 males and 15 females) Wistar WKAH/HOK rats, respectively. In each case the subjects were representative of 6 litters. Animals were born and reared at the vivarium of the Instituto de Investigacion Medica Mercedes y Martin Ferreyra (Cordoba, Argentina) under conditions of constant room temperature (22 ± 1.0 °C), on a 12-hour light 12-hour dark cycle. The day of parturition was considered postnatal day 0 (PD0). All procedures were in accordance with the guidelines for animal care and use established by the National Institute of Health (1986).
Procedures
Experiment 1a: locomotor activity as a function of ethanol and social condition
On PD 15, one subject from each litter was assigned randomly to one of the social conditions [homecage, paired (paired with a littermated), or isolation] defined for this experiment. Pups from the paired or isolation conditions were separated from their mothers and placed in a holding maternity cage (45 × 20 × 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 37° C (± 1° C) through the use of a heating pad. Subjects assigned to the paired condition remained with a littermate, while subjects assigned to the isolated condition remained without littermate until the testing phase. As in all experiments included in the present study, in no case was more than one subject from a given litter and with the same sex assigned to the same condition. Pups assigned to the homecage condition remained in the homecage with their respective mother. Four hours later, pup’s body weights were individually recorded (± 0.01 g) and they immediately received an intragastric (i.g.) administration of ethanol (0 or 2.5 g/kg). The volume administered was equivalent to 0.015 ml per gram of body weight of a 21 % v/v ethanol solution, respectively. An equivalent volume of water was administered to pups assigned to the vehicle control group. Intragastric administrations were performed using a 10-cm length of polyethylene tubing (PE-10 Clay Adams, Parsippany, New Jersey) attached to a 1 ml syringe with a 27 G × 1/2 needle. This tubing was gently introduced through the mouth and slowly guided into the stomach. The entire procedure took less than 20 seconds per pup.
Five and 35 minutes later, locomotor activity was assessed for 5 minutes in a novel environment (all subjects were evaluated at both testing intervals). These post-administration intervals were selected based on prior studies. We recently observed that 2.5 g/kg ethanol exerted locomotor stimulation in the first post-administration interval, and induced locomotor sedation in the later one in preweanling rats (Arias et al., 2008). The floor of the openfield (40 cm × 49 cm × 40 cm) utilized for locomotor assessment was divided in 9 quadrants, and two trained researchers blind to the experimental treatments estimated horizontal activity in terms of the number of quadrants crossed. A given subject was considered to cross a specific quadrant when the two forepaws and the head passed through the line that divided the quadrants.
Experiment 1b: Determination of blood ethanol concentration (BEC) as a function of ethanol and social condition
Procedures of this experiment were similar to those described for Experiment 1a. After four hours of social treatment (homecage, paired or isolation) body weights were individually recorded (± 0.01 g) and pups received an i.g. administration of 2.5 g/kg ethanol. Pups were sacrificed 7.5 or 37.5 minutes after receiving the corresponding ethanol dose, time points which coincide with the middle of the testing intervals selected for Experiments 1a. Blood samples were subjected to headspace gas chromatography analysis. Samples were placed in microvials containing 50 µl of a butanol solution (51 mg/100 ml) that served as an internal standard. The manipulation of the samples was performed in containers filled with crushed ice. Microvials were hermetically sealed and then incubated in a water bath at 60°C for 30 min. Gas-tight syringes (Hamilton, Reno, Nevada, 10 µl) were used to collect the volatile component of the samples and to inject them into the gas chromatograph (model 5890, Hewlett-Packard, Palo Alto, CA). Column (Hewlett Packard HP 20M, Carbowax 20 M; 10 m × 0.53 mm × 1.33 m film thickness), injector, and detector temperatures were as follows: 60, 150, and 250°C, respectively. Nitrogen served as the carrier gas (flow rate, 15 ml/min). BECs were expressed as milligrams of ethanol per 100 ml of blood.
Experiment 2a: Locomotor activity as a function of ethanol and corticosterone treatments
For this experiment we selected the homecage condition utilized in Experiment 1. Since the goal of this experiment was to analyze whether corticosterone administration potentiates the effect of ethanol, the homecage condition was ideal to test our hypothesis since subjects from this social condition in Experiment 1 did not show ethanol-mediated stimulation. In the present experiment, on PD 15 one subject from a given litter was assigned to one of the 6 independent groups derived from the 2 factors that defined the design [ethanol treatment (0 or 2.5 g/kg) and corticosterone treatment (0, 3 or 6 mg/kg). Corticosterone (0, 3 or 6 mg/kg) was administered 30 minutes before ethanol. Corticosterone was dissolved in a solution of saline and 0.25% acetic acid (volume administered was 10 ml per kg). Pups given 0 g/kg received the same amount of vehicle. Thirty minutes after corticosterone administration subjects received the corresponding ethanol administration (0 or 2.5 kg). Locomotor activity was evaluated 5 and 35 minutes after ethanol administration following the procedures described in Experiment 1.
Experiment 2b: Determination of BEC as a function of ethanol and corticosterone treatments
Procedures of this experiment were similar to those described for Experiment 1b. Ppups were sacrificed 7.5 or 37.5 minutes after receiving the corresponding doses of corticosterone (0, 3 or 6 mg/kg 30 minutes before ethanol) and ethanol (2.5 g/kg). Blood samples were collected to determine BECs.
Experiment 3a: Locomotor activity as a function of ethanol and adrenalectomy treatments
For this Experiment we selected the isolated condition utilized in Experiment 1, which showed the largest activating effect of ethanol. This social condition was selected because our hypothesis is that removing corticosterone ethanol-mediated stimulation in stressed subjects will be eliminated. On PD 15 one subject from a given litter were assigned to one of the 6 independent conditions derived from the factorial combination of the following factors: ethanol treatment (0 or 2.5 g/kg) and adrenalectomy treatment (adrenalectomy, sham or untreated). Adrenalectomy was performed on PD10 following the procedures described for young rats (Moriceau et al., 2004). Briefly, after being anesthetized, adrenal glands were removed through dorsal incisions made in the back of the infant. Sham controls also received dorsal incisions, but the adrenal glands were left intact. A third group of untreated animals was also included in the experiment. Following recovery from surgery (approximately 1 hour), pups were returned to the mother until the day of assessment. On postnatal day 15 pups received the corresponding ethanol administration (0 or 2.5 kg). Locomotor activity was evaluated 5 and 35 minutes after ethanol administration following the procedures described in Experiment 1.
Experiment 3b: Determination of BECs as a function of ethanol and adrenalectomy treatments
For this experiment subjects were adrenalectomized on PD 10. On PD 15, adrenalectomized, sham and untreated controls were sacrificed 7.5 or 37.5 minutes after ethanol administration (2.5 g/kg) to measure BECs (see procedures section of Experiment 1b).
Experiment 4a: Locomotor activity as a function of ethanol and CP154,526 treatments
In Experiment 4a we tested whether ethanol-mediated locomotor activitity in isolated pups is reduced by CP154,526, a CRH1 antagonist. Hence, all rat pups in this experiment were isolated individually from the dam, as in the isolation condition of Experiment 1. On PD 15 subjects from a given litter were assigned to one of the 4 independent groups derived from the 2 orthogonal factors that defined the design [ethanol treatment (0 or 2.5 g/kg) and CP154,526 treatment (0 or 10 mg/kg). CP154,526 was dissolved in a solution of saline and 0.25% acetic acid (Volumen administered was 10 ml per kg) and administered 30 minutes before ethanol. Pups given 0 g/kg received an equivalent volume of vehicle. After this treatment pups were returned to their homecage and, 30 minutes after corticosterone administration, given the corresponding ethanol administration (0 or 2.5 kg). Locomotor activity was evaluated 5 and 35 minutes after ethanol administration following the procedures described in Experiment 1.
Experiment 4b: Determination of BECs as a function of ethanol and CP154,526 treatments
On PD15, after receiving the corresponding CP154,526 (0 or 10 mg/kg) and ethanol treatments pups were sacrificed 7.5 or 37.5 minutes later to measure BECs.
Data analysis
Data from the present study were statistically analyzed using an analysis of variance (ANOVA). No significant effect of sex or interaction with the remaining factors was found in any of the analyses performed in the present study. Hence, for the inferential analysis and descriptive presentation of the results, data were collapsed across sex. In Experiment 1 locomotor activity data (Experiments 1a) were analyzed with a 3 (social condition: homecage, paired or isolated) × 2 (ethanol treatment: 0 or 2.5 g/kg) × 2 (post-administration interval: 5 and 35 min) mixed ANOVA. Blood ethanol levels (Experiment 1b) were analyzed by means of a 3 (social condition: homecage, paired or isolated) × 2 (post-administration time: 7.5 or 37.5 min) between-factor ANOVA. Activity data from Experiments 2a were analyzed with a 3 (corticosterone: 0, 3 or 6 mg/kg) × 2 (ethanol treatment: 0 or 2.5 g/kg) × 2 (post-administration interavl: 5 and 35 min) mixed ANOVA while blood ethanol levels obtained in Experiment 2c were analyzed by means of a 3 (corticosterone treatment: 0, 3 or 6 mg/kg) × 2 (post-administration time: 7.5 or 37.5 min) between-factor ANOVA. Locomotor activity data registered in Experiment 3a were analyzed with a 3 (adrenalectomy treatment: untreated, sham or adrenalectomy) × 2 (ethanol treatment: 0 or 2.5 g/kg) × 2 (post-administration interval: 5 and 35 min) mixed ANOVA, and blood ethanol levels from Experiment 2d were analyzed by means of a 3 (adrenalectomy) × 2 (post-administration time: 7.5 or 37.5 min) between-factor ANOVA. Finally, activity data from Experiment 4a were analyzed with a 2 (CP154,526: 0 or 10 mg/kg) × 2 (ethanol treatment: 0 or 2.5 g/kg) × 2 (post-administration interval: 5 and 35 min) mixed ANOVA, and blood ethanol levels from Experiment 4b were analyzed by means of a 2 (CP154,526: 0 or 10 mg/kg) × 2 (post-administration time: 7.5 or 37.5 min) between-factor ANOVA. Significant main effects or interactions indicated by the ANOVAs were further analyzed through post-hoc tests (Newman Keuls post-hoc test with a Type I error set at 0.05).
Results
Experiment 1
Experiment 1a: locomotor activity as a function of ethanol and social condition
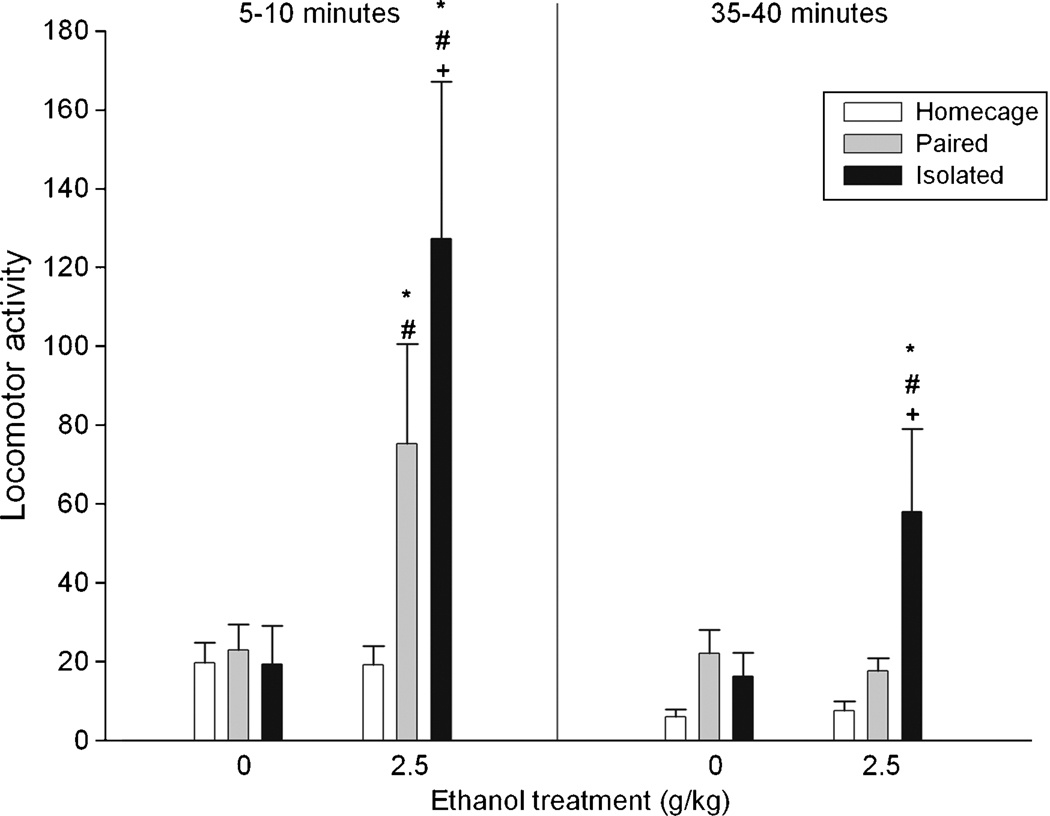
Figure 1 depicts locomotor activity scores as a function of ethanol and social treatment. The ANOVA indicated significant main effects of ethanol treatment [F(1, 41) = 9.30, p < 0.005], social condition [F(2, 41) = 4.97, p < 0.05] and post-administration interval [F(1, 41) = 13.09, p < 0.001]. The interactions between ethanol treatment and post-administration interval, and between ethanol treatment and social condition also reached statistical significance [F(1, 41) = 7.85, p < 0.01, and F(2, 41) = 4.02, p < 0.05, respectively]. Post-hoc analyses revealed that, regardless the testing interval, pups isolated and treated with ethanol showed more locomotor activity than their corresponding water-controls and than those subjects which remained at the homecage or were paired with a littermate and given ethanol. Water-treated subjects did not differ as a function of the social condition.
Figure 1.
Locomotor activity (operationalized through the number of quadrants crossed) in preweanling rats as a function of ethanol (0 or 2.5 g/kg), social isolation (homecage, paired or isolated), and postadministration time (5–10 and 35–40 minutes). Vertical lines illustrate standard errors of the means. * Significant difference with the corresponding water control. # Significant difference with group given ethanol from the homecage condition. + Significant difference with group given ethanol from paired condition.
Experiment 1b: Determination of blood ethanol concentration (BEC) as a function of ethanol and social condition
The ANOVA utilized to analyze BECs revealed a main effect of post-administration interval [F(1, 41) = 7.85, p < 0.01], indicating that BECs were higher at 37.5 min than at 7.5 minutes after ethanol administration. Social condition did not affect BECs (see Table 1).
Table 1.
Blood ethanol concentration as a function of time after ethanol administration (7.5 or 37.5 min) and the different treatments employed in the present study: social condition (Experiment 1b: Homecage, paired or isolated), corticosterone (Experiment 2b: 0, 3 or 6 mg/kg), adrenalectomy (Experiment 3b: untreated, sham or adrenalectomy) and CP154,526 (Experiment 4b: 0 or 10 mg/kg). Values represent blood ethanol levels (mg%) ± SEM.
| Experiment 1b | ||
| 7.5 min | 37.5 min | |
| Homecage | 111,91 ± 9.2 n=8 |
197,36 ± 8.3 n=8 |
| Paired | 114,20 ± 9.2 n=8 |
223,24 ± 18.8 n=7 |
| Isolated | 111,43 ± 14.64 n=6 |
220,62 ± 17.9 n=7 |
| Experiment 2b | ||
| 7.5 min | 37.5 min | |
| 0 mg/kg cort | 124.04 ± 10.7 n=7 |
189.13 ± 7.01 n=6 |
| 3 mg/kg cort | 124.95 ± 10.8 n=8 |
188.10 ± 5.6 n=7 |
| 6 mg/kg cort | 102.01 ± 10.2 n=6 |
181.34 ± 8.8 n=7 |
| Experiment 3b | ||
| 7.5 min | 37.5 min | |
| Untreated | 138.21 ± 14.4 n=11 |
239.58 ± 15.7 n=5 |
| Sham | 121.01 ± 12.3 n=11 |
234.82 ± 6.5 n=5 |
| Adrenalectomy | 117.79 ± 14.8 n=8 |
288.89 ± 13.9 n=7 |
| Experiment 4b | ||
| 0 mg/kg CP154,526 | 123.82 ± 8.7 n=7 |
237.41 ± 11.4 n=8 |
| 10 mg/kg CP154,526 | 136.85 ± 7.6 n=9 |
216.63 ± 9.5 n=8 |
Summarizing, pups from the homecage condition did not show ethanol-mediated stimulation in any post-administration interval, while pups paired with a littermate showed stimulation in the first post-administration interval. Interestingly, isolated pups showed simulation in both testing intervals. The interaction between ethanol and social condition cannot be explained by differences in BECs.
Experiment 2
Experiment 2a: Locomotor activity as a function of ethanol and corticosterone treatments
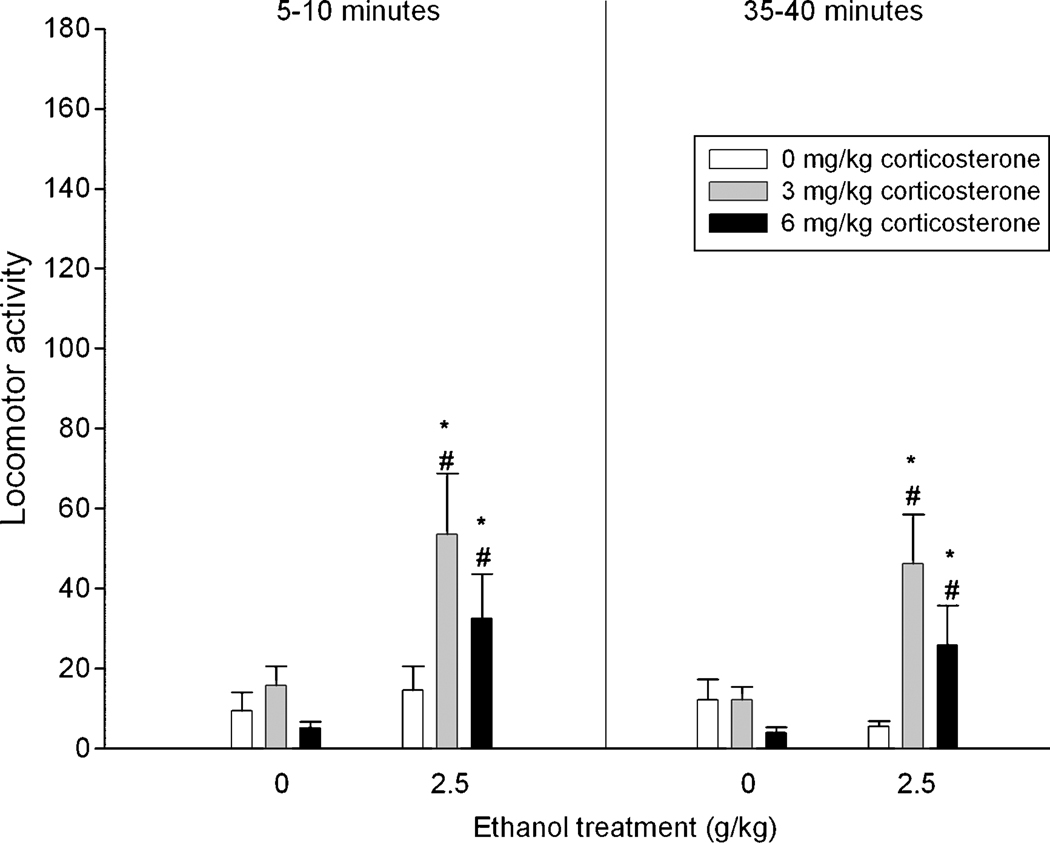
Figure 2 represents locomotor activity as a function of corticosterone and ethanol treatments. The ANOVA revealed significant main effects of ethanol [F(1, 49) = 12.69, p < 0.001] and corticosterone [F(2, 49) = 5.17, p < 0.01] treatments, as well as a significant interaction between corticosterone and ethanol treatment [F(2, 49) = 3.66, p < 0.05]. Post-hoc analyses revealed that pups given corticosterone (3 or 6 mg/kg) and ethanol showed more locomotor activity than their corresponding water controls and those subjects treated with ethanol and vehicle.
Figure 2.
Locomotor activity (operationalized through the number of quadrants crossed) in preweanling rats as a function of ethanol (0 or 2.5 g/kg), corticosterone treatments (0, 3 or 6 mg/kg) and time of assessment (5–10 and 35–40 minutes). Vertical lines illustrate standard errors of the means. * Significant difference with the corresponding water control. # Significant difference with group given ethanol and 0 mg/kg corticosterone.
Experiment 2b: Determination of BEC as a function of ethanol and corticosterone treatments
The ANOVA utilized to analyze BECs revealed a main effect of post-administration interval [F(1, 35) = 82.92, p < 0.001], indicating that BECs were higher at 37.5 min than at 7.5 minutes after ethanol administration. Corticosterone treatment did not affect BECs (see Table 1).
In summary, ethanol itself did not induce stimulation in the absence of isolation-induced stress, a result that replicates findings from Experiment 1. Corticosterone did not modify locomotor activity patterns of water-treated subjects. However, there was a clear synergism between ethanol and corticosterone. Pups given ethanol and corticosterone (3 or 6 mg/kg) showed significant locomotor activation compared to their corresponding controls. Corticosterone administration did not affect BECs at the time pups were tested for locomotor activity.
Experiment 3
Experiment 3a: Locomotor activity as a function of ethanol and adrenalectomy treatments
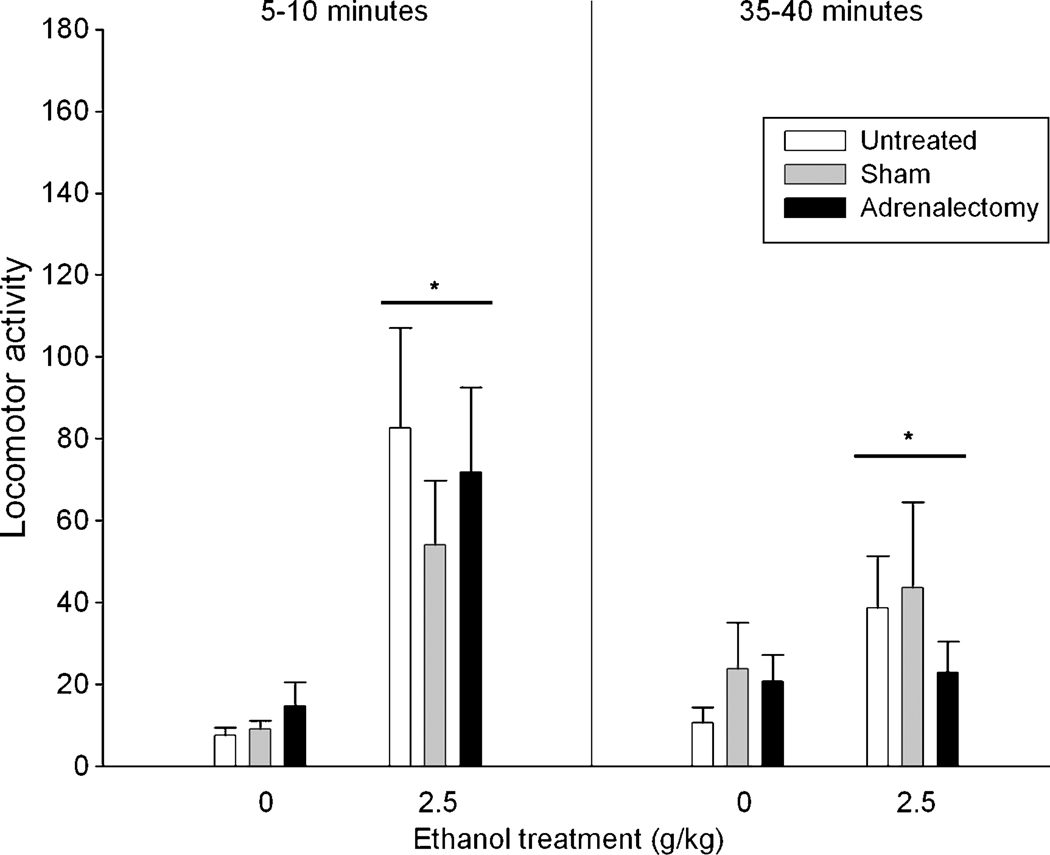
As suggested by the differences shown in Figure 3, the ANOVA revealed significant main effects of ethanol [F(1, 44) = 25.83, p < 0.001] and post-administration interval [F(1, 44) = 4.83, p < 0.05]. The interaction between ethanol treatment and post-administration interval also was significant [F(1, 44) = 10.51, p < 0.005]. Follow-up one-way ANOVAs including ethanol treatment as the only factor were run to find the locus of this interaction. For these analyses locomotor activity data from each post-administration interval were analyzed separately. These analyses revealed that pups given ethanol showed more locomotor activity than their corresponding water controls regardless of the adrenalectomy treatment. This effect was significant for both post-administration intervals [F(1, 48) = 33.12, p < 0.001, and F(1, 48) = 4.68, p < 0.05, respectively].
Figure 3.
Locomotor activity (operationalized through the number of quadrants crossed) in preweanling rats 5–10 and 35–40 minutes after ethanol administration as a function of ethanol (0 or 2.5 g/kg) and adrenalectomy treatments (adrenalectomy, sham or untreated). Vertical lines illustrate standard errors of the means. * Significant difference with subjects given water.
Experiment 3b: Determination of BEC as a function of ethanol and adrenalectomy
The ANOVA utilized to analyze BECs revealed a main effect of post-administration interval [F(1, 41) = 130.24, p < 0.0001], indicating that BECs were higher at 37.5 min than at 7.5 minutes after ethanol administration. This analysis also revealed a significant interaction between adrenalectomy treatment and post-administration interval, [F(2, 41) = 3.71, p < 0.05]. Post-hoc analyses indicated that BECs induced by 2.5 g/kg were higher in adrenalectomized subjects than in the sham or untreated controls, but only in the second post-administration interval (see Table 1).
Contrary to our expectation, ethanol induced locomotor stimulation regardless of the adrenalectomy treatment. One possibility to explain this result is that the mechanism by which stress potentiates ethanol-induced activation is independent of corticosterone. An alternative component of the stress-response that might modulate the activating effects of ethanol in isolated subjects is the CRF. This hormone modulates the locomotor effects of ethanol in adult mice. For example, ethanol-mediated locomotor sensitization is attenuated in adult rats by blocking the CRF1 receptor (Fee, Sparta, Picker, & Thiele, 2007). Pastor and colleagues also reported that mice lacking CRF1 receptors do not show psychomotor sensitization mediated by EtOH (Pastor et al., 2008). Based on these antecedents we hypothesize that by blocking the CRF1 receptor in maternally isolated rat pups, ethanol-mediated stimulation will be attenuated.
Experiment 4
Experiment 4a: Locomotor activity as a function of ethanol and CP154,526
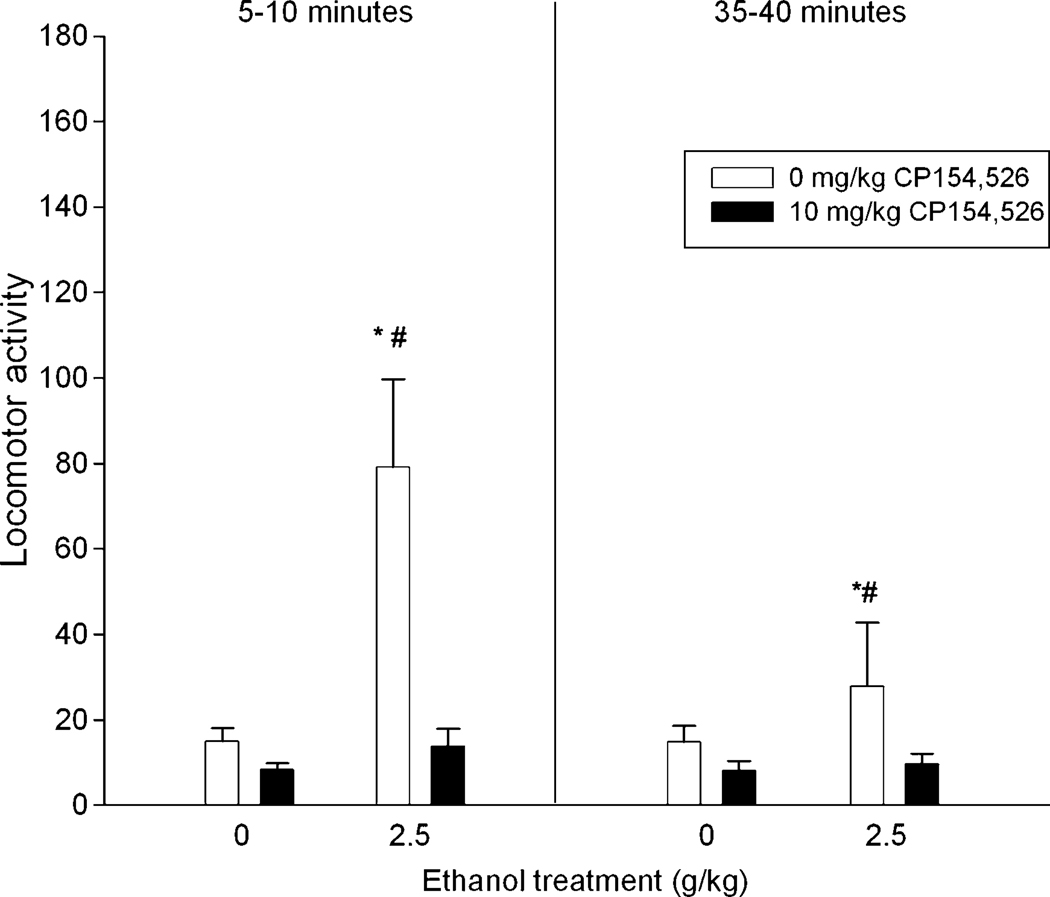
As can be observed in Figure 4, ethanol-induced locomotion was drastically attenuated by the CRF1 antagonist. The ANOVA revealed significant main effects of ethanol [F(1, 43) = 12.63, p < 0.001] and CP154,526 [F(1, 43) = 16.69, p < 0.001]. The interaction between ethanol treatment and CP154,526 was also significant [F(1, 43) = 8.75, p < 0.005]. Post-hoc analyses revealed that pups given ethanol after only saline injection showed more locomotor activity than their corresponding water controls, but this effect was not observed in subjects given ethanol after the CRF1 antagonist, CP154,526, although CP154,526 did not affect locomotion in water-treated controls.
Figure 4.
Locomotor activity (operationalized through the number of quadrants crossed) in preweanling rats as a function of ethanol (0 or 2.5 g/kg), CP154,526 (0 or 10 mg/kg) and testing interval (5–10 and 35–40 minutes). Vertical lines illustrate standard errors of the means. * Significant difference with the corresponding water control. # Significant difference with group given ethanol and 10 mg/kg CP154,526.
Experiment 4b: Determination of BECs as a function of ethanol and CP154,526
The ANOVA utilized to analyze BECs revealed a main effect of post-administration interval [F(1, 28) = 108,41, p < 0.001], indicating that BECs were higher at 37.5 min than at 7.5 minutes after ethanol administration. The CRF1 antagonist, CP154,526, did not affect BECs (see Table 1).
Discussion
The main results obtained in the present study can be summarized as follows: First, ethanol-mediated locomotor stimulation requires maternal isolation (Experiment 1). Second, exogenous corticosterone can potentiate the locomotor effects of ethanol in non-isolated rat pups (Experiment 2), but on the other hand, ethanol-induced locomotor stimulation in isolated pups is not affected when corticosterone production is virtually eliminated by adrenalectomy (Experiment 3). Finally, locomotor activity induced by ethanol is eliminated in isolated pups by blocking the CRF1 receptor (Experiment 4).
In Experiment 1 only pups given ethanol and separated from the mother (isolated or paired with a littermate) showed hyperlocomotion after ethanol administration. Since pups that remained in the homecage could be fed by the mother, absorption of ethanol could be different in such pups than in those separated from the homecage. However, two aspects of our data ruled out the possibility that the differences in ethanol-mediated activation as a function of the social condition could be associated with differences in ethanol pharmacokinetics. First, BECs did not differ as a function of social condition at the time points at which pups were evaluated. The absence of these differences were clearer at the first post-administration time-point (7.5 min), when the strongest difference was observed in terms of ethanol-mediated stimulation as a function of the social condition. Second, isolated pups also showed more locomotion after ethanol administration than those that had remained in pairs, but BECs did not statistically differ between these conditions.
Maternal separation is a well-known procedure for inducing stress in preweanling rats. Separation from the mother induces HPA activation with accumulation of corticosterone and CRF hormones. We hypothesize that locomotor activation induced by ethanol in preweanling rats is the result of a synergism between ethanol and stress. Ethanol by itself is not sufficient to induce activation (see subjects from the homecage condition in Experiment 1 or 2). In Experiment 1 control pups given only the vehicle but differing in the stressfulness of their social condition did not differ in terms of locomotor activity, indicating that in these circumstances the present stressor by itself does not modify activity at this age. Yet the combination of ethanol and stress induced a marked increase in locomotion (Experiment 1, 3 and 4). In adult rats stress induces dopamine release in many brain areas, such as prefrontal cortex (Abercrombie, Keefe, DiFrischia, & Zigmond, 1989), nucleus accumbens (Kalivas & Duffy, 1989) or amygdala (Inglis & Moghaddam, 1999). We recently reported that ethanol-mediated locomotor stimulation in preweanling rats is mediated by the dopaminergic system (Arias, Mlewski, Hansen et al., 2009). So, it is possible that the synergism between stress and ethanol is associated with the effect of the stress response upon the dopaminergic system.
In Experiment 2 a synergism was also observed between corticosterone and ethanol. Although pups (non-isolated) given ethanol or corticosterone separately did not differ in their locomotor activity, the combination of both treatments induced a clear hyperactivity. The present study has a clear limitation in that corticosterone was administered peripherally and we didn’t explore the specific neural substrate in which corticosterone (or another correlate of stress) and ethanol interacted to produce the increase in locomotion. However, some hypotheses raised in the following discussion might guide future studies.
Glucocorticoid receptors are present and functional in the brain in preweanling rats (Moriceau et al., 2004). Corticosterone at the doses employed (3 or 6 mg/kg) has been shown to have central effects that include activation of, for example, basolateral amygdala (Moriceau et al., 2004). Additionally, previous studies from our laboratory showed that ethanol-mediated stimulation in preweanling rats depends on the activity of dopamine receptors (Arias, Mlewski, Hansen et al., 2009). In adult rats glucocorticoid receptors are present in many brain areas, including the dopaminergic system (de Jong & de Kloet, 2004; Tye, Miller, & Blaha, 2009). Exogenous corticosterone administration in adult rats has enhanced locomotion and dopamine released by psychostimulants (Cador, Dulluc, & Mormede, 1993; Piazza et al., 1996; Roberts et al., 1995), whereas adrenalectomy in some cases reduces stimulation induced by these drugs (Marinelli, Rouge-Pont, De Jesus-Oliveira, Le Moal, & Piazza, 1997). Hence, glucocorticoid receptors in the dopaminergic system are a possible target to explain the interaction between ethanol and corticosterone in our study.
Since maternal separation induces corticosterone release (Levine, 2001, 2002,2005), and corticosterone and ethanol together induce locomotor stimulation (Experiment 2a), we expected that removing corticosterone would reduce the stimulating effect of ethanol observed in isolated pups (Experiment 1). Contrary to our expectation adrenalectomized pups still showed ethanol-mediated hyperlocomotion that did not differ from sham or untreated controls. This unexpected result led us to the hypothesis that the interaction between stress (induced by maternal separation) and ethanol may be associated with another component of the stress response rather than corticosterone. A candidate for this interaction was CRF acting upon the CRF1 receptor. Brain CRF receptors are functional early in postnatal life (Insel, Battaglia, Fairbanks, & De Souza, 1988). When preweanling rats are isolated, CRF levels increase (Hennessy, Collier, Griffin & Schwaiger, 1988; Harvey & Hennessy, 1995; Insel & Harbaugh, 1989). The CRF1 receptor is important for the development and expression of locomotor sensitization induced by ethanol in adults (Fee et al., 2007; Pastor et al., 2008). Intracerebroventricular administration of CRF sensitizes the locomotor response to amphetamine via its action at central CRF receptors (Cador et al., 1993). The CRF also interacts with the dopaminergic system. Tagliaferro and Morales (2008) have shown that CRF1 receptors are expressed in axons and terminals of the ventral tegmental area (VTA). These authors raised the possibility that stress induces activation of CRF-glutamatergic neurons innervating the VTA, leading to CRF and/or glutamate release that could activate dopaminergic neurons in the VTA. This hypothesis is supported by the facts that acute mild stressors cause CRF release as well as dopamine release in the ventral tegmental area and that CRF induces activation of dopamine neurons in vitro (see Tagliaferro & Morales, 2008). In our study the CRF1 antagonist CP154,526 eliminated the synergism between ethanol and stress. This result is consistent with the role of CRF in mediating stress responses in preweanling rats. Endogenous CRF increases during stress (Chappell et al., 1986), while intracerebroventricular administration of alpha-helical CRF9_41 (a CRF antagonist) reverses endocrine, physiological, and behavioral changes induced by stressors such as restraint and novelty (Dunn & Berridge, 1990a, 1990b).
In a previous study we reported that novelty is necessary for ethanol to induce locomotor stimulation in preweanling rats (Arias, Mlewski, Miller et al., 2009). In the present study we observed that ethanol treated pups evaluated in a novel environment don’t show stimulation unless isolated before testing. These data suggest that novelty is necessary, but not sufficient for ethanol to induce locomotor stimulation. In fact, it seems to be necessary certain degree of stress (maternal separation). In our prior studies showing stimulation induced by ethanol in preweanling rats, subjects were separated from the homecage for at least one hour before testing (Arias, Mlewski, Hansen et al., 2009; Arias, Mlewski, Miller et al., 2009; Arias, Mlewski, Molina et al., 2009a, 2009b).
Our preceding studies also indicate that infants with higher baseline activity are more sensitive to ethanol-mediated stimulation and more resistant to ethanol’s aversive effects (Arias, Molina, & Spear, 2009). On the basis of results obtained in the present study, differential response of the HPA axis to stress and ethanol may explain differences in sensitivity to ethanol-mediated stimulation. When preweanling rats are in an inescapable novel environment, they show increased locomotor activity that seems to reflect distress or fear rather than exploration (Campbell & Raskin, 1978). Differential sensitivity to stress may be associated in this case with differential response to a novel environment and to ethanol. In adult rats, high and low responders to novelty have been shown to differ in HPA sensitivity (Dellu, Mayo et al., 1996; Dellu, Piazza, Mayo, Le Moal, & Simon, 1996; Piazza, Deminiere, Le Moal, & Simon, 1989).
As mentioned, recent results from our laboratory suggest a possible mechanistic association between ethanol-induced stimulation and positive reinforcement. Ethanol-mediated locomotor stimulation and conditioned place preference is blocked in preweanling rats by mu-opioid antagonists (Arias et al., 2010; Nizhnikov et al., 2009). In addition, the dopamirgic and GABA B systems, which mediate ethanol-induced conditioned place preference in adult rodents (Matsuzawa et al., 1999; Bechtholt & Cunningham, 2005), also participate in ethanol-mediated locomotor stimulation in preweanling rats (Arias, Mlewski, Hansen et al., 2009). Beyond this mechanistic association, another important question is whether a positive affective state accompanies the activating effect of ethanol. In the present study ethanol-mediated activity was increased by the stress of maternal separation, which has been established as an aversive treatment (see, for example, Smith, Kucharski & Spear, 1984). Additionally, corticosterone, which also enhanced the stimulating effect of ethanol, mediates acquisition of fear conditioning in preweanling rats (Moriceau et al., 2004). These results make difficult to assign a positive motivational state to the stimulating effect of ethanol. However, at least in adult rodents, stress can also potentiate ethanol-mediated conditioned place preference (Song, Wang, Zhao, Wang, Zhai, & Lu, 2007; Matsuzawa & Suzuki, 2002). An alternative interpretation is to consider stimulation of locomotion induced by drugs, through dopamine mediation, as a behavior related to the approach required in any instrumental learning either positively or negatively reinforced. This interpretation is closer to recent theoretical approaches in which activity of the dopaminergic system is viewed as mediating motivation or learning processes generally, rather than just the pleasurable effects of drugs of abuse (see, for example, Berridge et al., 2009; Kelley et al., 2005; Robbins and Everitt, 2007; Salamone et al., 2009). But this interpretation does not exclude consideration of the analysis of mechanisms underlying ethanol-mediated stimulation as a valuable vehicle for furthering understanding of ethanol-mediated affective or motivated states.
Summarizing, in the present study we have shown a synergism between stress or corticosterone and ethanol in preweanling rats. The interaction between stress (induced by social isolation) and ethanol seems to be mediated by CRF, since blockade of CRF1 receptors cancelled the effect of ethanol in isolated pups. This study highlights the importance of considering stress as a possible intervening variable in studies evaluating ethanol effects in developing animals when maternal separation is used in the experimental procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Ader R, Friedman SB. Plasma corticosterone response to environmental stimulation: effects of duration of stimulation and the 24-hour adrenocortical rhythm. Neuroendocrinology. 1968;3(6):378–386. doi: 10.1159/000121726. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006a;120(3):710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006b;40(1):51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Hansen C, Molina JC, Paglini MG, Spear NE. Dopamine receptors modulate ethanol's locomotor-activating effects in preweanling rats. Dev Psychobiol. 2009;52(1):13–23. doi: 10.1002/dev.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Miller S, Molina JC, Spear NE. Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav. 2009;92(3):448–456. doi: 10.1016/j.pbb.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009a;43(1):13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009b;123(1):172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Ethanol-mediated aversive learning as a function of locomotor activity in a novel environment in infant Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92(4):621–628. doi: 10.1016/j.pbb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Differential role of micro, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav. 2010;99(3):348–354. doi: 10.1016/j.physbeh.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119(1):213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19(7):1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993;56(4):981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Raskin LA. Ontogeny of behavioral arousal: the role of environmental stimuli. J Comp Physiol Psychol. 1978;92(1):176–184. doi: 10.1037/h0077423. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6(10):2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behavioural Pharmachology. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- de Jong IE, de Kloet ER. Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci. 2004;1018:192–198. doi: 10.1196/annals.1296.022. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, Simon H. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly--a life-span study in rats. Psychoneuroendocrinology. 1996;21(5):441–453. doi: 10.1016/0306-4530(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann N Y Acad Sci. 1990a;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990b;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, Suchecki D. Disruptions of the mother-infant relationship and stress-related behaviours: Altered corticosterone secretion does not explain everything. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Picker MJ, Thiele TE. Corticotropin releasing factor-1 receptor antagonist, CP-154,526, blocks the expression of ethanol-induced behavioral sensitization in DBA/2J mice. Neuroscience. 2007;150(1):14–21. doi: 10.1016/j.neuroscience.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SB, Ader R, Grota LJ, Larson T. Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosom Med. 1967;29(4):323–328. doi: 10.1097/00006842-196707000-00003. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Hennessy MB. Corticotropin-releasing factor modulation of the ultrasonic vocalization rate of isolated rat pups. Brain Res Dev Brain Res. 1995;87(2):125–134. doi: 10.1016/0165-3806(95)00064-k. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Collier AC, Griffin AC, Schwaiger S. Plasma corticosterone fluctuations in an infant-learning paradigm. Behav Neurosci. 1988;102(5):701–705. doi: 10.1037//0735-7044.102.5.701. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72(3):1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8(11):4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Central administration of corticotropin releasing factor alters rat pup isolation calls. Pharmacol Biochem Behav. 1989;32(1):197–201. doi: 10.1016/0091-3057(89)90233-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25(7):913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: the role of maternal behavior. Neurotox Res. 2002;4(5–6):557–564. doi: 10.1080/10298420290030569. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16(3):387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rouge-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV. Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology. 1997;16(2):156–161. doi: 10.1016/S0893-133X(96)00169-8. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of dopamine D(1) and D(2) receptors in the ethanol-associated place preference in rats exposed to conditioned fear stress. Brain Res. 1999;835(2):298–305. doi: 10.1016/s0006-8993(99)01606-6. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T. Psychological stress and rewarding effect of alcohol. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37(3):143–152. [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Washington, DC: Government Printing Office; Guide for the care and use of laboratory animals (DHEW Publication No. 86-23) 1986
- Nizhnikov ME, Pautassi RM, Molina JC, Spear NE. Conditioned preferences and aversions in infant rats mediated through ethanol inhalation. Alcohol. 2009;43(1):1–12. doi: 10.1016/j.alcohol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A. 2008;105(26):9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30(3):448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93(16):8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275(2):790–797. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25(9):1301–1308. [PubMed] [Google Scholar]
- Smith GJ, Kucharski D, Spear NE. Conditioning of an odor aversion in preweanlings with isolation from home nest as the unconditioned stimulus. Dev Psychobiol. 1984;18(5):421–434. doi: 10.1002/dev.420180507. [DOI] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31(12):2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102(5):692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57(2):204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506(4):616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye SJ, Miller AD, Blaha CD. Differential corticosteroid receptor regulation of mesoaccumbens dopamine efflux during the peak and nadir of the circadian rhythm: a molecular equilibrium in the midbrain? Synapse. 2009;63(11):982–990. doi: 10.1002/syn.20682. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]