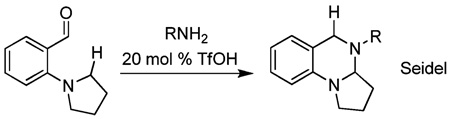
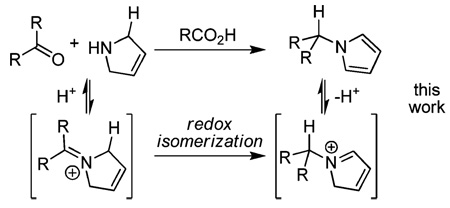
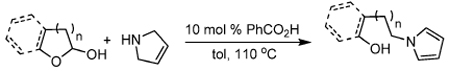
Redox isomerization reactions are of particular interest because they exhibit perfect atom economy, and they often utilize the inherent reducing power of hydrogen that is embedded in molecules to effect reduction of other functional groups.1–3 In doing so, redox isomerizations are able to circumvent the requirement for exogenous reducing agents, which tend to be high energy reagents. The power of redox isomerizations is arguably increased when it is used in conjunction with C–X bond-forming reactions. The Tishchenko reaction is a classic example of such a coupling reaction that has been proposed to proceed via an intermediate redox isomerization.2 More recently, Seidel has demonstrated several intriguing reactions where an intramolecular redox reaction is used to effect a reductive amination in concert with a second C–N bond forming reaction (eq 1).3 Herein, we report a related, acid-catalyzed intermolecular redox amination that takes advantage of the inherent reducing power of 3-pyrroline (eq 2). Ultimately, redox isomerization can be used to form N-alkyl pyrroles via reductive amination, a reaction that cannot typically occur since pyrrole is a weak N-nucleophile. Moreover, the mild conditions, atom-economy, and operational simplicity of the redox amination reported herein make redox amination a viable alternative to more standard syntheses of N-alkyl pyyroles.4
 |
(1) |
 |
(2) |
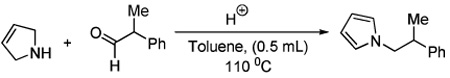
Initially, we were interested in performing an intramolecular variant of a rearrangement reaction described by Murahashi.5 However, rather than the intended product, the alkyl pyrrole 1a was isolated in 25% yield (eq 3). Since such a reductive amination to form aromatic amines is a potentially powerful synthetic method, we chose to investigate the reaction further. Literature searches reveal that Cook discovered an analogous thermal condensation of 3-pyrroline with cyclohexanone at 140 °C in xylene to produce N-cyclohexylpyrrole in 47% yield.6,7 Unfortunately, other ketones provided even poorer yields. Since our reaction appeared to take place under milder conditions, we set our sights on developing a catalytic reaction that would have broad utility.
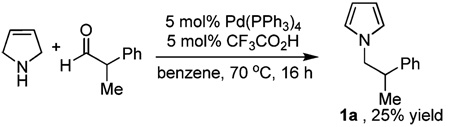
To begin, reactions were performed to determine which species in the original reaction mixture was responsible for catalyzing this transformation. As can be seen from Table 1, CF3CO2H effected the reaction alone (entry 3), and Pd(PPh3)4 was somewhat detrimental to the reaction (entry 2). Moreover, performing the reaction under air was problematic due to background oxidation of 3-pyrroline to pyrrole (entry 4). Having established that the reaction was catalyzed by Brønsted acids, we turned our attention to the use of milder acids. Indeed, acetic acid and benzoic acid are both competent catalysts for the transformation and can be used interchangeably. While reactions that were run with 1:1 pyrroline/aldehyde did not completely consume the aldehyde within the allotted reaction time, the yields of the reactions were still good (entries 5, 8, and 11). Ultimately, the use of 1.5 equiv of pyrroline allowed full conversion of starting material within a shorter time and provided a slightly higher product yield (entry 10 vs 11).8
Table 1.
Bronsted Acid Catalyzed Redox Amination
 | |||
|---|---|---|---|
| entry | conditions | time (h) | yield (%)a |
| 1 | no catalyst | 14 | <5 |
| 2 | Pd(PPh3)4 (5 mol %)TFA (10 mol %) | 16 | 25 |
| 3 | TFA (50 mol %), N2 | 14 | 83 |
| 4 | TFA (50 mol %), air | 24 | <5 |
| 5 | TFA (10 mol %), 0.2 mL tol | 24 | 85b |
| 6 | CH3CO2H (50 mol %) | 5 | 25 |
| 7 | CH3CO2H (50 mol %) | 24 | 85 |
| 8 | CH3CO2H (10 mol %), 0.2 mL tol | 12 | 85b |
| 9 | PhCO2H (10 mol %) | 12 | 86 |
| 10 | PhCO2H (10 mol %), 0.2 mL tol | 12 | 88 |
| 11 | PhCO2H (10 mol %), 0.2 mL tol | 24 | 80b |
| 12 | Amberlyst-15 (10 mol %) | 24 | <5 |
| 13 | p-TsOH | 24 | 50 |
Isolated yields of reaction between 3-pyrroline (0.75 mmol) and aldehyde (0.5 mmol) in 0.5 mL of toluene unless otherwise stated.
0.5 mmol pyrroline and aldehyde.
 |
(3) |
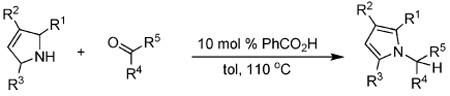
Next, the scope of the benzoic acid catalyzed reaction was investigated. It was gratifying to find that both aliphatic and aromatic aldehydes were good partners for the redox amination (entries 1–5, Table 2). Moreover, an α,β-unsaturated aldehyde formed the allylic pyrrole, albeit in moderate yield (entry 5). In addition to aldehydes, aliphatic and aromatic ketones are excellent substrates (entries 6–16). However, the ketone substrates usually required longer reaction times than the corresponding aldehydes. It is noteworthy that the mild acidic conditions are compatible with a variety of functional groups including alkene, nitrile, nitro, ether, CF3, and acetal groups. Lastly, the redox amination avoids stoichiometric strong bases that are typically associated with the N-alkylation of pyrroles.4 Thus, we can access products that would be highly prone to elimination if alkylation of a basic pyrrole anion with a bromoalkane were attempted (entries 6–16). Finally, while we have initially focused on the scope of aldehydes and ketones that undergo redox amination with 3-pyrroline, the concept applies to other pyrrolines as well. For example, 3-phenyl-3-pyrroline readily participates in the reaction, providing the substituted pyrrole 1q in high yield (entry 17). Dehydroproline methyl ester and 2,5-dimethyl-3-pyrroline provide high yields in the reaction with aldehydes as well (entries 18–20); however the sterically bulky 2,5-dimethylpyrroline failed to react with ketone substrates such as acetophenone and 4-phenyl-2-butanone.
Table 2.
Reaction Scopea
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | product | time | yield | entry | product | time | yield |
| 1 | 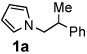 |
12 h | 88% | 11 | 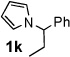 |
12 h | 86% |
| 2 | 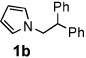 |
15 h | 83% | 12 | 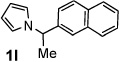 |
6 h | 82% |
| 3 | 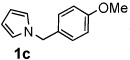 |
5 h | 85% | 13 | 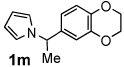 |
12 h | 70% |
| 4 | 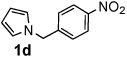 |
4 h | 94% | 14 | 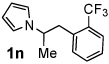 |
24 h | 71% |
| 5 | 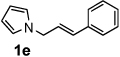 |
6 h | 50% | 15 | 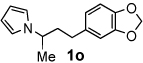 |
24 h | 83% |
| 6 |  |
12 h | 65% | 16 | 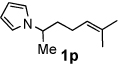 |
24 h | 60% |
| 7 | 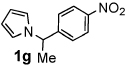 |
8 h | 90% | 17 | 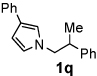 |
12 h | 92% |
| 8 | 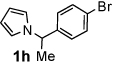 |
12 h | 65% | 18 | 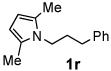 |
16 h | 80% |
| 9 | 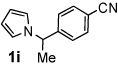 |
12 h | 93% | 19 | 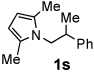 |
16 h | 86% |
| 10 | 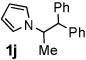 |
24 h | 80% | 20 | 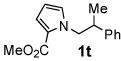 |
12 h | 92% |
Reactions between 0.5 mmol of aldehyde or ketone and 0.75 mmol of 3-pyrroline in 0.2 mL toluene with 0.05 mmol of PhCO2H at 110 °C.
Having demonstrated that the reaction has significant scope, it was posited that such a reaction may allow us to engage lactols to form ring-opened pyrrole conjugates (Table 3). Interestingly, treatment of five- and six-membered lactols under our standard reaction conditions produced the δ-hydroxy pyrroles in good yield (entries 1–3). Next, we turned our attention to the redox amination of chromanols (entries 4–6). It was particularly gratifying to observe good yields of the pyrrolyl phenols since our previous efforts to generate compounds of this chemotype by more standard acyl substitution and reduction chemistry failed.9
Table 3.
Redox Amination of Lactols and Chromanolsa
 | ||||
|---|---|---|---|---|
| entry | substrate | time (h) | product | yield (%)a |
| 1 | 24 | 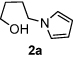 |
80 | |
| 2 | 24 | 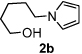 |
90 | |
| 3 | 12 | 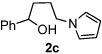 |
75 | |
| 4 | 24 | 85 | ||
| 5 | 24 | 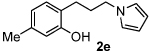 |
73 | |
| 6 | 24 | 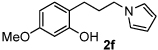 |
80 | |
All reactions were run with 0.5 mmol of lactol, 0.75 mmol of 3-pyrroline, and 0.05 mmol of PhCO2H in 0.2 mL of toluene at 110 °C.
In conclusion, a wide variety of aldehydes, ketones, and lactols undergo redox amination when allowed to react with 3-pyrrolines in the presence of a mild Brønsted acid catalyst. This reaction utilizes the inherent reducing power of 3-pyrroline to perform the equivalent of a reductive amination to form alkyl pyrroles. In doing so, the reaction avoids stoichiometric reducing agents that are typically associated with reductive aminations. Moreover, the redox amination protocol allows access to alkyl pyrroles that are not available via standard reductive amination.
Supplementary Material
Acknowledgment
We the National Institute of General Medical Sciences (1R01GM079644) and the KU Chemical Methodologies and Library Development Center of Excellence, (P50 GM069663). M.P. thanks the NSF-REU program (CHE-0649246) for support.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.l2;&-3q
References
- 1.Recent reactions that proceed via redox isomerization: Verboom W, Reinhoudt DN, Visser R, Harkema S. J. Org. Chem. 1984;49:269–276. Mátyus P, Éliás O, Tapolcsányi P, Polonka-Bálint A, Halász-Dajka B. Synthesis. 2006;16:2625–2639. McQuaid KM, Long JZ, Sames D. Org. Lett. 2009;11:2972–2975. doi: 10.1021/ol900915p. McQuaid KM, Sames D. J. Am. Chem. Soc. 2009;131:402–403. doi: 10.1021/ja806068h. Trost BM, Livingston RC. J. Am. Chem. Soc. 2008;130:11970–11978. doi: 10.1021/ja804105m. Han SB, Kim IS, Han H, Krische MJ. J. Am. Chem. Soc. 2009;131:6916–6917. doi: 10.1021/ja902437k..
- 2.(a) Mahrwald R. Curr. Org. Chem. 2003;7:1713–1723. [Google Scholar]; (b) Seki T, Nakajo T, Onaka M. Chem. Lett. 2008;35:824–829. [Google Scholar]
- 3.(a) Zhang C, Murarka S, Seidel D. J. Org. Chem. 2009;74:419–422. doi: 10.1021/jo802325x. [DOI] [PubMed] [Google Scholar]; (b) Zhang C, De CK, Mal R, Seidel D. J. Am. Chem. Soc. 2008;130:416–417. doi: 10.1021/ja077473r. [DOI] [PubMed] [Google Scholar]; (c) Murarka S, Zhang C, Konieczynska MD, Seidel D. Org. Lett. 2009;11:129–132. doi: 10.1021/ol802519r. [DOI] [PubMed] [Google Scholar]
- 4.(a) Schmuck C, Rupprecht D. Synthesis. 2007:3095–3110. [Google Scholar]; (b) Ferreira VF, De Souza MCBV, Cunha AC, Leticia OR, Ferreira ML. Org. Prep. Proc. Int. 2001;33:411–454. [Google Scholar]; (c) Lea Z-G, Chen Z-C, Hu Y, Zheng Q-G. Synthesis. 2004:1951–1954. [Google Scholar]; (d) Zeng DX, Chen Y. Synlett. 2006:490–492. [Google Scholar]; (e) Rao HSP, Jothilingam S, Scheeren HW. Tetrahedron. 2004;60:1625–1630. [Google Scholar]; (f) Okada E, Masuda R, Hojo M, Reiko Y. Heterocycles. 1992;34 1435–1431. [Google Scholar]; (g) Evans DA, Borg G, Scheidt KA. Angew. Chem., Int. Ed. 2002;41:3188–3191. doi: 10.1002/1521-3773(20020902)41:17<3188::AID-ANIE3188>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Murahashi S-I, Makabe Y, Kunita K. J. Org. Chem. 1988;53:4489–4495. [Google Scholar]
- 6.Cook AG, Switek KA, Cutler KA, Witt AM. Lett. Org. Chem. 2004;1:1–5. [Google Scholar]
- 7.A similar reaction has been proposed for the condensation of 3-pyrroline with a p-quinone. Lee Y, Ling K-Q, Lu X, Silverman RB, Shepard EM, Dooley DM, Sayre LM. J. Am. Chem. Soc. 2002;124:12135–12143. doi: 10.1021/ja0205434..
- 8.3-Pyrroline is commercially available but is expensive (97% $129/g; 65% $52/g) and readily oxidized by air.
- 9.Li K, Tunge JA. J. Org. Chem. 2008;73:8651–8653. doi: 10.1021/jo801627z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


