Abstract
Developed in 1992, the flow-mediated dilation test is now the most commonly utilized non-invasive assessment of vascular endothelial function in humans. Since its inception, scientists have refined their understanding of the physiology, analysis, and interpretation of this measurement. Recently, a significant growth of knowledge has added to our understanding and implementation of this clinically relevant research methodology. Therefore, this tutorial provides timely insight into recent advances and practical information related to the ultrasonic assessment of vascular endothelial function in humans.
Keywords: FMD, endothelial function, reactive hyperemia, shear stress
INTRODUCTION
This tutorial provides an easy to follow reference for researchers interested in performing flow-mediated dilation (FMD) testing for the first time, but also, due to the numerous recent advancements in this field, an important and timely updated summary of current practices for established researchers in the area. As vascular endothelial dysfunction represents an initial step towards hypertension and cardiovascular disease, the accurate assessment of vascular endothelial function is an essential tool which will assist in our understanding of the etiology of these vascular related diseases and determine the efficacy of therapeutic treatments that target vascular health. Beyond a short introduction of the history and physiology behind the ultrasonic assessment of FMD, there follows comprehensive, evidence-based, technical, and interpretive strategies for performing the ultrasonic assessment of endothelial function in humans.
Historical Perspective
In 1992, Celermajer and colleagues1 developed the FMD technique as a non-invasive method to measure vascular endothelial function. Since this time, the ultrasonic assessment of FMD in response to occlusion-induced hyperemia has been established as a reliable, non-invasive measurement of endothelial function 2 and has been documented to correlate with invasively assessed endothelial function in the coronary arteries 3. In an effort to standardize this measurement among investigators, in 2002 Corretti and colleagues 4 published the initial guidelines for the ultrasonic assessment of FMD of the brachial artery, which to date, have been referenced over 1,000 times. Since then, an ongoing effort has been made to adapt the original methodology, introduced by Celermajer et al. 5, to a more robust assessment of a true nitric oxide (NO)-dependent measurement of vascular endothelial function. In 2005, a meta-analysis was conducted on 250 studies that utilized the measurement of FMD and revealed that technical aspects of the measurement (i.e. occlusion location and duration) may explain the differences in FMD observed among studies 6. At this time, Deanfield and colleagues published their recommendations for global endothelial function testing with a specific section highlighting the non-invasive FMD technique 7. Most recently, Pyke and Tschakovsky 8 provided an update to the guidelines presented by Corretti et al. 4 that specifically targeted the issue of the shear stress stimulus and have provided important recommendations which are now common practice for FMD testing. Despite these considerable advancements in the understanding and application of the FMD technique, this comprehensive tutorial offers up-to-date technical instructions for the performance and interpretation of FMD.
The Endothelium
The endothelium plays multiple pathologic and physiologic roles including the regulation of smooth muscle tone, control of thrombosis, inhibition of leukocyte and platelet cell adhesion, and promotion of intra-arterial permeability 9–11. In addition, there are numerous vasoactive substances released from the endothelium, including prostacyclins, endothelins, endothelial cell growth factors, interleukins, plasminogen inhibitors and nitric oxide (NO). The latter is, perhaps, the major mediator of vasodilation 12, and has thus been intensely studied since its discovery in 1980 13. After almost 30 years of NO-related research, reduced NO bioavailability has become synonymous with the condition broadly described as “endothelial dysfunction” 14. In addition to being proposed as the primary etiology of atherosclerosis 15, endothelial dysfunction is the earliest identifiable event in the process of atherosclerotic cardiovascular disease, the leading cause of morbidity and mortality in the United States 16. It follows that the assessment of endothelial function has become an area of considerable interest to the medical and research communities.
Flow-Mediated Dilation (FMD)
When measured appropriately, the assessment of endothelial function via FMD has been proposed to represent a functional bioassay for endothelium-derived NO bioavailability in humans 14. During a FMD test, vasodilation occurs following an acute increase in blood flow, typically induced via circulatory arrest in the arm (supra-systolic cuff occlusion) for a period of time. Specifically, this hyperemia increases laminar shear forces parallel to the long axis of the vessel 17 which is transduced via luminal mechanoreceptors to the endothelial cell. This event increases G-protein expression of phosphokinase A, signaling an increase of endothelial nitric oxide synthase (eNOS) activity which catalyzes the conversion of L-arginine to NO 18. NO then diffuses into the tunica media where it activates soluble guanylate cyclase which converts guanosine triphospate into guanosine monophospate to induce relaxation of the smooth muscle and subsequent vasodilation. In its traditional form, the increase in arterial diameter, as a consequence of the reactive hyperemia, is compared to the baseline diameter and expressed simply as a percentage of this baseline diameter (% FMD). Despite this intuitive and “attractive” link between FMD testing and NO bioavailability, it should be noted that 1) vessel type and size may influence the relative contribution of NO19, and 2) there is still some debate about this in the literature with data both for14, 20, 21 and against the concept that vasodilation mediated by the endothelium is predominantly a consequence of NO22, 23.
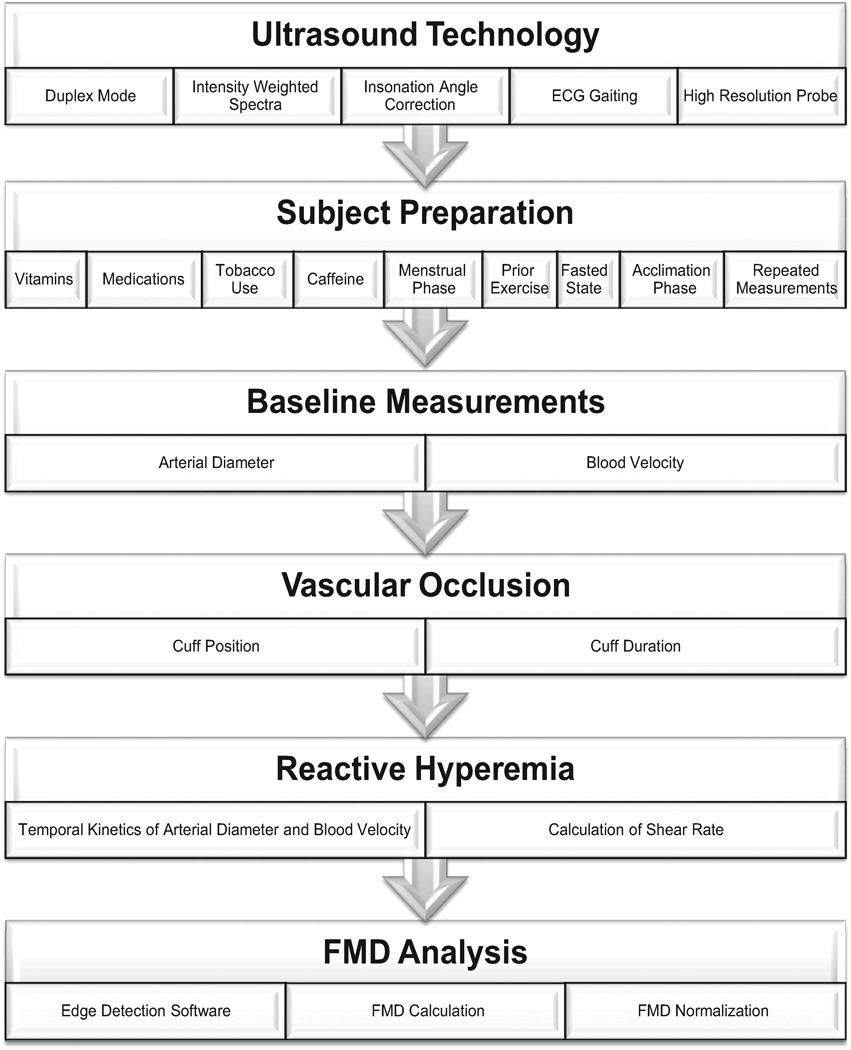
MEASUREMENT OF FMD: THE ESSENTIAL ELEMENTS (Figure 1)
Figure 1.
Schematic of the essential elements for the ultrasound assessment of FMD.
A. Appropriate Ultrasound Technology
FMD assessed by Doppler ultrasound has emerged as the most popular clinical research method of assessing vascular endothelial function, likely due to the relatively simple methodology and non-invasive nature. However, the appropriate ultrasound equipment, in conjunction with the high level of skill required, are essential for accurate and reliable measurements as detailed below.
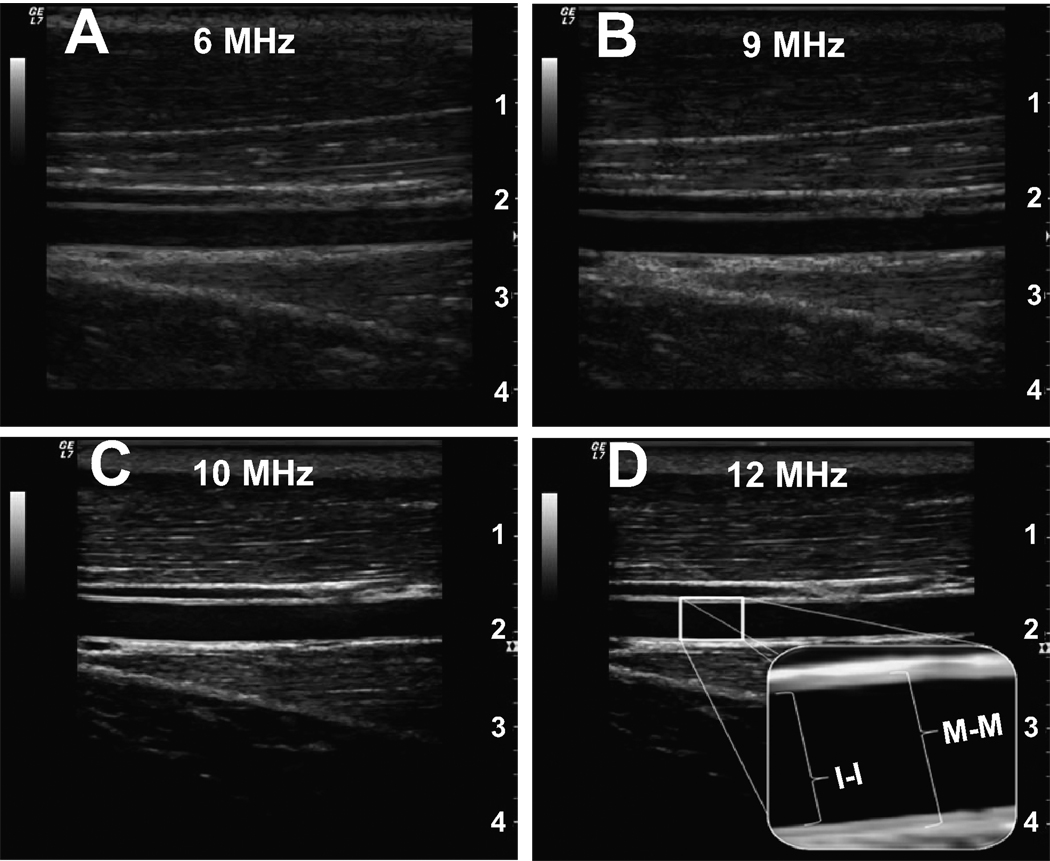
High Resolution, Multi-frequency Linear Ultrasound Doppler Probe: The accurate measurement of FMD is highly dependent on identification of a defined arterial wall, which requires a highly resolved ultrasound image. Each ultrasound probe is classified according to a frequency range in megahertz (MHz) which is inversely proportional to the depth of optimal imaging. Figure 2 demonstrates the quality of B-mode images utilizing different frequency probes in the brachial artery at a depth of approximately 2 cm. It is clear that for superficial vessels, a 10–14 MHz linear array probe, currently considered “high resolution”, is optimum. However, as previously recommended 4, it is also apparent that a much lower frequency probe will allow the detection of changes in diameter in all but the most superficial of vessels.
Duplex Mode: Duplex mode allows the simultaneous acquisition of B-mode and Doppler for determination of vessel diameter and blood velocity, respectively. These collective measurements enable the calculation of shear rate (see below), for any given time period from which the integral of shear across time (i.e. shear rate area under the curve, AUC) may be calculated. This is a key advantage of Duplex scanning, as it is the cumulative exposure to shear experienced by the artery which is proposed to be the major stimulus for the FMD response 24.
-
Angle Steer and Insonation Angle Correction: With a blood vessel that runs parallel to the surface of the skin (e.g. brachial artery), the simple placement of a high frequency linear ultrasound probe has the potential to yield an excellent image because the ultrasound beam will bisect the vessel at 90 degrees. However, this is the worst case scenario for the Doppler assessment of blood velocity, as the accuracy of this measurement technique varies from minimal error at 0 degrees (parallel with the blood flow) to virtually no signal (0 velocity) when the beam bisects the artery at 90 degrees (the optimum angle for imaging). Some compromising of image quality by rocking the transducer (probe) up on one end more than the other (known as heel/toe adjustments) will make the vessel appear to run diagonally across the monitor and bring the Doppler beam to an angle of less than 90 degrees. Even with excellent technique, to actually attain the accepted insonation angle of ≤60 degrees in the brachial artery is certainly challenging, if not impossible. This issue is resolved through use of “angle steer”, achieved by asynchronous firing of the phased array of pizo-electric crystals that transmit and receive the Doppler signal. With the beam “steered” left or right approximately 20–30 degrees, an insonation angle of 60 degrees can be achieved with far less heel/toe movements of the probe and sacrifice of image quality.
As angle steer is not always available, there have been a significant number of studies that have settled for an insonation of greater than 60 degrees 25–27. However, it should be noted that a 60 degree angle of insonation, is in fact, the “best of the worst” angles that should be acceptable and although this value itself introduces some error, this error is still far less than higher degrees of insonation 28–30. The significant impact of the angle of insonation determination of blood velocity and the subsequent calculation of blood flow may be seen in the Doppler shift equation:
where fD is the Doppler shift of the reflected ultrasound, fo is the transmitted frequency, v is the blood velocity, c is the sound velocity in tissue, and α is the insonation angle between the ultrasound beam and the velocity vector. To illustrate the impact of insonation angle on the measurement of blood velocity, Table 1 displays the velocity, blood flow and subsequent error among different angles of insonation studied sequentially in 5 individuals. The velocities associated with 70° and 80° angles are significantly (p<0.05) elevated when compared to the standard 60° insonation angle; however, only the blood flow calculated from an insonation angle of 80° is significantly augmented when compared to 60°. Although an insonation angle of 60° is recommended, the greatest angle typically accepted in literature is 70° 27. Electrocardiogram (ECG) Gating: Depending upon pulse pressure and vascular stiffness, arterial diameter may vary quite considerably across a single cardiac cycle 31. In some subjects, the change in diameter may be as much as one millimeter, which, if unaccounted for, may completely confound the assessment of FMD. Most ultrasound Doppler systems have an integrated electrocardiogram facilitating the assessment of diameter according to the cardiac cycle (e.g. end-diastole). However, if this feature is not available on the ultrasound system itself, an external electrocardiogram can be used to trigger an external image capture/analysis system 32.
Intensity Weighted Velocity Calculations: The simplest method of assessing blood velocity utilizes the outer envelope of the Doppler spectra to determine mean peak velocity, while the slightly more complex approach integrates the area under this envelope to calculate mean peak velocity. However, neither of these calculations accurately reflects the complex and varying range of velocities and their relative distributions within the Doppler spectra. Therefore, to accurately assess blood velocity it is recommended that intensity weighted calculations of the time-averaged mean be performed to most accurately reflect the contribution from red cells moving at differing speeds within the vessel. Basing velocity measurements on the peak envelope of the Doppler spectra will not only overestimate the actual blood velocity, but also yields subsequent calculations of shear profiles and blood flows that are inaccurate. If consistently used throughout the investigation, the peak velocity envelope approaches do offer a surrogate for true mean velocity measures, though results may conflict with others in the literature using intensity weighted velocity calculations 33.
Figure 2.
The image quality of B-mode images using different frequency linear probes. A) 6 MHz, B) 9 MHz, C) 10 MHz, and D) 12 MHz. The magnification illustrates both the intima to intima (I-I) and the media to media (M-M) interfaces. Note: Although there is definitely a visible layer among all images above, using at least a 10 MHz probe offers a very clear identification of the endothelium.
Table 1.
Evidence of alterations in blood velocity and blood flow as a consequence of insonation angle
| Theoretical | ||||||
|---|---|---|---|---|---|---|
| Insonation Angle |
Velocity (cm/s) |
Blood Flow (ml/min) |
% Δ from 60° | FMD (%) | Shear (s−1) | FMD/Shear |
| 40° | 3.52±0.46 | 24.8±8.3 | −49% | 7.0 | 33.52 | 0.21 |
| 50° | 4.24±0.57 | 29.5±9.9 | −25% | 7.0 | 40.38 | 0.17 |
| 60° | 5.27±0.50 | 36.7±11.1 | 0% | 7.0 | 50.19 | 0.14 |
| 70° | 7.97±1.07* | 55.5±18.7 | 51% | 7.0 | 75.90 | 0.09 |
| 80° | 15.69±2.12* | 109.4±36.8* | 197% | 7.0 | 149.43 | 0.05 |
Significant (p< 0.05) from 60°. Values are mean ±SD.
This table documents the actual blood velocity and blood flow as a function of different insonation angles and the theoretical degree of potential error associated with different angles of insonation that will occur when normalizing FMD for shear.
In summary, an ultrasound system offering Duplex mode, angle steer and insonation angle correction, intensity weighted spectra measurements, ECG monitoring and a high resolution linear array probe is optimal.
B. Subject Preparation
To ensure an accurate measurement of FMD, there are several subject-specific factors worth consideration.
Vitamin Supplementation: Just as the in vivo pro and antioxidant balance plays a clear role in vascular endothelial function 34, 35, there is direct evidence of a reduction in circulating free radicals following oral antioxidant supplementation (Vitamin C, Vitamin E, and α-lipoic acid) 34. Additionally, the intra-arterial administration of ascorbic acid has been documented to augment FMD 36. Therefore, subjects should abstain from vitamin supplementation for up to 72 hours prior to FMD assessment. Although more difficult to control, it should be noted that a diet high in naturally occurring antioxidants may also influence the results of an FMD study 37.
Medications: As many medications have both direct and indirect vascular effects, if possible, subjects should refrain from taking all medications for at least four half-lives of the drug prior to FMD measurements 4. In particular, special attention should be paid to medications that target the cardiovascular system (i.e. beta blockers, nitrates, calcium channel blockers, etc.) and although cessation of these medications may not be feasible, their potential to confound the results should at the very least be recognized and documented. Based on the half-life of non steroidal anti-inflammatory agents and aspirin, it is recommended these be discontinued 1 and 3 days, respectively, prior to an FMD measurement.
Tobacco Use: Smoking is a classic modifiable risk factor of cardiovascular disease which has been documented to attenuate endothelial function 38. In addition, even the exposure to second hand smoke has been shown to attenuate FMD 39. Thus, it is recommended that subjects refrain from both smoking and smoke exposure for at least 12 hours prior to FMD measurements.
Caffeine: Although there are other pharmacologically active beverages, coffee is the most common source of caffeine. Not only does caffeine inhibit soluble guanylate cyclase, a step in the NO mediated process that results in vasodilation40, caffeinated coffee has been documented to attenuate FMD41. Accordingly, caffeine ingestion should be avoided for at least 12 hours prior to FMD testing.
Menstrual Phase: The increased endogenous production of estrogen, concurrently with progesterone, across the menstrual cycle has been documented to increase eNOS activity 42 and antioxidant capacity 43 in both human and animal models, thus potentially influencing the vasodilatory response. Consequently, when studying pre-menopausal women, measurements should be performed at the same time of the menstrual cycle. To minimize the impact to these hormonal changes or when the research focus is upon sex differences, menses (days 1–7 of the menstrual cycle) offers the lowest attainable levels of both estrogen and progesterone in women and is therefore the optimum time for FMD studies 4, 44.
Prior Exercise/ Rested State: A single bout of exercise has been documented to improve FMD in apparently healthy adults 45, overweight men 26, and post-menopausal women 46. Therefore, it is important to be cognizant of the subject’s physiological state, thus it is recommended that subjects abstain from exercise for at least 12 hours prior to an FMD measurement.
Fasted State: There is considerable evidence describing the impact of the post-prandial state on the FMD response. Indeed, the consumption of a single high fat and high carbohydrate meal has been shown to attenuate FMD in apparently healthy subjects 47, 48 and in patients with Type 2 diabetes 49, in which oxidative stress and hyperglycemia, respectively, have been implicated. In contrast, it has been documented that the ingestion of a low-fat meal (i.e. a corn flake cereal with skimmed milk) does not influence the FMD measurement 47, 48. Therefore, it is recommended that FMD assessments are performed under fasting conditions; however, if fasting is not possible, a standardized low-fat meal may be consumed prior to the FMD measurement.
Adequate Acclimatization: As the goal of the FMD measurement is to compare the peak vasodilatory response to the baseline diameter, it is important that a true baseline be accurately assessed. Therefore, prior to an FMD test, it is recommended that subjects remain in the position in which the study will be performed (i.e. supine, semi-supine, seated, etc.) for at least 20 minutes in a quiet, climate controlled room (22–24°C) to control for orthostatic changes. Additionally, a separate familiarization visit of the procedures is recommended to limit stress-induced sympathetic activity on the day of actual measurement.
Repeated Measurements: With respect to either having to repeat an FMD test or the nature of the study design (i.e. repeated measures), it has been documented that multiple FMD tests can be validly performed if at least 30 minutes separates each measurement 25. However, an important phenomenon to acknowledge is that FMD measurements exhibit diurnal variation 50, thus comparisons between and within subjects should be performed as consistently as possible with regards to the time of day.
In summary, appropriate subject preparation is essential to the successful ultrasonic assessment of FMD. Therefore, strict compliance in terms of limiting vitamin supplementation, cessation (or at least documentation) of medications, tobacco and caffeine use, phase of the menstrual cycle, prior exercise, being fasted and rested prior to the FMD measurement is essential. Finally, if repeated measurements are necessary, an adequate amount of vessel recovery time should be allotted, but also recognizing that there is diurnal variation in the FMD response.
C. Baseline Measurements
Once the investigator confirms the subject has established a resting state (i.e. several repeated and consistent measurements of blood pressure, blood velocity, and arterial diameter), baseline measurements should be performed. Additionally, blood velocities provide an indication that a true resting state has been achieved and act as the starting point for the shear rate AUC calculations. The collection of accurate baseline diameters is essential for the valid FMD and shear rate calculations.
Baseline Arterial Diameter: The determination of baseline arterial diameters plays a pivotal role in the calculation and assessment of FMD. There is evidence to support similar diameters between resting and cuff occlusion conditions; however, recent data has emerged to indicate the impact of age 51 and cuff duration 52 on cuff occlusion induced changes in arterial diameter. Additionally, there is evidence to indicate a systemic difference in vascular endothelial function which appears to be dependent on the initial size of the artery 53. In an effort to standardize the FMD methodology, it is recommended that at least 10 cardiac cycles be used in the calculation of baseline diameter. As FMD is based upon change in diameter, within reason, the actual borders (e.g. intima-media or media-adventitia, Figure 2) that are utilized to determine the baseline and subsequent diameter are not as important as the need for consistency from baseline until maximal dilation. This having been said, it should be recognized that measuring from adventitia to adventitia rather than intima to intima will yield larger values for diameter (not actually representative of the vessel lumen), reducing FMD % change (as the baseline will be larger) and shear rate documented for the vessel being studied.
-
Baseline Blood Velocity: Blood velocity at rest plays an important role in the calculation of the shear response to cuff release, especially if employing the AUC approach to assessing shear rate 54. With the growing recognition that shear stress is the predominant stimulus for the FMD response, accurate assessment of resting blood velocity is essential. Even at rest, in an unperturbed scenario, blood velocity over time can vary substantially (often due to heart rate variability), therefore, it is recommended that baseline blood velocity be averaged over at least a 10–20 second period 55. In subjects who reveal a clear respiratory arrhythmia, this may need to be extended significantly, depending on respiratory rate, to reflect a true average basal blood velocity.
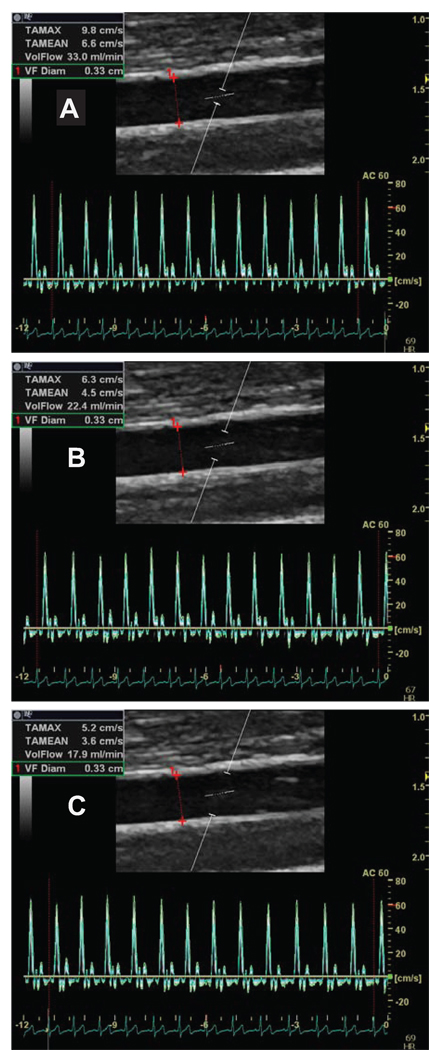
In addition to the clear need to optimize the insonation angle to accurately assess baseline blood velocities, placement and size of the sample volume (gate width) is of critical importance, especially when velocities are low. Figure 3 illustrates the impact of the Doppler sample gate size on the measurement of resting blood velocity and blood flow. In accordance with the hydraulic properties of Newtonian fluids, this figure identifies approximately 80% overestimation of volume flow when smaller sample volume sizes are utilized along the center of the vessel (top panel, A) compared to a sample volume size spanning intima-to-intima (bottom panel, C), owing to the laminar nature of blood flow and the reduction in blood velocity near the vessel wall. Thus, it is recommended that the sample volume be as wide as possible, but without encompassing the vessel walls and allowing for a slight margin for error in case of movement (figure 3B). Although the width of the gate will vary between laboratories, emphasis should be placed on maintaining consistency at the very least between pre and post cuff release within individual subjects and repeated measurements on the same subject.
Figure 3.
The determination of blood velocity and blood flow using different placements of the Doppler sample gate. A) Outer, B) Middle, C) Inner. Note: the difference in velocity and blood flow among the different placements of the sample gate.
In summary, the baseline measurements are an extremely important component of an FMD study, as these assessments are built upon following cuff release. Therefore, the accurate assessment of an average diameter and concurrent blood velocities, with appropriate sample volume, for at least 10 cardiac cycles is recommended prior to vascular occlusion (cuff inflation).
D. Vascular Occlusion
The initial stimulus for any FMD test relies upon temporary vascular occlusion, which creates a region of ischemic tissue distal to the point of occlusion 5. The metabolic byproducts of cellular respiration, in the absence of circulating blood, promote an increase in vascular conductance that allows a robust hyperemia upon eradication of the “upstream” occlusion. This reactive hyperemia, and the associated shear stress experienced by the conduit vessels upstream from the area of occlusion, is the primary stimulus for FMD. Accordingly, both cuff position (proximal cuff = greater volume of tissue experiencing ischemia = greater hyperemia) and the duration of occlusion (longer occlusion = greater degree of ischemia = greater hyperemia) play an integral role in shear-mediated vasodilation.
The Cuff: The size of the cuff utilized for vascular occlusion should be appropriate for the area being occluded. Although a conventional hand inflated cuff is adequate, it is both convenient and methodologically sound to almost instantaneously inflate and deflate the cuff, which can be achieved using a commercially available rapid (0.3 seconds) cuff inflator. It should be noted that a potential down fall of this rapid inflation approach is the “jolt” that can accompany both inflation and deflation, which can be avoided by supporting the arm in such a way that there is adequate space under the arm for the cuff to inflate and deflate
Cuff Position: Although there is no consensus regarding the placement of the occlusion cuff relative to the site of measurement, there is growing support in favor of placement distal to the ultrasound probe, as this approach is thought to yield a predominantly endothelium-dependent vasodilation 56. Positioning the cuff proximal to the imaging site elicits a greater peak hyperemic response and subsequent FMD 57 likely due to ischemia-induced hypoxia in the area being imaged. Additionally, the decreased hyperemic decay observed following cuff deflation proximal to the site of measurement (Doppler probe) suggests that mechanisms in addition to NO-mediated vasodilation play a role in this scenario 52, 56.
Cuff Duration : Although it has previously been documented that a 10 minute cuff occlusion results in no greater maximal arterial dilation than a five minute occlusion 4, for the sake of consistency and subject comfort it is recommended that a 5 minute suprasystolic cuffing period (200–250 mm Hg) be employed for FMD tests. While the link between vascular function in the brachial artery, NO, and the duration of cuff occlusion is still a matter of some debate 14, 22, recent evidence supports the use of a 5-min over 10-min occlusion, as sustained (> 5-min) occlusion may include more non-NO, ischemia-induced vasodilators 58. Consistent with this, our laboratory has recently documented a 50% greater brachial artery vasodilation following 10 versus 5 minutes of occlusion, even after normalizing FMD for shear rate 32, suggestive of non endothelium-dependent, NO-mediated vasodilators playing a significant role following longer bouts of ischemia.
In summary, when performing an FMD test, the cuff should be the appropriate size for the limb being studied, positioned distal to the ultrasound probe, and inflated at least 25–50 mm Hg above systolic arterial pressure for 5 minutes to elicit a reactive hyperemic stimulus that is considered to be predominantly endothelium mediated and NO-dependent.
E. Reactive Hyperemia (Post-Cuff Release) Measurements
The measurements following vascular occlusion (i.e. post cuff release) are just as important, if not more so, than the baseline measurements. The following observations and recommendations apply to measurements during the post-cuff release time period.
-
Temporal Kinetics of Arterial Diameters and Blood Velocities: As discussed above, it is recommended that Duplex mode on the ultrasound system be utilized. To ensure no anomalies in arterial diameter occur during cuff occlusion and to capture the immediate hyperemic response, it is recommended that post-cuff measurements be initiated at least 10 seconds prior to cuff release. Although the peak velocity occurs within the first 15 seconds, the peak vasodilation can be expected to occur 45–80 seconds following cuff release, and may differ between populations 59. Thus, it is also now recommended that the true peak diameter be determined on an individual basis (not simply the diameter in a given window of time) and the time to peak vasodilation be reported.
To capture the kinetics of reactive hyperemia induced shear and subsequent vasodilation, it is recommended that blood velocity and diameter measurements be performed for at least 2 minutes following cuff release. Because the velocity profile following occlusion is characterized by a parabolic shape with exponential decay, it is generally agreed upon that the integral of shear rate over time (i.e. area-under-the-curve) is the optimal method to quantify the accumulated shear that contributes to the FMD response 54. AUC is conventionally calculated using the trapezoidal rule, according to the equation:
where x is time, y is shear, xi is initial time point, yi is initial blood velocity.Although Duplex mode is required to calculate the total shear rate (AUC), the previous recommendation to capture the peak hyperemic velocity during the first 15 seconds 4 before switching back to 2D imaging is still a feasible method, acknowledging the limitations of such an approach.
Calculation of Shear Rate: As described in the baseline velocities section and illustrated in Figure 3, the measurement of blood velocity with Doppler is influenced by the width of the sample volume and the placement of this gate within the vessel. This is the consequence of the parabolic velocity profile within un-branched sections of conduit vessels. This same concept has an impact upon the calculation of shear rate, which is derived from Poiseuilles’s law, dependent upon Doppler sample volume size and placement:- Large, centered sample volume: 8 × mean blood velocity / internal diameter
- Small, centered sample volume: 4 × mean blood velocity / internal diameter
The difference in numerator (8 versus 4) of this calculation being explained by the failure to account for slower moving red cells at the edge of vessel and therefore a bias toward an artificially elevated mean blood velocity as the sample volume becomes smaller, but still located in the center of the vessel. Based upon this information, it is recommended, that the Doppler sample volume be kept wide and when shear rate is calculated the factor of 8 be employed in the numerator of this equation.
In summary, it is recommended that both diameter and velocity data be acquired for at least 10 seconds prior to cuff release and continue this data collection for at least 2 minutes post release. This method will not only allow the documentation of the “true” peak diameter, but it will also allow the quantitative analysis of shear AUC, the stimulus thought to be predominantly responsible for the FMD response. Additionally, documenting the time to peak vasodilation may more appropriately assess endothelial function when making comparisons among different groups and/or clinical populations.
F. FMD Analyses
Recently, it has become apparent that the measurement of FMD may not be as simple as assessing vessel diameter both before and after cuff release and reporting a percentage increase in vessel caliber. Indeed, over the past two decades both the methodology and analysis of FMD has received significant attention, evolving into what is recommended today. For example, it is now acknowledged that when using the traditional percentage change calculation, the initial baseline diameter has the potential to introduce mathematical bias into the FMD assessment, with smaller vessels appearing more reactive and vice versa 8. Therefore, it is now recommended that in addition to FMD expressed as a percentage, researchers document baseline diameters, absolute change in diameter, and shear rate (AUC).
-
Edge Detection Software: The use of edge detection software for offline analysis is recommended for the measurement of baseline and post cuff release diameters. Using this approach facilitates more objective and accurate diameter measurements and also permits synchronization with the ultrasound system and ECG to allow sequential end-diastolic images to be stored, avoiding artifacts due to pulse-related changes in vessel diameter. Currently, the most commonly used, commercially available, edge detection software is that developed by Medical Imaging Applications LLC (Coralville, IA). This edge detection software has been independently validated 60 and is now commonly found in the literature 26, 32, 36, 61–63.
If edge detection software is unavailable, it is recommended that data (diameter and velocity) be collected every 4 seconds for the first 20 seconds following cuff release, followed by every 10 seconds for the remaining 2 minute data collection period. Table 2 illustrates the variability and absolute differences in baseline diameter, peak diameter, and calculated FMD between careful manual (calipers on the ultrasound) and edge detection software evaluation in a range of subjects. Although strong correlations and low variability exist for baseline diameter (r=0.98; CV=1.9%), peak diameter (r=0.98; CV=1.9%), and calculated FMD (r=0.89; CV=15.2%), it does appear that using edge detection software not only provides a more robust and sensitive assessment of FMD, it removes any subjective error component from the data analysis.
-
Calculation of FMD: The calculation of FMD as a percentage change utilizes the peak diameter in response to reactive hyperemia in relation to the baseline diameter, and is calculated utilizing the following equation:
and when multiplied by 100, FMD is expressed as a percent change in vessel caliber.It is recommended that the average diameter assessed during end diastole (identified by the R wave on the ECG) over at least 10 cardiac cycles be utilized to represent the baseline diameter. With the introduction of the edge detection software, some debate has developed as to the optimum time resolution necessary to accurately determine peak diameter 59. The difference in FMD and determinants using 3, 5, and 10 second data smoothing averages are presented in Table 3. Indeed, if diameters are averaged (data smoothing) over some period of time (i.e. 10 sec), the “exact” peak will lose resolution. However, data smoothing with a time period that is too short (i.e. 3 sec) inevitably increases the noise of the measurement and may result in identification of an aberrant value for peak diameter. Accordingly, it is recommended that the peak diameter be determined over as short a period of time as possible (i.e. 5 seconds), but never relying upon peak diameter data that is obtained from the average of less than 3 measurements (i.e. 3 cardiac cycles). The data smoothing time period should be reported and blood velocities should be analyzed during the same time frame as the diameters (i.e. Duplex mode).
-
Normalization of FMD (FMD/Shear): As FMD is thought to be evoked by shear stress and thus proportional to reactive hyperemia, consideration of the heterogeneity of blood flow responses across subjects deserves greater attention than it received at the outset of such endothelial function measurements 4. Indeed, it has recently been suggested that FMD should be normalized by dividing the % FMD by shear rate (AUC) 54. While this mathematical correction for shear stimulus is theoretically simple, experimental evidence supporting normalization is equivocal. In the study of vascular aging, it has been reported that healthy older adults have a preserved endothelial function when FMD is normalized for their reduced post cuff release shear rates 33. Additionally, Padilla and colleagues 63 demonstrated that normalizing FMD for shear rate (AUC) eliminates the influence of differing shear profiles created by varying periods of cuff ischemia. Further data to support normalization has been indicated by the removal of limb-specific differences in FMD when shear rate was used to normalize the FMD response 64. In contrast, a recent study has concluded that normalizing FMD for shear is age dependent and only appropriate when investigating young adults 65. Therefore, the use of Duplex ultrasound and the subsequent ability to 1) assess FMD, 2) measure reactive hyperemia, and 3) calculate shear AUC, is emerging as an important component of FMD measurements made with ultrasound Doppler. In addition, reactive hyperemia alone has been documented to have high clinical prognostic value66.
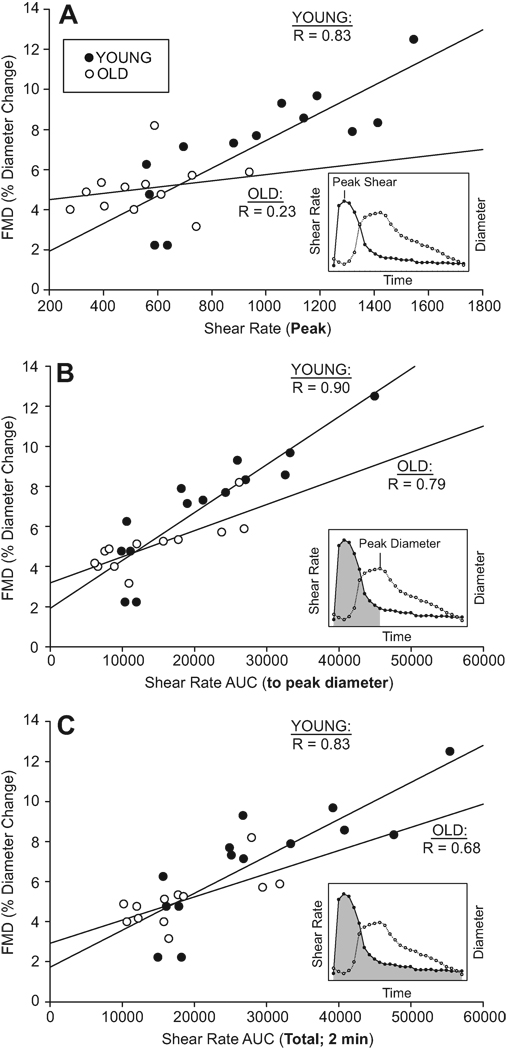
In statistical terms, the appropriate method by which to take differing shear rates into account during an FMD study is complex. In order to legitimately apply such a correction factor to a variable, the relationship between the two variables must satisfy three assumptions: 1) a significant correlation, 2) the y-intercept of this relationship must be zero, and 3) the data should be normally distributed. From our own experience and that of others (Figure 4, 54, 65) the relationship between vessel dilation and shear rate often violates at least 1, if not all 3, of these assumptions, leaving serious doubt as to whether a simple mathematical normalization should be categorically performed. If, as has been suggested, there is simply a modest correlation between FMD and shear, and the shear stimulus differs between independent variables, the proper method of taking into account the covariance of shear rate with FMD may be through the analysis of covariance 67, although, as of yet, this method is also not completely accepted.
Figure 4 illustrates the relationships between FMD and A) Peak shear, B) shear AUC up until the time of peak dilation, and C) total shear AUC (2 min) in both young and old subjects. Although all relationships for the young population appear to be very strong, it is important to note that only the shear AUC until peak vasodilation (Figure 4, plot B) yields the strongest relationship in both age groups. These data are in agreement with previous work 54 and provides further evidence that shear rate (AUC) until the time of peak dilation may be the most appropriate method of quantifying shear forces.
Table 2.
Absolute difference and variability between manual and software evaluations of FMD determinants
| Baseline Diameter(cm) | Peak Diameter(cm) | FMD (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Manual | Software | diff(cm) | CV(%) | Manual | Software | diff(cm) | CV(%) | Manual | Software | diff(cm) | CV(%) |
| 1 | 0.42 | 0.4000 | 0.015 | 2.6 | 0.44 | 0.4200 | 0.020 | 3.3 | 6.02 | 5.00 | 1.02 | 13.1 |
| 2 | 0.43 | 0.4497 | −0.022 | 3.6 | 0.45 | 0.4689 | −0.019 | 2.9 | 5.26 | 4.27 | 0.99 | 14.7 |
| 3 | 0.40 | 0.3951 | 0.002 | 0.4 | 0.41 | 0.4027 | 0.007 | 1.3 | 3.14 | 1.92 | 1.22 | 34.1 |
| 4 | 0.34 | 0.3475 | −0.007 | 1.5 | 0.35 | 0.3595 | −0.010 | 1.9 | 2.94 | 3.45 | −0.51 | 11.3 |
| 5 | 0.31 | 0.3200 | −0.010 | 2.2 | 0.35 | 0.3511 | −0.001 | 0.2 | 12.90 | 11.42 | 1.48 | 8.6 |
| 6 | 0.30 | 0.3029 | −0.003 | 0.7 | 0.32 | 0.3244 | −0.004 | 1.0 | 6.67 | 8.13 | −1.47 | 14.0 |
| 7 | 0.33 | 0.3200 | 0.010 | 2.2 | 0.37 | 0.3600 | 0.010 | 1.9 | 12.12 | 12.50 | −0.38 | 2.2 |
| 8 | 0.24 | 0.2400 | 0.000 | 0.0 | 0.27 | 0.2900 | −0.020 | 5.1 | 12.50 | 20.83 | −8.33 | 35.4 |
| 9 | 0.29 | 0.2833 | 0.009 | 2.3 | 0.31 | 0.3100 | 0.000 | 0.0 | 5.98 | 9.41 | −3.43 | 31.5 |
| 10 | 0.33 | 0.3204 | 0.006 | 1.4 | 0.36 | 0.3483 | 0.012 | 2.3 | 10.20 | 8.71 | 1.50 | 11.2 |
| 11 | 0.29 | 0.2733 | 0.017 | 4.2 | 0.31 | 0.2900 | 0.020 | 4.7 | 6.90 | 7.41 | −0.51 | 5.1 |
| 12 | 0.25 | 0.2500 | 0.003 | 0.9 | 0.27 | 0.2600 | 0.010 | 2.7 | 6.58 | 5.41 | 1.17 | 13.8 |
| 13 | 0.38 | 0.3900 | −0.010 | 1.8 | 0.39 | 0.3974 | −0.007 | 1.3 | 2.63 | 2.03 | 0.60 | 18.3 |
| 14 | 0.38 | 0.3800 | 0.000 | 0.0 | 0.38 | 0.3807 | −0.001 | 0.1 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15 | 0.30 | 0.3000 | 0.000 | 0.0 | 0.31 | 0.3100 | 0.000 | 0.0 | 3.33 | 3.33 | 0.00 | 0.0 |
| Mean | 0.33 | 0.3315 | 0.001 | 1.6 | 0.35 | 0.3515 | 0.001 | 1.9 | 6.48 | 6.92 | −0.4 | 15.2 |
Note: There is greater accuracy of diameter measurements and the subsequent determination of FMD using software analysis
Table 3.
Difference in FMD and determinants using 3, 5, and 10 second data smoothing averages
| Variable | 3 seconds | 5 seconds | 10 seconds |
|---|---|---|---|
| Baseline diameter | 3.25±0.15 | 3.25±0.15 | 3.25±0.15 |
| Peak diameter | 3.45±0.15 | 3.43±0.15* | 3.42±0.15* |
| FMD (%) | 6.7±0.9 | 6.0±0.8 | 5.6±0.7* |
| Time to peak (s) | 44±6 | 43±4 | 44±6 |
| # frames | 2.4±0.3 | 4.0±0.4* | 7.7±0.7*† |
Data are presented as mean±sem
Significant (p< 0.05) from 3 seconds
Significant (p< 0.05) from 5 seconds
Figure 4.
The relationships between flow-mediated dilation (FMD) and different assessments of shear rate to be considered when normalizing FMD. A) FMD vs. peak shear, B) FMD vs. shear AUC until peak diameter, C) FMD vs. total shear AUC for the entire 2 minutes. Inlays for each panel illustrate the corresponding shear rate (shaded) used in the analysis.
In summary, edge detection software has been independently validated and is recommended for the measurement of arterial diameter. To standardize the FMD technique, the recommended procedure for obtaining diameters is through continuous digital data recording and off line analysis utilizing edge detection software. To identify and calculate the FMD response, the “true” peak diameter is obtained and expressed as an increase in vasodilation above baseline values. Although normalization of FMD for shear has been embraced by many researchers, uncertainty currently exists as to how to properly normalize FMD. It is currently recommended that FMD normalized for shear rate (AUC) be calculated and reported, but that the raw shear (AUC up to peak vasodilation) and FMD data also be readily available to allow alternative analyses or interpretations. Additionally, the time it takes to obtain peak vasodilation may be an important indicator of stimulus sensitivity that should be incorporated into an evaluation of endothelial function by an FMD test.
CONCLUSION
The measurement of FMD is often mistaken as a simple non-invasive method of assessing vascular endothelial function which anyone with access to an ultrasound Doppler can perform 14. However, the appropriate ultrasound technology, subject preparation, and knowledge of the method are required to perform an accurate assessment of endothelial function utilizing the FMD technique. The recommendations proposed in this comprehensive tutorial represent the most recent advancements in the ultrasonic measurement of FMD and are presented in an attempt to standardize this measurement across research sites and subsequently facilitate the use of FMD as a clinically relevant research tool.
Acknowledgments
SOURCES OF FUNDING
The authors of this tutorial were supported by National Institute of Health grant PO1-HL-0918830, Tobacco-Related Disease Research Program grant 15RT-0100, The Parker B. Francis Family Foundation, American Heart Association Scientist Development Grant 08535209N, and the Salt Lake City Veterans Affairs Medical Center Geriatric Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
NONE.
REFERENCES
- 1.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 2.Uehata A, Lieberman EH, Gerhard MD, Anderson TJ, Ganz P, Polak JF, Creager MA, Yeung AC. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vascular Medicine. 1997;2:87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD. Close relationship of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gehard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the Ultrasound Assessment of Endothelial-Dependent Flow-Mediated Vasodilation of the Brachial Artery. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Gooch VM. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 6.Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. European heart journal. 2005;26:363–368. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- 7.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Journal of hypertension. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. The Journal of physiology. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celermajer D. Endothelial dysfunction: does it matter? is it reversible? Journal of American College of Cardiology. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 10.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and disease. Journal of Cardiovascular Pharmacology. 1993;22:S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 11.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular enodothelium. The New England Journal of Medicine. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 12.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annual review of physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 13.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 14.Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1233–1234. doi: 10.1152/japplphysiol.00601.2005. discussion 1237–1238. [DOI] [PubMed] [Google Scholar]
- 15.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 16.Ross R. Atherosclerosis - An inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 17.Niebauer J, Cooke JP. Cardiovascular effects of exercise: Role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 18.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 19.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. Journal of cardiovascular pharmacology. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–635. [PubMed] [Google Scholar]
- 21.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circulation research. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 22.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1235–1237. doi: 10.1152/japplphysiol.00607.2005. [DOI] [PubMed] [Google Scholar]
- 23.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. American journal of physiology. 2010;298:H119–H126. doi: 10.1152/ajpheart.00571.2009. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 25.Harris RA, Padilla J, Rink LD, Wallace JP. Variability of flow-mediated dilation measurements with repetitive reactive hyperemia. Vascular medicine (London, England) 2006;11:1–6. doi: 10.1191/1358863x06vm641oa. [DOI] [PubMed] [Google Scholar]
- 26.Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity (Silver Spring, Md. 2008;16:578–584. doi: 10.1038/oby.2007.87. [DOI] [PubMed] [Google Scholar]
- 27.Wright SA, O'Prey FM, Rea DJ, Plumb RD, Gamble AJ, Leahey WJ, Devine AB, McGivern RC, Johnston DG, Finch MB, Bell AL, McVeigh GE. Microcirculatory hemodynamics and endothelial dysfunction in systemic lupus erythematosus. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2281–2287. doi: 10.1161/01.ATV.0000238351.82900.7f. [DOI] [PubMed] [Google Scholar]
- 28.Jogestrand T, von Arbin M, Bergqvist D, Lilja A, Lindqvist M, Matzsch T, Norrving B, Nowak J, Troeng T, Wahlgren NG. [Recommendations by the Swedish Quality Board for Carotid Surgery. Ultrasound good preoperative method for evaluating degree of carotid stenosis] Lakartidningen. 2003;100:2443–2445. [PubMed] [Google Scholar]
- 29.Logason K, Barlin T, Jonsson ML, Bostrom A, Hardemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50–69% and 70–99% carotid artery stenosis. Eur J Vasc Endovasc Surg. 2001;21:311–313. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo RJ, Sandager G, Astleford P, Payne K, Peterson-Kennedy L, Flinn WR, Yao JS. Mesenteric flow velocity variations as a function of angle of insonation. J Vasc Surg. 1990;11:688–694. doi: 10.1067/mva.1990.19707. [DOI] [PubMed] [Google Scholar]
- 31.Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. The American journal of physiology. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- 32.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. European journal of applied physiology. 2009;107:445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 33.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. American journal of physiology. 2006;290:H1271–H1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- 34.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. American journal of physiology. 2007;292:H1516–H1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 35.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009;116:433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 36.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. The Journal of physiology. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens. 2005;18:510–516. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 39.Kato T, Inoue T, Morooka T, Yoshimoto N, Node K. Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Canadian journal of physiology and pharmacology. 2006;84:523–529. doi: 10.1139/y06-030. [DOI] [PubMed] [Google Scholar]
- 40.Strinden ST, Stellwagen RH. Inhibition of guanylate cyclases by methylxanthines and papaverine. Biochemical and biophysical research communications. 1984;123:1194–1200. doi: 10.1016/s0006-291x(84)80259-4. [DOI] [PubMed] [Google Scholar]
- 41.Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 2005;109:55–60. doi: 10.1042/CS20040358. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochemical and biophysical research communications. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 43.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. The New England journal of medicine. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 45.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 46.Harvey PJ, Morris BL, Kubo T, Picton PE, Su WS, Notarius CF, Floras JS. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. Hypertension. 2005;23:285–292. doi: 10.1097/00004872-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. European journal of applied physiology. 2006;98:256–262. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 48.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on enothelial function in healthy subjects. The American journal of cardiology. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 49.Ceriello A, Cavarape A, Martinelli L, Da Ros R, Marra G, Quagliaro L, Piconi L, Assaloni R, Motz E. The post-prandial state in Type 2 diabetes and endothelial dysfunction: effects of insulin aspart. Diabet Med. 2004;21:171–175. doi: 10.1111/j.1464-5491.2004.01101.x. [DOI] [PubMed] [Google Scholar]
- 50.Jarvisalo MJ, Jartti L, Marniemi J, Ronnemaa T, Viikari JS, Lehtimaki T, Raitakari OT. Determinants of short-term variation in arterial flow-mediated dilatation in healthy young men. Clin Sci (Lond) 2006;110:475–482. doi: 10.1042/CS20050333. [DOI] [PubMed] [Google Scholar]
- 51.Thijssen DH, van Bemmel MM, Bullens LM, Dawson EA, Hopkins ND, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. The impact of baseline diameter on flow-mediated dilation differs in young and older humans. American journal of physiology. 2008;295:H1594–H1598. doi: 10.1152/ajpheart.00669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. American journal of physiology. 1995;268:H1397–H1404. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- 53.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. American journal of physiology. 2008;295:H1927–H1934. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 55.Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol. 1985;11:625–641. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- 56.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. American journal of physiology. 2004;286:H442–H448. doi: 10.1152/ajpheart.00314.2003. [DOI] [PubMed] [Google Scholar]
- 57.Berry KL, Skyrme-Jones RA, Meredith IT. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clin Sci (Lond) 2000;99:261–267. [PubMed] [Google Scholar]
- 58.Kooijman M, Thijssen DHJ, de Groot PCE, Bleeker MWP, van Kuppevelt HJM, Green DJ, Rongen GA, Smits P, Hopman MTE. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. The Journal of physiology. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51:203–210. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 60.Mancini GB, Yeoh E, Abbott D, Chan S. Validation of an automated method for assessing brachial artery endothelial dysfunction. The Canadian journal of cardiology. 2002;18:259–262. [PubMed] [Google Scholar]
- 61.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. American journal of physiology. 2004;286:H1528–H1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- 62.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. The Journal of physiology. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovascular ultrasound. 2008;6:44. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishiyama SK, Wray DW, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: The role of shear rate. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 65.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. American journal of physiology. 2009;296:H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris RA, Padilla J. Proper "normalization" of flow-mediated dilation for shear. J Appl Physiol. 2007;103:1108. doi: 10.1152/japplphysiol.00518.2007. author reply 1109. [DOI] [PubMed] [Google Scholar]