Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is a widely diagnosed psychiatric disorder of childhood that may continue to manifest itself during adulthood. Across adults and children, inattention appears to be the most developmentally stable symptomatology of ADHD. To determine the neural systems that may be linked to such symptoms, the association between brain activation in a group of young adults in the face of an attentional challenge (the Stroop task) and inattentive symptoms was examined with functional magnetic resonance imaging. The results implicated a broad array of brain regions that are linked to behaviors compromised in ADHD, including executive function/cognitive control (prefrontal cortex, dorsal striatum), reward and motivational circuitry (ventral striatum), and stimulus representation and timing (posterior cortex and cerebellum). Also implicating these regions as being important for the manifestation of ADHD symptoms, the variability in the size of the BOLD signal across individuals was significantly higher for the ADHD group than for the control group, and variability across the time series in individuals with ADHD was linked to symptom severity and behavioral performance. The results suggest that a diverse set of brain structures is linked to ADHD symptoms and that the variability of activation within these regions may contribute to compromised attentional control.
Keywords: fMRI, ADHD, Symptoms, Inattention
1. Introduction
Various brain regions are implicated as dysfunctional in individuals with Attention Deficit Hyperactivity Disorder (ADHD), although many can be profitably grouped into functional sets. Frontal cortex consistently shows reduced activity and volume in individuals with ADHD, including lateral prefrontal cortex (PFC; Booth et al., 2005; Valera et al., 2005; Rubia et al., 2005), inferior frontal cortex (IFC; Booth et al., 2005; Rubia et al., 2005), anterior cingulate cortex (ACC; Bush et al., 1999) and orbital frontal cortex (OFC; Hesslinger et al., 2002). These frontal regions, thought to underlie cognitive control, modulate the ability to hold task-relevant information online, allocate attention, inhibit distraction, and process reward contingencies, all of which have been previously found to be impaired in individuals with ADHD (Bush et al., 1999; Barkley, 1997; Casey et al., 1997; Schweitzer et al., 2000). A second functional set, comprising the striatum and/or basal ganglia, is often discussed in tandem with frontal regions in ADHD because of dense connections between these regions (Alexander et al., 1990). Reduced activation and/or volume in striatal regions are observed in individuals with ADHD, including the caudate (Rubia et al., 1997; Vaidya et al., 1998; Castellanos et al., 2002), putamen (Konrad et al., 2006), and ventral striatum (Scheres et al., 2007). The striatal connections with PFC via cortical loops (Alexander et al., 1990) are thought to provide important signals related to cognitive control, such as signaling of the updating of working memory and information about the contingencies and regularities of events. A third region often identified is the cerebellum, as numerous studies show reduced activation (Valera et al., 2005; Durston et al., 2008) and decreased volume (Castellanos, 1997; Berquin et al., 1998) of the cerebellum in individuals with ADHD. The cerebellum has been suggested as being important for stimulus expectancy and detection, which is dependent on stimulus timing (Rubia et al., 2007a). As proposed by Nigg and Casey (2005), these sets of results taken together suggest that the neural dysfunction in individuals with ADHD is likely to involve a variety of brain regions or circuits rather than being limited to a couple of key nodes or areas. The goal of the current study is to identify such circuits and their relationship to behavioral symptomatology in young adults with ADHD.
In typical neuroimaging studies involving a population with a psychiatric disorder, the strategy used to identify neural dysfunction in the clinical group (e.g., ADHD) is to compare brain activation in that group with a control group during performance of an experimental task that taps a function compromised in the clinical group (e.g. attentional control). The logic is that the clinical group has symptoms, while the control group does not. Hence, the difference in activation between the two groups should isolate critical regions involved in the disorder. Indeed, we have previously performed just such an analysis on portions of the data reported in the current study in which we compare the level of brain activation in young adults with ADHD vs. control individuals during performance of the attentionally-demanding Stroop task. In particular, the young adults with ADHD exhibit less activation than non-ADHD individuals in regions involved in attentional control, such as the DLPFC and ACC (Banich et al., 2009).
Such an approach, however, may not identify all regions involved in a disorder. For example, a group difference may not emerge using such an approach because of variability of the severity of symptoms within the clinical group. For example, compared to controls, some individuals with ADHD may exhibit increased activation in an area to compensate for attentional difficulties, while other individuals with ADHD, whose disorder may be more severe, will show decreased activation because they have a reduced ability to recruit such an area in the face of attentional demand. Hence, areas that may relate meaningfully to an important manifestation of the disorder, symptom severity, may not be detected in a standard clinical-control group contrast.
Therefore, the approach taken in the present study is to identify the neural circuitry related to ADHD symptoms by determining the set of brain regions whose activity during performance of an attentionally-demanding task in young adults with ADHD is correlated with symptom severity. In particular, symptomatology was correlated with brain activation during performance of the Stroop task under three different attentional experimental conditions, as these conditions index many key aspects of attentional control that are likely to be disrupted in ADHD (Bush et al., 1999; Konrad et al., 2006). These processes include the ability to maintain a top–down attentional set, to select among competing representations used to guide behavior, and response-related aspects of attentional control (Banich et al., 2000). Accordingly, this task is likely to (i) index processes that are highly related to attentional symptomatology, and hence (ii) be an effective challenge to the neural structures that underlie attentional control.
If this approach is indeed helpful in identifying the circuitry underlying ADHD, one would then expect that characteristics of activation in the regions so identified should differ in significant ways from (non-affected) control individuals. Rather than simply examining levels of activation in these regions (for the reasons discussed above), the current study focused on the variability of activation in these regions, both between (inter-individual) and within individuals (intra-individual) (Bellgrove et al., 2005; Simmonds et al., 2007). Such an approach may be especially fruitful in understanding the neural underpinnings of ADHD, as one of the key deficits that have emerged between ADHD and control individuals is increased variability in behavioral responses (Rubia et al., 2007a). Response variability is found to be exaggerated in ADHD populations during attention (Leth-Steenson et al., 2000), executive control (Rubia et al., 2001), and timing tasks (Rubia et al., 1999, 2003), and is one of the most predictive measures of impaired functioning and severity of ADHD diagnosis (Rubia et al., 2007a,b). In addition, it shows some of the largest effect sizes in group comparisons (Klein et al., 2006; Castellanos et al., 2005; Sergeant et al., 2003). Moreover, there is some initial evidence that such variability may also be reflected in brain functioning. In particular, low oscillatory fluctuation in the brain’s default network during the resting state (Helps et al., 2008) and intrusions of the default mode during cognitive tasks (Sonuga-Barke and Castellanos, 2007) are linked to attentional lapses and increased within group (intra-individual) response variability in ADHD individuals.
Our hypothesis therefore was that the severity of symptomatology related to attention across ADHD individuals would predict the level of activity in brain regions that are involved in attentional control (LPFC) as well as potentially predicting activity in brain regions whose function might be affected by or contribute to reduced control (e.g., stimulus–response linkage; rIFG; stimulus association and timing inferior temporal lobe and cerebellum). Furthermore, the characteristics (i.e., variability) of brain activation in regions linked to symptom severity in the ADHD group would differ significantly from that of a control group. We predicted that the ADHD group would exhibit more variability in brain activation in these regions than a control group and that such variability would be linked to variability of behavioral performance on the Stroop task.
2. Methods
2.1. Participants
Participants included twenty-three young adults with combined-type ADHD (14 male, 9 female) and 23 healthy controls (14 males, 9 females) between 18 and 23 years of age.
2.1.1. Participant selection
All details for participant selection can be found in the Supplementary material: S1.
2.2. Stimuli and experimental design
Participants performed a variant of the Color-Word Stroop task in the scanner. Participants saw a series of words printed in one of four ink colors (red, blue, green, or yellow), and indicated the ink color via a manual keypress. There were three trial types: congruent words, which matched the ink color (e.g., “red” in red ink), incongruent words, which did not match the ink color (e.g. “red” in green ink), and neutral words, which did not name a color (e.g. “bond” in red ink). A mixed blocked/event-related functional magnetic resonance imaging (fMRI) design was utilized to allow the estimation of both blocked and event-related effects. Three types of task blocks were utilized–congruent, incongruent, and neutral–in addition to resting fixation blocks. Half of the trials in each task block were drawn from block-specific trials (incongruent, congruent, or neutral) and half were drawn from neutral words that were identical to all three blocks (referred to as NI, NC, and NN trials, depending on the block in which they were presented).
Participants performed three runs, with each run comprising 163 volumes. Seven null trials were dropped from the beginning of each run to ensure steady-state magnetization, leaving 156 volumes in each run. Each run was composed of 13 blocks of 12 volumes: four blocks of null trials (i.e., fixations) and nine blocks of task trials. Fixation blocks (F) alternated with triads of task blocks. Each triad consisted of one incongruent block (I), one neutral block (N), and one congruent block (C). For example, the block order for one run could be F-INC-F-NCI-FCIN-F. The order of blocks was counterbalanced across triads (e.g., INC for the first run, NCI for the second run and CIN for the last run), and the triad order was counterbalanced across participants. Each task block consisted of 12 trials (2 s each), one volume per trial. In total, there were 324 task trials, with 108 trials in each of the three task block conditions.
2.3. fMRI data acquisition
Functional images were acquired with a GE Signa (3T) MRI scanner using a T2*-weighted gradient-echo, echo-planar imaging (repetition time [TR]=2000 ms, echo time [TE]=40 ms, flip angle=90°, 29 slices parallel to the AC-PC line, thickness=4 mm, gap=0 mm, 64×64 in-plane resolution, in-plane FOV=22 cm). T1-weighted 3D IR-SPGR anatomical images were also collected along the coronal plane (TR=9 ms, TE=2.0 ms, flip angle=10°, inversion time=500 ms; 220 mm FOV, 256×256 matrix, 0.87×0.87 mm2 in-plane resolution, 124 slices, 1.7-mm slice thickness).
2.4. Image preprocessing
Image processing and statistical analyses were conducted using the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl/index. html) (Version 4.0.2). Images were motion corrected using MCFLIRT, and brain extracted using BET to remove all non-brain tissue from the images. Before statistical analysis, each participant’s images were spatially smoothed using a Gaussian kernel (FWHM=8 mm); mean based intensity normalized; high-pass temporal filtered with a cut-off period of 100 s to remove low-frequency noise.
2.5. Statistical analyses
Statistical analyses were conducted using FILM. Analyses on the BOLD timecourses were run separately for each participant and for blocked vs. event-related analyses. Blocked and event-related regressors were modeled by convolving predictors with a double-gamma hemodynamic response function. For comparisons across individuals and groups, parameter and variance estimates from each participant were registered to Montreal Neurological Institute standard stereotaxic space (MNI152) using the two-stage registration procedure implemented in FLIRT. FLAME (1 + 2) was used to model the mixed-effects variance for each contrast of interest, taking into account both fixed effects (within-participants variability) and random effects (between-participants variability).
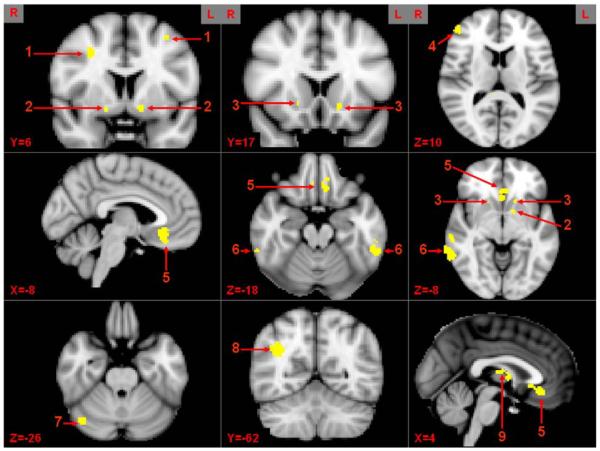
For the ADHD group only, we performed an analysis in which the covariate of lifetime inattentive and hyperactive scores on a Likert scale (range = 0–3), mean deviated, provided by the diagnostic interview described in S1 were used as a correlate to predict brain activation during the Stroop task for each of the three blocked conditions (I, N, C) separately, errors removed. The individual voxel threshold was set at Z =2.58 P<0.005 for significance for the correlation of brain activation with symptomatology measures. AlphaSim software of AFNI with our analysis parameters (e.g., FWHM of 8 mm) was used to determine the significant cluster size that would provide a false positive rate at P<0.05, which in this case was 120 voxels to protect at P<0.05. Furthermore, because sub-cortical areas tend to have smaller volumes than cortical regions, these structures were investigated using a mask (provided by Montreal Neurological Institute) for each specific region (e.g., nucleus accumbens) and selecting the appropriate cluster-wise threshold (P b 0.05) dependent on the total number of voxels within each mask. Thirteen ROIs that yielded a significant relationship with symptomatology across all three conditions were interrogated as described next (Fig. 1).
Fig. 1.
Brain regions correlated with inattentive symptomatology across condition. 1 = middle frontal gyrus (MFG), 2 = putamen (Puta), 3 = nucleus accumbens (NAC), 4 = inferior frontal gyrus (IFG), 5 = medial orbital frontal cortex (mOFC), 6 = inferior temporal gyrus (ITG), 7 = cerebellum (Cereb), 8 = lateral inferior parietal (LIP), 9 = thalamus (Thal).
2.5.1. ROI calculations
ΔS analyses were performed using FSL’s Featquery signal change processing tool to extract parameter estimates for the 13 significant ROIs. To summarize the data, average parametric maps of brain activation across the three conditions (I, N, C) vs. fixation baseline were averaged to create a single mean Z average parametric map across conditions. This method ensures that each of the contrasts has an equal likelihood of contributing to the results. Next, the associated parameter estimate was calculated using a 5 mm3 sphere around the peak of activation within the ROIs based on this mean Z volume. Parameter estimates were then converted to percent signal change (ΔS) values and correlated with symptom severity across individuals.
2.5.2. Between and within-individual calculation of variability
Between-individual or inter-individual variability was examined by extracting the average percent signal change (ΔS) of each ROI (13) computed across trials from each individual within each group (ADHD, controls). As described above, the average ΔS was derived from a statistical parametric map of brain activation that averaged the three conditions (I, N, C vs. fixation baseline). To determine whether the variance of BOLD signal varied between groups, Levene’s tests for equality of variance were computed. Within-individual or intraindividual calculation of variance was performed in exactly the same manner except that values were extracted from an average variance map (var_cope) of the parameter estimates from the mean Z average parametric map across the three conditions (I, N, C vs. fixation baseline). These variance maps (var_copes) contain the within-individual variance surrounding the amplitude of the BOLD signal. These variability estimates were then correlated with an individual’s standard deviation of their overall reaction time [RT; calculated across all blocks in the experiment (I, N, C)].
3. Results
Behavioral results of the Stroop task can be found in Supplementary material: S2.
3.1. Identifying brain regions in which task-induced activity is predicted by inattentive symptomatology: ADHD individuals only
Our first step was to identify the set of brain regions within ADHD individuals alone whose activity in the face of attentional demand was predicted by lifetime inattentive symptomatology. Likert scores (range=0–3) for each of the 18 symptoms assessed by DSM-IV were used to remove the skewness inherent in symptomatology data. Brain activity during the attentionally-demanding Stroop task was examined separately for three blocked conditions–Incongruent, Congruent and Neutral–relative to a fixation baseline. Activity for each of these contrasts was then correlated with the severity of inattentive symptomatology. To determine the core set of brain regions that are consistently linked to symptomatology across variations in attentional demand, those regions that yielded significant correlations with inattentive symptomatology across each of the three trial blocks separately (Z=2.58, P<0.005, cluster-wise correction, P<0.05) were identified. This approach yielded 13 brain regions (ROIs), which serve as the basis for the additional analyses described in subsequent sections.
Because a multitude of regions correlated with inattentive symptomatology we wished to summarize the data and explore whether each of the 13 ROIs were indeed activated by each task condition vs. baseline. To do so, parametric maps of brain activation for each of the three conditions (I, N, C) vs. fixation baseline were tested to determine whether each ROI passed a threshold of Z =2.58. All 13 ROIs produced significant brain activation above threshold for each condition. Thus, we averaged the three conditions (I, N, C) to create a single mean Z average parametric map, with which the average percent signal change (ΔS) could be extracted. Table 1 presents the average Z score across individuals for the peak location within each ROI for the Z average map as well as the individual Z score for each specific contrast (I, N, C vs. baseline). For each individual, the average ΔS was extracted at the group peak for each ROI. The ΔS from each peak was then correlated separately with inattentive symptomatology. As can be seen in Table 1, the direction of these correlations across all 13 regions was negative, indicating that the greater the severity of inattentive symptoms an individual with ADHD exhibits, the less likely he or she is to recruit these brain regions in the face of attentional demand. Scatter plots showing correlations with each ROI can be found in S3. Correlations of activation in these brain regions with lifetime hyperactivity score (also measured using a Likert scale) yielded no significant results.
Table 1.
The 13 ROIs in which the level of brain activity yielded a significant correlation with symptom severity across ADHD individuals. The columns from left to right indicate specific Z value by individual contrast (I, N, C vs. fixation baseline) and average Z score for task activation across all conditions in the average parametric map, the MNI coordinates for the peak of activation in the average parametric map, the correlation coefficient of activity in that region with the severity of inattentive symptoms (Inatt r), Bonferroni corrected probability scores for that correlation (Inatt P), the significance of an unpaired t-test comparing the percentage signal change between ADHD and control individuals for the relevant ROI (Signal P), and significance of a Levene’s test to examine whether the variance across members of the ADHD in percent signal change was significantly greater than across members of the control group for the given ROI (Lev P).
| Region | INC | CON | NEU | Avg | x | y | z | Inatten r | Inatten P | Signal P | Levene P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| lMFG | 2.83 | 2.65 | 2.63 | 2.70 | −34 | 6 | 58 | −0.63 | <0.001 | 0.42 | 0.007 |
| rMFG | 3.34 | 2.72 | 2.74 | 2.93 | 32 | 7 | 41 | −0.51 | <0.01 | 0.71 | 0.006 |
| lPuta | 2.84 | 2.67 | 2.66 | 2.72 | −16 | 13 | −8 | −0.5 | <0.01 | 0.95 | 0.11 |
| rPuta | 2.72 | 2.62 | 2.62 | 2.65 | 20 | 13 | −6 | −0.52 | <0.01 | 0.15 | 0.07 |
| lAccu | 2.71 | 3.12 | 3.05 | 2.96 | −13 | 6 | −11 | −0.52 | <0.01 | 0.31 | 0.04 |
| rAccu | 2.73 | 2.71 | 2.62 | 2.69 | 18 | 6 | −12 | −0.53 | <0.01 | 0.49 | 0.02 |
| lITG | 3.16 | 3.04 | 2.93 | 3.04 | −54 | −44 | −18 | −0.55 | <0.01 | 0.94 | 0.01 |
| rITG | 3.24 | 3.08 | 2.97 | 3.10 | 60 | −42 | −20 | −0.51 | <0.01 | 0.98 | 0.004 |
| Cereb | 2.81 | 3.12 | 3.53 | 3.15 | 42 | −72 | −26 | −0.71 | <0.0001 | 0.91 | 0.02 |
| Thal | 2.96 | 2.72 | 2.83 | 2.84 | 6 | −13 | 9 | −0.64 | <0.001 | 0.66 | 0.01 |
| rLIP | 3.32 | 3.43 | 3.41 | 3.39 | 34 | −56 | 28 | −0.68 | <0.001 | 0.51 | 0.0001 |
| rIFG | 3.33 | 2.85 | 2.94 | 3.04 | 52 | 42 | 16 | −0.51 | <0.01 | 0.47 | 0.01 |
| mOFC | 3.02 | 3.26 | 3.21 | 3.16 | −6 | 30 | −14 | −0.64 | <0.001 | 0.46 | 0.03 |
3.2. BOLD signal comparison with matched controls within the ROIs
The approach used in this paper of identifying brain regions whose activity is correlated with symptom severity is motivated, in part, by the idea that these regions may not be identical to those that show a difference in level of activation between a symptomatic group (i.e., individuals with ADHD) and a non-symptomatic group. To examine this issue, ΔS was extracted from the average parametric map for the peak activation within each ROI for each individual within the ADHD and control groups. An unpaired t-test was used to compare the average ΔS between the groups and they were found not to differ (see Table 1; Signal P), suggesting that both groups show the same degree of percent increase in BOLD signal in these regions to a similar degree during performance of the Stroop task. Hence, any group differences in subsequent analyses examining the variability of these brain regions cannot be due to group differences in average ΔS.
3.3. Variability of task-induced brain activation
As the degree of activity in the 13 ROIs did not differ between ADHD and the control group, the next set of analyses examined whether inter-individual variability in the average BOLD signal (averaged over the time series) was higher for the ADHD than control group. Additionally, in an attempt to identify an association between brain and behavior in variability, the variability in BOLD signal across the time series for each individual was correlated with variability in reaction time on the Stroop task (intra-individual) for each group (ADHD, controls) separately. The size of these correlations was examined to see if they differed across the groups.
3.3.1. Between-individual variability: task-induced brain activity
Levene’s tests for equality of variance between the two groups revealed that the variance in average BOLD signal across members of the ADHD group was greater as compared to members of the control group for each of the 13 ROIs (Table 1; Lev P). As discussed above, because there are no significant group differences in the amplitude of the BOLD signal (averaged across conditions; I, N, C vs. baseline) in these same regions (Table 1), these results indicate that across members of the ADHD group there is more variability in the average BOLD signal than was observed across the control group.
To establish that the increased variability of activity across members of the ADHD group is somewhat specific to these ROIs and not simply due to more variable brain activation in general, the variance of the average BOLD signal was also examined for a set of “control” regions not associated with symptomatology. Six cortical areas (bilateral posterior lateral and medial visual cortex, and motor cortex) served as control regions. These areas met the criteria that they were significantly activated above baseline for all three Stroop conditions but their degree of activation did not correlate with inattentive symptomatology in the ADHD group. Levene’s tests revealed that for these 6 areas, the variance in average BOLD signal across members of each group did not differ significantly (see S4, Table S2).
To further assess whether the increased variability in BOLD response across members of the ADHD group is specific to those brain regions correlated with the severity of inattentive symptomatology, we performed a similar analysis for four ROIs that exhibited significant differences in ΔS between the groups in a standard group comparison analysis (Banich et al., 2009) and have been implicated in numerous studies as being involved in cognitive control. The ROIs selected were three regions within DLPFC and one region within the ACC. These four regions exhibited no significant difference between the groups in the variability of the average BOLD signal across members within a group (see S4, Table S3). These results suggest that for the regions whose activity correlates with inattentive symptomatology, there is greater variability in BOLD signal across individuals in the ADHD group compared to the control group, and that such variation is distinct from regions that are defined by differences between the groups in average levels of activation.
3.3.2. Within-individual variability: task-induced brain activity and behavioral performance
To determine the impact on behavior of variability in the BOLD signal across the time series within each individual (intra-individual variation), the variability in the BOLD signal across the time series for each of the 13 ROIs was correlated with an individual’s variability in reaction time (RT) across all trials of the Stroop task. Behavioral results of RT variability, for each group, can be found in S2. More variability across time of the BOLD signal in lMFG, rMFG (r = 0.40, r = 0.46), lAccu (r = 0.39), and lITG (r = 0.46) predicted significantly more variability in overall RT across trials in ADHD individuals, with a trend observed for 5 additional regions (see Table 2). The same analysis yielded no significant correlations for control individuals. Furthermore, the four regions that exhibited group differences in ΔS previously discussed (3 regions within DLPFC and 1 region within the ACC) also yielded no correlations between variability in the BOLD signal across the time series with variability in overall RT across the time series (see S4, Table S3). Hence, variability in the brain regions of ADHD individuals that are linked to symptom severity appears to be directly linked to variability in behavioral performance.
Table 2.
Correlations of intra-individual BOLD signal variability and intra-individual RT variability in the 13 ROIs identified by inattentive symptomatology.
| Region | BOLD var–RT var ADHD | BOLD var–RT var CON |
|---|---|---|
| lMFG | 0.39** | −0.13 |
| rMFG | 0.38** | 0.26 |
| lPuta | 0.29* | −0.09 |
| rPuta | 0.32* | 0.08 |
| lAccu | 0.38** | 0.03 |
| rAccu | 0.28* | 0.03 |
| lITG | 0.46** | 0.26 |
| rITG | 0.32* | −0.07 |
| Cereb | −0.11 | −0.15 |
| Thal | 0.27* | 0.07 |
| rLIP | −0.15 | −0.05 |
| rIFG | 0.13 | 0.05 |
| mOFC | 0.23 | 0.15 |
Indicates Bonferroni corrected correlations at Pb0.05 (df=21).
Indicates a trend towards significance at Pb0.10 (df=21).
3.4. Using intra-individual variability to predict group classification
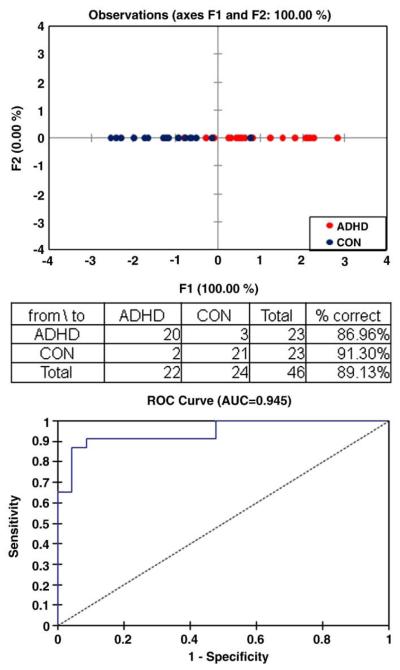
To determine if variability in the BOLD signal within an individual across the time series in the 13 ROIs can predict group membership, a discriminant analysis (DA) was performed. This analysis correctly identified 20 of 23 (87%) individuals as ADHD, 21 of 23 (91%) individuals as Controls, and 42 of 46 (89%) individuals of the entire sample (see Fig. 2). The receiver operating characteristics (ROC) curve indicates a 0.95 probability of correctly identifying an individual with ADHD. This analysis further illustrates that variability in the BOLD signal is a useful measure of characterizing ADHD, above and beyond simple analysis of ΔS.
Fig. 2.
Results of the discriminant analysis for BOLD signal variability. Receiver operating characteristic curves for the discriminant analysis indicating general fit of themodel.
4. Discussion
The current study yielded important new insights regarding the neural underpinning of ADHD in young adults, as well as the type of approach that can be fruitfully used to reveal them. The primary result indicated that a large group of brain regions spanning both cortical and sub-cortical regions, as well as anterior and posterior regions, exhibited activity that was significantly negatively correlated with the severity of ADHD inattentive symptomatology. Consistency of the findings with these brain regions across the different blocks of the Stroop task (Incongruent, Congruent, and Neutral) indicates the stability of their involvement across different levels of attentional demand. While many of these brain regions have been identified as being potentially dysfunctional in previous studies of individuals with ADHD, to our knowledge they have not been shown to be functionally associated in a single empirical study. Hence, the current study has identified a network of brain regions that are is related to symptom severity in young adults with combined-type ADHD.
There are a number of notable aspects to these findings. First, it is likely that the effects observed in this study are related to ADHD. We carefully excluded individuals with comorbid psychiatric and/or learning disorders that can influence attentional behavior, and our ADHD and control individuals were matched in IQ and academic skills, thereby ruling out these variables as likely sources of group differences. Second, the strong correlations of inattentive symptoms with activity within this set of brain regions were specific. It was not observed in relation to activity in other brain regions that are less related to attentional processing but which were nonetheless activated in response to task demands (e.g., visual and motor areas), nor to a set of regions that yielded group differences in overall signal change (i.e., regions of DLPFC and ACC). Third, activity in this set of brain regions was not significantly correlated with hyperactivity. Thus, the functioning of this set of brain regions, when challenged by an attentionally-demanding task, appears to be related somewhat specifically to inattentive symptoms in young adults with ADHD.
Furthermore, the results indicated that the variability in BOLD signal across time in these brain regions in individuals with ADHD is related to their performance on a task of attentional control. This variability is manifested in two main ways. First it is evident across individuals, as the average BOLD signal in these regions was more variable across members of the ADHD group than across members of the control group. Importantly, this group difference is not dependent on the level of ΔS, which was similar for each group. Second, within-individual variability in the BOLD signal across the time series in four distinct brain regions (with a trend in an additional 5 regions) predicted variability in RTs across trials on the Stroop task in ADHD individuals, whereas no correlation was found for controls. Interestingly, regions that did show differences between the two groups in the degree of BOLD activation (based on our previous analyses; Banich et al., 2009)didnotexhibit differences in either, inter-individual variability nor the correlation of intra-individual variability in the BOLD signal with variability across trials in RT. This set of findings suggests that brain regions predicted by inattentive symptomatology isolates a unique set of regions that are characterized by greater variability in responding across time. Moreover, this approach of examining the variability of the neural response under conditions of attentional demand provides a novel and powerful way of discriminating ADHD and control individuals. Discriminant analysis using intra-individual variability in BOLD signal achieved an 89% level of correct prediction, perhaps indicating that variability of neuronal response is a particularly strong index of brain dysfunction in ADHD.
Two implications of our results regarding research on ADHD are noteworthy. First, most of the 13 regions that composed the brain network whose activity is related to symptom severity are targets of dopaminergic innervation. Regions of dorsolateral prefrontal and orbitofrontal cortex are inervated by mesolimbic and mesocortical DA pathways (Depue and Collins, 1999). In addition, the cerebellum (Anderson et al., 2002), inferior temporal gyrus (Goldsmith and Joyce, 1996), and parietal lobe have also been linked to each of these DA pathways. Such a finding is not surprising as DA pathways have been implicated as being dysfunctional in individuals with ADHD, and alterations in both the DA transporter (DAT) and DA synthesis have been found in the striatum (Hesse et al., 2006; Volkow et al., 2007), PFC (Ernst et al., 1998) and midbrain (Ernst et al., 1999). Whether either between or within group variability in ADHD is intimately related to variability in DA function would appear to be worthy of further investigation.
Second, our results are also interesting in light of basic research regarding attentional control. As was initially suggested by Mesulam (1981), attentional control is exerted by a series of networks that act in a somewhat specialized but distributed manner. In the present study we found that there was a diverse set of cortical and sub-cortical brain regions that were related to symptomatology. What appears to link them together is that they have moment-to-moment variability of response in the face of attentional demands. Hence, assessing the dynamics of brain activation over time may provide additional insights into the nature of attentional control both in normal and clinical populations.
Our results also have implications for how brain imaging data can be used to identify brain regions that are dysfunctional in a clinical group. As argued in the Introduction, examining the correlation between symptom severity and brain activation may provide distinct information from looking for group differences in the level of activation. In fact, the regions that were identified with the current approach are not regions that yielded group differences in activity as shown by the comparison of ΔS in the present analysis and more standard group comparisons using a GLM approach (Banich et al., 2009). Moreover, the regions implicated in standard GLM analysis for group differences in activation levels exhibited no difference in either the inter-individual variability, or in the correlation between intra-individual variability in BOLD and intra-individual variability in RT. Hence, correlating inattentive symptomatology with brain activation appears to isolate brain regions distinct from those that show group differences in activation. These brain regions appear to be characterized by (1) exhibiting higher degrees of variability of the average BOLD signal across individuals within the ADHD group than controls and (2) exhibiting a relationship of intra-individual variability of the BOLD signal over time and variability in mean RT overtime within the ADHD group.
An interesting issue to consider is what explains the relative lack of overlap between the brain regions identified in the current study as being related to ADHD and those identified using a group-differences GLM approach (Banich et al., 2009). We speculate that those regions identified by the group-differences GLM approach are consistently affected across all individuals with ADHD, and as such identify core and critical regions for the disorder. In contrast, the regions identified in the current study may represent regions that show dysfunction in a dose response manner–the more severe the disorder, the greater the dysfunction of these regions. Because ADHD is thought to have multi-factorial etiology (Pennington, 2009), it may be that dysfunction of the regions identified in the current study represents an additive or multiplicative effect of these factors on brain organization and behavioral performance.
The current study has several limitations that need to be considered. First, a significant difference emerged for age between ADHD and control groups. Although the difference was significant, the mean values of age are only different by one year (19 vs. 20) and we think that it is highly unlikely that this difference made a significant contribution to our results. Second, although our discriminant analysis strongly dissociated ADHD from control individuals, it is unclear if this would be useful clinically. Furthermore, it is not clear if other psychiatric populations would exhibit similar variations in BOLD signal. This finding needs replication, with an emphasis on establishing thresholds for variability and examining additional psychiatric populations. Finally, while attentional dysfunction in ADHD, as indexed by variability in brain activation, manifested itself in the Stroop task, the extent to which the findings generalize to other types of attentional tasks will need to be explored.
Taking the above findings together, our results indicate that a major set of brain regions associated with ADHD (i) correlates with inattentive but not hyperactive symptomatology; (ii) shows enhanced variability in BOLD response over time, which is correlated with more variable behavioral performance; and (iii) that variability of brain activation is a particularly powerful predictors of ADHD diagnosis. These findings are concordant with a conception of ADHD neural dysfunction as reflecting altered functioning within a set of brain regions, which may form a functional circuit(s). Because adequate functioning of these circuits is dependent on DA transmission, the possibility is raised that increased variability of neural activity in these sets of regions may reflect alterations in DA neurotransmission, which has been suggested to be causally linked to the expression of the disorder.
Supplementary Material
Acknowledgements
NIMH grant R01 MH 070037 (Banich, P.I.) provided support for data collection and analysis as well as salary support for all but the fourth author. We thank Bruce Pennington for helpful discussion of this data. In addition, appreciation is also given to Deb Singel for her assistance with data collection and MR technical issues. Finally, we thank Anonymous Reviewer #2 for insightful comments in our review process.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2009.11.011.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Progressive Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. American Journal of Psychiatry. 2002;159:1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Burgess GC, Depue BE, Ruzic L, Bidwell LC, Hitt-Laustsen S, Willcutt EG. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47:3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin and Review. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of American Academy of Child Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clinical Pediatrics. 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue R, Collins P. Neurobiology and the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz M, Van Engelend H. Dopamine transporter genotype conveys familial risk of attention-deficit/ hyperactivity disorder through striatal activation. Journal of American Academy of Child Adolescent Psychiatry. 2008;47:61–67. doi: 10.1097/chi.0b013e31815a5f17. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults: a [fluorine-8] fluorodopa positron emission tomographic study. Journal of Neuroscience. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. American Journal of Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Goldsmith K, Joyce JN. Dopamine D2 receptors are organized in bands in normal human temporal cortex. Neuroscience. 1996;74:435–451. doi: 10.1016/0306-4522(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Helps S, James C, Debener S, Karl A, Sonuga-Barke EJ. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. Journal of Neural Transmission. 2008;115:279–285. doi: 10.1007/s00702-007-0825-2. [DOI] [PubMed] [Google Scholar]
- Hesse S, Ballaschkle O, Barthel H, von Cramon D, Sabri O. The striatal dopamine transporter availability is reduced in adults with attention-deficit/hyperactivity disorder. Journal of Nuclear Medicine. 2006;47:142. [Google Scholar]
- Hesslinger B, van Elst L.Tebartz, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Konrad S, Neufang C, Hanisch G, Fink R, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Leth-Steenson C, Elbaz ZK, Douglas VI. Mean response times, variability and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Diagnosing Learning Disorders. The Guilford Press; New York: 2009. [Google Scholar]
- Rubia K, Schuri U, van Cramon DY, Poeppel E. Time estimation as a neuronal network property: a lesion study. Cognitive NeuroReport. 1997;8:1273–1276. doi: 10.1097/00001756-199703240-00043. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A. Hypofrontality in attention deficit hyperactivity disorder (ADHD) during higher-order motor control: a study using fMRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith A, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001;79:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphe-nidate on motor timing. Journal of Abnormal Child Psychology. 2003;3:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naïve boys with Attention-Deficit/Hyperactivity Disorder during attention allocation and its relation to response variability. Biological Psychiatry. 2007a;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery for impulsiveness. Child Neuropsychology. 2007b;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. American Journal of Psychiatry. 2000;157:278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar J, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.