Abstract
Background
Aging and diabetes are major risk factors for poor wound healing and tissue regeneration that reflect an impaired ability to respond to ischemic insults. The authors explored the intrinsic neovascular potential of adipose-derived stromal cells in the setting of advanced age and in type 1 and type 2 diabetes.
Methods
Adipose-derived stromal cells isolated from young, aged, streptozotocin-induced, and db/db diabetic mice were exposed to normoxia and hypoxia in vitro. Vascular endothelial growth factor (VEGF) expression, proliferation, and tubulization were measured. Conditioned media harvested from adipose-derived stromal cell cultures were assessed for their ability to stimulate human umbilical vein endothelial cell proliferation (n = 3 and n = 3).
Results
Young adipose-derived stromal cells demonstrated significantly higher levels of VEGF production, proliferation, and tubulogenesis than those derived from aged, streptozotocin-induced, and db/db mice in both normoxia and hypoxia. Although aged and diabetic adipose-derived stromal cells retained the ability to up-regulate VEGF secretion, proliferation, and tubulogenesis in response to hypoxia, the response was blunted compared with young controls. Conditioned media derived from these cells cultured in normoxia in vitro also had a significantly greater ability to increase human umbilical vein endothelial cell proliferation compared with media harvested from aged, streptozotocin-induced, and db/db adipose-derived stromal cells. This effect was magnified in conditioned media harvested from hypoxic adipose-derived stromal cell cultures.
Conclusions
This study demonstrates that aging and type 1 and type 2 diabetes impair intrinsic adipose-derived stromal cell function; however, these cells may still be a suitable source of angiogenic cells that can potentially improve neovascularization of ischemic tissues.
Multipotent mesenchymal stromal cells are capable of osteogenic, chondrogenic, myogenic, and adipogenic differentiation.1,2 Although mesenchymal stromal cells have been harvested primarily from bone marrow, these cells can also be isolated from several other tissue compartments, particularly adipose tissue.3 A comparative analysis of mesenchymal stromal cells obtained from bone marrow and adipose tissue clearly demonstrated that adipose-derived stromal cells are equivalent to bone marrow–derived mesenchymal stromal cells with regard to morphology, cell surface receptor profile, and differentiation capacity.4–6 In addition, adipose-derived stromal cells offer distinct advantages over bone marrow–derived mesenchymal stromal cells because they are readily accessible, plentiful, and expandable. Therefore, the accessibility, abundance, and multilineage differentiation capacity of adipose-derived stromal cells has stimulated tremendous interest in using this cell population for regeneration and replacement of mesenchymal-derived tissues such as bone, cartilage, and muscle.7
Recent reports describing the ability of adipose-derived stromal cells to differentiate into vascular/endothelial cells has inspired many researchers to investigate the use of adipose-derived stromal cells to enhance neovascularization for the treatment of ischemic disorders.8 Postnatal neovascularization was previously thought to occur only by angiogenesis, which is the formation of new blood vessels through the proliferation and remodeling of differentiated endothelial cells derived from existing blood vessels.9 It is now well established that postnatal neovascularization also occurs by vasculogenesis, which is the de novo formation of blood vessels through the recruitment, proliferation, and differentiation of stem/progenitor cells.10,11
A significant amount of data have been published regarding the neovascular potential of bone marrow–derived mesenchymal stromal cells, and recently adipose-derived stromal cells have also been reported to possess similar vascular capabilities.12,13 Human and murine adipose-derived stromal cells have been shown to release many potent angiogenic factors, differentiate into endothelial cells, and form tubules on Matrigel in vitro.14–16 Similarly, in vivo studies have demonstrated that human and murine adipose-derived stromal cells can incorporate into blood vessels by differentiating into endothelial cells and subsequently enhance the recovery of perfusion in a murine model of hind-limb ischemia.14–16 However, these previous reports have studied only wild-type adipose-derived stromal cells and have not yet explored adipose-derived stromal cells derived from aged or diabetic populations, as would be important for human clinical application.
Advanced age17,18 and diabetes19 are major risk factors for vascular complications such as cardiovascular disease, peripheral vascular disease, and impaired wound healing. The extent of ischemic damage resulting from these complications is greatly increased by the impaired ability to form new blood vessels by means of angiogenesis and vasculogenesis following a hypoxic injury.20–22 Our study aims to explore the in vitro vascular biology of adipose-derived stromal cells and investigate whether the intrinsic neovascular potential of adipose-derived stromal cells is altered with advanced age or diabetes mellitus type 1 or 2.
MATERIALS AND METHODS
Animals
All experiments were performed in accordance with Stanford University Animal Care and Use Committee guidelines. Young (3-month-old) C57BL6 mice and db/db mice (BKS.Cg-m+/+Leprdb) were obtained from Jackson Laboratories (Bar Harbor, Me.). Homozygous db/db mice possess a genetic mutation of the leptin receptor and represent a model of type 2 diabetes mellitus characterized by hyperglycemia, obesity, hyperinsulinemia, and impaired wound healing. Aged (2 year old) C57BL6 mice were obtained from the National Institute of Aging (Bethesda, Md.). Type 1 diabetes was induced in C57BL6 mice through five consecutive daily intraperitoneal injections of 50 mg/kg streptozotocin (Sigma-Aldrich, St. Louis, Mo.) in 7.5 mg/ml sodium citrate (pH, 4.5). Six weeks after the final injection, glucose levels were assessed by a glucometer (Roche Diagnostics, Indianapolis, Ind.) to confirm successful induction of diabetes. Only db/db and streptozotocin-induced mice with sustained blood glucose levels above 350 g/dl were included in the study.
Adipose-Derived Stromal Cell Isolation and Culture
Inguinal mouse fat pads were excised, washed sequentially in povidone-iodine and phosphate-buffered saline (Sigma-Aldrich), and minced finely. The adipose tissue was digested with 0.075% type II collagenase (Sigma-Aldrich) and incubated in a 37°C shaking water bath for 30 minutes. The collagenase was neutralized with an equal volume of Dulbecco’s Modified Eagle Medium (Gibco Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (Gibco Invitrogen) and 1% antibiotic-antimycotic (Gibco Invitrogen). The tissue mixture was centrifuged at 1200 g for 5 minutes to separate mature adipocytes from the remaining stromal vascular fraction. The pellet was resuspended in growth media and passed through a 100-μm Falcon nylon cell strainer (Becton Dickinson, Bedford, Mass.) to remove undigested tissue fragments. The adipose-derived stromal cells were plated in a tissue culture dish and maintained at 37°C in 21% oxygen and 5% carbon dioxide. Growth media was changed 24 hours after the initial plating and every 3 days thereafter. Adipose-derived stromal cells were allowed to grow to subconfluence before detachment using 0.25% trypsin–ethylenediaminetetraacetic acid (Sigma-Aldrich) and subcultured 1:4. Adipose-derived stromal cells from passages 3 to 5 were used for all subsequent experiments.
Adipose-Derived Stromal Cell Characterization
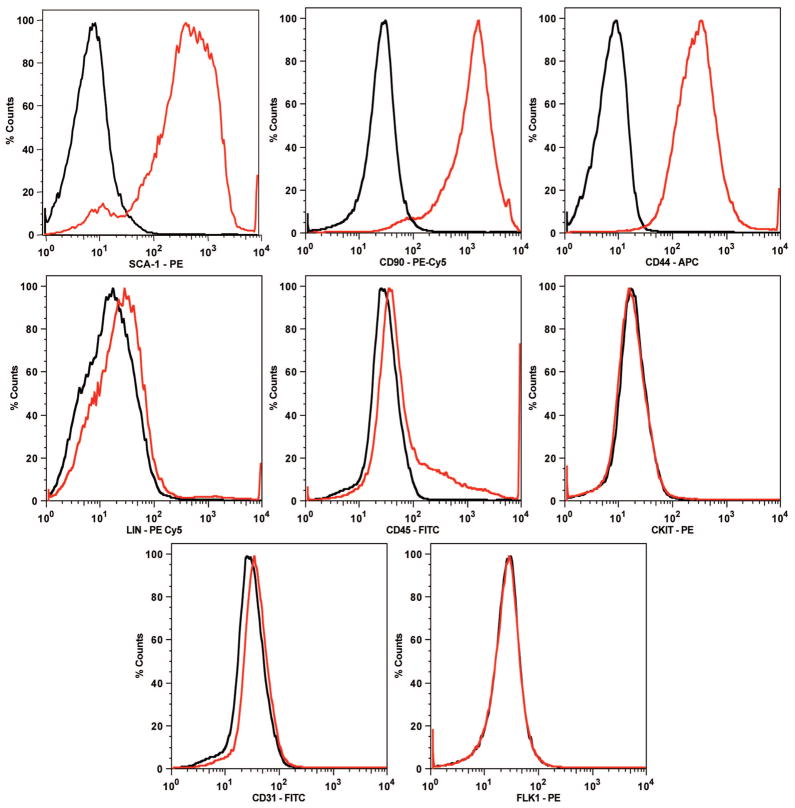
Cultured adipose-derived stromal cells were analyzed by fluorescence-activated cell sorting using the LSR flow cytometer (Becton Dickinson). Cells were detached, centrifuged at 1200 g for 5 minutes, and resuspended in phosphate-buffered saline containing 2% fetal bovine serum. Adipose-derived stromal cells were incubated with rat anti-mouse monoclonal antibodies against fluorescein isothiocyanate–conjugated CD31or CD45; phycoerythrin-conjugated Sca-1, c-kit/CD117, or Flk-1; phycoerythrin-Cy5–conjugated CD90 or lineage markers (CD4, CD8, CD11b, B220, GR-1, and TER-119); or allophycocyanin-conjugated CD44 for 30 minutes at 4°C in the dark. All antibodies were purchased from Becton Dickinson, except lineage antibodies, which were purchased from eBiosciences (San Diego, Calif.), and phycoerythrin-Cy5 anti-rat secondary antibody, which was purchased from Invitrogen/Caltag Laboratories (Carlsbad, Calif.). For each sample, a minimum of 50,000 events were acquired using CellQuest software (Becton Dickinson) and analyzed by FlowJo software (Tree Star, Ashland, Ore.). The events were acquired and analyzed under identical conditions and gated to exclude cellular debris from analysis. Cell surface marker expression was determined by comparison with an isotype control on a histogram plot.
Adipose-Derived Stromal Cell Angiogenic Cytokine Production
Murine vascular endothelial growth factor (VEGF) sandwich enzyme immunoassay (R&D Systems, Minneapolis, Minn.) was used to measure the levels of angiogenic cytokine secretion from adipose-derived stromal cells. Adipose-derived stromal cells were exposed to 24 hours of in vitro normoxic (21% oxygen tension) or hypoxic (1% oxygen tension) conditions before cell supernatants were collected for analysis. In vitro hypoxia experiments were performed in a customized hypoxic incubator (Reming BioInstruments, Red-field, N.Y.) that was maintained at an oxygen tension of 1%. The collected cell culture supernatants were analyzed according to the manufacturer’s instructions. Results were corrected for differences in proliferation rates and reported in nanograms per milliliter.
Adipose-Derived Stromal Cell Proliferation
Adipose-derived stromal cells were seeded onto 96-well plates and allowed to adhere for 8 hours before serum starvation in Dulbecco’s Modified Eagle Medium containing 1% fetal bovine serum for 24 hours. After starvation, fully supplemented Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum was replaced, and adipose-derived stromal cells were cultured in in vitro normoxic (21% oxygen) or hypoxic (1% oxygen) conditions for 24 hours. Bromodeoxyuridine colorimetric cell proliferation assay was then performed according to the manufacturer’s instructions (Roche, Mannheim, Germany).
Endothelial Cell Proliferation
Conditioned media was harvested from adipose-derived stromal cells after a 72-hour exposure to in vitro normoxia (21% oxygen) or hypoxia (1% oxygen). Human umbilical vein endothelial cells (Clonetics, San Diego, Calif.) were exposed to a 24-hour incubation in either normoxic or hypoxic conditioned adipose-derived stromal cell media before proliferation rates were determined using the bromodeoxyuridine colorimetric cell proliferation assay as described previously.
Adipose-Derived Stromal Cell Tubulization Assay
Thawed Matrigel (Sigma-Aldrich) was added to chamber slides (Nalge Nunc, Rochester, N.Y.) and allowed to solidify at 37°C for 1 hour. Adipose-derived stromal cells were resuspended in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and seeded on top of the solidified Matrigel. Adipose-derived stromal cells were incubated for 6 hours in in vitro normoxia (21% oxygen) or hypoxia (1% oxygen) before analysis. Tubulogenesis was analyzed using a Zeiss Axioplan 2 light-fluorescent microscope (Carl Zeiss Vision, Aalen, Germany) equipped with Zeiss AxioCam HR digital imaging software (Carl Zeiss Vision). Tubulogenesis, the alignment of endothelial cells and generation of a patent lumen, was defined on Matrigel as a structure exhibiting a length four times its width and was quantified in five random 10× fields by two blinded observers.
Statistical Analysis
A t test or two-way analysis of variance was performed to compare data sets. All experiments were performed with a minimum of n = 3 and n= 3. All data are reported as the mean ± SD. Differences were reported as statistically significant for two-tailed values of p < 0.05.
RESULTS
Adipose-Derived Stromal Cell Characterization
The cell surface marker profile of our isolated adipose-derived stromal cells was determined to confirm that the cell population isolated using the described protocol was comparable to that described in the published literature. Adipose-derived stromal cells from passages 3 to 5 expressed identical cell surface antigens when analyzed by fluorescence-activated cell sorting. Adipose-derived stromal cells were positive for the cell surface markers Sca-1, CD90, and CD44 and negative for all lineage markers (CD4, CD8, CD11b, B220, GR-1, and TER-119), CD45, c-kit, CD31, and Flk-1 (Fig. 1). These data are congruent with the marker profile of mouse-derived stromal cells found in previous studies.8,23,24
Fig. 1.
Adipose-derived stromal cells from passages 3 to 5 expressed identical cell surface antigens when analyzed by fluorescence-activated cell sorting. Adipose-derived stromal cells were positive for the cell surface markers Sca-1, CD90, and CD44 and negative for all lineage markers (CD4, CD8, CD11b, B220, GR-1, and TER-119), CD45, c-kit/CD117, CD31, and Flk-1. These data are congruent with the marker profile of murine-derived stromal cells described in previous studies. Data are expressed as a histogram plot, with black representing isotype control and red representing experimental.
Adipose-Derived Stromal Cell Angiogenic Cytokine Production
VEGF is a potent angiogenic growth factor known to initiate neovascularization and increase capillary density in ischemic areas.25 Prior studies have demonstrated that adipose-derived stromal cells secrete increased levels of VEGF relative to terminally differentiated cells and also up-regulate VEGF expression under hypoxia.8,25,26 Consequently, we investigated whether aged or diabetic adipose-derived stromal cells are impaired in their ability to secrete VEGF under in vitro normoxic and hypoxic conditions in comparison with young adipose-derived stromal cells. This analysis revealed that significantly higher levels of VEGF secretion were seen in adipose-derived stromal cells derived from young mice than were seen in adipose-derived stromal cells derived from aged, streptozotocin-induced, and db/db mice under in vitro normoxia (young, 261.41 ± 13.69 pg/ml; aged, 185.28 ± 6.05 pg/ml; streptozotocin-induced, 143.68 ± 10.50 pg/ml; db/db, 129.97 ± 27.92 pg/ml VEGF; p < 0.05) and hypoxia (young, 451.37 ± 16.48 pg/ml; aged, 379.37 ± 8.48 pg/ml; streptozotocin-induced, 324.72 ± 17.21 pg/ml; db/db, 279.92 ± 12.21 pg/ml VEGF; p < 0.05) (Fig. 2). Of note, although aged, streptozotocin-induced, and db/db adipose-derived stromal cells were able to up-regulate VEGF secretion in reaction to hypoxia, their response was diminished compared with young controls (p < 0.05).
Fig. 2.
Adipose-derived stromal cell supernatants were analyzed for VEGF production by enzyme-linked immunosorbent assay after a 24-hour exposure to either normoxia (21% oxygen; red bars) or hypoxia (1% oxygen; blue bars). Significantly higher levels of VEGF secretion were seen in adipose-derived stromal cells derived from young mice than were seen in adipose-derived stromal cells derived from aged, streptozotocin-induced, and db/db mice under normoxic (**p < 0.05) and hypoxic conditions (***p < 0.05). However, aged, streptozotocin-induced, and db/db adipose-derived stromal cells retained the ability to up-regulate VEGF secretion in response to hypoxia but not to the extent seen in young adipose-derived stromal cells (*p < 0.05) (n = 3 and N = 3).
Adipose-Derived Stromal Cell and Endothelial Proliferation
Adipose-derived stromal cells have been shown to exert both autocrine and paracrine effects, thereby increasing their own proliferation rate and promoting the proliferation of neighboring endothelial cells.25,27 Although adipose-derived stromal cells are able to augment cellular proliferation under in vitro normoxic conditions, the effect is more pronounced under hypoxic conditions.25,27 The proliferation of adipose-derived stromal cells in hypoxia and their ability to increase the proliferation rate of surrounding endothelial cells is an important aspect of ischemic neovascularization. Therefore, we examined the proliferation rate of adipose-derived stromal cells derived from aged and diabetic mice and their ability to up-regulate the proliferation of human umbilical vein endothelial cells through the proangiogenic cytokines they secrete under in vitro normoxic and hypoxic conditions. Our data demonstrate that aging and diabetes impair neovascularization not only directly through decreased adipose-derived stromal cell proliferation but also indirectly, as they exhibit a diminished ability to stimulate endothelial cell proliferation compared with young controls.
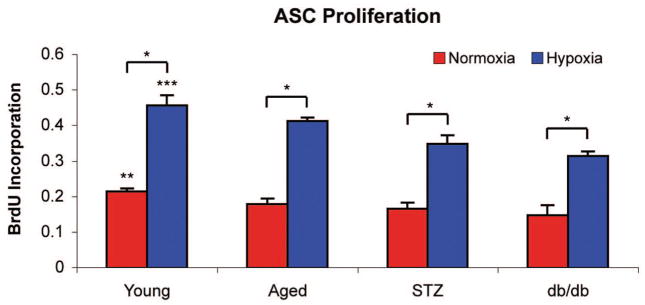
Adipose-derived stromal cells derived from young mice demonstrated a significantly higher proliferation rate than that seen in adipose-derived stromal cells derived from aged, streptozotocin-induced, and db/db mice under in vitro normoxia (young, 0.214 ± 0.009; aged, 0.178 ± 0.016; streptozotocin-induced, 0.166 ± 0.016; db/db, 0.147 ± 0.029 bromodeoxyuridine incorporation; p < 0.05) and hypoxia (young, 0.456 ± 0.029; aged, 0.412 ± 0.010; streptozotocin-induced, 0.348 ± 0.024; db/db, 0.313 ± 0.013 bromodeoxyuridine incorporation; p < 0.05) (Fig. 3). Importantly, despite a modest proliferative response in hypoxia, aged, streptozotocin-induced, and db/db adipose-derived stromal cells continue to demonstrate impaired intrinsic functional capacity compared with young controls (p < 0.05) (Fig. 3).
Fig. 3.
Adipose-derived stromal cell proliferation rates were measured by bromodeoxyuridine incorporation after a 24-hour exposure to either normoxia (21% oxygen, red bars) orhypoxia (1%oxygen, blue bars). Adipose-derived stromal cells derived from young mice demonstrated significantly increased bromodeoxyuridine incorporation than that seen in adipose-derived stromal cells derived from aged, streptozotocin-induced, and db/db mice under normoxic (**p < 0.05) and hypoxic conditions (***p < 0.05). However, aged, streptozotocin-induced, and db/db adipose-derived stromal cells retained the ability to up-regulate the rate of proliferation in response to hypoxia but not to the extent seen in young adipose-derived stromal cells (*p < 0.05) (n = 3 and N = 3).
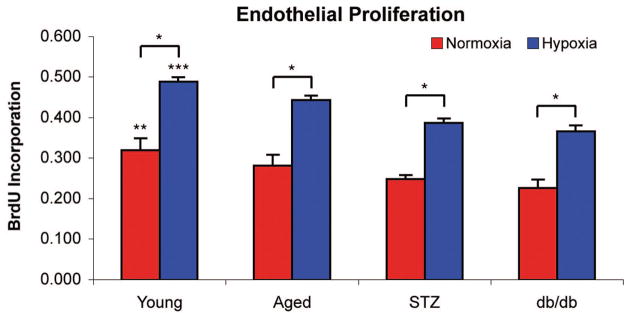
As predicted, conditioned media harvested from young adipose-derived stromal cells had a significantly greater impact on human umbilical vein endothelial cell proliferation than conditioned media derived from aged, streptozotocin-induced, or db/db adipose-derived stromal cells in in vitro normoxia (young, 0.319 ± 0.030; aged, 0.281 ± 0.027; streptozotocin-induced, 0.248 ± 0.010; db/db, 0.226 ± 0.021 bromodeoxyuridine incorporation; p < 0.05) (Fig. 4). When conditioned media harvested from adipose-derived stromal cells cultured in hypoxia was applied to human umbilical vein endothelial cells, the effect on human umbilical vein endothelial cell proliferation was even more dramatic (young, 0.488 ± 0.011; aged, 0.443 ± 0.011; streptozotocin-induced, 0.387 ± 0.011; db/db, 0.366 ± 0.014 bromodeoxyuridine incorporation; p < 0.05) (Fig. 4).
Fig. 4.
Proliferation rates of human umbilical vein endothelial cells were measured by bromodeoxyuridine incorporation after 24 hours of incubation in conditioned media harvested from adipose-derived stromal cells that were exposed to normoxia (21% oxygen, red bars) or hypoxia (1% oxygen, blue bars) for 72 hours. Normoxic conditioned media harvested from young adipose-derived stromal cells had a significantly greater ability to increase proliferation of human umbilical vein endothelial cells than conditioned media harvested from aged, streptozotocin-induced, or db/db adipose-derived stromal cells (**p < 0.05). Conditioned media from hypoxic cultures also generated a significant increase in human umbilical vein endothelial cell proliferation that was even more pronounced than normoxia-exposed conditioned media (***p < 0.05) (n = 3 and N = 3).
Tubulogenesis
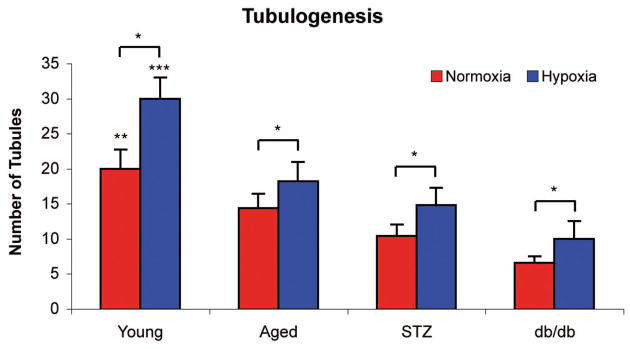
Adipose-derived stromal cells have previously been shown to form vascular tubules on Matrigel.14 We consequently used this functional assay to assess the induction of tubulogenesis by adipose-derived stromal cells derived from aged and diabetic mice. The left panels of Figure 5 show that adipose-derived stromal cells derived from young mice demonstrated a superior ability to undergo tubulogenesis on Matrigel than that seen in aged, streptozotocin-induced, or db/db adipose-derived stromal cells under in vitro normoxic conditions (young, 20 ± 2.7; aged, 14.4 ± 2.1; streptozotocin-induced, 10.4 ± 1.7; db/db, 6.6 ± 0.9 tubules per 10× field; p < 0.05) (Fig. 6). Young adipose-derived stromal cells formed thick, three-dimensional tubules that interconnected between cell clusters, whereas aged adipose-derived stromal cells formed thin, cord-like tubules connecting cell clusters. Streptozotocin-induced and db/db adipose-derived stromal cells formed significantly fewer tubules with markedly less intricate interconnecting networks between cell clusters than young controls. Interestingly, exposure to hypoxia (Fig. 5, right panels) further increased tubule formation in young, aged, streptozotocin-induced, and db/db adipose-derived stromal cells; however, as expected, aged and diabetic adipose-derived stromal cells failed to form the degree of tubulization seen in young adipose-derived stromal cells (young, 30.0 ± 3.0; aged, 18.2 ± 2.8; streptozotocin-induced, 14.8 ± 2.5; db/db, 10.0 ± 2.5 tubules per 10× field; p < 0.05) (Fig. 6).
Fig. 5.
Representative images of tubulization of adipose-derived stromal cells harvested from young (first row), aged (second row), streptozotocin-induced type I diabetic (third row), and db/db type II diabetic mice (last row) in normoxic (left panels) and hypoxic (right panels) conditions.
Fig. 6.
Quantification of adipose-derived stromal cell tubulization after seeding on Matrigel for 6 hours in either normoxia (21% oxygen) or hypoxia (1% oxygen). Adipose-derived stromal cells derived from young mice demonstrated a significantly superior ability to undergo tubulogenesis on Matrigel than that seen in aged, streptozotocin-induced, or db/db adipose-derived stromal cells under normoxic conditions (red bars; **p < 0.05). Hypoxia exposure increased tubule formation in all groups (blue bars); however, the response in young adipose-derived stromal cells far exceeded the degree of tubulization seen in other groups (***p< 0.05). Of note, the diabetic adipose-derived stromal cells demonstrated the most severe impairment in hypoxia-induced tubulogenesis (n = 3 and N = 3).
DISCUSSION
Adipose-derived stromal cells offer an attractive option and have tremendous potential in the field of therapeutic neovascularization. Not only are adipose-derived stromal cells easily harvested, readily available, and abundant in high numbers, they posses the ability to differentiate into cells of mesenchymal and nonmesenchymal origin. In particular, adipose-derived stromal cells have been shown to have unlimited self-replicating capacity and the ability to secrete potent proangiogenic cytokines, differentiate into endothelial cells, and form functional vascular tubules in vitro and in vivo.14–16 Consequently, these properties make adipose-derived stromal cells an attractive candidate for tissue engineering and cell-based therapies.
To our knowledge, this is the first report that adipose-derived stromal cells derived from aged and diabetic mice exhibit impairments in their ability to respond to hypoxic stimuli. However, despite a reduction in function, adipose-derived stromal cells harvested from aged and diabetic mice do retain a certain degree of their intrinsic vascular potential, similar to adipose-derived stromal cells derived from young controls. Specifically, adipose-derived stromal cells derived from aged and diabetic mice exhibit significantly reduced baseline levels of cellular proliferation, VEGF expression, and tubulization compared with young adipose-derived stromal cells in in vitro normoxic and hypoxic conditions. It is therefore not surprising that conditioned media harvested from young adipose-derived stromal cells containing proneovascular cytokines including VEGF have a more dramatic effect on endothelial cells than conditioned media derived from aged and diabetic adipose-derived stromal cells. It is also important to note that the defects observed in proliferation, cytokine secretion, and tubulization were more pronounced in diabetic adipose-derived stromal cells than in aged adipose-derived stromal cells. However, despite a significant reduction in their baseline intrinsic function, their ability to mount a response in the setting of hypoxia was not completely eliminated. Because aged and diabetic adipose-derived stromal cells are still able to manifest a hypoxic response, autologous adipose-derived stromal cells may still potentially be harvested from elderly and diabetic patients as a source of regenerative stem cells for the treatment of chronic wounds and ischemic tissues, pending confirmation by future in vivo studies that are underway.
From a clinical perspective, if aged and diabetic adipose-derived stromal cells do exhibit deficiencies in their neovascular potential, it is critical to identify the underlying mechanism for these impairments if their clinical applicability is to come to fruition. Controversy exists over whether a deficiency exists within the stem cells themselves (“the seed”) or whether the deficiency results from impairments in the environmental signals (“the soil”) necessary to recruit those stem cells. Heiss et al. reported that aging may result in a primary “seed” dysfunction in terms of endothelial progenitor cell proliferation, migration, and survival.28 However, our laboratory has recently shown that impaired neovascularization seen in aged endothelial progenitor cells results primarily from an inability of the soil to produce the hypoxia-induced signals necessary for endothelial progenitor cell recruitment.20 We have also previously demonstrated that endothelial progenitor cells derived from patients with type 2 diabetes exhibit alterations in functions important for blood vessel formation, including proliferation, adhesion, and incorporation into vascular structures.21,22 However, recent studies from our laboratory also suggest that diabetes results in defects in the soil and the seed.29
Our laboratory and others have identified hypoxia as playing a fundamental role in ischemia-induced neovascularization.22,30 Endothelial progenitor cells, bone marrow–derived mesenchymal stem cells, and recently adipose-derived stromal cells have been shown to be capable of responding to ischemic injury in a manner proportional to the degree of hypoxic stress.21,22,26,31–33 This response to a hypoxic stimulus is tightly regulated by a family of hypoxia-responsive transcription factors, most notably hypoxia inducible factor (HIF)-1.30 The α-subunit of HIF-1 is stabilized under low oxygen tension and regulates expression of several genes that are critical to the neovascular process, including VEGF.30 We have previously demonstrated that downstream effectors of HIF-1α such as VEGF and SDF-1α are critical for the mobilization and trafficking of progenitor cells to sites of neovascularization, and promote the adoption of functional characteristics necessary for new blood vessel growth and maturation.22,33,34
Considered together, these data implicate HIF-1α as one potential mechanism explaining why elderly and diabetic patients have impairments in blood vessel formation and why endothelial progenitor cells derived for these populations may not be ideal sources for cell-based therapy. However, because adipose-derived stromal cells are significantly more abundant than endothelial progenitor cells and may play similar roles in the process of neovascularization, we investigated whether aged or diabetic adipose-derived stromal cells could potentially be used in cell-based therapies for the treatment of vascular ischemic diseases. We have demonstrated that although aged and diabetic adipose-derived stromal cells do exhibit some inherent dysfunction in proliferation, VEGF secretion, and tubulization, they are able to mount a hypoxic response nonetheless. However, their ability to facilitate wound healing and prevent tissue necrosis in vivo remains to be elucidated and is an area of active investigation in our laboratory to assess their therapeutic potential in autologous cell-based therapies. As we have previously identified the mechanism for similar deficiencies in a similar population of progenitor cells, namely, endothelial progenitor cells, we are also poised to explore the mechanism underlying the adipose-derived stromal cell phenomenon as well. In light of our previous studies identifying deficiencies in the soil in aging and diabetes, we are actively exploring therapeutic strategies targeting HIF-1α to correct this deficiency. Thus, strategies aimed to augment the HIF cascade or increase HIF-1α stabilization may have similar effects on adipose-derived stromal cells, and could potentially be used as a therapeutic adjunct to cell-based therapies.
Our study suggests that therapeutic neovascularization techniques based solely on aged or diabetic adipose-derived stromal cells alone may be insufficient to augment neovascularization and stimulate tissue regeneration in those patients. If such cell-based therapy techniques are to reach clinical application, the functionality of these transplanted cells must be corrected. This represents a focus of intense research in our laboratory and many others, and the culmination of these results could have a dramatic impact on the management of chronic wounds, decubitus pressure sores, and diabetic ulcers in these patients.
CONCLUSIONS
Our study is the first to identify in vitro intrinsic impairments in the population of adipose-derived stromal cells in the setting of advanced age and particularly in the setting of type 1 and type 2 diabetes. Thus, caution must be exercised before cell-based strategies relying solely on adipose-derived stromal cells can be fully adopted and integrated into clinical application. Further investigation into the in vivo implications and the underlying mechanism for these impairments is certainly warranted before adipose-derived stromal cells can used for the treatment and prevention of chronic wounds in high-risk populations.
Acknowledgments
This study was supported by National Institute of Aging grant R01-AG025016 (G.C.G.), National Institute of Diabetes and Digestive and Kidney Diseases grant DK-074095 (G.C.G.), National Institute of Dental and Craniofacial Research grant R01-DE14526 (M.T.L.), the National Institutes of Health LRP (E.I.C.), and the Oak Foundation (M.T.L. and G.C.G.)
Footnotes
Disclosure: None of the authors has a financial interest to disclose in relation to the content of this article.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1626. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner W, Wein F, Seckinger A. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Kern S, Eichler H, Stroeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 6.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 7.De Ugarte DA, Ashjian PH, Elbarbary A, Hedrick MH. Future of fat as raw material for tissue regeneration. Ann Plast Surg. 2003;50:215. doi: 10.1097/01.SAP.0000029661.38066.15. [DOI] [PubMed] [Google Scholar]
- 8.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931. [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow–derived cells. Blood. 2005;105:1068. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Kim HK, Cho HH, Bae YC, Suh KT, Jung JS. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20:867. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 13.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2000;109:656. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 15.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 16.Moon MH, Kim SY, Kim YJ, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 17.Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci. 2003;8:s119–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DR. Age-related changes in wound healing. Drugs Aging. 2001;18:607–620. doi: 10.2165/00002512-200118080-00005. [DOI] [PubMed] [Google Scholar]
- 19.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 20.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1α stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 21.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 22.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 23.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 24.Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008;121:50. doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed] [Google Scholar]
- 25.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 26.Thangarajah H, Vial IN, Chang E, et al. IFATS series: Adipose stromal cells adopt proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2008 Oct 30; doi: 10.1634/stemcells.2008-0276. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 27.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 28.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Yao D, Grogan RH, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 31.Hamou C, Callaghan MJ, Thangarajah H, et al. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg. 2009;123(2 Suppl):45S–55S. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 33.Annabi B, Lee YT, Turcotte S, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 34.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]