Abstract
The neural crest (NC) lineage gives rise to a wide array of cell types ranging from neurons and glia of the peripheral nervous system to skeletal elements of the head. The mechanisms regulating NC differentiation into such a large number of cell types remains largely unknown. MicroRNAs (miRNAs) play key roles in regulating developmental events suggesting they may also play a role during NC differentiation. To determine what roles miRNAs play in differentiation of NC-derived tissues, we deleted the miRNA processing gene Dicer in NC cells using the Wnt1-Cre deleter line. We show that deletion of Dicer soon after NC cells have formed does not affect their migration and colonization of their targets in the embryo. However, the post-migratory NC is dependent on Dicer for survival. In the head, loss of Dicer leads to a loss of NC derived craniofacial bones while in the trunk, cells of the enteric, sensory and sympathetic nervous systems are lost during development. We found that loss of Dicer does not prevent the initial differentiation of NC but as development progresses, NC derivatives are lost due to apoptotic cell death. When Dicer is deleted, both Caspase-dependent and -independent apoptotic pathways are activated in the sensory ganglia but only the Caspase-dependent apoptotic program was activated in the sympathetic nervous system showing that the specific endogenous apoptotic programs are turned on by loss of Dicer. Our results show that Dicer and miRNAs, are required for survival of NC-derived tissues by preventing apoptosis during differentiation.
Keywords: Dicer, miRNA, neural crest, peripheral nervous system, apoptosis
INTRODUCTION
How animals generate the vast cellular diversity that accompanies development is a fundamental question in developmental biology. One lineage that gives rise to a diverse array of cell types is the neural crest (NC). Neural crest cells (NCCs) form as a small population of cells at the boundary between the neural plate and the prospective epidermis through the orchestrated action of several morphogenic proteins and transcription factors (Basch and Bronner-Fraser, 2006). After undergoing an epithelial to mesenchymal transformation, NCCs migrate throughout the embryo contributing to the formation of a large number of tissues (Morales et al., 2005). There are four main divisions of the NC: (i) the cranial NC which gives rise to numerous components in the head including most of the facial bones and several of the bones forming the cranial vault, (ii) the vagal NC which contributes to the cardiac outflow tracts and the majority of the enteric nervous system, and the (iii) trunk and (iv) sacral NC which gives rise to melanocytes and the peripheral autonomic and sensory nervous systems (Graham et al., 2004; Le Douarin and Teillet, 1973; Verberne et al., 2000). In the peripheral nervous system, the NC derived neurons differentiate with phenotype diversity equal to that found in the central nervous system. For the NC to differentiate into such varied cell types the developmental programs must be under numerous levels of control. One mechanism that may contribute to generating this assortment is through the action of microRNAs (miRNAs).
MiRNAs are small RNA molecules that repress translation of mRNAs by binding target sequences that lie within the 3′ UTR of mRNAs (Zhao and Srivastava, 2007). Transcribed miRNAs are processed in the nucleus into a hair-pin structure that are transported to the cytoplasm for further processing by Dicer, an RNase III endonuclease, to produce the functional single stranded miRNAs (Agrawal et al., 2003; Nilsen, 2007; Zhao and Srivastava, 2007). These miRNAs are loaded onto the RNA Induced Silencing Complex (RISC) that directs them to their mRNA targets (Nilsen, 2007).
Blocking miRNA processing by deletion of Dicer in mice leads to loss of the inner cell mass of the blastocyst resulting in early embryonic lethality (Bernstein et al., 2003). Generating a conditional Dicer allele circumvented the early lethality allowing for functional studies of Dicer and miRNA function during development and adulthood (Harfe et al., 2005). Tissue-specific deletion of Dicer shows that it plays diverse roles during development including maintenance of tissues such as lungs, skin, bone, heart, the immune system and neurons of the central nervous system (Andl et al., 2006; Chen et al., 2008; Cobb et al., 2006; Cobb et al., 2005; Cuellar et al., 2008; Davis et al., 2008; Harfe et al., 2005; Koralov et al., 2008; O’Rourke J et al., 2007). Limb specific deletion of Dicer results in a decrease in limb size in correlation with an increase in cell death (Harfe et al., 2005) while deletion of Dicer in lung epithelium results in branching defects with a concurrent increase in cell death (Harris et al., 2006). During skeletal muscle development, the loss of Dicer leads to an increase in cell death producing muscle hypoplasia as well as abnormal myofiber morphology (O’Rourke J et al., 2007). In the CNS, deletion of Dicer results in region specific defects. In the cortex and hippocampus, Dicer is needed for cell survival and dendritic branching (Davis et al., 2008). In the neocortex, the loss of Dicer results in neurogenic progenitor cell death but not neuroepithelial progenitors (De Pietri Tonelli et al., 2008). These studies suggest that Dicer may be required for cell survival during the switch from uncommitted to committed neuronal progenitors. In addition to its early role in cell survival, Dicer is also required for post-mitotic Purkinje cell survival following terminal differentiation (Schaefer et al., 2007). However, Dicer is not required for survival of all differentiated cells. Dicer ablation in post-mitotic dopaminoceptive neurons show that Dicer is required for maintenance of cell size but not cell survival (Cuellar et al., 2008).
Here, we investigate the role of Dicer during development of NC derived tissues by deleting Dicer in NC using Wnt1-Cre. Our results show that loss of Dicer in NCCs results in developmental defects in all NC derived tissues. Dicer loss does not prevent colonization of target tissues or initial differentiation of NCCs, however, as differentiation progresses cells are lost through apoptosis. In the head, where NC differentiate to form a number of lineages including bone, deletion of Dicer leads to the loss of facial and cranial vault structures. In the trunk, NC form the peripheral nervous systems. Deletion of Dicer does not effect initial formation of the sympathetic, sensory or enteric nervous systems, but as the nervous systems begin to terminally differentiate, neurons undergo apoptotic cell death.
MATERIALS AND METHODS
Generation of mutant embryos
Dicer was deleted in the NC lineage by crossing mice carrying the conditional allele of Dicer (Harfe et al., 2005) with a transgenic line expressing Cre under the control of the Wnt1 promoter (Danielian et al., 1998). To generate breeding adults, Dicerfx/fx females were crossed with Wnt1-Cre males to generate Dicerfx/+; Wnt1-Cre males. To generate Dicer conditional knockout embryos, Dicerfx/+; Wnt1-Cre males were crossed with Dicerfx/fx females. Genotyping was performed as previously described (Harfe et al., 2005). To trace the NC lineage, the Rosa26R line (Mao et al., 1999) was crossed into the Dicerfx/fx females.
Immunohistochemistry
Embryos were collected in PBS and fixed overnight in 4% paraformaldehyde at 4°C. Heads were removed and the trunks were equilibrated with 30% sucrose in PBS at 4°C overnight. 10 μm cryosections were blocked with 10% goat serum in PBST (PBS, 0.1% Tween-20) for 1 hour and incubated with primary antibody at 4°C overnight. Primary antibodies and dilutions used in this study are as follows: mouse monoclonal anti-β-III-tubulin (Tuj1) (Covance) at 1:1000 dilution; rabbit polyclonal anti-Tyrosine Hydroxylase (TH) (Chemicon) 1:200 dilution; rabbit polyclonal anti-active Caspase-3 (Abcam) 1:200 dilution. Samples were washed with PBST for 3 times and incubated with secondary antibodies at room temperature for 2 hours. Secondary antibodies used in this study are as follows: fluorescein conjugated goat anti-rabbit (Vector Laboratories) 1:200 dilution; rhodamine conjugated goat anti-mouse (Chemicon) 1:200 dilution. After incubation with secondary antibody, tissues were washed in PBS followed by a second wash with PBS plus 0.5 μg/ml DAPI for 5 min and slides were mounted in glycerol/PBS (1:1). TUNEL assays (Promega) were performed according to manufacturers instructions.
β-Galactosidase staining
Embryos were fixed in 4% paraformaldehyde for 15 min at room temperature and washed three times for 5 minutes each in PBS. Fixed embryos were incubated overnight at room temperature in β-galactosidase staining solution (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2mM MgCl2 and 1mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) and post fixed in 4% paraformaldehyde.
Alcian Blue-Alizarin Red Staining
Skinned and eviscerated embryos were fixed and dehydrated in 95% ethanol for 24 hrs at room temperature. Embryos were stained in Alcian Blue staining solution (0.03% Alcian Blue, 80% ethanol, 20% Acetic Acid) for 2 days, washed overnight in 95% ethanol followed by incubation in 2% KOH solution for 24 hrs prior to staining with Alizarin Red (0.03% Alizarin red, 1% KOH) overnight. Embryos were cleared for documentation in 1% KOH/20% Glycerol solution and prepared for storage by passing embryos through graded glycerol/ethanol solutions (50%, 80% and 100%).
Imaging and Quantification
For quantification of ganglia size, 3 embryos and a minimum of 15 sections along the length of each embryo of each genotype were used for analysis. Sections were photographed and areas measured in pixels using Adobe Photoshop CS3 and area values were averaged for each animal and these data were used for statistical tests. To quantify the active Caspase-3 and TUNEL positive cells, 9 sections were photographed and immuno-positive cells were counted in Image J. The Student’s t-test was used for statistical analysis.
RESULTS
Dicer is required for survival of NC derived craniofacial structures
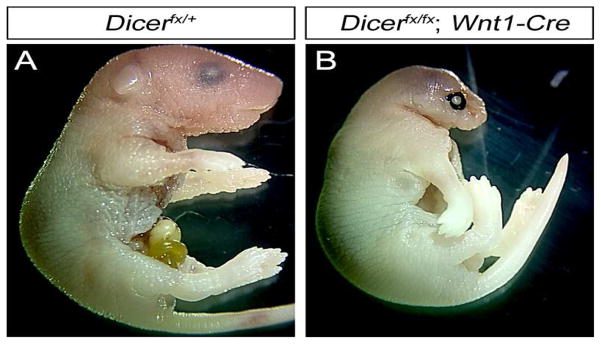
The NC lineage contributes to several structures of the head including bones, smooth muscle, glia and connective tissue (Graham et al., 2004; Santagati and Rijli, 2003). To investigate the roles of Dicer during cranial NC development, we deleted the Dicer gene in NC by crossing a conditional allele of Dicer with the Wnt1-Cre deleter mouse line. Genotype analysis of neonates showed that all genetic backgrounds survived to birth at the expected Mendelian ratio. Mice with the genotypes Dicerfx/+, Dicerfx/fx, and Dicerfx/+; Wnt1-Cre mice did not have morphological defects and were used as the control embryos. All Dicerfx/fx; Wnt1-Cre neonates have severe craniofacial malformations and extended forelimbs (Fig. 1). Aanalysis of mutant embryos did not show skeletal abnormalities outside the head (data not shown), suggesting that the extended forelimb phenotype in mutant embryos is due to neurological defects. The severity of craniofacial defects was most pronounced in the anterior region of the head corresponding to the region where NCCs contribute to the head. The loss of the anterior cranial vault resulted in displacement of the brain and neonates are unable to breath resulting in death immediately after birth.
Fig. 1. Loss of Dicer in the NC lineage results in severe cranial malformations.
Dicer was deleted in the NC lineage using the Wnt1-Cre deleter line and newborn pups were analyzed for gross phenotypic changes. (A) Dicerfx/+ newborn pups are phenotypically normal. (B) Dicerfx/fx; Wnt1-Cre pups show severe growth retardation of the head with the most pronounced defects in the anterior craniofacial region.
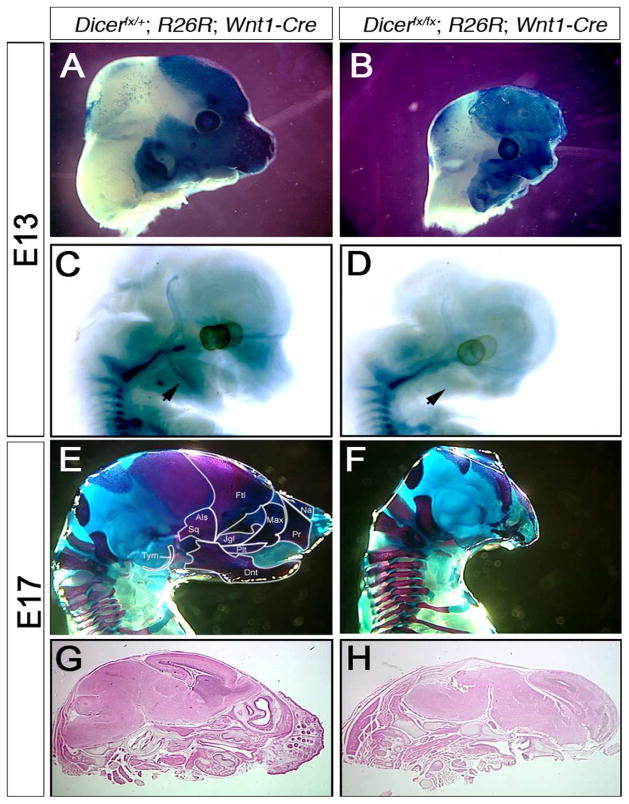
Although Cre expression does not commence until after formation of the NC, it is possible that the morphological defects in the head caused by deletion of Dicer results from defective NC migration and colonization of the head. To address these possibilities, the NC lineage was traced by Cre dependent β-galactosidase expression from the R26R locus (Fig. 2). The heads of Dicerfx/fx; R26R; Wnt1-Cre embryos at E11 are morphologically indistinguishable from their control littermates as is the level of β-galactosidase expression, suggesting that migration of NC into the head is not affected by loss of Dicer (data not shown). By E13, mutant embryos exhibit a reduced head size and retardation of facial structure outgrowth (Fig. 2A–B). NCCs in mutant heads are present, as shown by expression of β-galactosidase, but the level and area with β-galactosidase positive cells are reduced showing that the number of NCCs is reduced relative to control littermates (Fig. 2A–B).
Fig. 2. Dicer expression in NCCs is required for craniofacial bone formation.
(A–B) NCCs were traced by -galactosidase expression in E13 Dicerfx/+; R26R; Wnt1-Cre and (B) Dicerfx/fx; R26R; Wnt1Cre embryos. (A) At E13 the NCCs have migrated to their target locations in the head. (B) The loss of Dicer does not prevent NC migration and colonization of the head but the number of cells is reduced. (C–D) To detect differentiation of NCCs into cartilage, E13 embryos were stained with Alcian Blue. (C) Control embryos show cartilage formation in the vertebrae and mandible while in the absence of Dicer, Meckel’s cartilage is not formed (D) (arrowheads). (E–F) Cartilage and bone formation in embryos was examined in E17 embryos by staining with Alcian Blue-Alizarin Red. Bones that are NC derived are outlined in the control embryo (E). In mutant embryos, all NC derived craniofacial bones are either absent or severely reduced (F). (G–H) Heamotoxylin & eosin stained parasagital sections of control (G) and mutant (H) heads. In addition to loss of NC derived bones, the soft tissues of the face are lost including the whisker pads of mutant embryos. Tym: Tympanic, Als: Alisphenoid, Sq: Squomosal, Ftl: Frontal, Jgl: Jugal, Na: Nasal, Pr: Premaxilla, Plt: Palatine, Dnt: Dentary.
Most bones of the head are derived from the cranial NC lineage including the tympanic, alisphenoid, squomosal, frontal, jugal, nasal, premaxilla, palatine and dentary bones (Santagati and Rijli, 2003). To determine if Dicer is required for differentiation of NCCs into cartilage, embryos were stained with Alcian Blue. Meckel’s cartilage begins to form in the mandibular process at E13 (Fig. 2C). In Dicer mutant embryos, Meckel’s cartilage failed to form (Fig. 2D). To determine if the loss of cartilage is due to a delay in development or an inability of mutant NCCs to differentiate, cartilage and bone formation was examined in E17 embryos by Alician Blue - Alizarin Red staining (Fig. 2E, F). In control embryos all bones of the head have begun to form with the facial bones being more developed than the posterior bones that are not NC derived (Fig. 2E). In mutant embryos all NC derived facial and cranial vault bones are either absent or severely reduced in size (Fig. 2F). The bones of the cranial vault that remain appear to be the mesodermally derived exoccipital, supraoccipital and parietal. The size of the occipital bones are not affected by loss of the NC derived bones while the parietal bone is reduced in size. Our results show that Dicer is essential for survival of the NC derived cartilage and bones of the head.
A histological examination was undertaken to determine the effect of Dicer loss on the soft tissues of the head (Fig. 2G, H). E17 Hematoxylin-Eosin stained head sections show that the whisker pads, nasal cavities and lower jaw of the mutant embryos were lost (Fig 2H). On a gross level all regions of the brain appear to form, however, the mid-brain region is thinner and the lack of bones in the face results in the neocortex projecting forward. In the absence of bone, the brain projects outside the head at birth.
Dicer is required for enteric nervous system development
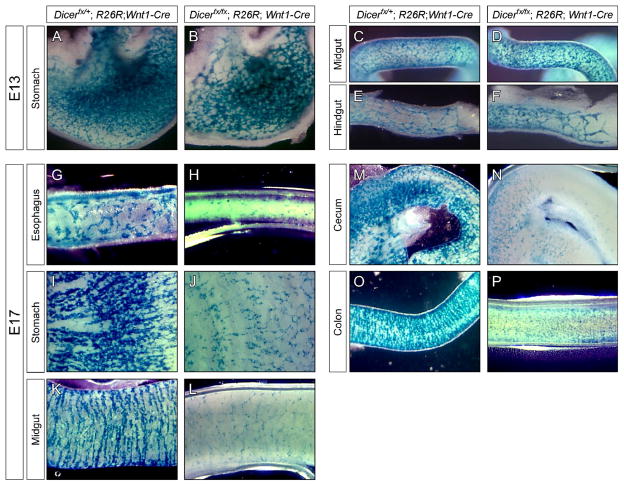
The vagal NC gives rise to the majority of the enteric nervous system (ENS) (Le Douarin and Teillet, 1973) with the sacral NC making a small contribution to the hindgut (Burns and Douarin, 1998; Kapur, 2000). Vagal NCCs enter the foregut at E9.5 and migrate along the developing gut to populate its entire length by E14.5 (Kapur, 2000). To determine if Dicer is required for NC to populate the gut, NCCs were traced in E13 embryos using β-galactosidase expression from the R26R locus to mark NC derived cells (Fig. 3A–F). At E13, NCCs colonize the length of the stomach with the mutant embryos showing a slight decrease in the number of NC and altered organization of the ENS (Fig. 3A–B). Since colonization of enteric NC occurs in a rostral to caudal manner, the decrease in ENS cells in stomach is not due to defective colonization of NC. The midgut of both control and mutant embryos is fully populated by similar numbers of NCCs (Fig. 3C–D). At E13, NCCs have entered the developing colon in similar numbers between control and mutant embryos but have not reached the terminal bowel (Fig. 3E–F). Our results show that loss of Dicer does not affect the colonization of the gut.
Fig. 3. Dicer is not required for colonization and formation of the enteric nervous system.
Development of the enteric nervous system was examined in E13 (A–F) and E17 (G–P) embryos by lineage tracing NCCs using β-galactosidase activation by Wnt1-Cre. In the stomach, Dicerfx/+; R26R; Wnt1-Cre control embryos (A) contain more cells in the ENS than Dicerfx/fx; R26R; Wnt1-Cre mutant embryos (B). In the midgut (C–D) and hindgut (E–F), the number of cells in the developing ENS are comparable. Comparison of control and mutant embryos at E17 shows that in the esophagus (G–H), stomach (I–J), midgut (K–L), cecum (M–N) and colon (O–P), the number of cells contributing to the ENS is dramatically reduced in mutant embryos relative control.
To determine if Dicer is required for maintenance of the ENS, NCCs were traced in Dicerfx/fx; R26R; Wnt1-Cre embryos at E17 (Fig. 3G–P). Loss of Dicer dramatically decreased ENS cell density along the length of the gut. The esophagus (Fig. 3G–H), stomach (Fig. 3I–J), midgut (Fig. 3K–L), cecum (Fig. 3M–N) and colon (Fig. 3O–P) all show a large decrease in the number of NC derived cells that form the ENS. The number of ENS cells in the stomach and midgut at E17 is decreased relative to E13 showing that as the ENS differentiates, loss of Dicer leads to cell loss.
Dicer is required for maintenance of the sensory nervous system
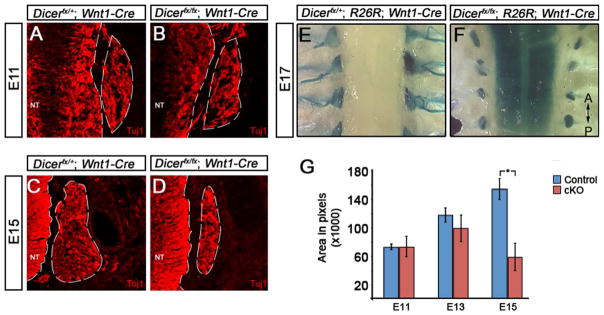
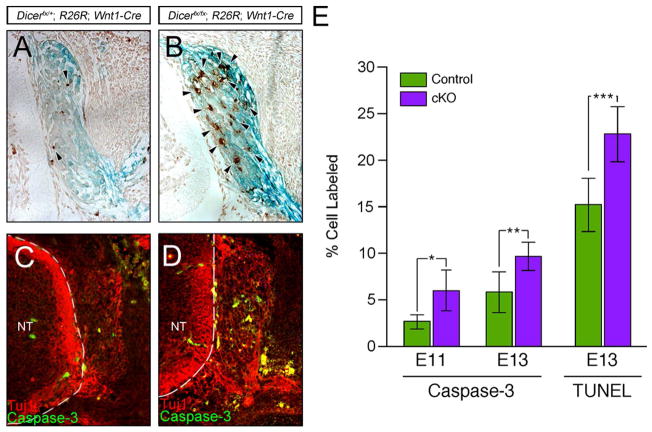
Trunk NCCs give rise to the dorsal root ganglia (DRG), the peripheral component of the sensory nervous system (Raible and Ungos, 2006). To determine if loss of Dicer affects DRG development, we undertook an immunohistochemical and anatomical examination of DRG formation and survival (Fig. 4). To determine if Dicer plays a role during ganglia formation and early neuronal differentiation, DRG of E11 throughout the trunk were analyzed for expression of the pan-neuronal marker Tuj1. The DRG express Tuj1 showing that differentiation of NC progenitors into neurons is not dependent on Dicer (Fig. 4A–B). To determine if Dicer is required for maintenance of the neuronal phenotype, DRG were examined for the expression of Tuj1 in E15 embryos (Fig. 4C–D). Mutant DRG express Tuj1 but in a smaller number of cells relative to control embryos. To determine if the organization of the DRG late in development is impacted by deletion of Dicer, DRG organization was examined in E17 embryos by tracing NCCs using β-galactosidase expression. A dorsal view of the DRG in control embryos shows they are aligned lateral to the neural tube and have extensive axonal projections (Fig. 4E). Loss of Dicer does not affect DRG organization, however, ganglia size is reduced and axons fail to project (Fig. 4F).
Fig. 4. Dicer is required for sensory nervous system survival but not neuronal differentiation.
(A–D) Neuronal differentiation in the DRG was examined by immunofluorescent analysis of expression of the pan-neuronal marker Tuj1 at E11 and E15 of development. At E11, the DRG of (A) control and (B) Dicerfx/fx; Wnt1-Cre embryos have formed and express Tuj1 (A). At E15, the DRG of control embryos (C) continues to grow while DRG of Dicerfx/fx; Wnt1-Cre embryos (D) fail to expand. (E–F) The result of Dicer loss on DRG survival and patterning late in development was examined in E17 embryos by tracing NC derived cells using β-galactosidase expression from the R26R locus. A comparison of a dorsal view of the DRG from control (E) and Dicer mutant (F) embryos shows that the DRG are maintained in mutant embryos but the ganglia size of mutant embryos is reduced and project axons. (G) To determine when during development the DRG fails to expand in mutant embryos, the cross sectional area of ganglia was calculated using the pan-neural marker Tuj1 to mark neurons. At E11 and E13, there is no significant difference between the control and mutant ganglia (P=0.967 and P=0.209 respectively). By E15, there is a significant decrease in the size of the ganglia in mutant embryos (P=0.003).
Immunohistochemical and anatomical examination show that the size of DRG in mutant embryos is reduced. To determine at which developmental stage loss of Dicer affects size of the DRG, the area of the DRG was quantified using expression of Tuj1 to mark the ganglia boundaries in E11, E13 and E15 embryos (Fig. 4G). At E11, the DRG of mutant embryos are comparable in size to control embryos (P=0.967) suggesting that early development does not require Dicer. At E13, the size of the developing DRG in mutant embryos is not significantly reduced relative to control embryos (P=0.209), however at E15, the size of the DRG in mutant embryos is reduced in size by approximately 2.5 fold relative to control DRG (P=0.003). The DRG of conditional Dicer mutant embryos at E15 are also reduced in size by approximately 2 fold relative to those of control and mutant at E13 embryos (P=0.0015) showing that DRG neurons are lost during development. These results show that Dicer and miRNAs are not required for formation of DRG, or differentiation of NC into neurons, but are required for maintenance of neurons during development.
Sensory neurons undergo apoptosis in the absence of Dicer
The decrease in size of DRG in mutant embryos at E15 relative to E13 embryos could be due to a combination of decreased proliferation and cell death. To determine if proliferation is affected by loss of Dicer, the proliferation rate was analyzed by measuring the number of cells in S-phase by BrdU incorporation. A comparison of control and mutant DRG showed that proliferation was unaffected (P=0.904) suggesting that decrease in size of the ganglia during development is due to cell death. To determine if loss of Dicer in NCCs leads to apoptotic cell death in the DRG, embryos were analyzed by TUNEL analysis (Fig. 5A–B). Relative to control DRG, mutant DRG have increased numbers of apoptotic cells showing that loss of Dicer enhances apoptotic cell death in sensory ganglia during development. At E13, number of TUNEL positive cells increased to about 50% in mutant (P=0.009).
Fig. 5. Loss of Dicer results in increased apoptosis in sensory ganglia.
(A–B) E13 DRG were marked by tracing NCCs using β-galactosidase expression from the R26R locus and apoptotic cells were identified by TUNEL analysis. Compare to control DRG (A), mutant DRG show an increase in the number of cells undergoing apoptosis (B). (C–D) To determine if apoptosis is occurring in a Caspase-dependent or -independent manner, DRG of E13 embryos were examined for expression of activated Caspase-3. Fewer cells in control DRG contain activated Caspase-3 (C) compared to mutant DRG (D). (E) Quantification of the number of cells with activated Caspase-3 and TUNEL shows a significant increase in the both activated Caspase-3 and TUNEL positive cells in mutant DRG (*P=0.001, **P=0.0007, ***P=0.009).
Apoptosis can occur through Caspase-dependent and -independent mechanisms (Krantic et al., 2007). During development of the DRG, apoptosis occurs by both mechanisms (Jiang et al., 2005; Kouroku et al., 1998). To determine the mechanism of apoptosis in DRG neurons, activated Caspase-3 was co-localized to neurons by co-labeling with the pan-neuronal marker Tuj1 (Fig 5C–D). In the absence of Dicer, an increase in the number of cells with activated Caspase-3 was observed. Quantification of active Caspase-3 immunoreactivity shows that at E11 the number of cells with activated Caspase-3 increase significantly in the absence of Dicer (P=0.001) (Fig 5E). At E13 Dicer mutant DRG, there is significant increase in the number of cells containing activated Caspase-3 (P=0.0007) compared to the controls showing that Dicer is required for DRG neuron survival during differentiation. When the number of activated Caspase-3 positive neurons was compared with the total number of cells undergoing apoptosis (Fig 5E), both control and mutant embryos show a significant difference in the number of TUNEL positive cells and activated Caspase-3 positive cells (control P=0.0004, mutant P=0.000007). Approximately 50% of apoptotic cells are activated Caspase-3 positive in both genotypes. The increase in apoptosis due to loss of Dicer increases both Caspase-dependent and –independent apoptosis in the DRG.
Dicer is required for survival of differentiating sympathetic neurons
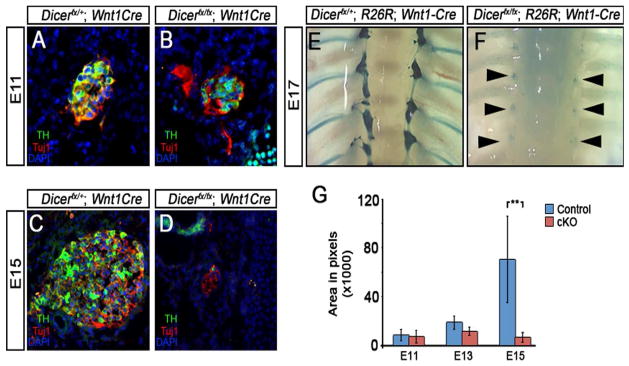
The sympathetic nervous system (SNS) develops from trunk NC that arrive at the dorsal aorta by E10 and begin to aggregate to form the sympathetic ganglia (Goridis and Rohrer, 2002; Huber, 2006). Within 12 hrs a subset of sympathetic precursors begin differentiating into neurons and express the norepinephrine biosynthetic enzyme tyrosine hydroxylase (TH). To address the role of Dicer during SNS development, we examined how deletion of Dicer in the NC lineage affects SNS formation and differentiation (Fig. 6). To determine if Dicer is required for SNS ganglia formation, neuronal differentiation or phenotype selection, the expression of Tuj1 and TH were examined in E11 embryos (Fig. 6A–B). At E11, SNS ganglia form and express Tuj1 and TH in embryos lacking Dicer at levels comparable to control liter mates showing that Dicer does not play an essential role in ganglia formation or activation of the noradrenergic differentiation program in sympathetic neuroblasts. To determine if Dicer plays a role in maintenance of neurons and noradrenergic differentiation of the SNS, we examined if Dicer loss affects Tuj1 and TH expression in E15 embryos (Fig. 6C–D). In control embryos, the SNS continues to grow and neurons co-express Tuj1 and TH (Fig. 6C). In mutant embryos, the size of the ganglia is reduced and the few remaining neurons express Tuj1 but not TH (Fig. 6D). To determine if the loss of Tuj1 expression is due to loss of cells, NCCs were traced by β-galactosidase expression from the R26R locus and the SNS was examined by whole mount staining in E17 embryos (Fig. 6E–F). Loss of Dicer does not affect the organization of the SNS but results in severe hypoplastic ganglia showing that the NC-derived cells are lost in the SNS.
Fig. 6. Dicer is required for maintenance of the sympathetic nervous system.
To determine the role of Dicer during SNS development, sympathetic ganglia were analyzed for neuronal and noradrenergic differentiation and neuronal survival. (A–D) Neuronal and noradrenergic differentiation of the SNS was determined by expression of Tuj1 and TH. Immunohistochemical analysis shows that sympathetic ganglia form and differentiate into sympathetic neurons in control (A) and the mutant embryos (B). At E15, control sympathetic ganglia increase in size and continue expressing Tuj1 and TH (C) while ganglia in Dicer mutant embryos are dramatically reduced in size and do not express TH (D). (E–F) Whole-mount lineage tracing in E17 embryos was used to determine the late effect of Dicer loss on patterning and survival of sympathetic ganglia. Relative to control embryos (E), ganglia (arrow heads) of mutant embryos contain few cells but maintain correct patterning (F). (G) The sizes of the developing SNS ganglia were quantified using the pan-neuronal marker Tuj1 to mark ganglia boundaries. There is no significant difference in the size of ganglia at E11 and E13 but the SNS ganglia dramatically decreases in size in mutant embryos by E15 (P=0.037).
To determine at what stage of SNS development Dicer is required for maintenance of sympathetic neurons, the area of the sympathetic ganglia at different stages of development was quantified using Tuj1 expression to mark ganglia boundaries (Fig. 6G). The area of control and mutant ganglia is not significantly different at E11 or E13. At E15, the area of the mutant SNS is dramatically decreased relative to control (P=0.037). When the size of mutant E15 ganglia is compared to mutant E13 ganglia, there also is a significant reduction in size (P=0.0413) showing that neurons are lost by E15. These results show that Dicer is not required for initial formation of noradrenergic SNS neurons but is required for maintenance of neurons after they have begun to terminally differentiate.
Sympathetic neurons undergo apoptosis in the absence of Dicer
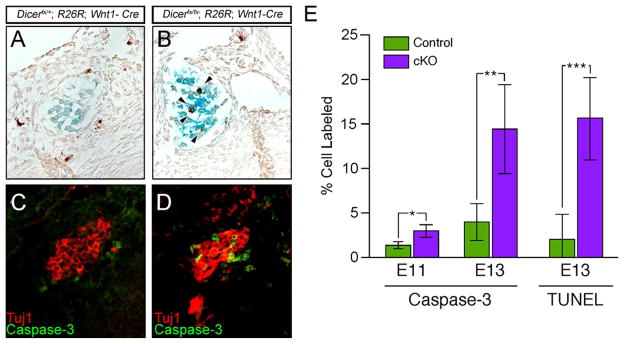
Our results suggest that loss of Dicer results in a progressive loss of SNS neurons starting at mid-gestation. To determine if reduced proliferation contributes to the reduction in cell number during development, we examined if the proliferation rate was affected by measuring the number of cells in S-phase by BrdU incorporation. A comparison of the number of BrdU positive cells in the SNS in control and mutant embryos showed that proliferation was unaffected (P=0.928), suggesting that decrease in size of the ganglia during development is due to cell death. To determine if cell death by apoptosis accounts for the loss of sympathetic neurons, the developing SNS was analyzed by TUNEL (Fig. 7A–B). At E13, control sympathetic ganglia have few or no TUNEL positive cells (Fig. 7A) whereas TUNEL positive nuclei are increased in conditional Dicer mutant ganglia (P=0.0004) (Fig. 7B).
Fig. 7. Dicer is required to suppress apoptotic cell death in sympathetic ganglia.
(A–B) Cells undergoing apoptosis in the sympathetic ganglia were determined by TUNEL analysis in E13 embryo sections containing β-galactosidase tracing to mark ganglia. Few cells were found to apoptose in control sympathetic ganglia (A), while mutant ganglia show increased TUNEL positive cells (B). (C–D) To determine if cell death is Caspase-dependent, SNS ganglia were marked by Tuj1 expression and examined for expression of activated Caspase-3 by immunohistochemistry. Compared to controls (C) increased expression of activated Caspase-3 is seen in the mutant ganglia (D). (E) Quantification of activated Caspase-3 and TUNEL positive cells in the sympathetic ganglia shows a dramatic increase (*P=0.007, **P=0.00004, ***P=0.0004).
To determine if the enhanced apoptosis due to Dicer loss is through the Caspase-dependent pathway, we examined Caspase-3 activation in SNS neurons (Fig. 7C–D). To mark neurons, sections were co-labeled with Tuj1. In control SNS ganglia few neurons contain activated Caspase-3 (Fig. 7C) while ganglia lacking Dicer contain numerous neurons containing activated Capsase-3 (Fig. 7D). Quantification of active Caspase-3 positive cells shows that in the absence of Dicer, increased numbers of activated Caspase-3 positive cells begin to be observed at E11 (P=0.007) with the number of cells expressing active Caspase-3 increasing dramatically by E13 (P=0.00004). Neurons in the SNS undergo refinement in a Caspase-dependent manner during development (Deshmukh et al., 1996; Parlato et al., 2007). To determine if loss of Dicer activates Caspase-independent apoptosis in addition to Caspase-dependent apoptosis, the number of activated Casspase-3 positive cells was compared with the number of TUNEL positive apoptotic neurons. The number of activated Caspase-3 and TUNEL positive cells was the same in both control (P=0.158) and mutant (P=0.672) showing that loss of Dicer does not activate Caspase-independent apoptosis.
DISCUSSION
Dicer is required for miRNAs processing to generate functional miRNAs that can regulate translational suppression of mRNA (Agrawal et al., 2003; Nilsen, 2007; Zhao and Srivastava, 2007). Although the main function of Dicer is to process miRNAs that act to repress translation, Dicer plays additional roles including processing RNAs that regulate chromatin structure to regulate transcription (Djupedal and Ekwall, 2009). In this study, we show that Dicer and miRNAs are required for survival of NC derived tissues during development. Using Wnt1-Cre to delete Dicer soon after NC form, we show that Dicer and newly processed miRNAs are not required for NC migration and their correct distribution in the embryos. However, Dicer is required for survival of all NC lineages examined as they begin to differentiate.
The roles of Dicer in cranial NC
Loss of Dicer does not affect NC migration or colonization of the head in early stage embryos but results in a near complete loss of frontal bones resulting in the brain extending into the region normally occupied by the nasal cavity. The loss of all the NC derived bones demonstrates that their survival is dependent on Dicer.
The NC does not contribute directly to the brain and on a gross level, all regions of the brain in conditional Dicer mutant embryos appear to form, however, the mid-brain appears much thinner. Since the Wnt1-Cre transgene is expressed in the dorsal neural tube including the midbrain region (Brault et al., 2001), the loss of cells at the mid-brain may be directly due to a loss of Dicer in a cell autonomous manner. Alternatively, cranial NCCs have been reported to influence midbrain development and their loss may affect signaling between these lineages (Creuzet et al., 2006).
Dicer is essential for peripheral nervous system survival but not initial differentiation
NCCs form the neurons and glia of the enteric, sensory, and sympathetic nervous systems. Loss of Dicer did not affect formation of ganglia suggesting that after NCCs have formed, newly synthesized miRNA may not be required for colonization, formation of PNS ganglia or initial differentiation of neurons. This suggests that either miRNA synthesized prior to Dicer deletion by Wnt1-Cre play a role or that miRNA are not required for these processes. The pan neuronal marker Tuj1 is expressed in the sensory and sympathetic neurons showing that neuronal differentiation does not require Dicer.
In the SNS, expression of TH, an enzyme essential for noradrenergic differentiation, was not affected by loss of Dicer suggesting that neuronal phenotype selection in the SNS does not require Dicer. Norepinephrine synthesized from the SNS is essential for embryonic survival past E12 (Morikawa and Cserjesi, 2008; Thomas et al., 1995). Although expression of other genes required for norepinephrine synthesis and secretion were not examined, the survival of conditional Dicer mutant embryos to birth suggesting that all enzymes required for noradrenergic synthesis were also expressed. In addition, loss of Dicer did not affect the expression of the transcription factors Hand2 and Gata3 (data not shown), which are essential for regulating SNS noradrenergic differentiation (Lim et al., 2000; Moriguchi et al., 2006; Morikawa et al., 2007). Taken together, our results show that the neuronal differentiation of the sensory and sympathetic nervous systems and phenotype selection of the SNS is not dependent on Dicer hence it does not require synthesis of miRNA in their precursors.
The mechanisms of cell death mediated by Dicer loss
During development, loss of Dicer in the NC results in a progressive loss of the enteric, sensory, and sympathetic nervous systems. At mid-gestation, when neuroblasts of the PNS are undergoing terminal differentiation and exiting the cell cycle, the size of the ganglia failed to expand in Dicer mutant embryos due to apoptotic cell death. Cell death in the PNS normally occurs late in development during remodeling (Oppenheim, 1991). Loss of Dicer results in apoptosis occurring early in development suggesting that Dicer and newly synthesized miRNAs are critical in PNS survival by preventing premature apoptotic dependent cell death. Our analysis of the mechanism of cell death shows that it occurs through both Caspase-dependent and –independent apoptosis in the DRG but only Caspase-dependent apoptosis in the SNS. During development, the DRG undergo apoptosis by both Caspase-dependent and –independent mechanisms (Jiang et al., 2005; Kouroku et al., 1998) while apoptosis in the SNS is Caspase-dependent (Deshmukh et al., 1996; Parlato et al., 2007). This suggests that loss of Dicer does not activate apoptotic programs in general but activates the pathways endogenous to the cell lineage. Since the apoptotic pathways are activated at the level of transcription, loss of Dicer is not activating apoptosis by relieving translational suppression of apoptotic mRNA. The cause of apoptotic program activation may be due to enhanced translation of a large number of proteins normally regulated by miRNAs during differentiation resulting in cell suicide.
Alternatively, since loss of Dicer results in apoptosis at the time that cells transition from proliferation to cell cycle exit, it is possible that Dicer and miRNAs are required to regulate cell cycle exit and the disruption of this transition leads to cell suicide. Conditional deletion of Dicer in other developing tissues also results in apoptosis (Davis et al., 2008; Harfe et al., 2005; Kim et al., 2007; O’Rourke J et al., 2007) suggesting that expression of Dicer and miRNAs may be a general requirement in suppressing apoptosis during differentiation. Our results suggest that Dicer and miRNAs are required to prevent activation of the apoptotic program but miRNAs are also known to be required for regulation of apoptosis after apoptotic genes are activated. In Drosophila, the miRNA bantam regulates expression of a pro-apoptotic protein hid and prevents hid-dependent apoptosis (Brennecke et al., 2003) while in Xenopus retina, miR-24a represses two pro-apoptotic proteins Caspase-9 and APAF-1 to regulate retinal size development (Walker and Harland, 2009). Combined with our results, it appears that apoptosis is regulated by miRNA at multiple levels.
Acknowledgments
We wish to thank Michael McManus (University of California, San Francisco) for providing the conditional Dicer mouse line and Henry Sucov (University of Southern California, Los Angele) for providing the Wnt1-Cre mouse line. We wish to thank David Clouthier (University of Colorado, Denver) for help with the analysis of the head phenotype. This work was supported by grants from AHA (SDG) to Y. M. and AHA (Grant in Aid), NSF (IOS-0529746, MCB-0529746) to P. C. and NIH (NS15547).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basch ML, Bronner-Fraser M. Neural crest inducing signals. Adv Exp Med Biol. 2006;589:24–31. doi: 10.1007/978-0-387-46954-6_2. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–30. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pietri Tonelli D, Pulvers J, Haffner C, Murchison E, Hannon G, Huttner W. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol. 1996;135:1341–54. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djupedal I, Ekwall K. Epigenetics: heterochromatin meets RNAi. Cell Res. 2009;19:282–95. doi: 10.1038/cr.2009.13. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Graham A, Begbie J, McGonnell I. Significance of the cranial neural crest. Dev Dyn. 2004;229:5–13. doi: 10.1002/dvdy.10442. [DOI] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–13. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298:335–43. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang JS, Jakobsen J. Differential effect of p75 neurotrophin receptor on expression of pro-apoptotic proteins c-jun, p38 and caspase-3 in dorsal root ganglion cells after axotomy in experimental diabetes. Neuroscience. 2005;132:1083–92. doi: 10.1016/j.neuroscience.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Urase K, Fujita E, Isahara K, Ohsawa Y, Uchiyama Y, Momoi MY, Momoi T. Detection of activated Caspase-3 by a cleavage site-directed antiserum during naturally occurring DRG neurons apoptosis. Biochem Biophys Res Commun. 1998;247:780–4. doi: 10.1006/bbrc.1998.8815. [DOI] [PubMed] [Google Scholar]
- Krantic S, Mechawar N, Reix S, Quirion R. Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol. 2007;81:179–96. doi: 10.1016/j.pneurobio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96:5037–42. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AV, Barbas JA, Nieto MA. How to become neural crest: from segregation to delamination. Semin Cell Dev Biol. 2005;16:655–62. doi: 10.1016/j.semcdb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–9. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- O’Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD. Essential role for Dicer during skeletal muscle development. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Parlato R, Otto C, Begus Y, Stotz S, Schutz G. Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development. 2007;134:1663–70. doi: 10.1242/dev.02838. [DOI] [PubMed] [Google Scholar]
- Raible DW, Ungos JM. Specification of sensory neuron cell fate from the neural crest. Adv Exp Med Biol. 2006;589:170–80. doi: 10.1007/978-0-387-46954-6_10. [DOI] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–18. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–8. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32:189–97. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]