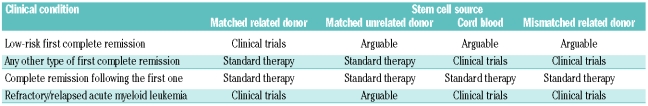
Allogeneic hematopoietic stem cell transplantation is a curative treatment for patients with both malignant and non-malignant hematologic diseases and nowadays represents the most widely available form of stem cell therapy. More than 50 years have elapsed since the first report by E. Donnall Thomas on this new radical and innovative approach1 and since then the field has improved enormously, particularly in the last decade. The recent recommendations of the European LeukemiaNet for management of acute myeloid leukemia in adults have highlighted the value of allogeneic stem cell transplantation in this setting; Table 1 summarizes the indications to this procedure provided by the international experrts panel.2 In individual clinical decision making, both the disease risk (cytogenetic and molecular profile) and the risk associated with the transplant itself as assessed by comorbidity and other transplant-related risk-indices, should be taken into account.
Table 1.
Current indications to allogeneic stem cell transplantation in acute myeloid leukemia according to disease status and stem cell source.
Hematopoietic stem cell transplantation was initially developed for two purposes. First, it was a strategy to replace an abnormal hematopoietic system with one from a healthy donor. Second, it allowed the delivery of myeloablative doses of radiation and/or chemotherapy to cure hematologic malignancies. The delivery of high doses of myeloablative conditioning resulted in an unacceptable treatment modality for patients over 50 years of age and/or with co-morbidities because of the high rate of transplant related toxicity and mortality. Over the years, the relevance of the role of the allogeneic immune system in the eradication of the underlying malignancy became more and more apparent. In fact, the relapse rate after hematopoietic stem cell transplantation appeared lower in patients with graft versus host disease while it was the highest in identical twin transplant recipients. This observation led to the pivotal question on the respective contribution of the conditioning regimen and the immunological graft versus leukemia effect of the graft in the final generation of the anti-tumor activity of hematopoietic stem cell transplantation.
Since the late 1990s, new conditioning regimens have been developed, markedly less intense but still sufficiently immunosuppressive to ensure engraftment of allogeneic cells. Reduced intensity conditioning regimens allowed hematopoietic stem cell transplantation to be performed safely in patients up to 70 years old or with relevant co-morbidities. Since 2006, approximately 40% of hematopoietic stem cell transplantations use reduced conditioning regimens. Unfortunately, reduced conditioning regimen transplants failed to demonstrate a real survival advantage compared to myeloablative conditioning transplants because the resultant reduction in toxicity was gained at the price of an increased incidence of relapse. In addition, despite a reduction in tissue damage provided by the reduced intensity of the conditioning, this approach translated into graft versus host disease rates not inferior to myeloablative conditioning. While absolute numbers of allohematopoietic stem cell transplantation are steadily increasing, thanks to the wide applicability of reduced conditioning regimen transplants in fragile patients, graft versus host disease and the consequent toxicities related to the necessary immunosuppressive treatments still represent its biggest limitation. Therefore, major efforts are being made to optimize graft versus host disease prevention and treatment while preserving a graft versus leukemia effect.
The report by Craddock et al.3 published in this issue, describes the UK experience on hematopoietic stem cell transplantation in acute myeloid leukemia using an in vivo T-cell depletion by alemtuzumab associated to a reduced intensity regimen based on fludarabine and melphalan. In a large retrospective analysis, the authors confirm the efficacy of in vivo T-cell depletion in graft versus host disease prevention while the major cause of treatment failure in T-cell depleted reduced intensity regimen transplants is disease relapse. In the authors’ analysis, three factors predict an increased risk of disease relapse: disease status at transplant, adverse cytogenetics at diagnosis and increased intensity of post-transplant immunosuppression. The 3-year overall survival varies between 50% for patients in first complete remission to 15% for those with active disease at transplant. While no clinical intervention can modify the biological characteristics of the original disease, some pre- and post-transplant factors influencing the outcome can be modulated.
However, the most critical aspect to increase the proportion of high-risk acute myeloid leukemia patients receiving allo-hematopoietic stem cell transplantation is timely initiation of donor search as soon as diagnosis is established. Allo-hematopoietic stem cell transplantation was initially limited to the approximately 25% of patients with a matched sibling; in the late 1970s, the Seattle group performed the first successful marrow grafting from a matched, unrelated donor in a patient with leukemia. Methods for HLA testing have dramatically improved over the past 15 years, and today patients receiving a well matched unrelated donor in experienced transplant centers have similar outcome to HLA-identical sibling recipients.4 Furthermore, the organization of hematopoietic stem cell donor registries has improved dramatically in recent years, resulting in a successful recruitment of a matched donor in 50–80% of patients in an appropriate time according to disease status. However, patients from ethnicities less represented in world-wide registries still have a significantly lower chance of finding a well matched donor. In recent years, a third source of stem cells, umbilical cord blood, has become more and more popular. Cord blood has several potential advantages, including rapid availability and lower risk of graft versus host disease, resulting in less stringent HLA-matching requirements. Nowadays in the US, umbilical cord blood transplants represent around a third of all transplants for children with acute leukemia; the use of umbilical cord blood is also increasing in adults, particularly following the advent of double-unit transplants to augment graft cell dose.5 The Perugia group6 carried out seminal work on profound T-cell depletion in the setting of HLA-haploidentical hematopoietic stem cell transplantation associated to infusion of large numbers of purified CD34+ cells resulting in high engraftment rate and low incidence of graft versus host disease. Since then, many achievements have been made in this setting. HLA-haploidentical transplantation offers an immediate source of stem cells to almost every patient because of easy and rapid donor availability, but the delayed recovery of immune response against pathogens results in high transplant related mortality, limiting the spread of the procedure.7 Different strategies to speed up the immunoreconstitution have been developed, such as the infusion of genetically modified lymphocytes post-transplant8 or different strategies of in vivo T-cell depletion i.e. CD3/CD19 negative selection.9 In the last few years, the infusion of un-manipulated haploidentical stem cells has also been investigated, using alternative strategies of post-transplant immunosuppression; among these the administration of rapamycin to promote in vivo T-regulatory cell expansion10 or the use of cyclophosphamide on day 3 after graft infusion to reduce alloreactive lymphocytes.11 All these advances in the field of alternative donors and multiple options in stem cell sources and content are now expected to translate into a higher rate of patients undergoing hematopoietic stem cell transplantation in an early disease stage according to the intention-to-treat.
One prognostic factor of hematopoietic stem cell transplantation outcome amenable to intervention pre-transplantation is iron overload. Several studies have documented that pre-transplant red blood cell transfusion-dependence and/or high serum ferritin level, surrogate markers of iron overload, are associated to poorer survival due to higher transplant related mortality in patients with myelodysplastic syndromes or acute myeloid leukemia.12 Ongoing studies are focusing on the predictive value of iron overload at transplantation using hepatic magnetic resonance imaging as non-invasive evaluation of liver iron concentration. In the near future, it will be vital to prospectively determine whether pre-transplantation chelation therapy with oral drugs such as deferasirox can safely and effectively reduce the deleterious impact of transfusional iron overload on the outcome of hematopoietic stem cell transplantation.
The choice of the conditioning regimen is now relying on multiple available options. Although reduced intensity conditioning has resulted in a reduction in transplant related mortality, it is less effective in tumor killing. The Standford group pioneered a combination of total lymphoid irradiation with anti-thymocyte globulin as minimal toxic conditioning, and demonstrated that this procedure not only allows hematopoietic stem cell transplantation in older patients, more heavily pre-treated or with co-morbidities, but also has a protective effect on acute graft versus host disease with a rate of grade 2–4 graft versus host disease of less than 5%. This preparation modality before hematopoietic stem cell transplantation relies completely on the graft versus leukemia effect for disease control which can, however, take some months to be fully active.13 Also the field of standard myeloablation is improving. The combination of fludarabine with newer formulations of old drugs, like intravenous busulphan, or with different alkylating agents like treosulfan are under evaluation in prospective comparative trials (MC-FludT.14/L, EudraCT-No.: 2008-002356-18) depicting a new reduced toxicity regimen option. Another way to intensify the conditioning could be the substitution of fludarabine with clofarabine, a purine analog with marked direct anti-leukemia properties. Finally, also different modalities of radiation delivery are being investigated; a very promising way to reduce toxicity maintaining a high anti-leukemia power is the application of tomotherapy to total body irradiation, obtaining a total marrow irradiation, sparing deleterious effect of total body irradiation on lung, liver and other organs.15
Also the intensity of post-transplant immunosuppression can be modulated affecting graft versus host disease and morbidity. Craddock et al.2 show that increased exposure to cyclosporine A in the first 21 days post-transplant is strongly associated to an increased risk of relapse and poorer overall survival. Curiously the authors do not find any association between acute graft versus host disease and cyclosporine A levels, and there is only a trend towards an increased risk of chronic graft versus host disease in patients with lower cyclosporine A levels. These findings probably depend on the concomitant use of alemtuzumab. Tuning cyclosporine A exposure in the first post-transplant days appears to be a potentially valuable strategy to improve the outcome of very high-risk patients, for example those with adverse cytogenetics at diagnosis. Manipulation of immunosuppressive therapy post-transplant should be performed not only according to patient characteristics, but also considering graft source and quantity of donor T cells infused, modality of T depletion (alemtuzumab, anti-lymphocyte globulins-ATG or others) and HLA matching. High resolution matching of HLA-A, -B, -C, -DRB1 and -DQB1 (10/10) can improve clinical outcome in terms of overall survival, transplant related mortality and acute graft versus host disease; but it is now emerging that also matching at HLA-DPB1 can be important. HLA-DPB1 displays weak linkage disequilibrium with the other class II loci; therefore, only approximately 15% of 10/10 matched pairs are also matched for HLA-DPB1 (12/12). HLA-DPB1 allele-mismatched transplantations permissive according to a new functional algorithm developed by Fleischhauer et al. have better outcome in terms of survival.15 Besides tuning cyclosporine A exposure, new immunosuppressive strategies are becoming available, above all the use of rapamycin as graft versus host disease prophylaxis.16 Rapamycin is an immunosuppressive drug that arrests cell cycle in G1 through the inhibition of DNA transcription, DNA translation and protein synthesis but, in contrast to calcineurin inhibitors, promotes the generation of T-regulatory cells (Tregs). Besides its intriguing effect on Tregs, rapamycin has also a potential antitumor activity in different hematologic malignancies,17 rendering it suitable for high-risk patients.
In conclusion, despite more than 50 years have passed since the first hematopoietic stem cell transplantation, this field is now evolving very rapidly in terms of stem cell sources, conditioning regimens, immunosuppression and supportive therapy, and the outcome of this type of transplant is improving year by year, providing a curative option to patients with diseases which still cannot be cured by chemotherapy.18
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–6. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. Epub 2009 Oct 30. Review. PubMed PMID: 19880497. [DOI] [PubMed] [Google Scholar]
- 3.Craddock C, Nagra S, Peniket A, Brookes C, Buckley L, Nikolousis E, et al. Factors Predicting Long Term Survival After T Cell Depleted Reduced Intensity Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Haematologica. 2009 Nov 30; doi: 10.3324/haematol.2009.013920. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacMillan ML, Davies SM, Nelson GO, Chitphakdithai P, Confer DL, King RJ, Kernan NA. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14 (9 Suppl):16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for haematological malignancies: conditioning regimens, double cord transplant and infectious complications. Br J Haematol. 2009;147(2):207–16. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 6.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–54. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 7.Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. Acute Leukemia Working Party (ALWP) of European Blood and Marrow Transplant (EBMT) Group. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112(9):3574–81. doi: 10.1182/blood-2008-02-140095. [DOI] [PubMed] [Google Scholar]
- 8.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 9.Bethge WA, Faul C, Bornhäuser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008;40(1):13–9. doi: 10.1016/j.bcmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Peccatori J, Clerici D, Forcina A, et al. In-vivo T-regs generation by rapamycin-mycophenolate-ATG as a new platform for Graft versus host disease prophylaxis in T-cell repleted unmanipulated haploidentical peripheral stem cell transplantation: results in 68 patients. Bone Marrow Transplant. 2010 Mar;35(suppl 1) [Google Scholar]
- 11.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–9. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreth J, Antin JH. Iron overload in hematologic malignancies and outcome of allogeneic hematopoietic stem cell transplantation. Haematologica. 2010;95(3):364–6. doi: 10.3324/haematol.2009.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohrt H, Lowsky R. Nonmyeloablative conditioning with total lymphoid irradiation and antithymocyte globulin: an update. Curr Opin Hematol. 2009;16(6):460–5. doi: 10.1097/MOH.0b013e3283319e8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73(1):273–9. doi: 10.1016/j.ijrobp.2008.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocchiolo R, Zino E, Vago L, Oneto R, Bruno B, Pollichieni S, et al. Gruppo Italiano Trapianto di Midollo Osseo, Cellule Staminale Ematopoietiche (CSE) e Terapia Cellulare; Italian Bone Marrow Donor Registry. Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood. 2009;114(7):1437–44. doi: 10.1182/blood-2009-01-200378. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GRAFT VERSUS HOST DISEASE prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098–105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010 May;95(5):819–28. doi: 10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–48. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]