Allogeneic stem cell transplant is a potentially curative treatment for patients with acute myeloblastic leukemia and myelodysplastic syndrome. In this issue of the journal, Craddock et al.1 report the largest reported series of T-cell depleted reduced intensity stem cell transplant for acute myeloblastic leukemia, with encouraging long-term survival. Although prevalence of extramedullary relapse was not reported separately, relapse accounted for 49% of mortality. Extramedullary relapse after stem cell transplant for acute myeloblastic leukemia is an under-reported long-term complication of this procedure. The pathogenesis of extramedullary relapse is not well described, but may be due to a less potent graft-versus-leukemia response than in the bone marrow.
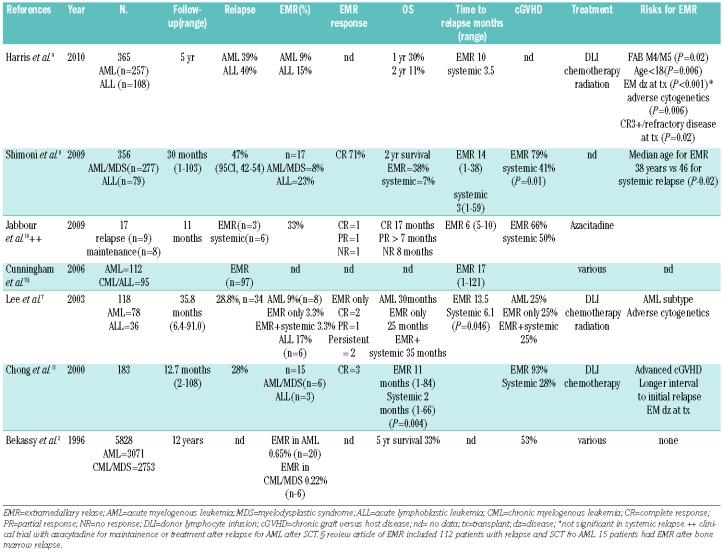
In a European group for Blood and Marrow Transplantation (EBMT) study, the incidence of extramedullary relapse after stem cell transplant was reported as 0.65% for acute myeloblastic leukemia, but the incidence in this cohort might have been underreported.2 Among long-term survivors the incidence has been reported to be over 20%.2–7 Recently, Shimoni et al. reported on 356 consecutive patients with acute myeloblastic leukemia/myelodysplastic syndrome (n=277) and acute lymphoblastic leukemia (n=79).8 Incidence of extramedullary relapse among the acute myeloblastic leukemia/myelodysplastic syndrome cohort was 8% with a median follow-up of 30 months. Another study of 365 consecutive patients with acute myeloblastic leukemia (n=257) or acute lymphoblastic leukemia (n=108) after stem cell transplant reported a 9% cumulative incidence of extramedullary relapse among acute myeloblastic leukemia patients with a follow-up of five years.9 The median time to diagnosis of extramedullary relapse is longer than to bone marrow only relapse; about 12–17 months versus 3–6 months, respectively (Table 1).2,3,7–9 Extramedullary relapse has been reported even 5–10 years after stem cell transplant.3 As supportive care improves and patients live longer after stem cell transplant, the cumulative incidence of extramedullary relapse may continue to increase over time.
Table 1.
Extramedullary relapse after allogeneic stem cell transplant.
Clinical presentation and prognosis
The risk factors for the development of extramedullary relapse after stem cell transplant are not well established but may include: age under 18 years at diagnosis, acute myeloblastic leukemia subtypes (FAB) M4/M5, extramedullary disease prior to stem cell transplant, adverse cytogenetics, and relapse/refractory disease at time of transplant (Table 1).3,9,11 In a retrospective analysis, Wilms’ tumor 1 (WT1) gene expression levels were monitored from peripheral blood and bone marrow in patients with extramedullary relapse and bone marrow only relapse. Patients with extramedullary relapse had abnormally high WT1 expression levels in peripheral blood as compared to WT1 expression levels in the bone marrow 11–46 days prior to diagnosis.12 Although prognosis of extramedullary relapse after stem cell transplant is poor and early detection of these tumors might improve treatment options, there are no established strategies for surveillance of extramedullary relapse and regular CT, MRI or PET/CT are not part of the routine long-term follow-up for these patients. As a result, extramedullary relapse is typically diagnosed only once the patient becomes symptomatic. Extramedullary relapse may be localized to a single site, or manifest more diffusely with multi-organ involvement.2,3,7,11,13,14,19,20 Extramedullary relapse is predisposed to develop within certain tissues including the known sanctuary sites of the testis, ovary and central nervous system. Other sites include bone, paranasal sinuses, breast tissue, skin, retroperitoneum, gastrointestinal tract and kidney.2,3,7,11,13,14,19,20 Once a single focus of disease becomes clinically evident, progression at other extramedullary sites and bone marrow typically follows within a year.11
Are extramedullary tissues sanctuary sites for graft-versus-leukemia effect?
It has long been thought that the graft-versus-leukemia effect associated with allogeneic marrow transplantation would protect patients from extramedullary relapse and bone marrow relapse.3,11,14 The increased incidence of graft-versus-host disease in patients with extramedullary relapse implies the graft-versus-leukemia surveillance preferentially maintains remission in the bone marrow while allowing leukemic cells in peripheral tissues to evade immune surveillance. In our experience at the National Institutes of Health (personal communication Barrett AJ, 2010, NHLBI 05-H-0130; ClinicalTrials.gov identifier NCT00106925), 5 patients developed extramedullary relapse beyond four years post allogeneic stem cell transplantation. All patients had a history of chronic graft-versus-host disease and 3 of 5 had concomitant chronic graft-versus-host disease at the time of extramedullary relapse. Among the larger cohort reported by Shimoni et al., 79% of patients with extramedullary relapse more than three months following stem cell transplantation had chronic graft-versus-host disease compared to 49% of those with systemic relapse, (P=0.01).8
The mechanism by which leukemic cells evade immune surveillance and recur as extramedullary relapse is not well understood. In vitro granulocytic sarcoma cell lines can bind to dermal fibroblasts.15 CD56 (NCAM) is a member of the immunoglobulin superfamily that is expressed on natural killer cells. About 20% of myeloid leukemia expresses CD56. CD56 expression has been associated with cutaneous involvement compared to CD56 negative myeloid leukemia.16 Cytotoxic CD8 positive T cells (CTLs), the main effector cell of graft-versus-leukemia are highly concentrated in the marrow compared to peripheral tissues. This may lead to a less potent response in soft tissue.11 T-cell homing is determined by a range of selectin molecules, “addressins”, which direct the T cell to specific tissues and such relapse may occur because of sanctuary sites not patrolled by antileukemic T cells.17 Clearly therapies aimed at routing the graft-versus-leukemia effect to extramedullary tissues might be the key to improving the outcome for patients with extramedullary relapse.
Management of extramedullary relapse
Due to the lack of sufficient data, there are no established guidelines for clinical decision making in the treatment of extramedullary relapse after allogeneic stem cell transplantation. The standard practice is a combination of localized radiation, systemic chemotherapy, immunotherapy with donor lymphocyte infusions and repeated transplant (Table 1). Although the prognosis is poor for extramedullary relapse, it is better than for systemic relapse, with a 2-year overall survival of 11–38%.2,8,9,18 Therefore, the goal of therapy should be to prevent systemic relapse. Many patients are already heavily pre-treated and may be unable to tolerate chemotherapy at potentially curative doses. In a recent case report, a 38-year old woman with chronic graft-versus-host disease after stem cell transplantation from a matched unrelated donor developed gastric extramedullary relapse. Her immunosuppression was stopped. She received high-dose cytarabine and amsacrine. Three months after her diagnosis of extramedullary relapse, she died of sepsis.19
Donor lymphocyte infusion
Donor lymphocyte infusion has been found to be successful for relapse involving the bone marrow; however, it has little effect at extramedullary sites.5 This may relate to the problem of lymphocyte homing described above.17 Although some patients have an initial, favorable response to this therapy, it is typically unsustained. In one example, a 24-year old male patient with acute myeloblastic leukemia relapsed in the left breast after CR3. After achieving CR4, he underwent stem cell transplantation from his HLA-matched brother, but relapsed one year later in the same breast. His extramedullary relapse was treated with donor lymphocyte infusion, local radiotherapy, and ICE (ifosfamide, carboplatin, and etoposide) chemotherapy, but the tumor progressed to involve other subcutaneous tissues.20 As this case illustrates, most patients receive concurrent chemotherapy with donor lymphocyte infusion making it difficult to determine the efficacy of donor lymphocyte infusion for the treatment of extramedullary relapse.
Second allogeneic stem cell transplantation
Second transplantation has also failed to eliminate extramedullary relapse. Kikushige et al. report a case in which a 49-year old man with acute myeloblastic leukemia relapsed in his inguinal lymph nodes 15 months after allogeneic stem cell transplantation. He underwent a second transplant from a separate donor. He had an extramedullary relapse 150 days later in the skin and central nervous system. Bone marrow aspirate revealed nor-mocellular marrow, with all three donor-derived cell lineages maturing normally.13 Szomor et al. reviewed 2 cases of extramedullary relapse in which re-induction chemotherapy was followed by second transplant; both patients eventually died of liver toxicity.14
Strategies to prevent extramedullary relapse and late relapse
Due to the lack of efficacious treatment strategies with systemic chemotherapy, donor lymphocyte infusion, and second stem cell transplant (Table 1), there is a need for novel approaches to manage extramedullary relapse after stem cell transplantation. A better understanding of the molecular genetics and risk factors that predispose individuals to developing extramedullary relapse after stem cell transplantation may result in increased surveillance of patients at high risk and novel regimens to augment the graft-versus-leukemia response. After a patient develops extramedullary relapse after stem cell transplantation, the aim of treatment is to trigger immune effector cells to kill antigen-expressing cancer cells in soft tissue. Gemtuzumab ozogamicin is a humanized anit-CD33 monoclonal antibody that selectively targets CD33 expressing tumors. T cells are potent effectors of graft-versus-leukemia that do not express CD33 and extramedullary relapse after stem cell transplantation effect should, therefore, be maintained. Two patients who received gemtuzumab ozogamicin for extramedullary relapse after stem cell transplantation have achieved a complete remission.21,22 One was a 54-year old male who developed multiple sites of extramedullary relapse 120 days after stem cell transplantation. He achieved a complete hematologic and radiographic response 21 days after gemtuzumab ozogamicin as a single agent.21 Azacitidine is a hypomethylating agent that may induce leukemic cell differentiation and increase the expression of tumor associated antigens. Jabbour et al. hypothesized that this may increase graft-versus-leukemia response after stem cell transplantation for salvage therapy or maintenance. Azacitidine was given to 9 patients who relapsed after stem cell transplantation. Of the 3 patients with extramedullary relapse in this group, 2 responded (Table 1).10 Furthermore, clinical evidence for the effectiveness of anti-leukemia immune response has been obtained in a number of pilot clinical studies with immunotherapeutic targeting tumor antigens. One of these tumor antigens is WT1. The expression of WT1-derived peptides on malignant cell surfaces and recognition of those peptides by cellular and humoral immune responses have identified WT1 as a promising target in immunotherapeutic trials in the post-transplant setting. Routine vaccination with WT1 peptides at one or two years post-transplant might decrease late transplant failure in high-risk populations.
In summary, extramedullary relapse is a long-term complication of stem cell transplantation with a poor prognosis and lack of efficacious treatment. Prospective studies are needed to define the true incidence, risk factors, and appropriate therapeutic strategies for extramedullary relapse after stem cell transplantation given the lack of robust data on this subject. Future management of extramedullary relapse after stem cell transplantation should focus on creating predictive models for early detection of extramedullary relapse and novel therapies that modulate the graft-versus-leukemia response.
Footnotes
No potential conflicts of interests relevant to this article were reported.
References
- 1.Craddock C, Nagra S, Peniket A, Brookes C, Buckley L, Nikolousis E, et al. Factors predicting long-term survival after T cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95(6):989–95. doi: 10.3324/haematol.2009.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Békássy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;17(5):801–8. [PubMed] [Google Scholar]
- 3.Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006;47(9):1754–67. doi: 10.1080/10428190600632857. [DOI] [PubMed] [Google Scholar]
- 4.Frassoni F, Barrett AJ, Grañena A, Ernst P, Garthon G, Kolb HJ, et al. Relapse after allogeneic bone marrow transplantation for acute leukaemia: a survey by the E.B.M.T. of 117 cases. Br J Haematol. 1988;70(3):317–20. doi: 10.1111/j.1365-2141.1988.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 5.Berthou C, Léglise MC, Herry A, Balcon D, Hardy E, Lessard M, Abgrall JF. Extramedullary relapse after favorable molecular response to donor leukocyte infusions for recurring acute leukemia. Leukemia. 1998;12(11):1676–81. doi: 10.1038/sj.leu.2401144. [DOI] [PubMed] [Google Scholar]
- 6.Koc Y, Miller KB, Schenkein DP, Daoust P, Sprague K, Berkman E. Extramedullary tumors of myeloid blasts in adults as a pattern of relapse following allogeneic bone marrow transplantation. Cancer. 1999;85(3):608–15. doi: 10.1002/(sici)1097-0142(19990201)85:3<608::aid-cncr11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32(8):835–42. doi: 10.1038/sj.bmt.1704223. [DOI] [PubMed] [Google Scholar]
- 8.Shimoni A, Rand A, Shem-Tov N, et al. Isolated Extra-Medullary Relapse of Acute Leukemia After Allogeneic Stem-Cell Transplantation (SCT); Different Kinetics and Better Prognosis than Systemic Relapse. Biol Blood Marrow Transplant. 2009;15 (2 supplement 1):59. doi: 10.1016/j.bbmt.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Harris AC, Mageneau J, Braun T, et al. Extramedullary Relapse In Acute Leukemia Following Allogeneic Hematopoietic Stem Cell Transplantation: Incidence, Risk Factors And Outcomes. Biol Blood Marrow Transplant. 2010;16 (2 supplement 1):S177–S178. [Google Scholar]
- 10.Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115(9):1899–905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong G, Byrnes G, Szer J, Grigg A. Extramedullary relapse after allogeneic bone marrow transplantation for haematological malignancy. Bone Marrow Transplant. 2000;26(9):1011–5. doi: 10.1038/sj.bmt.1702659. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara S, Tamaki H, Ikegame K, et al. Early prediction of extramedullary relapse of leukemia following allogeneic stem cell transplantation using the WT1 transcript assay. Biol Blood Marrow Transplant. 2006;12 (2 supplement 1):86. [Google Scholar]
- 13.Kikushige Y, Takase K, Sata K, Aoki K, Numata A, Miyamoto T, et al. Repeated relapses of acute myelogenous leukemia in the isolated extramedullary sites following allogeneic bone marrow transplantations. Intern Med. 2007;46(13):1011–4. doi: 10.2169/internalmedicine.46.6384. [DOI] [PubMed] [Google Scholar]
- 14.Szomor A, Passweg JR, Tichelli A, Hoffmann T, Speck B, Gratwohl A. Myeloid leukemia and myelodysplastic syndrome relapsing as granulocytic sarcoma (chloroma) after allogeneic bone marrow transplantation. Ann Hematol. 1997;75(5–6):239–41. doi: 10.1007/s002770050350. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Imamura M, Soga R, Tsuda Y, Maeda S, Iwasaki H, et al. Establishment of a novel granulocytic sarcoma cell line which can adhere to dermal fibroblasts from a patient with granulocytic sarcoma in dermal tissues and myelofibrosis. Br J Haematol. 1992;82(1):26–31. doi: 10.1111/j.1365-2141.1992.tb04589.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuwabara H, Nagai M, Yamaoka G, Ohnishi H, Kawakami K. Specific skin manifestations in CD56 positive acute myeloid leukemia. J Cutan Pathol. 1999;26(1):1–5. doi: 10.1111/j.1600-0560.1999.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 17.Sackstein R. A Revision of Billingham’s Tenets: The Central Role of Lymphocyte Migration in Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):2–8. doi: 10.1016/j.bbmt.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009;44(12):769–77. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 19.Choi ER, Ko YH, Kim SJ, Jang JH, Kim K, Kang WK. Gastric Recurrence of Extramedullary Granulocytic Sarcoma After Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. J Clin Oncol. 2010;28(4):e54–e55. doi: 10.1200/JCO.2009.23.7560. [DOI] [PubMed] [Google Scholar]
- 20.Au WY, Ma SK, Kwong YL, Lie AK, Shek WH, Chow WC, Liang R. Acute myeloid leukemia relapsing as gynecomastia. Leuk Lymphoma. 1999;36(1–2):191–4. doi: 10.3109/10428199909145963. [DOI] [PubMed] [Google Scholar]
- 21.Ando T, Mitani N, Matsunaga K, Nakazora T, Gondo T, Yujiri T, Tanizawa Y. Gemtuzumab ozogamicin therapy for isolated extramedullary AML relapse after allogeneic hematopoietic stem-cell transplantation. Tohoku J Exp Med. 2010;220(2):121–6. doi: 10.1620/tjem.220.121. [DOI] [PubMed] [Google Scholar]
- 22.Owonikoko T, Agha M, Balassanian R, Smith R, Raptis A. Gemtuzumab therapy for isolated extramedullary AML relapse following allogeneic stem-cell transplant. Nat Clin Pract Oncol. 2007;4(8):491–5. doi: 10.1038/ncponc0899. [DOI] [PubMed] [Google Scholar]