The study of factor V Leiden (FVL) has created many expectations but also engendered much controversy. Factor V Leiden is widely considered the first and most common prothrombotic polymorphism, but in 1965, the non-O blood group, present in 50% of the population, was associated with a 2-fold increased risk of venous thrombosis. Factor V Leiden may have developed through genetic drift or natural selection in Caucasians, possibly by conferring a reduced risk of bleeding and an evolutionary advantage, but no similar prothrombotic polymorphism has been described in other populations. The risk of venous thrombosis (OR: 4 for heterozygous) and the relatively high prevalence in Caucasians (4–10%), together with its simple genotyping explain why testing for factor V Leiden has been widely studied and is still commonly requested. However, the utility of such testing is under debate, as it might complicate more than facilitate the clinical management of carriers, particularly the prophylaxis of venous thrombosis in asymptomatic carriers. Moreover, factor V Leiden has a very mild effect on arterial thrombosis. These controversies may be explained by the moderate functional consequences of the activated protein C (APC) resistance caused by this polymorphism and the requirements of additional genetic and environmental risk factors and triggering factors that are ultimately responsible for the development of a thrombotic event. Additionally, there are two apparent paradoxes concerning the clinical consequences of factor V Leiden.
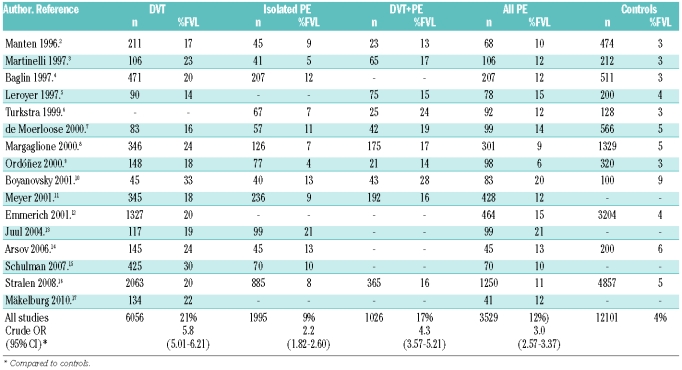
Pulmonary embolism (PE) is usually considered to be a complication of deep vein thrombosis (DVT) and therefore the genetic risk factors for both DVT and PE are believed to be the same. However, in 1996, Desmarais and co-workers first described that activated protein C resistance was associated with lower risk of pulmonary embolism than deep vein thrombosis.1 The repeated confirmation of this finding in different registries from diverse populations (Table 1),2–16 the thrombophilic family-cohort study by Mäkelburg and colleagues in this issue that is the first to report annual incidences of deep vein thrombosis and pulmonary embolism for carriers of factor V Leiden,17 the low prevalence of factor V Leiden among patients with fatal pulmonary embolism,18 and the higher incidence of deep vein thrombosis than pulmonary embolism in patients with factor V Leiden,19 are strong arguments for this paradox and do not support the hypothesis of a possible selection bias. A recent analysis of the RIETE registry also revealed a lower incidence of factor V Leiden among patients with pulmonary embolism, and interestingly cases of pulmonary embolism in factor V Leiden carriers were less severe than in non-carriers (M Monreal, personal oral communication, 2010). Despite the consistency observed in many epidemiological association studies, there are some limitations that question the reality of this paradox. The numbers of patients, particularly with pulmonary embolism, is low and no accurate multivariate analysis including environmental or genetic factors with potential modulating or confounding effect have been performed. Moreover, the paradox is moderated when combining isolated pulmonary embolism with concomitant deep vein thrombosis and pulmonary embolism (Table 1), so a diagnostic bias has to be considered, as asymptomatic pulmonary embolism can be found in about half the patients presenting with proximal deep vein thrombosis, while approximately 90% of the pulmonary emboli arise from thrombi in the deep veins of the lower limbs (40–50% asymptomatic DVT). Finally, two prospective studies that have addressed this issue found no differences in the prevalence of factor V Leiden between pulmonary embolism and deep vein thrombosis.20,21 Accordingly, there are too many weaknesses to sustain only with association studies a different role for a prothrombotic polymorphism on two clinically different entities of a single disease. Some differences between FVL-carriers and non-carriers have been described regarding thrombus location and number of affected veins, but none offered a clear explanation for the paradox.16
Table 1.
Prevalence of factor V Leiden in deep venous thrombosis (DVT), pulmonary embolism (PE), and controls.
The paradox might be explained by a reduced embolization risk induced by factor V Leiden. Two hypotheses have been proposed in this framework. The first one suggested that factor V Leiden may enhance local thrombin generation intensifying the local inflammatory process against the thrombus, and strengthen the clot structure by activation of thrombin-induced FXIII transglutaminase activity (Figure 1). The analysis of the effect on deep vein thrombosis and pulmonary embolism of other thrombophilic factors associated with high thrombin generation, such as antithrombin, protein C or S deficiencies might help to evaluate this hypothesis. This is an additional strength of the new study by Mäkelburg and colleagues. The similar risk of deep vein thrombosis and pulmonary embolism observed for all thrombophilic risk factors except factor V Leiden, makes this hypothesis unlikely.17
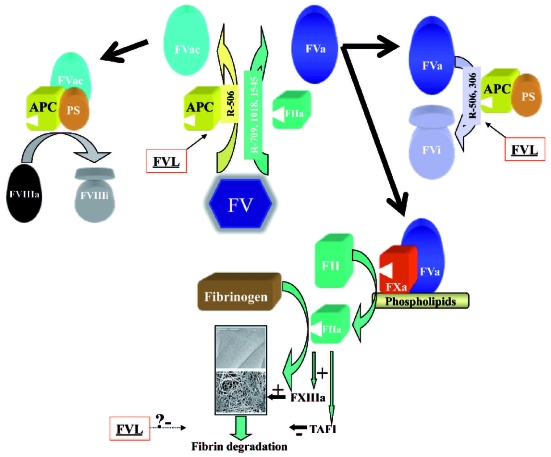
Figure 1.
Role of factor V (FV) on blood coagulation and effect of FV Leiden (FVL). Thrombin (IIa) cleaves FV at R709, R1018, and R1545 to activate FV (FVa). FVa together with factor Xa, forms the prothrombinase complex, which promotes prothrombin (FII) conversion to thrombin on the platelet surface. Thrombin generates the fibrin clot and also activates FXIII, which by means of its transglutaminase activity crosslinks fibrin fibers. Moreover, thrombin also activates the thrombin activatable fibrinolysis inhibitor (TAFI). These two effects contributed to the strength and resistance of the clot. Cleavage of FVa at R506 by activated protein C (APC) and protein S (PS) initiates the inactivation of FVa (FVi). Patients with FV Leiden (FVL) are resistant to FVa inactivation. Therefore, FVL activated has a longer half-life in plasma that results in increased thrombin generation. FV can also be cleaved by APC at R506, resulting in a molecule that acts as an anticoagulant (FVac) by stimulating APC- and PS–mediated inactivation of FVIIIa. FVL carriers also have this anticoagulant mechanism. Both FVL effects certainly contribute to the increased risk of venous thrombosis. However, it is necessary to identify any possible relationship of these two FVL-mediated mechanisms with a potential reduced embolization risk that could explain the minor incidence of pulmonary embolism in FVL-carriers.
The second hypothesis that sustains a specific effect of factor V Leiden on resistance to embolization, involves the antifibrinolytic effect of this polymorphism described in a few studies. Bajzar and co-workers22 determined the profibrinolytic effect of activated protein C on lysis time of plasma clots formed in vitro from 4 normal and 4 FVL homozygous subjects, and in a purified system reconstituted with either normal factor V or factor V Leiden in presence and absence of thrombin activatable fibrinolysis inhibitor (TAFI). Their results suggested an impaired TAFI-dependent profibrinolytic response to activated protein C in FVL-carriers.22 These results were confirmed by Parker and co-workers,23 who also injected radiolabeled clots into the jugular veins of wild-type and FVL heterozygous or homozygous mice. Pulmonary clot lysis was significantly reduced in homozygous FVL mice compared with wild-type supporting the hypothesis that factor V Leiden might inhibit fibrinolysis in vivo, although the relevance of TAFI is controversial.23 Against this hypothesis, FVL-carriers did not show an increased risk of post-thrombotic syndrome, which would be attributable to an increased clot resistance to fibrinolysis. Therefore, further pathophysiological studies are required to establish the mechanism(s) involved in the inhibition of fibrinolysis by factor V Leiden in vivo, including evaluation of plasma fibrin degradation products. The anticoagulant role of factor V might also be studied in this context, as it is also affected by factor V Leiden (Figure 1). Finally, it would be desirable to study the kinetics of thrombus formation and lysis, and the clot size and structure in FVL-carriers and non-carriers.
Could this paradox have any clinical relevance? The identification of hemostatic markers associated with the prognosis of venous thrombosis, particularly those involved in pulmonary embolism would be important in reducing mortality. The weak association between factor V Leiden and pulmonary embolism has given rise to a debate on whether the patients with factor V Leiden might not need a lengthy period of anticoagulant treatment. Indeed, this association was also initially used to explain the second paradox concerning factor V Leiden: the increased risk of a first venous thrombosis but not of a particularly high-risk of recurrence in FVL-carriers. A recent systematic review of this topic found a mild but significant risk of recurrence for factor V Leiden heterozygous (OR: 1.56; 95% CI: 1.14–2.12).24 Baglin illustrates different explanations for this paradox, which is basically sustained by limitations imposed by a restricted dichotomous testing strategy compounded by test inaccuracy, imprecision and multicausality.25
So what do we know now? On the basis of multiple association studies, among all thrombophilic defects, only factor V Leiden showed a mild risk factor for isolated pulmonary embolism whereas it substantially increases the risk of deep vein thrombosis. The suggested antifibrinolytic mechanism associated with factor V Leiden might contribute to explain this paradox. Thus, the thrombotic tendencies in subjects with factor V Leiden are caused by the inability of activated protein C to effectively inactivate the procoagulant factor V Leiden and the resistance of these thrombi to fibrinolysis (Figure 1). The last feature, however, might protect against embolization. Accordingly, carriers of factor V Leiden would have a better prognosis. However, there are many limitations in the understanding of the factor V Leiden paradox and its clinical implications. First, it would be desirable to clarify whether patients carrying factor V Leiden have lower incidence of proximal deep vein thrombosis than non-carriers. Secondly, more studies are required to verify the antifibrinolytic effect of factor V Leiden and to identify other element(s) involved (TAFI, PAI, FXIII, etc). Finally, additional large prospective studies are necessary with routine screening for both deep vein thrombosis and pulmonary embolism in all patients. Moreover, the identification of modifier or confounding factors and multivariate analysis are required. The minor risk of pulmonary embolism associated with factor V Leiden was initially associated with the low risk of recurrence described for carriers of this polymorphism. Now, it is clear that the recurrence is similar in deep vein thrombosis and pulmonary embolism, and the apparent paradox of lower recurrence rate for factor V Leiden, as for all other congenital thrombophilic defects compared with the first event risk, is indeed the wrong interpretation of two different pictures using inadequate diagnostic tests and designs. Independently of this controversy, all available data support the hypothesis that the diagnosis of factor V Leiden should not influence the secondary thromboprophylaxis.
The term paradox comes from the Greek paradoxos (para against, doxa opinion) and it is defined by the dictionary as “a situation or thing which is strange because it has or involves two opposite facts or qualities which could not both be true at the same time”. The history of science has many examples of apparent paradoxes, some of them legendary and have been created when new observations challenge existing paradigms. Paradoxes have many enthusiasts and have generated controversy (the word “paradox” generates 7,501 entries in pubmed). However, there are also many sceptics who consider that many paradoxes in science result from a lack of rigorous observation of the subtle interactions between “existence” and “knowing”. In other words, many of us consider that there are no paradoxes in science, only bad hypotheses, wrong interpretations or enigmas (“something that is mysterious or puzzling”). Only strong scientific evidence is required to explain factor V Leiden “enigmas”, which might help understand the complex interrelations between procoagulant, anticoagulant and fibrinolytic elements that makes the enigmatic (but not paradoxical) hemostatic system appropriate for the requirements of a closed and high pressure circulatory system.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Desmarais S, de Moerloose P, Reber G, Minazio P, Perrier A, Bounameaux H. Resistance to activated protein C in an unselected population of patients with pulmonary embolism. Lancet. 1996;347(9012):1374–45. doi: 10.1016/s0140-6736(96)91013-2. [DOI] [PubMed] [Google Scholar]
- 2.Manten B, Westendorp RGJ, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost. 1996;76(4):510–3. [PubMed] [Google Scholar]
- 3.Martinelli I, Cattaneo M, Panzeri D, Mannucci PM. Low prevalence of factor V:Q506 in 41 patients with isolated pulmonary embolism. Thromb Haemost. 1997;77(3):440–3. [PubMed] [Google Scholar]
- 4.Baglin TP, Brown K, Williamson D, Baker P, Luddington R. Relative risk of pulmonary embolism and deep vein thrombosis in association with the factor V Leiden in a United Kingdom population. Thromb Haemost. 1997;77(6):1219. [PubMed] [Google Scholar]
- 5.Leroyer C, Mercier B, Escoffre M, Férec C, Mottier D. Factor V Leiden prevalence in venous thromboembolism patients. Chest. 1997;111(6):1603–6. doi: 10.1378/chest.111.6.1603. [DOI] [PubMed] [Google Scholar]
- 6.Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost. 1999;81(3):345–8. [PubMed] [Google Scholar]
- 7.De Moerloose P, Reber G, Perrier A, Perneger T, Bounameaux H. Prevalence of factor V Leiden and prothrombin G20210A mutations in unselected patients with venous thromboembolism. Br J Haematol. 2000;110(1):125–9. doi: 10.1046/j.1365-2141.2000.02039.x. [DOI] [PubMed] [Google Scholar]
- 8.Margaglione M, Brancaccio V, De Lucia D, Martinelli I, Ciampa A, Grandone E, et al. Inherited thrombophilic risk factors and venous thromboembolism: distinct role in peripheral deep venous thrombosis and pulmonary embolism. Chest. 2000;118(5):1405–11. doi: 10.1378/chest.118.5.1405. [DOI] [PubMed] [Google Scholar]
- 9.Ordóñez AJ, Carreira JM, Alvarez CR, Rodríguez JM, Alvarez MV, Coto E. Comparison of the risk of pulmonary embolism and deep vein thrombosis in the presence of factor V Leiden or prothrombin G20210A. Thromb Haemost. 2000;83(2):352–4. [PubMed] [Google Scholar]
- 10.Boyanovsky B, Russeva M, Ganev V, Penev M, Baleva M. Prevalence of factor V Leiden and prothrombin 20210 A variant in Bulgarian patients with pulmonary thromboembolism and deep venous thrombosis. Blood Coagul Fibrinolysis. 2001;12(8):639–42. doi: 10.1097/00001721-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Meyer G, Emmerich J, Helley D, Arnaud E, Nicaud V, Alhenc-Gelas M, et al. Factor V leiden and II 20210A in patients with symptomatic pulmonary embolism and deep vein thrombosis. Am J Med. 2001;110(1):12–5. doi: 10.1016/s0002-9343(00)00653-7. [DOI] [PubMed] [Google Scholar]
- 12.Emmerich J, Rosendaal FR, Cattaneo M, Margaglione M, De Stefano V, Cumming T, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism--pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809–16. [PubMed] [Google Scholar]
- 13.Juul K, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med. 2004;140(5):330–7. doi: 10.7326/0003-4819-140-5-200403020-00008. [DOI] [PubMed] [Google Scholar]
- 14.Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J. 2006;47(3):433–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Schulman S. Thrombophilia and location of venous thromboembolism. J Thromb Haemost. 2007;5(10):2151–2. doi: 10.1111/j.1538-7836.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 16.van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol. 2008;28(10):1872–7. doi: 10.1161/ATVBAHA.108.169524. [DOI] [PubMed] [Google Scholar]
- 17.Mäkelburg AB, Veeger NJ, Middeldorp S, Hamulyák K, Prins MH, Büller HR, et al. Different risk of deep vein thrombosis and pulmonary embolism in carriers with factor V Leiden compared with non-carriers, but not in other thrombophilic defects. Results from a large retrospective family cohort study. Haematologica. 2010;95(6):1025–8. doi: 10.3324/haematol.2009.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper WC, De Staercke C. The relationship between FV Leiden and pulmonary embolism. Respir Res. 2002;3:8–10. doi: 10.1186/rr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinelli I, Battaglioli T, Razzari C, Mannucci PM. Type and location of venous thromboembolism in patients with factor V Leiden or prothrombin G20210A and in those with no thrombophilia. J Thromb Haemost. 2007;5(1):98–101. doi: 10.1111/j.1538-7836.2006.02291.x. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Cushman M, Tsai MY, Aleksic N, Heckbert SR, Boland LL, et al. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002;99(8):2720–5. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- 21.Juul K, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med. 2004;140(5):330–7. doi: 10.7326/0003-4819-140-5-200403020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Bajzar L, Kalafatis M, Simioni P, Tracy PB. An antifibrinolytic mechanism describing the prothrombotic effect associated with factor V Leiden. J Biol Chem. 1996;271(38):22949–52. doi: 10.1074/jbc.271.38.22949. [DOI] [PubMed] [Google Scholar]
- 23.Parker AC, Mundada LV, Schmaier AH, Fay WP. Factor V Leiden inhibits fibrinolysis in vivo. Circulation. 2004;110(23):3594–8. doi: 10.1161/01.CIR.0000148781.87906.C0. [DOI] [PubMed] [Google Scholar]
- 24.Segal JB, Brotman DJ, Necochea AJ, Emadi A, Samal L, Wilson LM, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–85. doi: 10.1001/jama.2009.853. [DOI] [PubMed] [Google Scholar]
- 25.Baglin T. Unraveling the thrombophilia paradox: from hypercoagulability to the prothrombotic state. J Thromb Haemost. 2010;8(2):228–33. doi: 10.1111/j.1538-7836.2009.03702.x. [DOI] [PubMed] [Google Scholar]