Abstract
Background
The hemangioblast is a bi-potential precursor cell with the capacity to differentiate into hematopoietic and vascular cells. In mouse E7.0–7.5 embryos, the hemangioblast can be identified by a clonal blast colony-forming cell (BL-CFC) assay or single cell OP9 co-culture. However, the ontogeny of the hemangioblast in mid-gestation embryos is poorly defined.
Design and Methods
The BL-CFC assay and the OP9 system were combined to illustrate the hemangioblast with lymphomyeloid and vascular potential in the mouse aorta-gonad-mesonephros region. The colony-forming assay, reverse transcriptase polymerase chain reaction analysis, immunostaining and flow cytometry were used to identify the hematopoietic potential, and Matrigel- or OP9-based methods were employed to evaluate endothelial progenitor activity.
Results
Functionally, the aorta-gonad-mesonephros-derived BL-CFC produced erythroid/myeloid progenitors, CD19+ B lymphocytes, and CD3+TCRβ+ T lymphocytes. Meanwhile, the BL-CFC-derived adherent cells generated CD31+ tube-like structures on OP9 stromal cells, validating the endothelial progenitor potential. The aorta-gonad-mesonephros-derived hemangioblast was greatly enriched in CD31+, endomucin+ and CD105+ subpopulations, which collectively pinpoints the endothelial layer as the main location. Interestingly, the BL-CFC was not detected in yolk sac, placenta, fetal liver or embryonic circulation. Screening of candidate cytokines revealed that interleukin-3 was remarkable in expanding the BL-CFC in a dose-dependent manner through the JAK2/STAT5 and MAPK/ERK pathways. Neutralizing interleukin-3 in the aorta-gonad-mesonephros region resulted in reduced numbers of BL-CFC, indicating the physiological requirement for this cytokine. Both hematopoietic and endothelial differentiation potential were significantly increased in interleukin-3-treated BL-CFC, suggesting a persistent positive influence. Intriguingly, interleukin-3 markedly amplified primitive erythroid and macrophage precursors in E7.5 embryos. Quantitative polymerase chain reaction analysis demonstrated declined Flk-1 and elevated Scl and von Willebrand factor transcription upon interleukin-3 stimulation, indicating accelerated hemangiopoiesis.
Conclusions
The hemangioblast with lymphomyeloid potential is one of the precursors of definitive hematopoiesis in the mouse aorta-gonad-mesonephros region. Interleukin-3 has a regulatory role with regards to both the number and capacity of the dual-potential hemangioblast.
Keywords: interleukin-3, hemangioblast, AGM, definitive hematopoiesis, hematopoietic stem cells
Introduction
There are generally two stages of early embryonic hematopoiesis in the mouse. The first stage, primitive hematopoiesis, is characterized by de novo generation of nucleated erythrocytes (large cells that produce embryonic hemoglobin) in the extra-embryonic yolk sac. The second stage, definitive hematopoiesis, is marked by autonomous formation of adult type hematopoietic stem cells (HSC) in E10.5 major vessels or highly vascularized structures, i.e. the aorta-gonad-mesonephros (AGM) region, umbilical/vitelline arteries, yolk sac, and placenta.1–8 Although hematopoietic activities are largely different in the two stages, in both stages there is close intertwining with the vascular lineage in two speculated forms: the hemangioblast (a common precursor of hematopoietic and endothelial cell lineages) and hemogenic endothelium (de-differentiation of mature endothelium into hematopoietic lineage).9–12
Eighty years ago, the term hemangioblast was proposed in the light of the finding that, in the yolk sac, the aggregated mesodermal cells simultaneously gave rise to central primitive erythrocytes and peripheral endothelial cells in close proximity, forming a distinct structure called a blood island. However, It was not until 1998 that two independent groups provided the first convincing evidence of the hemangioblast through in vitro differentiation assays of mouse embryonic stem cells. Nishikawa et al. used a collagen-based, two-dimensional differentiation system with an OP9 feeder layer to visualize blood budding and tube formation from single Flk-1+CD144+ cells.13 Choi et al., on the other hand, defined clonal blast colony-forming cells (BL-CFC) with remarkable responsiveness to vascular endothelial growth factor (VEGF) in early embryoid bodies.14 Recently, they revealed that the comparable BL-CFC (Brachyury+Flk-1+) predominantly arise in the posterior primitive streak of E7.5 embryos, not, as presumed, in the yolk sac.15 This finding suggests that: (i) the precursor represents a transient mesoderm population undergoing hematopoietic and vascular specification, and (ii) hematopoietic commitment initiates prior to the establishment of the yolk sac blood island. It is believed that embryonic stem cell-derived hematopoietic differentiation recapitulates yolk sac-stage hematopoiesis.16 Embryonic stem cell/hemangioblast models have helped in the understanding of the complicated molecular mechanisms underlying commitment to the mesoderm-hemangioblast-hematopoietic cascade. In contrast, the hemangioblastic origin and molecular modulation of definitive intra-embryonic hematopoiesis remain poorly understood. Because HSC derived from the AGM region possess nearly all the immunophenotypic features of endothelium (LDL receptor+, CD31+, CD144+, CD34+ and Tie2+), it is accepted that the hemogenic endothelium serves as the direct precursor of HSC.17–20 Recently, we found that high proliferation potential precursors in the mouse AGM region could be categorized into two types: one restricted to the hematopoietic lineage, and the other with hemangioblastic properties (referred to as HPP-HA).21 Of note, it remains unknown whether hemangioblasts (from either embryonic stem cells or embryos) possess lymphoid potential.
In this study, we combined the BL-CFC and OP9 systems to quantify hemangioblasts with B and T lymphoid potential in the mouse AGM region and screened candidate cytokines for a regulatory effect on hemangioblast development in the AGM region.
Design and Methods
Blast colony-forming cell assay
The BL-CFC medium was composed of 0.9% methylcellulose (Sigma), 2 mM glutamine (Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Hyclone), 5% protein-free hybridoma medium II (Gibco, Grand Island, NY, USA), 200 μg/mL iron-saturated transferrin (Sigma), 1% bovine serum albumin (Stem Cell Technologies, Vancouver, Canada), 0.45 mM monothio-glycerol (Sigma), 15% fetal bovine serum (Hyclone) and a cocktail of recombinant human (rh) and recombinant mouse (rm) cytokines including basic fibroblast growth factor (rhbFGF; 10 ng/mL), stem cell factor (rmSCF; 50 ng/mL), interleukin-6 (rhIL-6; 5 ng/mL), insulin-like growth factor-I (rhIGF-I; 50 ng/mL), leukemia inhibitory factor (rmLIF; 10 ng/mL) and rhVEGF-165 (5 ng/mL). Following 4–5 days of inoculation, blast colonies were scored.
OP9-based hematopoietic and endothelial progenitor potential assay
Individual blast colonies were plated on OP9 stromal cells in the presence of rhVEGF-165 (200 ng/mL), rhFlt3 ligand (rhFL; 20 ng/mL) and rhIL-7 (20 ng/mL). After 10 days of culture, they were fixed in 4% paraformaldehyde for 30 min, and incubated with monoclonal antibody against CD45 (phycoerythrin-conjugated, eBioscience, San Diego, CA, USA) overnight at 4°C. Thereafter, a two-step staining protocol was used to identify the endothelial development. Following incubation with a primary anti-CD31 antibody (BD Pharmingen), the culture was visualized by the indirect immunoperoxidase method using horseradish peroxidase–conjugated anti-rat IgG (Zymed, South San Francisco, CA, USA). The peroxidase activity was visualized using 3,38-diaminobenzidine (Zymed).
To determine B lymphoid potential, blast colonies were replated on fresh OP9 stromal cells in the presence of rhFL (20 ng/mL) and rhIL-7 (20 ng/mL). After 7–10 days of culture, B lymphocyte differentiation was assessed.
To generate T lymphocytes, blast colonies were replated onto OP9-DL1 stromal cells supplemented with rhFL (5 ng/mL), rhIL-7 (5 ng/mL) and thrombopoietin (rhTPO; 5 ng/mL). After 4 days of culture, TPO was withdrawn. T lymphocyte differentiation was analyzed after a further 11–16 days of culture.
Polymerase chain reaction analyses
Nested reverse transcriptase polymerase chain reaction (RT-PCR) for individual colonies was performed as described previously.21 For RT-PCR analysis, total RNA from E7.5 embryos or the E10.5 AGM region was extracted using TRIZOL reagent (Invitrogen) and reverse transcribed using the mRNA Selective PCR Kit (TaKaRa). Blast colonies were harvested and analyzed by quantitative real-time PCR as described previously.22 The specific primer sequences and amplified product size are listed in Online Supplementary Tables S1–S3.
Immunohistochemistry
Immunohistochemical studies were carried out as described elsewhere.22 The primary antibodies used were anti-CD31 (BD Pharmingen), anti-endomucin (eBioscience), and anti-platelet-derived growth factor receptor-β (anti-PDGFR β; Cell Signaling).
Other procedures
The methods of embryo dissection, the hematopoietic colony-forming cell assay, in vitro tube formation assay, flow cytometry, magnetic sorting, immunofluorescence staining, western blotting, and phase contrast and fluorescence imaging are described in the Online Supplementary Design and Methods.
Statistical analysis
Results are expressed as means ± s.e.m. The statistical significance of differences was determined using the Student’s t test.
Results
Characterization of the blast colony-forming cell in the mouse aorta-gonad-mesonephros region
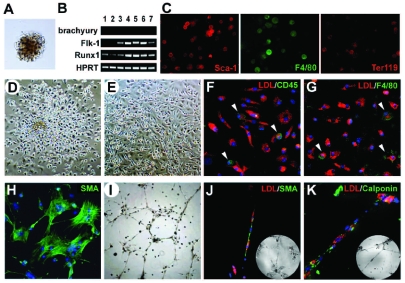
In order to carry out the clonal BL-CFC analysis, a single-cell suspension from mouse E10.5 AGM region (0.5–1×105 cells/mL) was plated in methylcellulose medium containing bFGF, SCF, VEGF, LIF, IGF-I and IL-6. After 4–5 days of incubation, the typical blast colonies with unique and identifiable morphology emerged. The blast colony was composed of a compact core and a loose peripheral layer with clear intercellular boundaries (Figure 1A). Under such conditions, the typical hematopoietic colonies were rare and could be easily identified. The blast colony predominantly consisted of immature cells with a high nuclear/plasma ratio as demonstrated by Wright-Giemsa staining (data not shown). Nested RT-PCR assays of individually plucked colonies revealed that most of them harbored the hemangioblast-like molecular pattern: Brachyury−, Flk-1+, and Runx1+ (Figure 1B). Immunofluorescence staining demonstrated that the colonies contained Sca-1+, F4/80+ and Ter119+ cells (Figure 1C). The data suggested that the blast colonies derived from the AGM region progressed beyond the mesoderm and underwent hemangioblastic commitment.
Figure 1.
Molecular characteristics, hematopoietic and vascular potential of BL-CFC derived from E10.5 mouse AGM region. (A) Morphology of a typical blast colony. (B) Expression of Brachyury, Flk-1 and Runx1 in seven representative blast colonies determined by nested RT-PCR. (C) Immunofluorescence staining of Sca-1, F4/80 and Ter119 in blast colonies. Morphology of individually plucked blast colonies in the liquid expansion system after 48 h (D) and 10 days (E) of incubation. (F–G) DiI-Ac-LDL incorporation (red) combined with CD45 or F4/80 staining (green) of the adherent cells. Arrowheads, CD45 positive in H or F4/80 positive in I. (H) α-SMA+ staining (green) of the adherent cells. (I) Tube-like structures in Matrigel of the adherent cells. (J–K) DiI-Ac-LDL incorporation (red) combined with α-SMA or Calponin staining (green) of the tube-like structures. Inserts show the corresponding bright fields. Original magnification: ×40 (I), ×100 (D, E), and ×200 (A, C, F–H, J, K).
We then attempted to determine the critical cytokines for generating blast colonies from the AGM region. By eliminating cytokines, one by one, from the original formula (SCF+bFGF+IL-6+IGF-I+LIF+VEGF), we found that bFGF and SCF had the strongest effect on colony growth (P<0.01). The removal of IL-6 had a modest effect on the number of colonies (P<0.05) (Online Supplementary Figure S1).
To validate the hematopoietic potential, the blast colonies were individually harvested and replated into a hematopoietic colony-forming cell (CFC) system containing SCF, IL-3, IL-6, IL-11, erythropoietin (Epo), TPO and granulocyte-macrophage colony-stimulating factor (GM-CSF). As demonstrated in Online Supplementary Figure S2A, they gave rise to CFU-E, CFU-GM, CFU-Mix, and BFU-E. Of 12 representative colonies, all could generate hematopoietic CFC (Online Supplementary Figure S2B). On average, a single BL-CFC yielded 5.7 CFU-E, 1.9 CFU-GM, and 5.7 CFU-Mix.
To further characterize the vascular potential, a single blast colony was cultured in a liquid expansion system with hematopoietic and endothelial cytokines (bFGF, VEGF, SCF, IL-3, IL-11, Epo, IGF-I and ECGS). After 24 h of incubation, the adherent cells were evident in over 97% (64/66) of the colonies. In a couple of days, they amplified significantly (Figure 1D). Meanwhile, non-adherent or semi-adherent hematopoietic clusters developed in the upper layer and retained hematopoietic colony-forming capacity (data not shown). At day 4, the non-adherent hematopoietic population was depleted and the culture was replaced with EGM-2 to support vascular differentiation and maturation. At day 10, adherent cells with cobble-stone or spindle-like morphology were predominant in the culture (Figure 1E). Most of them incorporated DiI-Ac-LDL, but occasionally expressed CD45 and F4/80 (Figure 1F, G). This indicated that the endothelial lineage (rather than hematopoietic/monocytic cells) prevailed in the culture. At the same time, α-SMA+ cells appeared in the adherent layer (Figure 1H). Next, an in vitro tube forming assay was used to evaluate whether the adherent population was functionally equivalent to the vascular cells. As shown in Figure 1I, the adherent cells from 45 of a total of 60 colonies were capable of forming tube-like structures in Matrigel. Alternatively, the adherent cells were labeled with DiI-Ac-LDL prior to replating. The participation of elongated LDL-incorporating endothelial cells and α-SMA+ or calponin+ cells in the capillary formation was visualized (Figure 1J,K). Of note, under appropriate stimuli, the blast colonies failed to generate osteogenic and adipogenic lineages in vitro (data not shown).
OP9, the bone marrow stromal cell line, is known to provide an appropriate microenvironment for endothelial and lymphocyte differentiation.13,23,24 To further validate hemangioblastic activity, 50 blast colonies were individually co-cultured with OP9 cells for 10 days. All of them demonstrated both hematopoietic and endothelial progenitor potential. As shown in Figure 2A,B, a representative colony simultaneously generated abundant CD45+ hematopoietic cells and CD31+ tube-like structures on OP9 stromal cells.
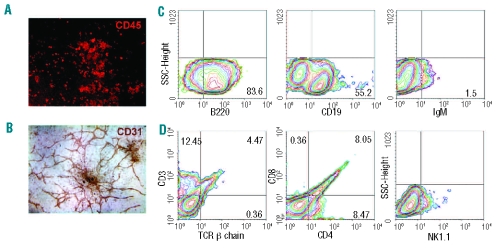
Figure 2.
Identification of hematopoietic and endothelial potential of BL-CFC by the OP9-based system. Individual blast colonies are co-cultured with OP9 for 10 days in a 48-well plate. In one representative well, both CD45+ cells (A) and CD31+ tube-like structures (B) are detected. (C) Flow cytometry analysis of B lymphocyte markers of blast colonies differentiated on OP9 stroma for 10 days. (D) Flow cytometry analysis of T lymphocyte markers and NK1.1 of blast colonies differentiated on OP9-DL1 stroma for 15 days. Representative data from three independent experiments are shown. Original magnification: ×40 (B), and ×100 (A).
To determine B lymphoid potential, blast colonies were incubated with OP9 in the presence of FL and IL-7 for 7–10 days. After 6 days of culture, significant proliferation of round non-adherent cells was detected. B220 (CD45R) is expressed at all stages of B-cell ontogeny. However, CD19 remains the most reliable marker for B-lineage commitment. As shown in Figure 2C, abundant B220+ and CD19+ populations were generated from blast colonies. Mature B cells were detected in approximately 1.5% of total cells by analyzing surface expression of IgM.
To further assay T lymphoid potential, blast colonies were co-cultured with OP9-DL1 cells (proven to support T lymphocyte development from hematopoietic stem progenitor cells and embryonic stem cells).24,25 After 8 days of culture, round non-adherent cells proliferated abundantly. After 15–20 days, CD3+TCRβ+ and CD4+/CD8+ T lymphocytes were generated (Figure 2D). NK1.1+ natural killer cells also emerged in the culture. Furthermore, six blast colonies were individually expanded and tested for B and T potential. All demonstrated both B (CD19+) and T (CD3+TCRβ+) lymphocyte potential (Online Supplementary Table S4). Compared with the OP9 co-culture, differentiation of CD19+ B lymphocytes was greatly suppressed in the OP9-DL1 system (data not shown). However, the blast colonies contained hematopoietic precursors with both myeloid and lymphoid potential, indicating their definitive hematopoietic identity.
As previously described,14,15 two steps were taken to validate the clonality of the AGM-derived blast colonies. First, cells from the AGM region were plated at different concentrations. As shown in Online Supplementary Figure S3A, the number of blast colonies was directly proportional to the input of AGM cells, indicating that a single cell corresponded to the growth of a colony. Second, a cell mixing analysis was carried out (Online Supplementary Figure S3B). An equal number of cells from the AGM region of male wild type (Sry+GFP−) and female GFP transgenic (Sry−GFP+) mice were mixed at the density of 0.5–1×105 cells/mL. After 4 days of culture, the blast colonies were picked out and expanded individually to determine the content of genomic DNA. Among 20 representative colonies analyzed, ten displayed exclusively the Sry+GFP− genotype and the other ten 10 Sry−GFP+ (Online Supplementary Figure S3B). Representative blast colonies from wild type and GFP transgenic AGM region in the cell mixing analysis are shown in Online Supplementary Figure S3C. The experiments suggest that the blast colony originated from a single cell.
Spatiotemporal distribution and surface markers of blast colony-forming cells in the mouse aorta-gonad-mesonephros region
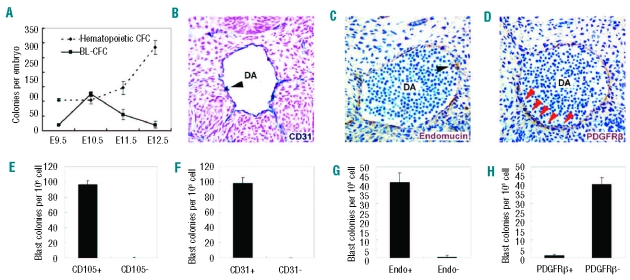
To characterize the developmental kinetics of BL-CFC in the caudal half (E9.5) or AGM region (E10.5–12.5), the tissues were analyzed daily between E9.5 and E12.5. As shown in Figure 3A, E9.5 caudal half contained 19.3±2.1 BL-CFC on average, reaching a maximum of 125.0±8.0 BL-CFC at E10.5, followed by a significant decline to 54.8±17.6 at E11.5 and 20.0±10.6 at E12.5. The kinetics of BL-CFC was different from that of hematopoietic CFC, which peaked in number at E12.5. A further separation of dorsal aorta with surrounding mesenchyme from gonad and mesonephros in the E11.5 AGM region revealed that over 95% of BL-CFC were concentrated in the former subregion (data not shown).
Figure 3.
Development kinetics and surface markers of BL-CFC. (A) BL-CFC and hematopoietic CFC derived from caudal half (E9.5) or AGM region (E10.5–12.5) are analyzed in parallel, showing different development kinetics. (B–D) Immunohistochemical analysis of E10 embryos. Transverse sections of dorsal aortas (DA) are stained with anti-CD31 (B), anti-endomucin (C) and anti-PDGFRβ (D) antibodies. The dorsal aspect is upward. The endothelial layer is positive for CD31 and endomucin, but negative for PDGFRβ (red arrowheads in D). CD31 and endomucin are also expressed in hematopoietic cell clusters (arrowheads in B and C) associated with endothelium. (E–H) The frequency of BL-CFC in CD105 (E), CD31 (F), endomucin (G), and PDGFRβ (H) positive cells sorted from E10.5 AGM region. The results are representative data expressed as means ± s.e.m from three of four independent experiments.
Next, four molecules were selected to determine the location of BL-CFC in the AGM region. As shown in Figure 3B, C and described in previous reports,26,27 CD31, endomucin, and CD105 have similar expression patterns (restricted to the endothelial layers and intro-aortic hematopoietic clusters). In contrast, PDGFRβ is expressed by mural cells (Figure 3D), and its response to PDGF secreted by endothelial cells results in migration and maturation of mural cells.28 As demonstrated in Figure 3E–G, the E10.5 AGM-derived BL-CFC were enriched in CD31, endomucin, and CD105 exclusively. Together with the finding that most BL-CFC were negative for PDGFRβ (Figure 3H), we concluded that the hemangioblasts were mainly located within the endothelium lining the vessel wall of dorsal aorta.
Of note, the blast colonies were not readily detected in cells from yolk sac (E9.5–E11.5), placenta (E9.5–E11.5), fetal liver (E10.5–E11.5) or the embryonic circulation (E10.5–E12.5) (Online Supplementary Figure S4).
Positive regulation of blast colony-forming cells derived from the aorta-gonad-mesonephros by interleukin-3
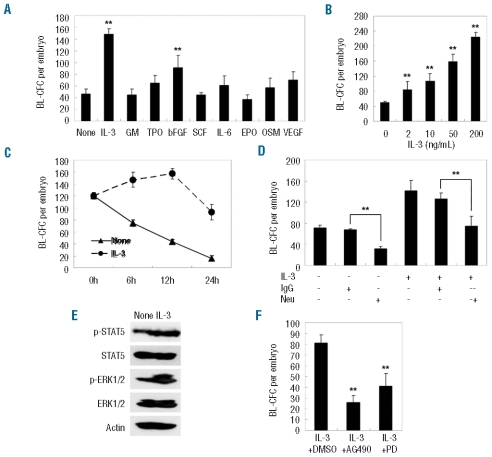
As the BL-CFC model was quantitative, a set of hematopoietic/vascular cytokines (i.e., bFGF, SCF, GM-CSF, IL-6, OSM, VEGF, TPO, IL-3, EPO) were screened for their modulation of hemangioblast development in the mouse AGM region. Initially, a single-cell suspension of the AGM region was incubated with the indicated cytokines for 12 h, and then collected for the BL-CFC assay. As expected, a significant expansion of BL-CFC was detected in the presence of bFGF (10 ng/mL). However, among the other cytokines, only IL-3 (50 ng/mL) was able to amplify the BL-CFC markedly (148.0±22.5 versus 46.7±8.6, P<0.01) and its effect appeared more pronounced than that of bFGF (Figure 4A). Interestingly, IL-3-stimulated BL-CFC formed typical blast colonies at day 2.5 of culture, 12 h earlier than the control. Furthermore, the effect was dose-dependent (Figure 4B). As shown in Figure 4C, without IL-3 treatment, the number of BL-CFC gradually decreased after in vitro incubation. In contrast, significant expansion was detected after incubation with IL-3 for 6 h (165.7±21.0 versus control of 93.3±23.5; P<0.01) and 12 h (210.3±41.2 versus control of 93.3±23.5, P<0.01). However, prolonged culture to 24 h resulted in a dramatic decrease (103.2±31.7), indicating that IL-3 failed to support hemangioblast expansion in vitro for a long duration.
Figure 4.
Influence of BL-CFC development in the E10.5 AGM region by IL-3-mediated signaling. (A) Among a panel of cytokines, IL-3 and bFGF significantly amplified the BL-CFC in the E10.5 AGM region. The IL-3-mediated effect was dose-dependent (B) and temporally regulated (C). Neutralizing tissue-derived IL-3 markedly reduced the number of BL-CFC in the AGM region. Neu denotes IL-3 neutralizing antibody (D). Phosphorylation of STAT5 and ERK1/2 in AGM cells was enhanced by IL-3 (E), and the corresponding pharmacological inhibitors abrogated IL-3-mediated BL-CFC expansion (F). The results represent the means ± s.e.m. Significance was determined using the Student’s t test: *P<0.05; **P<0.01, compared with the control data.
To determine whether IL-3 is physiologically required, we used IL-3 neutralizing antibody and an isotype control (IgG1). Compared with the IgG1, neutralization of tissue-derived IL-3 reduced the number of BL-CFC (31.3±4.5 versus 67.7±1.5, P<0.01) (Figure 4D). Of note, the result was obtained by continuous exposure to neutralizing antibody from the AGM incubation to the BL-CFC assay. This implies that the cytokines in the BL-CFC assay could compensate for a transient absence of endogenous IL-3 signals during incubation for 12 h.
The IL-3 signaling pathway has been well characterized.29 Following stimulation with IL-3, an immediate response of cells is tyrosine phosphorylation of Janus kinase-2 (JAK2)/STAT5 proteins as well as activation of Ras, Raf, MEK and ERK. Subsequently, these signals are transduced to the nucleus, where they bind specific DNA elements to activate transcription of target genes. As demonstrated in Figure 4E, IL-3 supplementation enhanced the phosphorylation of STAT5 and ERK1/2 in the AGM cells. To further investigate the IL-3-mediated signaling involved in hemangioblast expansion, AGM cells were stimulated with IL-3 for 12 h in the presence of pharmacological inhibitors or vehicle control (dimethyl sulfoxide). There was greater than 50% inhibition of BL-CFC amplification (P<0.01) when cells were incubated with 100 μM AG490 (an inhibitor of JAK2) and similar results with 50 μM PD98059 (an inhibitor of MEK1/2) (P<0.01). Thus, IL-3 expanded BL-CFC at least partially through JAK2/STAT5 and MAPK/ERK signaling (Figure 4F).
Increased hematopoietic and vascular potential of interleukin-3-treated blast colony-forming cells
We carried out differentiation assays to determine the regulatory roles of IL-3 in the hematopoietic/vascular potential of BL-CFC. As shown in Figure 5A, the IL-3-stimulated BL-CFC gave rise to increased number of CFC (43.7±3.5 versus 6.4±2.1 per blast colony, P<0.01),with this increase being more pronounced than that with bFGF treatment (P<0.01). Furthermore, quantification of in vitro tube-forming capacity on Matrigel revealed that IL-3-stimulated BL-CFC displayed significant increases in both tube number (2.0±0.1 fold, P<0.01) and tube length (2.3±0.3 fold, P<0.01) (Figure 5B–D). Alternatively, IL-3 was added during the formation of blast colonies. As shown in Figure 5E, the IL-3-treated blast colonies generated increased numbers of CD31+ tubes on OP9 stromal cells, confirming the enhanced endothelial progenitor capacity.
Figure 5.
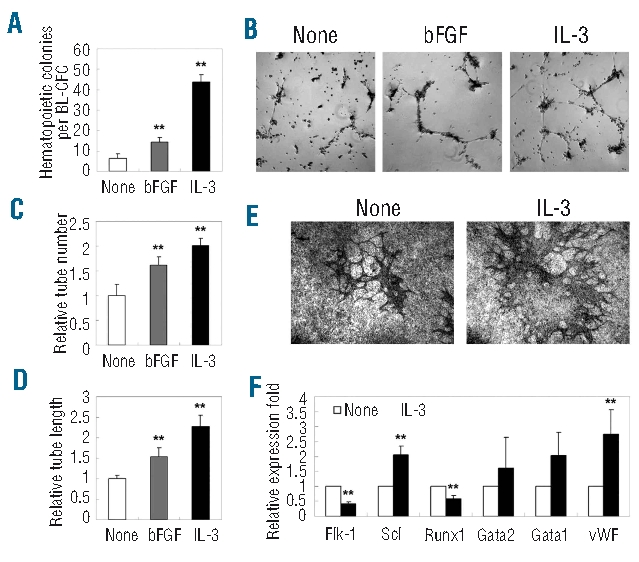
Increased hematopoietic and vascular potential of IL-3-treated BL-CFC from the E10.5 AGM region. (A) IL-3- and bFGF-stimulated BL-CFC gave rise to increased numbers of hematopoietic CFC. (B–D) A Matrigel-based test revealed that IL-3- and bFGF-stimulated BL-CFC have enhanced tube-forming capacity both with regards to the number and length of the tubes. (E) CD31 staining of blast colonies co-cultured with OP9 for 10 days. Increased tube formation was detected when IL-3 was added during blast colony formation. (F) Real-time PCR analysis of BL-CFC cultures to examine IL-3-mediated molecular changes. Original magnification: ×40 (B, E). The results represent means ± s.e.m. Significance was determined using the Student’s t- test: **, P<0.01, compared with the control data.
Subsequently, several hematopoietic- and vascular-related genes were selected to define the IL-3-mediated molecular changes in the AGM cells. Upon stimulation, up-regulated expression of Scl and vWF were detected, corroborating the enhanced hematopoiesis and endothelial differentiation seen in the BL-CFC assay (Figure 5F). Of interest, expression of Runx1 was significantly decreased, presumably reflecting its complicated and dose-dependent involvement in regulating embryonic hematopoiesis.
These data collectively indicated that incubation with IL-3 led to elevated numbers as well as intrinsic hemangiopoietic commitment of the hemangioblast.
Interleukin-3 promoted primitive hematopoiesis in E7.5 embryos
The first wave of hemangioblasts (BL-CFC) is detected mainly in the posterior primitive streak of E7.5 embryos. Of note, the number of BL-CFC in this stage is small, and the hypoxic culture condition is critical. We only detected typical hematopoietic other than BL-CFC colonies in E7.5 embryos. Once transferred to the liquid expansion medium, these hematopoietic-like colonies remained non-adherent and failed to expand as an adherent population. As shown in Online Supplementary Figure S5A, the expression of two types of IL-3 receptors (IL-3Rα/βC, IL-3Rα/βIL-3) was detected by RT-PCR in both E10.5 AGM and E7.5 embryos. After incubation with IL-3 for 12 h, a single-cell suspension of E7.5 embryos was subjected to the CFC assay. Hematopoietic colonies were classified as three types of primitive hematopoietic progenitors, i.e. CFU-EryP, CFU-Mac and CFU-EM (Online Supplementary Figure S5C). As shown in Online Supplementary Figure S5B,D IL-3 significantly amplified CFU-EryP (23.7±3.1 versus 6.3±2.5, P<0.01), CFU-M (17.0±4.0 versus 3.0±1.0, P<0.01) and CFU-EM (11.3±2.5 versus 2.0±0, P<0.01). Quantitative RT-PCR revealed that, upon IL-3 stimulation, transcriptional changes of the hematopoietic- and vascular-related genes were similar to those of E10.5 AGM, including down-regulation of Flk-1 and up-regulation of Scl and vWF. In contrast, expression of Runx1 and GATA1 was significantly elevated, demonstrating somewhat different mechanisms of IL-3 in the regulation of primitive and definitive hematopoiesis (Online Supplementary Figure S5E). Collectively, these data indicate that IL-3 is a positive modulator of the first wave of embryonic hematopoiesis.
Discussion
It remains unclear whether the hemangioblast serves as the precursor of definitive intra-embryonic hematopoiesis. Here, we show that AGM-derived BL-CFC are capable of generating various kinds of lymphomyeloid progenitors and vascular lineages in vitro, representing a canonical definitive hemangioblast. Based on the model, the novel role of IL-3 in governing ontogeny and differentiation of hemangioblast is demonstrated.
Characterization of the hemangioblast with lymphomyeloid potential in the mouse aorta-gonad-mesonephros region
The precursor of definitive hematopoiesis in the AGM region has been supposed to be hemogenic endothelium or differentiated mesoderm cells, i.e., the hemangioblast and mesenchyme. Thus, the site may harbor multiple hematopoiesis-related precursors, with their sequential maturation and migration under dynamic but stringent control.
The existence of the hemangioblast in the AGM region was initially implied by a bulk culture system in which hematopoietic clusters budded from proliferating endothelial layers in the presence of bFGF, SCF and OSM.30 Recently, the HPP-HA model has been used to quantify hemangioblasts with high proliferating potential in the AGM region.21 In comparison, the BL-CFC assay revealed more progenitors with hemangioblastic activity in the AGM region (E10.5: 125 BL-CFC versus 8 HPP-HA per tissue). More importantly, it demonstrated the lymphoid capacity of the hemangioblast in vitro.
Based on the analysis of four membrane proteins (CD31+, endomucin+, CD105+ and PDGFRβ−) and knowledge regarding their distribution in the AGM region, we concluded that the endothelial layer of dorsal aorta was the main location of BL-CFC. In particular, the identity of the AGM-derived BL-CFC as the authentic hemangioblast rather than more primitive precursors was reinforced by its failure to generate additional mesoderm progenies such as bone and fat. Interestingly, the BL-CFC was not detected in yolk sac or placenta. This may reflect the different nature of hematopoietic precursors in various blood-forming tissues. Alternatively, the AGM region (a hemogenic other than hematopoietic site) may provide a unique niche to maintain the hematopoietic precursors in a primitive undifferentiated state. The absence of BL-CFC in the circulation and fetal liver suggests that the AGM-derived hemangioblast may differentiate and become undetected upon intravasation.
Several lines of evidence support the hypothesis that hemogenic endothelium may be the direct precursor of definitive hematopoiesis in the AGM region. Firstly, morphological analysis has shown that cells in the hematopoietic clusters form tight junctions with the endothelial cells of the dorsal aorta, and appear to bud from the endothelium,31,32 Furthermore, these cells express either endothelial or hematopoietic markers, i.e. CD144, CD31, CD34 and CD45, indicative of their ongoing immunophenotypic transition from endothelium to blood. Of interest, North et al. pointed out that mesenchymal cells (CD45−CD34−) can function as HSC in Runx1+/− embryos, but not in normal embryos.33 Very recently, using an in vitro embryonic stem cell differentiation system, Lancrin et al. demonstrated that the hemangioblast generates hematopoietic cells through a hemogenic endothelium stage, providing the first direct link between the two types of precursors.34 It will, therefore, be intriguing to determine whether the BL-CFC in the AGM region is capable of generating hematopoietic lineages through hemogenic endothelium or other unknown intermediates. In close association with dorsal aorta, the mesoangioblast is identified as a common precursor for vascular and extra-vascular mesodermal derivatives. Compared with the BL-CFC, the mesoangioblast emerges at a higher frequency, is unable to proliferate in semisolid culture, and fails to generate typical hematopoietic colonies.35, 36 It is, therefore, challenging to illustrate relationships between hemogenic endothelium, the hemangioblast and the mesoangioblast in the AGM region.
Interleukin-3-mediated positive regulation of hemangioblast development in the mouse aorta-gonad-mesonephros region
Studies of in vitro differentiation of embryonic stem cells has provided substantial understanding of hemangioblast regulation by intrinsic transcriptional factors or microenvironment cues.9–12,37 In particular, a precise and stage-specific control of the differentiation cascade (Brachyury+ meseoderm/Flk-1+ hemangioblast/CD41+ or CD45+ hematopoietic cells) has been linked to multiple molecules. In striking contrast, little is known regarding the modulation of embryonic hemangioblast development. Among the currently tested cytokines, IL-3 appeared to be the most potent stimulator of AGM hemangiopoiesis. In line with this, Robin et al. found that IL-3 can amplify HSC with long-term reconstitution capacity in the AGM region up to 30-fold.38 They attempted to define which target gene could rescue the defective HSC ontogeny of Runx1−/− embryos, and found that IL-3 other than GM-CSF can fulfill such a rescue function by promoting survival and proliferation of HSC in the AGM region. Likewise, 12 h of IL-3 treatment was capable of preventing apoptosis of CD31+ cells in the AGM region (data not shown). Together with the temporal analysis of BL-CFC development in vitro after IL-3 stimulation, we concluded that IL-3 was more of a survival than an expanding factor on hemangioblasts. Taoudi et al. reported that the cytokine cocktail containing IL-3, SCF and FL can promote HSC generation within a novel reaggregate composed of CD144+CD45+ cells from the AGM region.39 While addition of IL-3 to the explant culture of E10 AGM leads to precocious HSC activity,38 IL-3-treated AGM cells form blast colonies 12 h earlier than control cells. The similarity suggests that IL-3 can fuel the intrinsic hematopoietic potential of pre-hematopoietic precursors in addition to increasing the number of hemangioblasts. In comparison, IL-3-induced expansion of HSC appears more drastic than that of hemangioblasts. This discrepancy may be ascribed to a longer duration of IL-3 incubation (3 days versus 12 h) and more importantly, to the three-dimensional organ culture, advantageous for maintaining supportive microenvironment cues. Of note, bFGF fails to promote HSC expansion, in striking contrast to its unambiguous function on avian and mouse hemangioblast.21,40 This may reflect that, unlike IL-3, bFGF-induced BL-CFC can produce more hematopoietic progenitors other than HSC. A previous study of bulk AGM cultures revealed that oncostatin M can promote hemogenesis from endothelial cells.30 However, oncostatin M does not expand either HSC or BL-CFC, suggesting that it may predominantly accelerate the generation of hematopoietic progenitors from hemogenic endothelium or the hemangioblast.38 Thus, the multi-step control of embryonic hemangiopoiesis by candidate molecules can be accurately analyzed by the BL-CFC assay.
Albeit dispensable, the regulatory roles of IL-3 in AGM hematopoiesis are physiologically important. The expression of IL-3 is detected by in situ hybridization mainly in the cells lining the lumen or attaching to the endothelium of dorsal aorta.38 Thus, in the mouse AGM region, the HSC and BL-CFC with an endothelial immunophenotype (CD31+endomucin+) are directly regulated by autocrine or paracrine IL-3. Consistently, the use of IL-3-blocking antibody leads to decreased activity of HSC as well as BL-CFC.38 Direct transplantation of E11 IL3−/− AGM cells into irradiated recipients resulted in undetectable donor-derived HSC activity.38 Indeed, IL3 acts as a target gene of Runx1, and can rescue the HSC defect in the Runx1−/−AGM region.38 Here, upon IL-3 stimulation, the expression of Runx1 in AGM cells was significantly down-regulated, implying a possible negative feedback. In line with our data, the hemangioblast and hematopoietic specification from mesodermal precursors is accelerated in Runx1 heterozygous embryoid bodies.41 In Runx1+/− embryos, HSC activity is reduced in the AGM region, contrasting with its increase in the yolk sac and the placenta. Therefore, the gene dosage of Runx1 markedly affects the spatiotemporal distribution of HSC in mid-gestation blood-forming tissues.33,42
Another novel finding is the positive role of IL-3 in the stage of primitive hematopoiesis. The dramatic amplification of primitive hematopoietic precursors by short-term stimulation with IL-3 may be largely ascribed to rapid hemogenesis from pre-existing precursors, such as Flk-1+ mesodermal cells or hemangioblasts. In agreement with this, the expression of Flk-1 declined in the presence of IL-3, with elevated transcription of a panel of transcriptional factors such as Scl and GATA1. As defective primitive hematopoiesis is undetected in gastrulating IL-3 mutants, these findings may reveal a cytokine redundancy.
It has recently been reported that IL-3 is capable of expanding angiogenic cells from adult CD45+ hematopoietic progenitors via STAT5 signaling and of promoting arterial specification.43 Taken together, these data suggest that IL-3 can exert functions on the hemangiopoietic system from active embryogenesis to homeostatic adulthood.
Footnotes
Funding: this study was supported by the National Natural Science Foundation (n. 30730043, 30770921, 30871437, 30800427), the National Key Basic Research Program of China (n. 2005CB522705, 2005CB522506 and 2006CB943601), the National 863 program (n. 2007AA021109), the Beijing Natural Science Foundation (n. 5092024), and the State Key Laboratory of Proteomics® grant n. SKLP-Y200807.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
W-YH: performed research, analyzed data, and wrote the paper; YL: performed research, analyzed data, and wrote the paper; H-YY: performed research, and analyzed data; ZL: performed research; X-YW: performed research; X-SL: performed research; J-YZ: analyzed data; YZ: designed research, and analyzed data; BL: designed research, analyzed data, and wrote the paper: NM: designed research, analyzed data, and wrote the paper.
The authors declare no competing financial interests.
References
- 1.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33(9):1041–7. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–75. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 3.McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33(9):1021–8. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8(3):377–87. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–85. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 6.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–61. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 7.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9(2):129–36. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–63. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacaud G, Robertson S, Palis J, Kennedy M, Keller G. Regulation of hemangioblast development. Ann NY Acad Sci. 2001;938:96–108. doi: 10.1111/j.1749-6632.2001.tb03578.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa SI. A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Curr Opin Cell Biol. 2001;13(6):673–8. doi: 10.1016/s0955-0674(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 11.Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C, de Bruijn M. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol. 2005;33(9):1029–40. doi: 10.1016/j.exphem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Park C, Ma YD, Choi K. Evidence for the hemangioblast. Exp Hematol. 2005;33(9):965–70. doi: 10.1016/j.exphem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125(9):1747–57. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 14.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 15.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–30. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 16.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–55. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 17.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16(5):673–83. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106(3):903–5. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132(18):4179–91. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- 21.Yao H, Liu B, Wang X, Lan Y, Hou N, Yang X, et al. Identification of high proliferative potential precursors with hemangioblastic activity in the mouse aorta-gonad-mesonephros region. Stem cells. 2007;25(6):1423–30. doi: 10.1634/stemcells.2006-0556. [DOI] [PubMed] [Google Scholar]
- 22.Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, et al. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol. 2007;27(21):7683–92. doi: 10.1128/MCB.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265(5175):1098–101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–7. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 26.Pimanda JE, Chan WY, Wilson NK, Smith AM, Kinston S, Knezevic K, et al. Endoglin expression in blood and endothelium is differentially regulated by modular assembly of the Ets/Gata hemangioblast code. Blood. 2008;112(12):4512–22. doi: 10.1182/blood-2008-05-157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brachtendorf G, Kuhn A, Samulowitz U, Knorr R, Gustafsson E, Potocnik AJ, et al. Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev Dyn. 2001;222(3):410–9. doi: 10.1002/dvdy.1199. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 29.Blalock WL, Weinstein-Oppenheimer C, Chang F, Hoyle PE, Wang XY, Algate PA, et al. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs. Leukemia. 1999;13(8):1109–66. doi: 10.1038/sj.leu.2401493. [DOI] [PubMed] [Google Scholar]
- 30.Mukouyama Y, Hara T, Xu M, Tamura K, Donovan PJ, Kim H, et al. In vitro expansion of murine multipotential hematopoietic progenitors from the embryonic aorta-gonad-mesonephros region. Immunity. 1998;8(1):105–14. doi: 10.1016/s1074-7613(00)80463-x. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Porrero JA, Godin IE, Dieterlen-Lievre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol. 1995;192(5):425–35. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- 32.North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–75. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 33.North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16(5):661–72. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 34.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–5. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, et al. The mesoangioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–83. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 36.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13(5):537–42. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V, et al. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11(2):171–80. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Pardanaud L, Dieterlen-Lievre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126(4):617–27. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- 41.Lacaud G, Kouskoff V, Trumble A, Schwantz S, Keller G. Haploinsufficiency of Runx1 results in the acceleration of mesodermal development and hemangioblast specification upon in vitro differentiation of ES cells. Blood. 2004;103(3):886–9. doi: 10.1182/blood-2003-06-2149. [DOI] [PubMed] [Google Scholar]
- 42.Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing RJ, et al. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13(4):423–31. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- 43.Zeoli A, Dentelli P, Rosso A, Togliatto G, Trombetta A, Damiano L, et al. Interleukin-3 promotes expansion of hemopoietic-derived CD45+ angiogenic cells and their arterial commitment via STAT5 activation. Blood. 2008;112(2):350–61. doi: 10.1182/blood-2007-12-128215. [DOI] [PubMed] [Google Scholar]