Abstract
Background
We previously established a mesenchymal stem cell line (FMS/PA6-P) from the bone marrow adherent cells of fetal mice. The cell line expresses a higher level of neural cell adhesion molecule and shows greater hematopoiesis-supporting capacity in mice than other murine stromal cell lines.
Design and Methods
Since there is 94% homology between human and murine neural cell adhesion molecule, we examined whether FMS/PA6-P cells support human hematopoiesis and whether neural cell adhesion molecules expressed on FMS/PA6-P cells contribute greatly to the human hematopoiesis-supporting ability of the cell line.
Results
When lineage-negative cord blood mononuclear cells were co-cultured on the FMS/PA6-P cells, a significantly greater hematopoietic stem cell-enriched population (CD34+CD38− cells) was obtained than in the culture without the FMS/PA6-P cells. Moreover, when lineage-negative cord blood mononuclear cells were cultured on FMS/PA6-P cells and transplanted into SCID mice, a significantly larger proportion of human CD45+ cells and CD34+CD38− cells were detected in the bone marrow of SCID mice than in the bone marrow of SCID mice that had received lineage-negative cord blood mononuclear cells cultured without FMS/PA6-P cells. Furthermore, we found that direct cell-to-cell contact between the lineage-negative cord blood mononuclear cells and the FMS/PA6-P cells was essential for the maximum expansion of the mononuclear cells. The addition of anti-mouse neural cell adhesion molecule antibody to the culture significantly inhibited their contact and the proliferation of lineage-negative cord blood mononuclear cells.
Conclusions
These findings suggest that neural cell adhesion molecules expressed on FMS/PA6-P cells play a crucial role in the human hematopoiesis-supporting ability of the cell line.
Keywords: neural cell adhesion molecule, cord blood, human hematopoiesis, mesenchymal stem cells
Introduction
Human umbilical cord blood (CB) has been used as an alternative source of hematopoietic stem cells (HSC) for various diseases, such as leukemia, aplastic anemia and autoimmune diseases. The advantages of CB transplantation over bone marrow (BM) or mobilized peripheral blood stem cell transplantation include the ease of stem cell collection, the less stringent requirement on human leukocyte antigen (HLA) matching between donors and recipients, as well as the low severity of graft-versus-host diseases.1–3 However, the low cell content in CB units is a major limiting factor, particularly for adult recipients, which has confined the use of CB transplants mostly to patients with low body weight.1,2 Some studies have demonstrated that successful engraftment can be achieved in CB transplantation with a cell dose of over 4×107 nucleated cells/kg body weight of the recipient.1–4 When insufficient numbers of cells are grafted, the consequent delay in reconstitution causes a high morbidity and mortality, due to systemic infections, accompanied by high costs due to hospitalization and blood cell transfusions. Thus, efforts are being made to generate large number sof HSC and progenitor cells by ex vivo expansion in order to improve the applicability and outcome of CB transplantation. Some clinical improvements have been observed in trials using expanded CB cells,5 BM cells,6 and peripheral blood stem cells.7,8 However, a major disadvantage of culturing HSC in the presence of hematopoietic growth factors is the accelerated differentiation from HSC to lineage cells, possibly at the expense of multipotent HSC with self-renewal and long-term engrafting potential.9 It has been reported that long-term hematopoiesis can be maintained only by co-culturing HSC with stromal cells in human and mouse hematopoietic systems.10–15 We have also found that successful BM transplantation depends on the co-transplantation of stromal cells obtained from donor mice;16–19 stromal cells migrate into the recipient BM and spleen, where they support hematopoiesis. These findings have shaped the view that stromal cell-hematopoietic cell interactions in the marrow microenvironment are crucial for physiological hematopoiesis.
We have recently obtained a mesenchymal stem cell line (FMS/PA6-P) from BM adherent cells of day-16 fetal mice.20,21 This cell line is highly positive for neural cell adhesion molecules (NCAM) and shows a higher hematopoiesis-supporting capacity in mice than other stromal cell lines (MS-512 and PA6).20 The human cDNA sequence encoding NCAM (145-kDa isoform) was reported by Saito et al. in 199422 and we found that there is 94% homology between human and murine NCAM. In the present study, therefore, we attempted to examine whether the FMS/PA6-P cells support human hematopoiesis and whether NCAM expressed on the FMS/PA6-P cells contributes greatly to the human hematopoiesis-supporting ability of the cell line.
Design and Methods
Purification of lineage-negative cord blood mononuclear cells from human cord blood
CB samples were collected from cord veins of uncomplicated full-term, vaginal deliveries. The samples were collected into bags containing citrate-phosphate-dextrose (Terumo, Japan) and processed within 24 h. Informed consent was obtained for all CB collections and this study was approved by the Ethics Committee for Clinical Research of Kansai Medical University. Low-density CB mononuclear cells were isolated by Ficoll-Paque PLUS density gradient centrifugation (<1.077g/mL, GE Healthcare, Uppsala, Sweden) and cryopreserved in IMDM medium containing 10% dimethyl sulfoxide and 20% fetal bovine serum (FBS) until use. Dead cells contained in the cryopreserved low-density CB mononuclear cells were depleted using the Ficoll-Paque PLUS density gradient centrifugation. Lineage-positive cells, expressing CD3, CD9, CD11b, CD14, CD15, CD16, CD19, CD20 and CD235a (glycophorin A) molecules, were then removed using a magnetic bead separation system; the low-density CB mononuclear cells were incubated with monoclonal antibody (mouse IgG class; BD Biosciences Pharmingen, San Diego, CA, USA) cocktails against the above-mentioned lineage markers, and then incubated twice with sheep anti-mouse IgG-conjugated immunobeads (#110.31; Dynal Inc., Oslo, Norway) with gentle agitation at 5:1 and 3:1 bead/cell ratios. The immunobead-rosetted cells were removed using a magnetic particle concentrator. The thus-prepared lineage-negative CB mononuclear cells (L−CBMC) were considered as a partially-HSC-enriched population. The L−CBMC were stained with fluorescent isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibodies against human CD34 (#348053), CD38 (#555459) and CD56 (#556647) (BD Biosciences Pharmingen). Cells stained with isotype-matched IgG served as a negative control. The stained cells were analyzed by a FACScan (BD, Mountain View, CA, USA).
Co-culture of lineage-negative cord blood mononuclear cells on FMS/PA6-P cells
The FMS/PA6-P cells were cultured in 12-well plates containing DMEM (low glucose) supplemented with 10% FBS at 37°C in 5% CO2 in air and confluent monolayers were prepared. After irradiation (20 Gy) of the FMS/PA6-P monolayers, the L−CBMC (7×103/well in 1 mL IMDM supplemented with 10% FBS and human cytokines [SCF, Flt3-L and TPO (20 ng/mL)] were inoculated. Flt3-L (#300-19) was purchased from PeproTech (Rocky Hill, NJ, USA). TPO and SCF were kindly donated by the Kirin Brewery Co. Ltd. (Tokyo, Japan). The L−CBMC were also cultured without the FMS/PA6-P cells. Weekly, half of the medium in the wells containing non-adherent cells was removed and replaced with fresh medium. At 1 and 2 weeks, all non-adherent cells in the well were collected, and the adherent cells (FMS/PA6-P cells + hematopoietic cells which adhered to or under the FMS/PA6-P cells) were collected by trypsin-EDTA treatment. The adherent and non-adherent cells obtained from the same well were mixed, and the number of hematopoietic cells was counted (the FMS/PA6-P cells are larger than hematopoietic cells and, therefore, easily distinguished from these latter).
The collected cells were stained with FITC- or PE-labeled monoclonal antibodies against human CD34 (#CD34-581-04) (Caltag), CD11b (#555388), CD38 (#555459), CD235a (#555570) (BD Biosciences Pharmingen), CD15 (#IM1954), CD14 (#IM0650) and CD41 (#IM1416) (Beckman Coulter, Fullerton, CA, USA). The stained cells were analyzed by a FACScan.
The number of colony-forming cells (CFU-C), including colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophage (CFU-M), colony-forming unit-granulocyte/macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E) and colony-forming unit-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM), were assessed in clonal cell culture using a methylcellulose assay (Methocult GF H3434, Stem Cell Technologies Inc., Vancouver, BC, Canada).
The collected cells were also spread on a glass slide using a cyto-centrifuge and stained with May-Giemsa reagent to observe their morphology.
The L−CBMC were also co-cultured on a monolayer of MS-5 cells12 (kindly donated by the Kirin Brewery Co. Ltd.) and the hematopoiesis-supporting ability of the MS-5 cells was analyzed using the methods described above.
Engraftment of culture-expanded cells into SCID mice
Seven- to 8-week-old Icr-Scid (SCID) mice were purchased from Japan Clear Experimental Animal Laboratory (Tokyo, Japan). All mice were maintained in a pathogen-free environment and were kept for at least 2 weeks before the initiation of experiments. Experiments using mice were conducted in accordance with protocols approved by the university’s committee for animal research. The L−CBMC (5×104/well) were cultured in the presence of the FMS/PA6-P cells for 2 weeks as described above. All the cells, including the FMS/PA6-P cells from one well, were collected using trypsin-EDTA and injected into 3 Gy-irradiated SCID mice (n=6) via the intravenous route (total 6 mice). The L−CBMC were also cultured in the absence of the FMS/PA6-P cells, and the culture-expanded cells were collected without trypsin-EDTA treatment and transplanted into SCID mice (n=6).
For the assessment of human CD45+ cells and subsets, the mice were sacrificed by cervical dislocation 8 weeks post-transplantation, and the femora and tibiae were removed and cleaned of all connective tissue. BM cells were collected by flushing the femora and tibiae with 2% FBS/PBS using a 26-gauge needle, filtered, and washed twice. Peripheral blood cells were collected by heart puncture. For flow cytometric analyses, contaminating red blood cells were lysed with BD Pharm Lyse™ Lysing Buffer (BD Biosciences Pharmingen) and washed with 2% FBS/PBS. These cells were then double-stained with anti-human CD45 (#0452 or #0454, Exalpha Biologicals, Inc., Watertown, MA, USA) and anti-human CD14, CD19 (#302205, BioLegend, San Diego, CA, USA), CD34, CD41 or CD235a monoclonal antibodies or with anti-human CD34 and anti-human CD38 monoclonal antibodies. The stained cells were analyzed by a FACScan.
Polymerase chain reaction (PCR) analysis using primers targeted to human specific DNA 17-α satellite gene was also performed to confirm the engraftment of human cells into the BM of the SCID mice. The sequences of the human-specific DNA 17-α satellite gene are as follows: Forward, gggATAATTTCAgCTgACTAAACAg; Reverse, TTCCgTTTAgTTAggTgCAgTTATC.
Non-contact culture of lineage-negative cord blood mononuclear cells on FMS/PA6-P cells
Confluent FMS/PA6-P monolayers were prepared in a 24-well plate and irradiated at a dose of 20 Gy. The L−CBMC (2.5×104/well) were loaded directly on the stromal layer (contact culture) or loaded into a culture chamber insert (pore size: 0.45 μm, Intercell, Kurabo, Osaka, Japan) placed above the stromal layer (non-contact). The same number of L−CBMC were cultured without the stromal layer. The culture medium consisted of 10% FBS/IMDM containing human cytokines [SCF, Flt3-L and TPO (20 ng/mL)]. After 2 weeks, all cells in the well were harvested, and flow cytometric analyses and clonal cell cultures were performed.
Addition of anti-neural cell adhesion molecule antibodies to co-culture of lineage-negative cord blood mononuclear cells and FMS/PA6-P cells
Confluent FMS/PA6-P monolayers prepared in a 24-well plate were irradiated at a dose of 20 Gy and the culture medium was replaced with fresh IMDM supplemented with 10% FBS and anti-mouse NCAM monoclonal antibody recognizing the three major isoforms (120, 140 and 180 kDa) (1 μg/mL, #556324, Clone: N-CAM 13, BD Bioscience Pharmingen) or corresponding isotype (mouse IgG2a)-matched monoclonal antibody. After 2 h, 3.4×103 L−CBMC were added to the wells and co-cultured. On day 7, half of the medium in the wells containing non-adherent cells was removed and replaced with fresh medium with anti-NCAM or isotype monoclonal antibodies. Two weeks later, all cells were collected and analyzed as described above.
Statistics
The engraftment experiment was carried out twice and the in vitro culture experiments three or more times. Reproducible results were obtained. Representative data are shown in the figures. Statistical differences in all experiments were analyzed by a Student’s two-tailed t test.
Results
Expansion of human hematopoietic stem cells and progenitor cells on FMS/PA6-P cells in vitro
To examine the human hematopoiesis-supporting ability of the FMS/PA6-P cells, we purified a human HSC-enriched population (L−CBMC) from a CB sample. May-Giemsa staining revealed that the L−CBMC showed HSC-like features (Figure 1A). The proportions of CD34+CD38−and CD34+CD56(NCAM)+ cells in the population were 23.8±3.8% and 4.2±0.6%, respectively. When the L−CBMC were inoculated on the FMS/PA6-P cell layer (7×103/well), the L−CBMC adhered rapidly to the stromal layer, and 2196.1±190.9 cells/well had adhered to the layer by 4 h after the inoculation. These cells then began to “crawl” under the stromal layer and 1880.2±242.6 cells/well showed pseudoemperipolesis to the FMS/PA6-P cells 18 h later. Cell division under the stromal layer was seen at and after 32 h. The proliferating cells demonstrated a cobblestone-like appearance on day 2–3 of culture and were referred to as “cobblestone area-forming” cells (CAFC) (Figure 1A).
Figure 1.

Human hemopoiesis-supporting capacity of FMS/PA6-P cells in vitro. (A) Morphology of L−CBMC and formation of cobblestone colonies in the co-culture of L−CBMC with FMS/PA6-P cells. The purified L−CBMC were stained with May-Giemsa reagent (left photograph). The L−CBMC (7×103/well) were inoculated to the monolayers of confluent FMS/PA6-P cells (20 Gy-irradiated) and the formation of cobblestone areas was observed on day 2–3 of culture (middle and right photographs). Phase-contrast images (B) Expansion of L−CBMC on the monolayer of FMS/PA6-P cells. The L−CBMC (7×103/well) were cultured with or without the FMS/PA6-P cells (20 Gy-irradiated). At 1 and 2 weeks, all cells in the well were collected by trypsin-EDTA treatment and the number of hematopoietic cells was counted. The number of CD34+ cells and CD34+CD38−cells per well was calculated from the hematopoietic cell number per well and the percentages of these populations obtained by flow cytometric analyses. The number of CFU-C per well was also calculated from the hematopoietic cell number per well and the number of CFU-C/104 cells obtained by clonal cell culture assay. Fold Increase = the number of hematopoietic cells per well after culture/the number of hematopoietic cells per well before culture. The fold increases of CD34+ cells, CD34+CD38− cells and CFU-C counts were also calculated from the number of these populations before and after culture, respectively. Each sample was run in triplicate. Representative data from three independent experiments. **P<0.01; *P<0.05. (C) Multi-lineage differentiation of L−CBMC in co-culture with FMS/PA6-P cells. At 2 weeks of culture, non-adherent cells were collected from the co-culture of the L−CBMC and FMS/PA6-P cells, and were stained with May-Giemsa reagent.
After 1 or 2 weeks of culture, adherent and non-adherent cells in the co-culture with the FMS/PA6-P cells were collected (the former by typsin-EDTA treatment) and assessed for cellularity and differentiation capacity. There were only non-adherent cells in the culture without the FMS/PA6-P cells, and these cells were, therefore, collected without trypsin-EDTA treatment. In the co-culture of L−CBMC with FMS/PA6-P cells, a significantly higher production of hematopoietic progenitor cells (CD34+ cells) as well as HSC-enriched population (CD34+CD38− cells) was observed at both 1 and 2 weeks (Figure 1B) than in the culture without FMS/PA6-P cells. The numbers of total hematopoietic cells, CD34+ cells and CD34+CD38− cells increased by 51-, 27-, and 25-fold of the original cell input in the co-cultures with the FMS/PA6-P cells at 1 week, whereas only 19-, 9- or 8-fold increases were found in the culture without FMS/PA6-P cells. An even more remarkable difference was observed at 2 weeks; total cells, CD34+ cells and CD34+CD38− cells increased by 526-, 205- and 189-fold, respectively, in the co-cultures with FMS/PA6-P cells and by 107-, 30- and 26-fold in the cultures without FMS/PA6-P cells.
The culture-expanded cells were then examined for their ability to form clonal hematopoietic colonies (CFU-C) using MethoCult GF H3434. Significantly higher CFU-C counts were observed at 1 week in co-cultures with FMS/PA6-P cells than in the cultures without FMS/PA6-P cells (Figure 1B). Although there was no evident difference in the total CFU-C counts between the two culture conditions at 2 weeks, the cell components of the CFU-C differed; a higher number of lineage-committed CFU-C (CFU-G, CFU-M and BFU-E, but not CFU-GM and CFU-GEMM) was detected in the cultures without the FMS/PA6-P cells than in the co-cultures with the FMS/PA6-P cells. Adherent cells in the co-cultures were collected using trypsin-EDTA treatment. Our preliminary experiments showed that trypsin-EDTA treatment of freshly-isolated and cultured L−CBMC did not affect the expression of cell surface markers (CD11b, CD14, CD15, CD34, CD38, CD41 and CD235a) or cell viability. In contrast, the total CFU-C counts were reduced to 73.2% in freshly-isolated L−CBMC and 74.5% in the cultured L−CBMC after the trypsin-EDTA treatment. There was no evident decrease in the CFU-M counts, but CFU-G, BFU-E, CFU-GM and CFU-GEMM counts were markedly reduced after the trypsin-EDTA treatment. Thus, the trypsin-EDTA treatment affects not only the total CFU-C count but also the cell components of CFU-C. It is, therefore, possible that the actual CFU-C counts in the co-culture with the FMS/PA6-P cells would have been much higher than the values shown in the present experiments. These findings suggest that FMS/PA6-P cells can support the proliferation of human HSC and progenitors in vitro.
Lineage-positive cells expressing CD11b, CD14, CD15, CD41 or CD235a molecules were also detected by flow-cytometric analysis in the co-culture of L−CBMC with FMS/PA6-P cells (data not shown). The production of mature hematopoietic cells was further confirmed by May-Giemsa staining of the non-adherent cells recovered from the co-culture; normoblasts and megakaryocytes were seen in addition to many granulocytes and macrophages (Figure 1C). These findings suggest that the FMS/PA6-P cells facilitate the proliferation of human HSC and progenitors, resulting in the production of mature myeloid, erythroid and megakaryocytic cells. A slightly, but not statistically significantly higher percentage of lineage-positive (CD11b, CD14, CD15, CD41 or CD235a-positive) cells was also seen in the culture without FMS/PA6-P cells than in the culture with FMS/PA6-P cells (data not shown).
It is known that the murine BM stromal cell line MS-5 has hematopoiesis-supporting ability for human cells.15 We previously showed that NCAM is expressed at lower levels on MS-5 than on FMS/PA6-P cells.20 Here we co-cultured L−CBMC on MS-5 cells and the hematopoiesis-supporting ability of these latter was compared with that of the FMS/PA6-P cells. At 1 week of culture, there was no significant difference in the number of total cells, CD34+ cells, CD34+CD38− cells or CFU-C per well between the two cultures. At 2 weeks, however, the number of CD34+ cells and CD34+CD38− cells per well was, respectively, 1.40 and 1.38 times higher in the culture on the FMS/PA6-P cells than in that on the MS-5 (both P<0.05). This finding indicates that the FMS/PA6-P cells have greater hematopoiesis-supporting ability than the MS-5 cells in the present experimental system.
Engraftment of ex vivo-expanded human hematopoietic stem cells and progenitor cells into SCID mice
To investigate whether the HSC and progenitor cells produced in the co-culture system with FMS/PA6-P cells are able to proliferate and differentiate in vivo, we injected the culture-expanded cells into sublethally-irradiated SCID mice. All cells in each culture well, including the expanded cells and FMS/PA6-P cells, were harvested using trypsin-EDTA treatment and injected intravenously into a single SCID mouse. Eight weeks after the transplantation, there was no significant difference in survival rates between the mice which had received the cells co-cultured with the FMS/PA6-P cells (hereafter described as the co-cultured group) (5/6) and those which had received cells cultured without the FMS/PA6-P cells (the non-co-cultured group) (6/6). However, as shown in Figure 2A, the proportion of human CD45+ cells, CD34+ cells and CD34+CD38−cells was significantly higher in the BM of the co-cultured group than in the BM of the non-co-cultured group. In the BM of the co-cultured group, mature human myeloid cells (CD14+: 0.57%), B cells (CD19+: 0.18%), erythroid cells (CD235a+: 3.31%) and megakaryocytic cells (CD41+: 0.21%) were also detected. A clear difference between both groups was also seen in human CD45+ cells in the peripheral blood (Figure 2A). Figure 2B shows a representative flow cytometric pattern (CD45/CD34 and CD38/CD34 stains) of BM cells from the SCID mice that received L−CBMC cultured with or without FMS/PA6-P cells.
Figure 2.
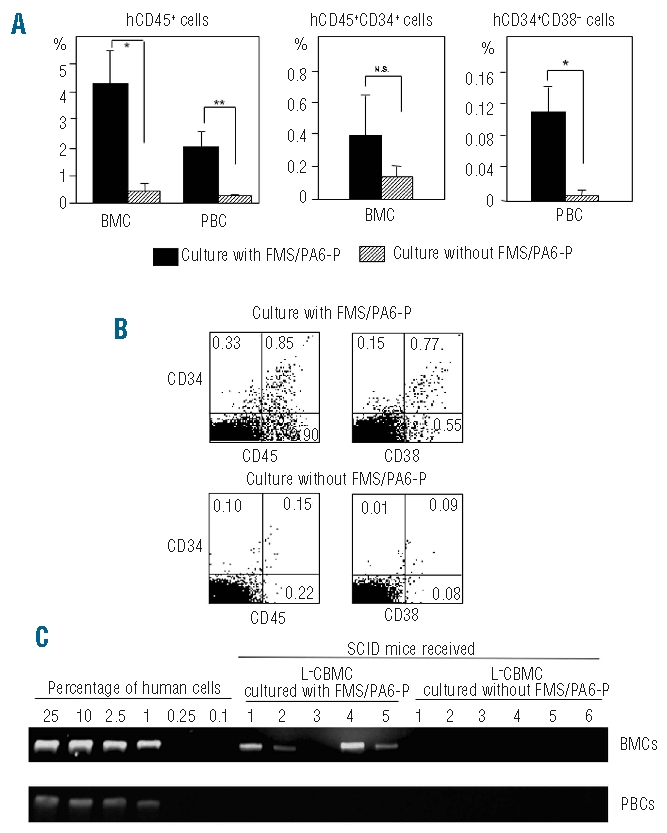
Reconstitution of human cells in SCID mice that received culture-expanded L−CBMC. (A) Higher engraftment of human cells in SCID mice that received L−CBMC co-cultured with FMS/PA6-P cells. The L−CBMC were cultured in a 12-well plate with the FMS/PA6-P cells for 2 weeks and all cells in one well, including the FMS/PA6-P cells and hematopoietic cells, were harvested and injected into a single SCID mouse via the intravenous route. All cells in one well, cultured without the FMS/PA6-P cells, were also collected and injected into a single SCID mouse. Eight weeks after the transplantation, BM and peripheral blood cells were collected and the percentages of human CD45+ cells, CD34+ cells and CD34+CD38− cells were measured using FACScan. **P<0.01; *P<0.05. (B) Representative flow cytometric pattern of BM cells from SCID mice that received L−CBMC cultured with or without FMS/PA6-P cells. BM cells collected from the SCID mice were double-stained with anti-human CD45 and anti-human CD34 monoclonal antibodies or with anti-human CD38 and anti-human CD34 monoclonal antibodies and analyzed by a FACScan. The values indicate the percentages of the population in whole BM cells. (C) Detection of human DNA in SCID mice that received L−CBMC co-cultured with FMS/PA6-P cells. Four of five mice, transplanted with the expanded cells produced in the co-culture of L−CBMC with FMS/PA6-P cells, contained human DNA in the BM, whereas no human DNA was detected in the BM of six mice transplanted with the expanded cells produced in culture of L−CBMC without FMS/PA6-P cells.
The presence of human cells was further proven in the SCID mice of the co-cultured group by PCR analysis using the human-specific DNA 17α-satellite gene; four out of five mice showed human DNA in their BM (Figure 2C). In contrast, no human DNA was detected in the non-co-cultured group. Even in those mice showing human DNA in their BM, we failed to detect human DNA in the peripheral blood of the animals, implying that human-type cells were under the detection level of PCR in the peripheral blood in contrast to the BM.
These findings indicate that HSC and progenitor cells contained in the culture-expanded cells homed to the BM, where they proliferated and differentiated into multi-lineage mature blood cells, suggesting that the FMS/PA6-P cells provide a suitable microenvironment for the proliferation of functional HSC and progenitor cells.
As mentioned above, we observed a decline in CFU-C counts after trypsin-EDTA treatment. The cells transplanted into the SCID mice were collected from the co-culture using trypsin-EDTA treatment, and it is possible that this treatment might damage the in vivo proliferation and differentiation activity of the transplanted cells.
Contribution of neural cell adhesion molecules to the human hematopoiesis-supporting ability of FMS/PA6-P cells
Since many studies have demonstrated that stromal cell-hematopoietic cell interactions in the marrow microenvironment are crucial for physiological hematopoiesis, we next investigated, using a culture chamber system, whether direct cell-to-cell interactions between human L−CBMC and FMS/PA6-P cells are also crucial for the expansion of human hematopoietic cells. As shown in Figure 3, the numbers of CD34+cells, CD34+CD38− cells and CFU-C were significantly lower in non-contact cultures than in contact cultures: L−CBMC in contact cultures showed approximately 98-, 92- and 2.6-fold increases in CD34+ cells, CD34+CD38− cells and total CFU-C, respectively, whereas L−CBMC in non-contact cultures showed 62-, 58- and 0.72-fold increases in CD34+ cells, CD34+ CD38− cells and CFU-C, respectively. However, there were no differences in CD34+ cells, CD34+CD38− cells and CFU-C counts between the non-contact culture and the culture without the FMS/PA6-P cells. These findings suggest that direct cell-to-cell contact between human HSC/progenitor cells and FMS/PA6-P cells is essential for the maximum expansion of human cells, and that the adhesion molecules mediating cell-to-cell contact may be crucial for this maximum expansion.
Figure 3.
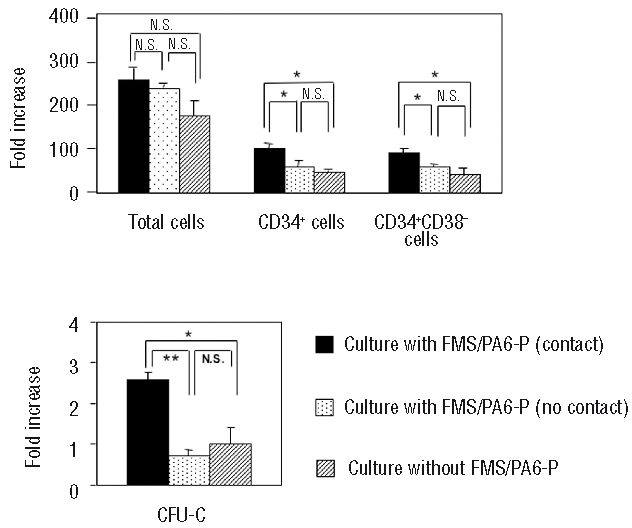
Higher proliferation and differentiation of L−CBMC in contact culture with FMS/PA6-P cells. The monolayer of FMS/PA6-P cells was prepared in a 24-well plate and the L−CBMC were loaded into the culture chamber inserts or directly into the FMS/PA6-P cell monolayer. As a negative control, L−CBMC were cultured without FMS/PA6-P cells. Two weeks later, all cells in the well were collected by trypsin-EDTA treatment, and the fold increases of total cells, CD34+ cells, CD34+CD38− cells and CFU-C counts were calculated, as mentioned in Figure 1B. Each sample was run in triplicate. Representative data from three independent experiments. ** P<0.01; *P<0.05, N.S.: not significant.
We have previously shown that FMS/PA6-P cells express a high level of NCAM, and that NCAM contributes greatly to murine hematopoiesis.20 It can, therefore, be speculated that NCAM may play a crucial role in the human hematopoiesis-supporting capacity of FMS/PA6-P cells. To address this, we examined whether the expansion of the L−CBMC on the FMS/PA6-P cells was inhibited by anti-mouse NCAM monoclonal antibody. The FMS/PA6-P cell layer was pre-incubated with the anti-mouse NCAM monoclonal antibody or an isotype control for 2 h and the L−CBMC were then added to the wells (3.4×103 cells/well). The number of L−CBMC adhering to the FMS/PA6-P cell layer was significantly lower (394.0±8.3 cells/well) in the co-cultures to which the anti-mouse NCAM monoclonal antibody had been added than in the co-cultures to which isotype control had been added (1078.3±315.4 cells/well) (P<0.005) (4 h after inoculation of the L−CBMC on the FMS/PA6-P cells). Two weeks later, the numbers of CD34+ cells, CD34+CD38−cells and CFU-C were markedly suppressed, in contrast to the numbers in the co-cultures to which isotype control was added (Figure 4). This finding clearly shows that NCAM on the FMS/PA6-P cells plays an important part in supporting human hematopoiesis.
Figure 4.
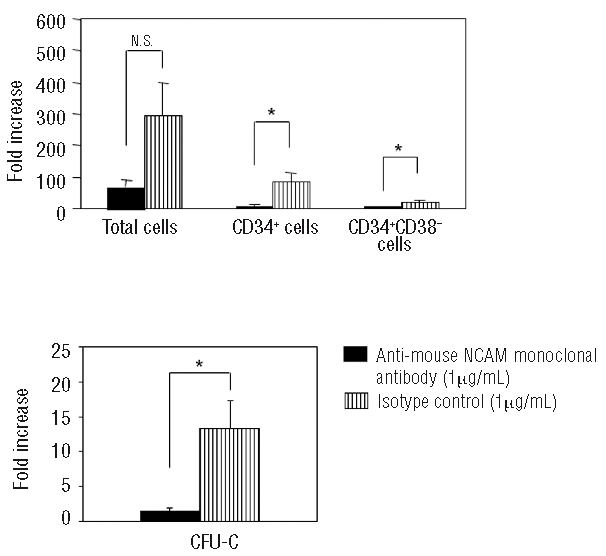
Inhibitory effect of anti-NCAM monoclonal antibody on the proliferation of L−CBMC. The L−CBMC were cultured on the FMS/PA6-P cells in the presence of anti-mouse NCAM monoclonal antibody (5 wells/sample). As a control, the same concentration of isotype control (mouse IgG2a) was added to the culture. Two weeks later, all cells in the well were collected by trypsin-EDTA treatment, and the fold increases of total cells, CD34+ cells, CD34+CD38− cells and CFU-C counts were calculated, as mentioned in Figure 1B. Mean ± SD of five wells. Representative data from three independent experiments. *P<0.05, N.S.: not significant.
We have found by flow cytometoric analyses that anti-mouse NCAM monoclonal antibody does not react with L−CBMC (data not shown), indicating that the monoclonal antibody reacted with murine NCAM expressed on the FMS/PA6-P cells but not with human NCAM expressed on the L−CBMC in the present co-culture system. It is, therefore, conceivable that the monoclonal antibody, which had reacted to mouse NCAM on the FMS/PA6-P cells during the pre-incubation, inhibited the contact between the L−CBMC and NCAM on the FMS/PA6-P cells, and resulted in the low proliferation of the L−CBMC.
Discussion
There is a clear need for ex vivo expansion of CB HSC and progenitor cells for successful CB transplantation. However, in vitro, it is difficult to enhance the self-renewal and/or expansion of HSC without stromal cells, even if all known exogenous growth factors and other materials are added to the cultures.23–26 In the present study, we found that mouse mesenchymal stem cells (FMS/PA6-P) show human hematopoiesis-supporting capacity.
When L−CBMC were co-cultured with FMS/PA6-P cells, significantly higher numbers of HSC-enriched population (CD34+CD38−) and progenitor cells (CD34+ cells and CFU-C) were obtained than when they were cultured without FMS/PA6-P cells (Figure 1B). Moreover, significantly higher percentages of human CD45+ cells and CD34+CD38−cells were detected in the BM of the SCID mice that had received the L−CBMC cultured with FMS/PA6-P cells than in the BM of SCID mice that had received Lin-CBMC cultured without FMS/PA6-P cells (Figure 2A). These findings suggest that FMS/PA6-P cells provide a microenvironment that promotes the proliferation of functional human HSC and progenitor cells. However, we cannot exclude the possibility that some multipotent HSC, which had been contained in the inoculated L−CBMC, were induced to differentiate into their progenitor cells under the influence of exogenous cytokines.
Stromal cells promote the growth, survival and differentiation of HSC and progenitor cells by expressing cell adhesion molecules in addition to producing various growth factors and matrix proteins.27–30 In the present experiments, we found that there were no differences in CD34+ cells, CD34+CD38− cells and CFU-C between the non-contact culture of L−CBMC with FMS/PA6-P cells and the culture of L−CBMC without FMS/PA6-P cells (Figure 3). However, in the contact culture, much higher numbers of CD34+ cells, CD34+CD38− cells and CFU-C counts were obtained. These findings clearly show that direct cell-to-cell contact between L−CBMC and FMS/PA6-P cells is essential for the maximum expansion of the L−CBMC.
We have previously demonstrated that NCAM is highly expressed by FMS/PA6-P cells and contributes greatly to the murine hematopoiesis-supporting capacity of the stromal cells; hematopoiesis was markedly suppressed when anti-mouse NCAM monoclonal antibody was added to the co-culture system of mouse HSC and FMS/PA6-P cells.21 In the present study, we also observed that the addition of anti-mouse NCAM monoclonal antibody to the culture significantly inhibited their contact and the proliferation of L−CBMC (Figure 4), suggesting the importance of the NCAM expressed on the FMS/PA6-P cells for human hematopoiesis. The fact that the cDNA sequence of murine NCAM exhibits 94% homology with that of human NCAM22 may be a reasonable explanation of why murine NCAM on the FMS/PA6-P cells can contribute to human hematopoiesis.
NCAM is known to interact with cell surface molecules by homophilic or heterophilic binding.31 N-syndecan,32 heparin sulphate,33 and fibroblast growth factor-receptor34 are proposed as ligands of the heterophilic binding. Recently, we found that NCAM is expressed on both a HSC-enriched population (lineage-negative, CD34-positive and blast-gated cells) and BM stromal cells (containing mesenchymal stem cells) in monkeys, and contribute greatly to monkey hematopoiesis.35 Although the expression of NCAM or its ligand on human HSC has not been well-elucidated, it is possible that NCAM may support human hematopoiesis through homophilic interaction. Our preliminary study showed that commercially-available human mesenchymal stem cells line (#PT-2501, Cambrex Bio Science, Walkersville, MD, USA and #Yub636, Human Science Research Resources Bank, Osaka, Japan) expressed NCAM molecules. The NCAM expression level on these human mesenchymal stem cell lines declined rapidly with the increase of cell passages, whereas there was no decline in other mesenchymal stem cell markers such as CD29, CD73 and CD105. In parallel with the decline in NCAM expression, the cell lines’ hematopoiesis-supporting ability was also reduced (unpublished data). We observed this phenomenon in the FMS/PA6-P cell line, although the decline in NCAM expression was much more moderate than that of the human mesenchymal stem cell lines.20 Our further investigation will focus on the molecular mechanisms underlying the interaction between HSC and stromal cells via NCAM in human and mouse hematopoietic systems.
So far, several murine stromal cell lines (e.g. MS-5 cells derived from the BM of adult mouse15 and embryonic stromal cell lines derived from the aorta-gonad-mesonephros region36,37) have been reported to have the ability to support human hematopoiesis. The embryonic stromal cell line expresses Sca-1, VCAM-1 and CD13 [but not PECAM-1, c-kit, CD49d (VLA4α) and CD34] and the VCAM-1/VLA4 pathway has been considered to be one of the most important interactions between HSC/progenitor cells and these stromal cell lines.37 Although the importance of direct interaction of human HSC and the MS-5 cells was demonstrated by Issaad et al.,15 Nishi et al. suggested that cytokines produced by MS-5 cells (granulocyte-colony stimulating factor and stem cell factor) contribute to the human hematopoiesis-supporting activity of the cell line.38 Here, for the first time, we have shown the importance of NCAM in the interaction between human HSC and the murine stromal cell line.
Multipotent HSC need to interact directly with niche cells in order to maintain their ‘stemness’. It has been reported that, in in vitro co-culture systems, a higher number of HSC and progenitor cells localize in the stromal cell layer than in the non-adherent cell population.12,15 Therefore, enzymatic digestion of the adherent cell layer is necessary to collect sufficient numbers of HSC and progenitor cells. However, this treatment is thought to damage these cells. Indeed, we observed decreases in the CFU-C counts after the trypsin-EDTA treatment in the present study. Recently, a unique culture system using temperature-sensitive dishes (RepCell), in which cell detachment is induced by a change in temperature, has been developed. This culture system might be useful for collecting culture-expanded cells from stromal cell layers.
In conclusion, we have shown that the FMS/PA6-P cells can support human hematopoiesis, and that NCAM plays a crucial role in the human hematopoiesis-supporting capacity of these cells. This finding will be of great advantage in improving the clinical application of CB transplantation and other human stem cell transplants. It is also of great importance in further understanding the mechanisms underlying the interaction between human HSC and stromal cells and the regulation of human hematopoiesis.
Acknowledgments
we thank Ms. Y. Tokuyama, K. Hayashi and A. Kitajima for their expert technical assistance. We also thank Mr. Hilary Eastwick-Field and Ms. K. Ando for their help in the preparation of the manuscript.
Footnotes
Funding: this work was supported by grants from the “Haiteku Research Center” of the Ministry of Education, the “Millennium” program of the Ministry of Education, Culture, Sports, Science and Technology, and the “Science Frontier” program of the Ministry of Education, Culture, Sports, Science and Technology, a grant-in-aid for scientific research (B) 11470062, grants-in-aid for scientific research on priority areas (A)10181225 and (A)11162221, and Health and Labor Sciences research grants (Research on Human Genome, Tissue Engineering Food Biotechnology), The 21st Century Center of Excellence Program (Project Leader), and The Ministry of Education, Culture, Sports, Science and Technology and also grants from the Department of Transplantation for Regeneration Therapy (Sponsored by Otsuka Pharmaceutical Company, Ltd.), the Molecular Medical Science Institute, Otsuka Pharmaceutical Co., Ltd., and Japan Immunoresearch Laboratories Co., Ltd. (JIMRO).
Authorship and Disclosures
XW and HH contributed to the conception and design of the study, and to the analysis and interpretation of data; XW performed the majority of the experiments and drafted the article; HH revised the article; SI profoundly revised the article and obtained the necessary funding. TM provided the study materials. The other authors contributed to some of the experiments.
The authors reported no potential conflicts of interest.
References
- 1.Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28(11):1197–205. doi: 10.1016/s0301-472x(00)00540-3. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Wagner JE, Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling: Eurocord and International Bone Marrow Transplant Registry Working Committeebon Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342(25):1846–54. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Rocha V, Chastang C, Arcese W, Michel G, Abecasis M, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia: Eurocord-Cord Blood Transplant Group. Blood. 1999;93(11):3662–71. [PubMed] [Google Scholar]
- 5.Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101(12):5061–7. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 6.Stiff P, Chen B, Franklin W, Oldenberg D, His E, Bayer R, et al. Autologous transplantation of ex vivo expanded bone marrow cells grown from small aliquots after high-dose chemotherapy for breast cancer. Blood. 2000;95(6):2169–74. [PubMed] [Google Scholar]
- 7.Paquette RL, Dergham ST, Karpf E, Wang HJ, Slamon DJ, Souza L, et al. Ex vivo expanded unselected peripheral blood: progenitor cells reduce posttransplantation neutropenia, thrombocytopenia, and anemia in patients with breast cancer. Blood. 2000;96(7):2385–90. [PubMed] [Google Scholar]
- 8.Reichle A, Zaiss M, Rothe G, Schmitz G, Andressen R. Autologous tandem transplantation: almost complete reduction of neutropenic fever following the second transplantation by ex vivo expanded autologous myeloid postprogenitor cells. Bone Marrow Transplant. 2003;32(3):299–305. doi: 10.1038/sj.bmt.1704126. [DOI] [PubMed] [Google Scholar]
- 9.Mcniece IK, Almeida-Porada G, Shpall EJ, Zanjani E. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30(6):612–6. doi: 10.1016/s0301-472x(02)00805-6. [DOI] [PubMed] [Google Scholar]
- 10.Kodama H, Sudo H, Koyama H, Kasai S, Yamamoto S. In vitro hemopoiesis within a microenvironment created by MC 3T3-G2/PA6 preadipocytes. J Cell Physiol. 1984;118(3):233–40. doi: 10.1002/jcp.1041180303. [DOI] [PubMed] [Google Scholar]
- 11.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of hematopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 12.Itoh K, Tzuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17(2):145–53. [PubMed] [Google Scholar]
- 13.Ye ZQ, Burkholder JK, Qiu P, Shahidi NT, Yang NS, et al. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91(25):12140–4. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa M, Ozawa K, Tojo A, Yoshikubo T, Okano A, Tani K, et al. Changes in hematopoiesis-supporting ability of C3H10T1/2 mouse embryo fibroblasts during differentiation. Blood. 1993;81(5):1184–92. [PubMed] [Google Scholar]
- 15.Issaad C, Croisille L, Katz A, Vainchenker W, Coulombel L. A murine stromal cell line allows the proliferation of very primitive human CD34++/CD38- progenitor cells in long-term cultures and semisolid assays. Blood. 1993;81(11):2916–24. [PubMed] [Google Scholar]
- 16.Hisha H, Nishino T, Kawamura M, Adachi S, Ikehara S. Successful bone marrow transplantation by bone grafts in chimeric-resistant combination. Exp Hematol. 1995;23(4):347–52. [PubMed] [Google Scholar]
- 17.Ishida T, Inaba M, Hisha H, Sugiura K, Adachi Y, Nagata N, et al. Requirement of donor-derived stromal cells in the bone marrow for successful allogeneic bone marrow transplantation. J Immunol. 1994;152(6):3119–27. [PubMed] [Google Scholar]
- 18.Hashimoto F, Sugiura K, Inoue K, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood. 1997;89(1):49–54. [PubMed] [Google Scholar]
- 19.Sugiura K, Inaba M, Hisha H, Borisov K, Sardina EE, Good RA, et al. Requirement of major histocompatibility complex-compatible microenvironment for spleen colony formation (CFU-S on day 12 but not on day 8) Stem Cells. 1997;15(6):461–8. doi: 10.1002/stem.150461. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Hisha H, Taketani S, Inaba M, Li Q, Cui W, et al. NCAM contributes to hemopoiesis-supporting capacity of stromal cell lines. Stem Cells. 2005;23(9):1389–99. doi: 10.1634/stemcells.2004-0343. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Hisha H, Taketani S, Adachi Y, Li Q, Cui W, et al. Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow. Stem Cells. 2006;24(3):482–93. doi: 10.1634/stemcells.2005-0219. [DOI] [PubMed] [Google Scholar]
- 22.Saito S, Tanio Y, Tachibana I, Hayashi S, Kishimoto T, Kawase I. Complementary DNA sequence encoding the major neural cell adhesion molecule isoform in a human small cell lung cancer cell line. Lung Cancer. 1994;10(5–6):307–18. doi: 10.1016/0169-5002(94)90660-2. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein ID, Andrews RG, Zsebo KM. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+ lin- cells, and the generation of colony-formation cell progeny from CD34+ lin- cells cultured with inter-leukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colonystimulating factor. Blood. 1991;77(11):2316–21. [PubMed] [Google Scholar]
- 24.Lowry PA, Zsebo KM, Deacon DH, Eichman CE, Quesenberry PJ. Effects of rhSCF on multiple cytokine-responsive HPP-CFC generated from Sca-1+ Linmurine hematopoietic progenitors. Exp Hematol. 1991;19(9):994–6. [PubMed] [Google Scholar]
- 25.Li CL, Johnson GR. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84(2):408–14. [PubMed] [Google Scholar]
- 26.Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K, et al. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988;7(5):1337–43. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homologue of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89(16):7350–4. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of hematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368(6472):643–8. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 29.Satoh M, Mioh H, Shiotsu Y, Ogawa Y, Tamaoki T. Mouse bone marrow stromal cell line MC3T3-G2/PA6 with hematopoietic-supporting activity expresses high levels of stem cell antigen Sca-1. Exp Hematol. 1997;25(9):972–9. [PubMed] [Google Scholar]
- 30.Ueno H, Sakita-Ishikawa M, Morikawa Y, Nakano T, Kitamura T, Saito M. A stromal cell-derived membrane protein that supports hematopoietic stem cells. Nature Immunol. 2003;4(5):457–63. doi: 10.1038/ni916. [DOI] [PubMed] [Google Scholar]
- 31.Crossin KL, Krushel LA. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev Dyn. 2000;218(2):260–79. doi: 10.1002/(SICI)1097-0177(200006)218:2<260::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Toba Y, Horie M, Sango K, Tokashiki A, Matsui F, Oohira A, et al. Expression and immunohistochemical localization of heparan sulphate proteoglycan N-syndecan in the migratory pathway from the rat olfactory placode. Eur J Neurosci. 2002;15(9):1461–73. doi: 10.1046/j.1460-9568.2002.01983.x. [DOI] [PubMed] [Google Scholar]
- 33.Gupta P, Oegema TR, Jr, Brazil JJ, Dudek AZ, Slungaard A, Verfaillie CM. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood. 1998;92(12):4641–51. [PubMed] [Google Scholar]
- 34.Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol. 2002;157(3):521–32. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato J, Hisha H, Wang X, Mizokami T, Okazaki S, Li Q, et al. Contribution of neural cell adhesion molecule (NCAM) to hemopoietic system in monkeys. Ann Hematol. 2008;87(10):797–807. doi: 10.1007/s00277-008-0513-9. [DOI] [PubMed] [Google Scholar]
- 36.Xu M, Tsuji K, Ueda T, Mukouyama Y, Hara T, Yang F, et al. Stimulation of mouse and human primitive hemopoiesis by murine embryonic aorta-gonado-mesonephros-derived stromal cell lines. Blood. 1998;92(6):2032–40. [PubMed] [Google Scholar]
- 37.Weisel KC, Moore MAS. Genetic and functional characterization of isolated stromal cell lines from the aorta-gonado-mesonephros region. Ann NY Acad Sci. 2005;1044:51–9. doi: 10.1196/annals.1349.007. [DOI] [PubMed] [Google Scholar]
- 38.Nishi N, Ishikawa R, Inoue H, Nishikawa M, Kakeda M, Yoneya T. Granulocyte-colony stimulating factor and stem cell factor are the crucial factors in long-term culture of human primitive hemopoietic cells supported by a murine stromal cell line. Exp Hematol. 1996;24(11):1312–21. [PubMed] [Google Scholar]


