Abstract
Background
The presence of minimal residual disease detected by polymerase chain reaction techniques prior to allogeneic hematopoietic stem cell transplantation has proven to be an independent prognostic factor for poor outcome in children with acute lymphoblastic leukemia.
Design and Methods
The aim of this study was to ascertain whether the presence of minimal residual disease detected by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation is related to outcome in children acute lymphoblastic leukemia. Minimal residual disease was quantified by multiparametric flow cytometry at a median of 10 days prior to hematopoietic stem cell transplantation in 31 children (age range, 10 months to 16 years) with acute lymphoblastic leukemia. Thirteen patients were transplanted in first remission. Stem cell donors were HLA-identical siblings in 8 cases and matched unrelated donors in 23. Twenty-six children received a total body irradiation-containing conditioning regimen. According to the level of minimal residual disease, patients were divided into two groups: minimal residual disease-positive (≥0.01%) (n=10) and minimal residual disease-negative (<0.01%) (n=21).
Results
Estimated event-free survival rates at 2 years for the minimal residual disease-negative and -positive subgroups were 74% and 20%, respectively (P=0.004) and overall survival rates were 80% and 20%, respectively (P=0.005). Bivariate analysis identified pre-transplant minimal residual disease as the only significant factor for relapse and also for death (P<0.01).
Conclusions
The presence of minimal residual disease measured by multiparametric flow cytometry identified a group of patients with a 9.5-fold higher risk of relapse and a 3.2-fold higher risk of death than those without minimal residual disease. This study supports the strong relationship between pre-transplantation minimal residual disease measured by multiparametric flow cytometry and outcome following allogeneic hematopoietic stem cell transplantation and concur with the results of previous studies using polymerase chain reaction techniques.
Keywords: minimal residual disease, hematopoietic stem cell transplantation, polymerase chain reaction
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) offers a survival advantage over chemotherapy for children with very high-risk, primary resistant or relapsed acute lymphoblastic leukemia (ALL). The curative effect of allogeneic HSCT is hampered by relapse, the most frequent cause of transplant failure. Some recent studies indicated that minimal residual disease (MRD) detected prior to allogeniec HSCT is a predictor of relapse and an independent prognostic factor of poor outcome in children with ALL.1–5 The MRD assays applied in those studies were based on amplification of antigen-receptor genes and immunoglobulin gene rearrangements by polymerase chain reaction. However, multiparametric flow cytometry is widely used to detect abnormal immunophenotypes in the diagnostic work-up of ALL and monitoring of MRD during treatment.6–9 The aim of this study was to quantify MRD prior to HSCT in children with ALL by multiparametric flow cytometry and ascertain whether a relationship exists between pre-transplantation MRD and outcome.
Design and Methods
Patients
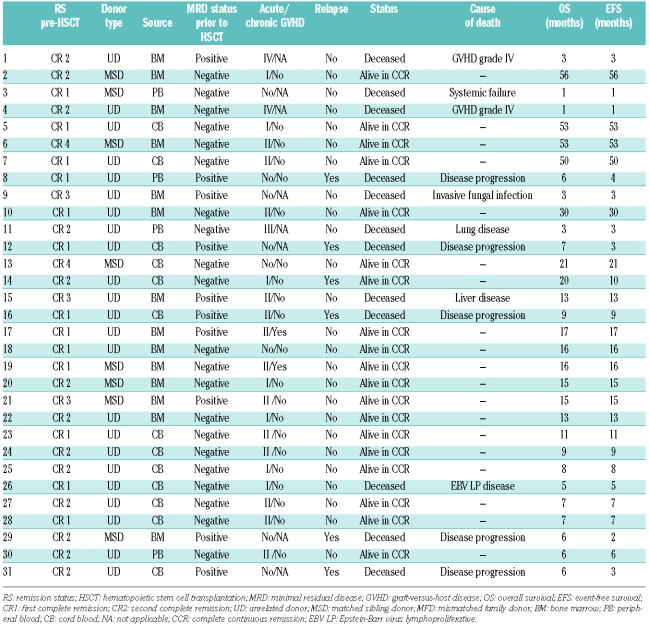
Between October 2002 and October 2007, 31 children (19 boys and 12 girls) underwent allogeneic HSCT for high-risk ALL at our Pediatric HSCT Unit in a study that was conducted in accordance with the Helsinki Declaration. The median age at transplantation was 7 years (range, 10 months to 16 years). The group consisted of 28 patients with B-cell precursor ALL and 3 with T-cell ALL. Seven of the 31 patients failed to achieve complete remission after first-line induction treatment and when remission was achieved with second-line treatment, were transplanted in first complete remission. Furthermore, six patients were transplanted in first complete remission: four infants with MLL-positive ALL (age range, 10–12 months) and two children with Philadelphia chromosome-positive B-ALL. Eighteen patients were transplanted in second or later remission. All patients were previously treated according to the Spanish ALL protocols: Pethema10,11 and SHOP.12 Hematopoietic stem cell donors were HLA-identical siblings in 8 cases and matched unrelated donors in 23. The source of stem cells was bone marrow in 14, cord blood in 13 and peripheral blood in 4. No graft was manipulated prior to infusion. In 26 cases the conditioning consisted of fractionated total body irradiation (total dose 12 Gy), etoposide (30 mg/kg) and cyclophosphamide (120 mg/kg). A busulphan/thiotepa/fludarabine conditioning regimen was used in four children under the age of 3 years and in one with cardiomyopathy. In addition, all recipients of transplants from unrelated donors received antithymocyte globulin as part of their conditioning regimen. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine A in HLA-identical sibling transplants and a combination of cyclosporine A and methotrexate in unrelated donor transplants.
Methods
At diagnosis of ALL, leukemia-associated aberrant immunophenotypes were identified by multiparametric flow cytometry and all patients had at least one useful phenotype for follow-up throughout leukemia treatment. The samples were assessed prior to transplantation to confirm morphological remission and MRD was studied by multi-parametric flow cytometry according to reported studies.7,13–15 Immunophenotypic analysis was performed with four-color flow cytometry. Briefly, cells were stained by direct immunofluorescence using monoclonal antibodies conjugated with the following fluorochromes: fluorescein isothiocyanate, phycoerythrin, the tandem phycoerythrin-Texas red and the tandem phycoerythrin-cyanin 5.1. Cells (106) were incubated for 10 min at room temperature in the dark with saturating amounts of fluorochrome-conjugated antibodies. Erythrocytes were then lysed with a one-step method. Cells were acquired in two steps in an FC-500 flow cytometer (Beckman-Coulter, Miami, FL, USA), with an argon laser tuned at 488 nm: first, 2–5×104 nucleated cells were acquired to determine the percentage of CD19+ lymphocytes (in B-ALL cases) or CD7+ lymphocytes (in T-ALL cases), and then a live-gate was applied to CD19+ or CD7+ events to acquire only the cell population of interest. A total of 0.5 to 1×106 cells were acquired for each antibody combination. SSC/CD19 and SSC/CD7 were used as the primary gating methods in B-ALL and T-ALL, respectively. Sequential gating was then used to identify the leukemia-associated immunophenotypes. The number of residual leukemic cells was calculated as the percentage of the total nucleated cells. The CXP software package (version 2.0) (Beckman Coulter, Miami, FL, USA) was used for the acquisition and analysis of data. This approach has a sensitivity of at least 10−4 for the detection of leukemic cells among normal cells.
Definitions
MRD-positive status was defined as a level of disease of 0.01% or more. High MRD burden was defined as a level more than 1%. MRD-negative status was defined as MRD less than 0.01%. Relapse-free survival was defined as the total length of time a patient survived without relapse. Event-free survival was defined as the length of time a patient remained free of relapse or death. Overall survival was defined as the time elapsed from the date of transplantation to death from any cause.
Statistical analysis
Data for analysis were obtained up to April 2008. The probabilities of event-free, relapse-free and overall survival were estimated by the Kaplan-Meier method. Bivariate analysis of independent variables such as sex, remission status prior to HSCT, type of HSCT, grade of acute GVHD, and presence of MRD pre-HSCT was performed to evaluate these variables as risk factors for relapse and death, using risk difference, risk ratio and odds ratio (95% confidence interval). The statistical program used was STATA.
Results
Minimal residual disease prior to allogeneic stem cell transplantation
Bone marrow samples were collected at a median of 10 days prior to the start of the conditioning regimen; however, in four patients samples were taken between 42 and 100 days pre-HSCT. No treatment was given between MRD assessment and HSCT. All 31 children were in complete morphological remission prior to transplantation. According to MRD status pre-HSCT, 21 patients were included in the MRD-negative group and ten in the MRD-positive group. MRD values were between 0.003% and 3.3%. One patient had a MRD level of 0.003% and was included in the MRD-negative group. Five patients had MRD levels between 0.01% and less than 0.1%, one between 0.1% and less than 1% and four had a high MRD burden of more than 1%. In patients with molecular markers at the time ALL was diagnosed, there were no discrepancies between the results of MRD determined by multiparametric flow cytometry and the detection of fusion genes by polymerase chain reaction pre-HSCT.
Minimal residual disease-positive group
At day 90 post-HSCT, six out of the ten children who had MRD prior to transplantation were alive without MRD. Two of the remaining four had relapsed and died due to progression and one had died from treatment-related causes. The fourth patient, on day 90, had 8% MRD. Between days 90 and 180, two of the seven survivors relapsed and died: the one who was MRD-positive and another who relapsed on day 127. During this same period, one patient died from treatment-related causes. At 180 days post-HSCT, four patients were alive: two with MRD and two without MRD. One patient, who showed MRD of 0.22% on day 180, relapsed on day 250. At 1 year post-HSCT three patients were alive: two were MRD-negative and one was MRD-positive. One MRD-negative patient died on day 395.
Table 1.
Main characteristics of the transplanted acute lymphoblastic leukemia children, minimal residual disease results prior to stem cell transplant and outcome.
In summary, five of ten patients had a hematologic relapse (on days 48, 77, 106, 127 and 250) and died from disease progression. The median time elapsed between relapse and death was 53 days (range, 8–128 days); no rescue treatment, only palliative care, was given. Treatment-related mortality occurred in three out of ten patients, with these patients dying of invasive fungal infection, severe GVHD and metabolic liver disease on days 71, 96 and 395, respectively. At the end of the study, two patients remained alive in continuous remission: one 14 months post-transplant with negative MRD and limited chronic GVHD and the other 17 months post-transplant with positive MRD (0.03%) and extensive GVHD.
Minimal residual disease-negative group
None of the evaluable patients in this group had detectable MRD at 3, 6, 12 or 24 months post-transplant except patient n. 14 who was MRD-negative at 3 and 6 months post-HSCT but relapsed on day +305. This patient had a Philadelphia-positive B-ALL; treatment with steroids and imatinib was started and the patient remains alive 10 months after relapse. Four of the 21 patients died from treatment-related causes without MRD: these deaths were due to severe GVHD, multiorgan failure, lung disease and fatal Epstein-Barr virus lymphoproliferative disease at days +30, +36, +103 and +150, respectively. Sixteen patients remain alive in remission with undetectable MRD by multiparametric flow cytometry, with a median follow-up of 16 months (range, 6–56).
Outcome
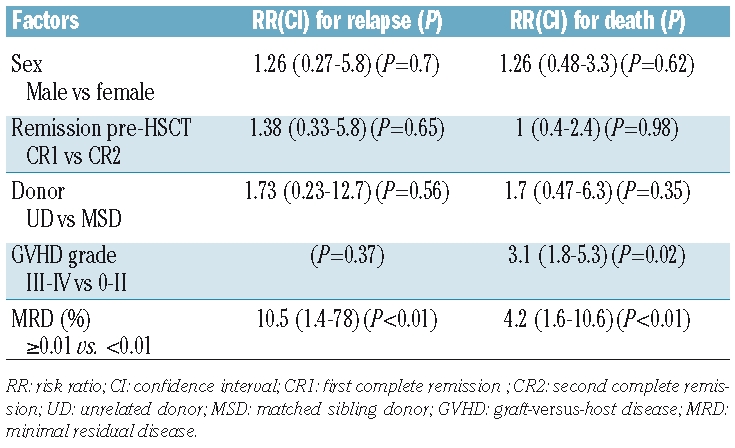
The median clinical follow-up was 9 months (range, 1–56 months). At 2 years, survival analysis was as follows: among all 31 patients the relapse-free survival rate was 75%, the event-free survival 55% and overall survival 60%. The relapse-free survival rate in MRD-negative patients was 91% compared with 42% in MRD-positive patients (P=0.001). The event-free survival rate in MRD-negative patients was 75% compared with 20% in MRD-positive patients (P=0.005). The overall survival rate for the MRD-negative and MRD-positive subgroups was 80% and 20%, respectively (P=0.004) (Figure 1). Bivariate analysis showed that the presence of MRD prior to transplantation was the only significant factor for relapse with a risk ratio of 10.5 (1.4–78) (P<0.01) and also a significant risk factor for death with a risk ratio of 4.2 (1.6–10.7) (P<0.01) (Table 2).
Figure 1.
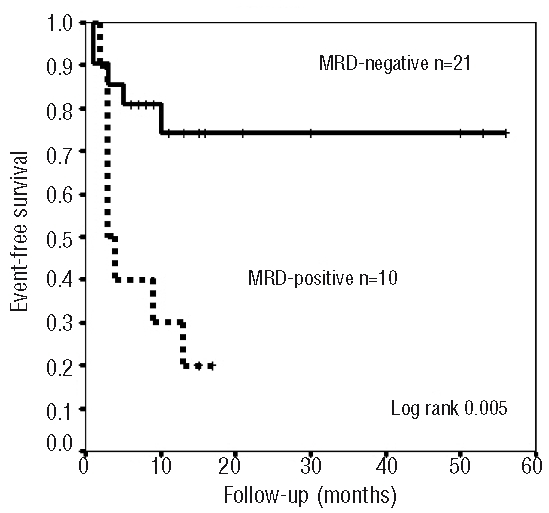
Kaplan-Meier analysis of event-free survival according to MRD levels prior to HSCT measured by multiparametric flow cytometry.
Table 2.
Bivariate analysis for risk factors for relapse and death post-HSCT.
Discussion
The detection of MRD is particularly useful for the evaluation of treatment response and, consequently, for improving therapy stratification in pediatric ALL patients. Given the strong correlation between MRD levels and risk of relapse, several ongoing regimens include treatment intensification for children in whom MRD is detected after induction and consolidation treatment. Current MRD assays are based on polymerase chain reaction amplification of antigen-receptor genes and detection of abnormal immunophenotypes by multiparametric flow cytometry.7,16,17 Multiparametric flow cytometry-based assays are rapid, readily available and provide accurate MRD quantification while simultaneously yielding information on normal hematopoietic status.9 Multiparametric flow cytometry and polymerase chain reaction amplification of the genes encoding immunoglobulin and T-cell receptor proteins estimate similar levels of MRD in most remission samples obtained from children with ALL,8,18–20 when the level of MRD is 0.01% or greater.
HSCT provides a survival advantage over chemotherapy in the treatment of children with high-risk ALL. The main barrier to successful HSCT is disease recurrence and the detection of MRD pre-transplantation has been studied as a predictive marker for relapse following allogeneic HSCT. Following a retrospective study of 56 children, Knechtli et al. reported that a high MRD burden, detected by real-time quantitative polymerase chain reaction analysis, before T-depleted allogeneic HSCT pointed to a significantly poorer outcome.2 Van der Velden and Bader confirmed these results.21,22 The International Pre-BMT MRD Study Group analyzed the presence of MRD pre-transplantation and outcome in 140 pediatric ALL patients. MRD, detected by polymerase chain reaction, proved to be a highly significant independent factor for event-free survival.3,23 Sramkova et al. assessed MRD level prior to allogeneic HSCT in 36 children with ALL using real-time quantitative polymerase chain reaction analysis and found that MRD was the only significant prognostic factor on multivariate analysis.4 Bader et al. studied MRD by real-time quantitative polymerase chain reaction in 91 children, finding that the 5-year event-free survival rate was 27% in those with a MRD burden of 10−4 or more, compared with 60% in those with a MRD level of less than 10−4.1 Our results, using multiparametric flow cytometry, concur with those using polymerase chain reaction24 and also support the strong relationship between pre-HSCT MRD and outcome following allogeneic transplantation. Patients with MRD of 0.01% or more had a higher risk of relapse and death. No statistical relationship was found between sex, remission status pre-HSCT, type and source of donor, or GVHD grade and relapse. Severe GVHD and positive MRD pre-HSCT were significant predictors for death.
Although patients with a high risk of relapse can be identified, little is known to date on how to prevent relapses. Different approaches to improving transplant outcome should include both pre- and post-transplant strategies, such as additional cytoreductive therapy prior to transplantation, monoclonal antibodies such as imatinib before and after HSCT in cases of Philadelphia-positive ALL, new purine analogs, and HSCT protocols favoring the graft-versus-leukemia effect.
Uzunel et al. showed that the combination of acute and chronic GVHD was significantly associated with a lower risk of relapse.26 On the other hand, Bader et al. reported that the administration of a low-dose of donor lymphocyte infusion in 31 children with mixed chimerism post-HSCT resulted in better outcomes.27–29 In the present study, 16 patients had acute GVHD grade II–IV and no statistical differences were found in terms of event-free survival. Two of ten MRD-positive patients who are currently alive have chronic GVHD. One has persistently low MRD levels 17 months post-transplant (0.03%) and in the other, MRD remains undetectable 14 months post-transplant. The presence of chronic GVHD could control MRD and avoid relapse; however, these two cases were only isolated observations.
This study has two main limitations: the low number of cases and the heterogeneity of the patients’ risk factors. Thus, it was not possible to carry out a multivariate analysis. The interest of this study lies in the fact that multiparametric flow cytometry was used to study MRD prior to HSCT. The results concur with those of previous studies using polymerase chain reaction techniques, and support the strong relationship between pre-transplant MRD and outcome following allogeneic HSCT. Further studies are required to confirm these results and determine whether specific protocols should be designed for patients found to have MRD prior to HSCT.
Acknowledgments
we thank Dr. J.J. Ortega for critical review of the manuscript, Ms. C. O′Hara for invaluable help with translation and Dr. Rodrigo for help with statistical analysis.
Footnotes
Authorship and Disclosures
IE: conception and design of the study collection, analysis and interpretation of data, drafting the article; CP: conception and design of the study, analysis and interpretation of data, critical review; JLD, LG: collection, analysis and interpretation of data; JSdT: critical review and final approval; CDdH: conception and design of the study, analysis and interpretation of data, critical review and final approval.
The authors reported no potential conflicts of interest.
References
- 1.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukaemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 2.Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris El, Garland RJ, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92(11):4072–9. [PubMed] [Google Scholar]
- 3.Krejci O, Van der Velden V, Bader P, Kreyenberg H, Goulden N, Hancock J, et al. Level of minimal residual disease prior to hematopoietic transplantation predicts prognosis in pediatric patients with acute lymphoblastic leukemia: a report of the Pre-BMT MRD study group. Bone Marrow Transplant. 2003;32(8):849–51. doi: 10.1038/sj.bmt.1704241. [DOI] [PubMed] [Google Scholar]
- 4.Sramkova L, Muzikova K, Fronkova E, Krejci O, Sedlacek P, Formankova R, et al. Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48(1):93–100. doi: 10.1002/pbc.20794. [DOI] [PubMed] [Google Scholar]
- 5.Uzunel M, Jaksch M, Mattsson J, Ringden O. Minimal residual disease detection after allogeneic stem cell transplantation is correlated to relapse in patients with acute lymphoblastic leukaemia. Br J Haematol. 2003;122(5):788–94. doi: 10.1046/j.1365-2141.2003.04495.x. [DOI] [PubMed] [Google Scholar]
- 6.Campana D, Coustan-Smith E, Janossy G. The immunologic detection of minimal residual disease in acute lymphoblastic leukemia. Blood. 1990;76(1):163–71. [PubMed] [Google Scholar]
- 7.Coustan-Smith E, Behm FG, Sánchez J, Boyett JM, Hancock ML, Raimondi SC, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351 (9102):550–1. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- 8.Kerst G, Kreyenberg H, Roth C, Well C, Dietz K, Coustan-Smith E, et al. Concurrent detection of minimal residual disease in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br J Haematol. 2005;128(6):774–82. doi: 10.1111/j.1365-2141.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- 9.Robillard N, Cavé H, Méchinaud F, Guidal C, Garnache-Ottou F, Rohrlich PS, et al. Four-color flow cytometry bypasses limitations of Ig/TCR polymerase chain reaction for minimal residual disease detection in certain subsets of children with acute lymphoblastic leukemia. Haematologica. 2005;90(11):1516–23. [PubMed] [Google Scholar]
- 10.Ortega JJ, Ribera JM, Oriol A, Bastida P, González ME, Calvo C, et al. Early and delayed consolidation chemotherapy significantly improves the outcome of children with intermediate risk acute lymphoblastic leukemia. Final results of the prospective randomized PETHEMA ALL-89 TRIAL. Haematologica. 2001;86(6):586–95. [PubMed] [Google Scholar]
- 11.Ribera JM, Ortega JJ, Oriol A, Bastida P, Calvo C, Pérez-Hurtado JM, et al. Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol. 2007;25(1):16–24. doi: 10.1200/JCO.2006.06.8312. [DOI] [PubMed] [Google Scholar]
- 12.Badell I, Muñoz A, Estella J, Fernández-Delgado R, Javier G, Verdeguer A, et al. Long-term results of two consecutive trials in childhood acute lymphoblastic leukaemia performed by the Spanish Cooperative Group for Childhood Acute Lymphoblastic Leukaemia Group (SHOP) from 1989 to 1998. Clin Transl Oncol. 2008;10(2):117–24. doi: 10.1007/s12094-008-0165-1. [DOI] [PubMed] [Google Scholar]
- 13.Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999;38(4):139–52. doi: 10.1002/(sici)1097-0320(19990815)38:4<139::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Nagler A, Condiotti R, Rabinowitz R, Schlesinger M, Nguyen M, Terstappen LW. Detection of minimal residual disease after bone marrow transplantation by multi-parametric flow cytometry. Med Oncol. 1999;16(3):177–87. doi: 10.1007/BF02906129. [DOI] [PubMed] [Google Scholar]
- 15.Dworzak MN, Gaipa G, Relei R, Veltroni M, Schumich A, Maglia O, et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: multiparametric assessment is feasible. Cytometry B Clin Cytom. 2008;74(6):331–40. doi: 10.1002/cyto.b.20430. [DOI] [PubMed] [Google Scholar]
- 16.Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96(8):2691–6. [PubMed] [Google Scholar]
- 17.Szczepanski T. Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia. 2007;21(4):622–6. doi: 10.1038/sj.leu.2404603. [DOI] [PubMed] [Google Scholar]
- 18.Campana D. Status of minimal residual disease testing in childhood haematological malignances. Br J Haematol. 2008;143(4):481–9. doi: 10.1111/j.1365-2141.2008.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan J, Quinn F, Meunier A, Boublikova L, Crampe M, Tewari P, et al. Minimal residual disease detection in childhood acute lymphoblastic leukaemia patients at multiple time-points reveals high levels of concordance between molecular and immunophenotypic approaches. Br J Haematol. 2009;144(1):107–15. doi: 10.1111/j.1365-2141.2008.07429.x. [DOI] [PubMed] [Google Scholar]
- 20.Malec M, van der Velden VH, Björklund E, Wijkhuijs JM, Soderhall S, Mazur J, et al. Analysis of minimal residual disease in childhood with acute lymphoblastic leukemia: comparison between RQ-PCR analysis of Ig/TcR gene rearrangement and multicolor flow cytometric immunophenotyping. Leukemia. 2004;18(10):1630–6. doi: 10.1038/sj.leu.2403444. [DOI] [PubMed] [Google Scholar]
- 21.van der Velden VH, Joosten SA, Willemse MJ, van Wering ER, Lankester AW, van Dongen JJ, et al. Real-time quantitative PCR for detection of minimal residual disease before allogeneic stem cell transplantation predicts outcome in children with acute lymphoblastic leukaemia. Leukemia. 2001;15(9):1485–7. doi: 10.1038/sj.leu.2402198. [DOI] [PubMed] [Google Scholar]
- 22.Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, et al. Minimal residual disease status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16(9):1668–72. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- 23.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time PCR: principles, approaches and laboratory aspects. Leukemia. 2003;17(6):1013–35. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 24.Schilham MW, Balduzzi A, Bader P. Is there a role for minimal residual disease levels in the treatment of ALL patients who receive allogeneic stem cell? Bone Marrow Transplant. 2005;35 (Suppl 1):S49–52. doi: 10.1038/sj.bmt.1704847. [DOI] [PubMed] [Google Scholar]
- 25.Vettenranta K, Saarinen-Pihkala UM, Cornish J, Steward C, Pamphilon D, Hori L, et al. Pediatric marrow transplantation for acute leukaemia using unrelated donors and T-repleted or deplete grafts: a case-matched analysis. Bone Marrow Transplant. 2000;25(4):395–9. doi: 10.1038/sj.bmt.1702162. [DOI] [PubMed] [Google Scholar]
- 26.Uzunel M, Mattsson J, Jaksch M, Remberger M, Ringder O. The significance of graft-versus-host disease and pretransplantation minimal residual disease status to outcome after allogeneic stem cell transplantation in patients with acute lymphoblastic leukemia. Blood. 2001;98(6):1982–4. doi: 10.1182/blood.v98.6.1982. [DOI] [PubMed] [Google Scholar]
- 27.Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P, et al. Prevention of relapse in paediatric patients with acute leukaemia and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single centre experience of 12 children. Leukemia. 1999;13(12):2079–86. doi: 10.1038/sj.leu.2401581. [DOI] [PubMed] [Google Scholar]
- 28.Choi SJ, Lee JH, Kim S, Lee YS, Seol M, Ryu SG, et al. Treatment of relapsed acute lymphoblastic leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a prospective study. Bone Marrow Transplant. 2005;36(2):163–9. doi: 10.1038/sj.bmt.1705024. [DOI] [PubMed] [Google Scholar]
- 29.Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavourable outcome in children with acute lymphoblastic leukaemia after allogeneic stem-cell transplantation: possible role for pre-emptive Immunotherapy? J Clin Oncol. 2004;22(9):1696–705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]