Abstract
Background
Chronic thromboembolic pulmonary hypertension after pulmonary embolism is associated with high morbidity and mortality. Understanding the incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism is important for evaluating the need for screening but is also a subject of debate because of different inclusion criteria among previous studies. We determined the incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism and the utility of a screening program for this disease.
Design and Methods
We conducted a cohort screening study in an unselected series of consecutive patients (n=866) diagnosed with acute pulmonary embolism between January 2001 and July 2007. All patients who had not been previously diagnosed with pulmonary hypertension (PH) and had survived until study inclusion were invited for echocardiography. Patients with echocardiographic suspicion of PH underwent complete work-up for chronic thromboembolic pulmonary hypertension, including ventilation-perfusion scintigraphy and right heart catheterization.
Results
After an average follow-up of 34 months of all 866 patients, PH was diagnosed in 19 patients by routine clinical care and in 10 by our screening program; 4 patients had chronic thromboembolic pulmonary hypertension, all diagnosed by routine clinical care. The cumulative incidence of chronic thromboembolic pulmonary hypertension after all cause pulmonary embolism was 0.57% (95% confidence interval [CI] 0.02–1.2%) and after unprovoked pulmonary embolism 1.5% (95% CI 0.08–3.1%).
Conclusions
Because of the low incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism and the very low yield of the echocardiography based screening program, wide scale implementation of prolonged follow-up including echocardiography of all patients with pulmonary embolism to detect chronic thromboembolic pulmonary hypertension does not seem to be warranted.
Keywords: Chronic thromboembolic pulmonary hypertension, pulmonary embolism, pulmonary hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a life-threatening condition characterized by intraluminal thrombus organization and fibrous stenosis or complete obliteration of the pulmonary arteries.1 CTEPH is commonly seen as a long-term sequel of acute pulmonary embolism (PE), although the pathogenesis of impaired clearance of acute thrombi and the resulting vascular remodeling is unknown, and there is no history of symptomatic venous thromboembolism (VTE) in 31–42% of the patients diagnosed with CTEPH.1–3
The incidence of CTEPH has been reported to be between 0.1% and 8.8% in patients after acute PE.1,4–7 This wide range can be explained by important differences in the inclusion and diagnostic criteria between these previous studies: selection of patients was often based on the etiology of the acute PE, patients with further comorbid conditions associated with pulmonary hypertension were frequently excluded and the diagnosis of CTEPH was not always confirmed by right heart catheterization.1,4–7
Since CTEPH is a very serious but potentially treatable disease, the exact incidence of CTEPH in the clinical course of acute PE is of particular interest. High frequencies of 3.8% to 8.8%4,6 would suggest the need for prolonged follow-up after discontinuation of anticoagulant therapy including specific screening programs for CTEPH by echocardiography, whereas lower frequencies would not. The purpose of this study was to evaluate the efficacy of a screening program for CTEPH in patients after acute PE. This evaluation was based on the overall incidence of CTEPH and the additional utility of this screening program on top of standard clinical care. Accordingly, we performed a prospective cohort screening study evaluating the occurrence of CTEPH in an unselected large series of patients diagnosed with acute PE.
Design and Methods
Patients
Consecutive patients diagnosed with an episode of acute PE in the period between January 1st 2001 and July 1st 2007 of an academic institute (Leiden University Medical Center, Leiden, the Netherlands) and affiliated teaching hospital (Medical Center Haaglanden, The Hague, the Netherlands) were eligible for study inclusion, irrespective of age, medical history or comorbid conditions. Neither of these 2 hospitals serves as a tertiary referral center for CTEPH. All patients diagnosed and treated for acute PE are registered in a database by physicians of all clinical specialities of both hospitals. For the purpose of this study, we crosschecked this database with data from the radiology department to ensure no patients were missing. The diagnosis of acute PE was verified for all registered patients according to predefined criteria which were intraluminal filling defects on pulmonary angiography or computed-tomography pulmonary-angiography (CTPA), high probability ventilation perfusion scintigraphy (VQ-scan) or intermediate probability VQ-scan in combination with objectively diagnosed deep venous thrombosis (DVT).8 All patients fulfilling these criteria were included in this analysis. Unprovoked PE was defined as PE occurring in the absence of the following risk factors: active malignancy, immobility for more than three days or recent long flight (over six hours), recent surgery or fracture of extremity, pregnancy or peri-partum period, hormone replacement therapy and use of oral contraception. Patients were initially treated with at least five days of either unfractioned heparin, aiming at a 1.5 to 2.5 prolongation of the activated partial thromboplastin time, or weight based therapeutic doses of LMWH, followed by vitamin K antagonists for a period of at least six months with a target international normalized ratio (INR) of 2.0 to 3.0.9 In patients with severe acute PE, anticoagulant treatment was preceded by administration of thrombolytic drugs, thrombosuction or surgical embolectomy according to the judgment of the attending clinician. Clinical follow-up and treatment monitoring after hospital discharge were performed in the local pulmonary, internal or vascular medicine outpatient clinic as well as in the anticoagulation clinic.
Outcome
Primary outcomes of this study were the incidence of CTEPH and the effectiveness of our screening program. Criteria for the diagnosis of CTEPH were mean pulmonary artery pressures assessed by right heart catheterization exceeding 25 mmHg and normal pulmonary capillary wedge pressure in combination with an abnormal perfusion scintigram and signs for distal or central CTEPH on conventional pulmonary angiography.10,11 CTEPH was considered excluded in case of a normal perfusion scintigram.10,11
Procedures
The original admission and outpatient medical charts of all patients diagnosed with acute PE in the registration period were systematically reviewed using predefined criteria. Only patients with geographical inaccessibility (living outside the Netherlands) precluding follow-up were excluded from the study. Data regarding diagnostic management, etiology, treatment and documented clinical course of the acute PE at registration as well as recurrent episodes, and established diagnosis of pulmonary hypertension were assembled. Only recurrent events that were objectively confirmed according to our predefined criteria were accounted for. For all eligible patients who had died before study inclusion (July 2007), time and cause of death were extracted from the autopsy report or verified with the treating physician or general practitioner. All surviving patients prior to diagnosis of pulmonary hypertension were interviewed by telephone to complete the data derived form their medical charts, to obtain information regarding the presence of clinical symptoms suggestive of pulmonary hypertension and, if applicable, the results of recent echocardiography. In addition, information on known risk factors for CTEPH was noted down for patients who had been previously diagnosed with CTEPH: these include large central emboli, unprovoked VTE, splenectomy, presence of lupus anticoagulant or antiphospholipid antibodies, chronic inflammatory conditions and ventriculo-atrial shunts.12 Furthermore, all surviving patients were invited for a single visit to our vascular medicine outpatient clinic for pulmonary hypertension screening by echocardiography. This visit was scheduled between July 1st 2007 and January 1st 2009 and planned at least one year after the index event, or one year after a recurrent thromboembolic episode, to rule out the initial effect of acute PE. All patients who responded to our invitation underwent physical examination and standardized transthoracic echocardiography performed by an experienced technician. This echocardiography was reviewed by an independent expert cardiologist, without knowledge of the patient’s medical condition. Echocardiographic criteria for suspected pulmonary hypertension were one or more of the following: 1) maximal tricuspid regurgitation velocity over 2.8 m/s; 2) estimated systolic pulmonary artery pressureof 35 mmHg or over (maximal pressure gradient across the tricuspid valve calculated by the modified Bernoulli equation plus the estimated right atrium pressure); 3) estimated mean pulmonary artery pressure of 25 mmHg or over (estimated systolic pressure plus 2 times end-diastolic pressure as estimated by pulmonary regurgitation end-diastolic velocity divided by 3; 4) borderline value of criterion 1 or 2 in combination with a right ventricular TEI index over 0.36 (isovolumic contraction time plus isovolumic relaxation time divided by ejection time); 5) secondary changes associated with pulmonary hypertension, e.g. systolic septal flattening, right ventricular hypertrophy or W-pattern in the right ventricular outflow curve; 6) AcT (acceleration time ) under 120 or AcT/RVET (right ventricular ejection time) under 0.40.11,13,14 All patients who met one or more of these 6 criteria were suspected of having pulmonary hypertension and underwent further standardized work-up including perfusion lung scintigraphy and right heart catheterization for pressure measurements. The final diagnosis was assessed by an independent expert panel according to our predefined criteria. This study was approved by the Institutional Review Board of both participating hospitals and all patients provided written informed consent.
Statistical analysis
Patients were categorized in 4 sub-groups according to their medical history of single or recurrent and provoked or unprovoked PE. The cumulative incidence of CTEPH after acute PE for all 4 study groups was calculated by the Kaplan-Meier life table method. In addition, we calculated the incidence rates of CTEPH. The number of patient years (py) for both analyses was calculated from the date of the index event until diagnosis of CTEPH was established or ruled out or else until death had occurred, whichever came first. Finally, for a more conservative estimation of the incidence of CTEPH after acute PE, we performed a second analysis including only the patients in whom objective testing for CTEPH was performed. SPSS version 14.02 (SPSS Inc, Chicago, IL, USA) was used for all analyses.
Results
Patients
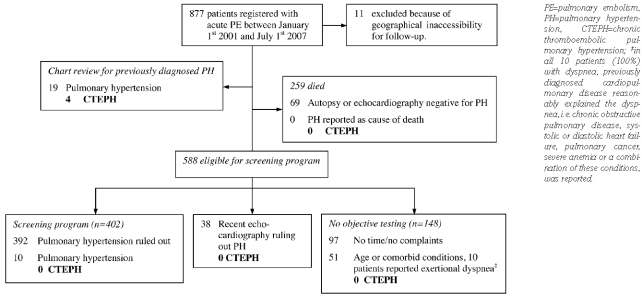
In 877 patients the diagnosis of acute PE had been established between January 1st 2001 and July 1st 2007. Eleven patients were excluded because of geographical inaccessibility, leaving 866 patients who were included in the study. General characteristics of these patients are shown in Table 1: mean age at registration was 56 years, 410 (47%) were males and 308 patients (36%) had had an unprovoked episode of PE. More than 90% of patients were initially treated with either unfractionated heparin or LMWH alone. A small number of patients additionally received thrombolytic therapy, had a vena cava filter inserted or had surgical embolectomy performed (Table 1). The average follow-up period was 2.8 years.
Table 1.
Characteristics of included patients.
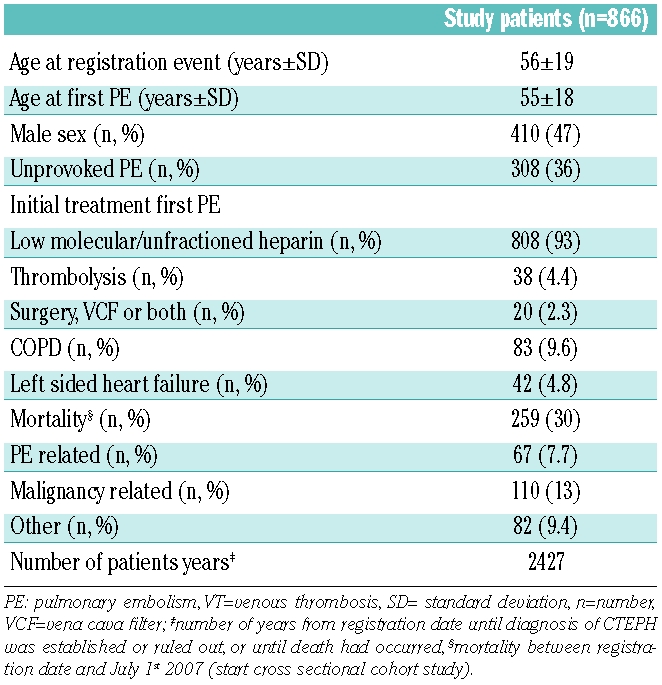
Chart review
After reviewing the medical charts of all patients, 19 cases of previously diagnosed pulmonary hypertension were identified (Figure 1) of whom 4 had CTEPH. During the study period, 259 patients died, 75 (8.7%) as a direct result of acute (recurrent) PE, 88 (10%) of malignant disease and 96 (11%) from other conditions. Of these 259 patients, 185 (71%) had died within the first year after the acute PE, 216 (83%) within two years, 238 (92%) within three years and 247 (95%) within four years. In 69 patients, autopsy reports or echocardiography performed before the patient’s death ruled out the presence of pulmonary hypertension. Furthermore, pulmonary hypertension was not adjudicated as cause of death in any of these patients. Therefore, and in accordance with criteria from previous studies,4–7 we assumed that none of these patients had developed CTEPH.
Figure 1.
Flow chart of the cohort study.
The remaining 588 patients were invited for our screening program. We were able to complete this program in 402 (68%) of them. Of these patients, 170 had symptoms suggestive of CTEPH. Echocardiographic criteria for suspected pulmonary hypertension were met by 25 patients. After further clinical work-up and right heart catheterization, pulmonary hypertension was diagnosed in 10 of these patients.
From the 186 patients who did not respond to our invitation, 38 had undergone echocardiography for clinical reasons other than our screening study. None of these patients was diagnosed with pulmonary hypertension after evaluating these echoes for our predefined criteria for suspected pulmonary hypertension. Objective testing for pulmonary hypertension was not performed in the remaining 148 surviving patients. Of these, 97 declared themselves to be in excellent health without any physical complaints and to have no time to be involved in any clinical trials. The final 51 patients were unable to visit our hospital due to old age or comorbid conditions. Most of these patients were over the age of 80 years and suffered from severe cancer. Of these latter 51 patients, 10 reported exertional dyspnea that was reasonably explained by previously diagnosed cardiopulmonary diseases, i.e. chronic obstructive pulmonary disease (COPD), systolic left-sided heart failure, pulmonary cancer, severe anemia or a combination of these conditions. Since none of these patients had unexplained dyspnea,4–6 we assumed that CTEPH was not present in these patients for the purpose of the incidence calculation.
Incidence of chronic thromboembolic pulmonary hypertension
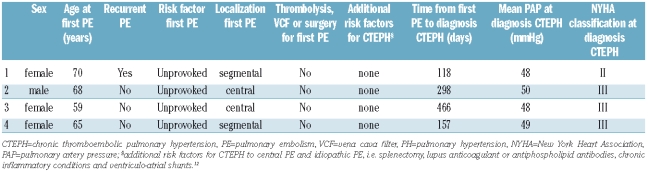
Upon study inclusion, 19 patients with a history of PE had already been diagnosed with pulmonary hypertension of various causes. Among these, 4 had been diagnosed with CTEPH by routine clinical care and CTEPH was ruled out by perfusion scintigraphy or pulmonary angiography in the remaining 15 patients. Our screening program identified an additional 10 patients with pulmonary hypertension, but distal or central CTEPH was ruled out in all of these patients after normal perfusion scintigraphy or pulmonary angiography. All 4 patients with CTEPH returned to their physician within two years after their first acute PE because of typical symptoms of CTEPH, including exertional dyspnea (Table 2). None of them was found to have additional risk factors for CTEPH or other comorbidities causing pulmonary hypertension. The diagnoses were confirmed by right heart catheterization and measurement of pulmonary artery pressure. Mean time to diagnosis was 260 days. The cumulative incidence of CTEPH in our cohort was 0.57% (4/866, 95% CI 0.02–1.2%) in the overall population, 1.5% (4/308, 95% CI 0.08–3.1%) in the patients with unprovoked PE and 0.0% (0/558) in the patients with provoked PE (Table 3 and Figure 2). Incidence rate of CTEPH was 0.16/100 py (95% CI 0.04–0.42/100 py) for the overall population, 0.44/100 py (95% CI 0.12–1.1/100 py) for patients with unprovoked PE and 0.0/100 py (95% CI 0.0–0.24/100 py) for patients with provoked PE (Table 3). There was no difference in incidences following a single event or recurrent disease between the study groups (Table 3). Lastly, to provide a more conservative estimation of the CTEPH incidence after acute PE, we calculated the separate incidence solely for the patients in whom CTEPH was objectively ruled out or established (incidence 0.80%, 95% CI 0.01–1.9).
Table 2.
Characteristics of patients with chronic thromboembolic pulmonary hypertension.
Table 3.
Cumulative incidence by the Kaplan-Meier life table method, and incidence rates of CTEPH in the study population.
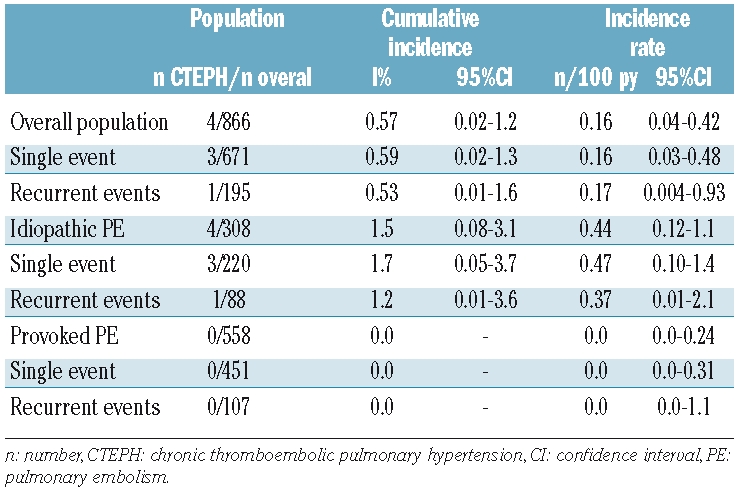
Figure 2.
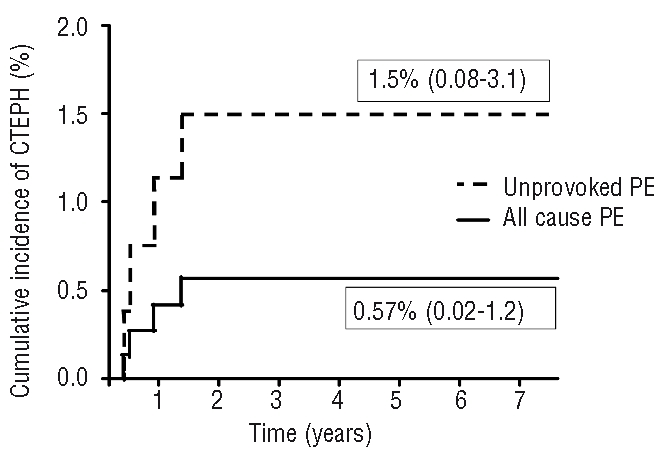
Cumulative incidence of chronic thromboembolic pulmonary hypertension.
Follow-up of patients with chronic thromboembolic pulmonary hypertension
Because of distal pulmonary artery involvement, only one of the 4 patients with CTEPH was considered suitable for pulmonary endarterectomy. However, this patient refused surgery for personal reasons and was treated with the oral dual endothelin receptor antagonist bosentan.15 Two additional patients were treated with bosentan of whom one developed severe elevation of transaminases and this treatment was consequently stopped. The final patient did not receive treatment because of the benign clinical presentation of CTEPH (NYHA Class II, satisfactory exercise tolerance without severe desaturation during maximal exercise). At the moment of drafting this paper (April 1st 2009) and after a mean follow-up of 43 months after diagnosis of CTEPH, the clinical condition of all 4 patients was stable.
Efficacy of the screening program
Ten patients with pulmonary hypertension were identified by the screening program in the 402 patients with a history of acute PE but without established pulmonary hypertension 2.8 years after the acute thromboembolic event. Pulmonary angiography did not reveal CTEPH in these 10 patients: the pulmonary hypertension was caused by left sided heart disease in 5 patients and by COPD in the remaining 5 patients. All patients with CTEPH from our total study population were previously diagnosed by routine clinical practice.
Discussion
This study has two main findings. First, we observed a 0.57% incidence of CTEPH after acute PE in an unselected large patient series. Second, the yield of a standard screening program to detect CTEPH in patients after acute PE is low, since additional cases of CTEPH to cases identified by routine clinical care were not detected in our study population.
Understanding of the incidence of CTEPH is important to guide the screening and diagnostic strategy in patients after acute PE. The incidence of CTEPH we observed challenges other studies reporting higher incidences ranging from 3.8% to 8.8%.4–7 There are several reasons for these discrepancies. These previous studies included selected patient cohorts, e.g. excluding patients with transient or permanent risk factors for PE5 and excluding patients with other conditions associated with pulmonary hypertension.4–6 In addition, the diagnosis of CTEPH was partly based on results from echocardiography alone without confirmation by right heart catheterization.6 In the study by Pengo et al. an incidence of 3.8% after two years was reported, which is considerably higher than the incidence observed in our population.4 The main differences between the two studies were the inclusion criteria: whereas we only excluded patients who were geographically inaccessible for follow-up, Pengo also excluded all patients with other diseases that could have caused non-thromboembolic pulmonary hypertension (e.g. severe emphysema) or had preexisting exertional dyspnea. Although given our study design we were unable to estimate precisely the prevalence of those latter patients in our cohort, they provide a considerable contribution to our sample size. The selection criteria applied by Pengo may have influenced their results, leading to a higher incidence of CTEPH. However, the duration to diagnosis (within two years after diagnosis of PE) was comparable between the 2 studies.
We consider our results to be representative for several reasons. First, we included all patients who presented to the participating hospitals, independently of etiology or severity of the acute PE or presence of comorbid conditions. Our study comprised 3 times the number of previous reports and only 11 patients (1.3%) were excluded. Second, we used very sensitive echocardiographic criteria for establishing the suspicion of pulmonary hypertension and confirmed all cases of CTEPH with heart catheterization.11,13,14 Third, the incidence of CTEPH in patients with unprovoked PE in our study was 1.5%. This is in accordance with or within the lower limit of the confidence interval of the incidences described in studies focusing solely on patients with unprovoked PE.4,5
It could be reasoned that our estimation of 0.57% represents an underestimation of the incidence of CTEPH since objective testing to confirm or reject this diagnosis was not performed in the whole study population. However, this same issue can be applied to all previous reports on this subject. The vast majority of the non-survivors in our study died within one year after the PE was diagnosed, and a reasonable alternative cause of death was reported in all of them. Furthermore, all cases of CTEPH presented with symptoms of cardiopulmonary impairment. Hence, it is unlikely that we missed cases of CTEPH in the asymptomatic patients who were not able to visit our outpatient clinic or in the patients who had died. Also, plausible alternative diagnoses for dyspnea were confirmed in all 10 patients who reported exertional dyspnea but did not visit our outpatient clinic. We nonetheless have provided an additional, more conservative estimation of the CTEPH incidence by calculating this incidence in a selected patient population who all underwent objective testing for CTEPH (incidence 0.8%).
Limitations of our study include the lack of accurate data on the time from complaints to diagnosis of PE, which is a hypothetical risk factor for CTEPH, and the different follow-up periods between the study patients, which could complicate the interpretation of our results. Even so, patients who completed our screening program underwent echocardiography at least one full year following the index acute PE, with a mean follow-up period of 3.7±1.4 years, which is well over the two year detection frame described by Pengo et al.4
The mortality as well as the recurrence rates in our cohort were relatively high. Since we did not apply any exclusion criteria except for geographical inaccessibility, patients who would not have been eligible for other studies because of poor prognosis or other reasons, were in fact included in the current analysis. Hence, our population consisted of relatively more patients with serious comorbidity explaining the high mortality rate. Also, we included patients with a first as well as with recurrent VTE. Some patients suffered form recurrent VTE during the follow-up period and were therefore also classified in the recurrence group, resulting in a relatively high number of patients with recurrent VTE.
In this study, a cardiopulmonary screening program to detect CTEPH was evaluated. Although completed by 402 patients, this screening program did not result in any additional patients being detected with a diagnosis of CTEPH beside those patients who were identified by routine clinical practice. In combination with the low frequency of CTEPH, this leads to the conclusion that wide scale implementation of screening programs for CTEPH after acute PE is not warranted and echocardiography to rule out or establish CTEPH should be restricted to patients presenting with characteristic symptoms.
We conclude that CTEPH is a rare complication of acute PE (incidence 0.57%) and that this diagnosis is more frequent in patients with unprovoked acute PE (incidence 1.5%). CTEPH becomes clinically apparent and is diagnosed within the first two years following acute PE. Wide scale screening for CTEPH after acute PE results in a very low yield. Although CTEPH occurs infrequently in the clinical course of acute PE, physicians should be aware of this potentially lethal but treatable disease, especially in those patients with unprovoked disease and persistent dyspnea. The direct clinical consequence of our study is that because of the very low incidence of CTEPH after PE, the implementation of extensive follow-up programs for the detection of CTEPH after acute PE seems to be unnecessary.
Footnotes
Funding: the study was supported by an unrestricted research grant from Actelion Pharmaceuticals Ltd.
Authorship and Disclosures
FAK: study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript; KWvK: analysis and interpretation of data, critical revision of the manuscript for important intellectual content; APJvD: study concept and design, critical revision of the manuscript for important intellectual content; JTT: critical revision of the manuscript for important intellectual content; FHH: critical revision of the manuscript for important intellectual content; HWV: critical revision of the manuscript for important intellectual content; MVH: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
The authors reported no potential conflicts of interest.
References
- 1.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345(20):1465–72. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 2.Egermayer P, Peacock AJ. Is pulmonary embolism a common cause of chronic pulmonary hypertension? Limitations of the embolic hypothesis. Eur Respir J. 2000;15(3):440–8. doi: 10.1034/j.1399-3003.2000.15.03.x. [DOI] [PubMed] [Google Scholar]
- 3.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, Goldsmith K, Coghlan JG, Pepke-Zaba J. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33(2):332–8. doi: 10.1183/09031936.00092008. [DOI] [PubMed] [Google Scholar]
- 4.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 5.Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, Ageno W. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130(1):172–5. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 6.Dentali F, Donadini M, Gianni M, Bertolini A, Squizzato A, Venco A, Ageno W. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009;124(3):256–8. doi: 10.1016/j.thromres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006;85(5):253–62. doi: 10.1097/01.md.0000236952.87590.c8. [DOI] [PubMed] [Google Scholar]
- 8.Huisman MV, Klok FA. Diagnostic management of clinically suspected acute pulmonary embolism. J Thromb Haemost. 2009;7(Suppl 1):312–1. doi: 10.1111/j.1538-7836.2009.03386.x. [DOI] [PubMed] [Google Scholar]
- 9.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ American College of Chest Physicians. Anti-thrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 10.Auger WR, Fedullo PF, Moser KM, Buchbinder M, Peterson KL. Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology. 1992;182(2):393–8. doi: 10.1148/radiology.182.2.1732955. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113(16):2011–20. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 12.Bonderman D, Jakowitsch J, Adlbrecht C, Schemper M, Kyrle PA, Schönauer V, Exner M, Klepetko W, Kneussl MP, Maurer G, Lang I. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005;93(3):512–6. doi: 10.1160/TH04-10-0657. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association; ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Vonk MC, Sander MH, van den Hoogen FH, van Riel PL, Verheugt FW, van Dijk AP. Right ventricle Tei-index: a tool to increase the accuracy of non-invasive detection of pulmonary arterial hypertension in connective tissue diseases. Eur J Echocardiogr. 2007;8(5):317–21. doi: 10.1016/j.euje.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Jaïs X, D’Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, et al. Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension Study Group. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–34. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]