Abstract
Background
We analyzed the outcome of 100 patients with acquired severe aplastic anemia undergoing an alternative donor transplant, after immune suppressive therapy had failed.
Design and Methods
As a conditioning regimen, patients received either a combination of fludarabine, cyclophosphamide, and antithymocyte globulin (n=52, median age 13 years) or this combination with the addition of low dose (2 Gy) total body irradiation (n=48, median age 27 years).
Results
With a median follow-up of 1665 and 765 days, the actuarial 5-year survival was 73% for the group that received fludarabine, cyclophosphamide, and antithymocyte globulin and 79% for the group given the conditioning regimen including total body irradiation. Acute graft-versus-host disease grade III–IV was seen in 18% and 7% of the groups, respectively. Graft failure was seen in 17 patients with an overall cumulative incidence of 17% in patients receiving conditioning with or without total body irradiation: 9 of these 17 patients survive in the long-term. The most significant predictor of survival was the interval between diagnosis and transplantation, with 5-year survival rates of 87% and 55% for patients grafted within 2 years of diagnosis and more than 2 years after diagnosis, respectively (P=0.0004). Major causes of death were graft failure (n=7), post-transplant-lymphoproliferative-disease (n=4) and graft-versus-host disease (n=4).
Conclusions
This study confirms positive results of alternative donor transplants in patients with severe aplastic anemia, the best outcomes being achieved in patients grafted within 2 years of diagnosis. Prevention of rejection and Epstein-Barr virus reactivation may further improve these results.
Keywords: aplastic anemia, unrelated transplants, graft failure, graft versus host disease, fludarabine
Introduction
Transplant-related mortality has been greatly reduced over the past decade in patients with acquired severe aplastic anemia undergoing an unrelated donor transplant, as shown in a report by a French cooperative transplant group.1 Factors contributing to this improved outcome include modifications of the conditioning regimen, with the introduction of low-dose total body irradiation (TBI),2,3 and more stringent criteria of HLA compatibility for donor selection.1 This improvement has not been confirmed in all published studies, and a high mortality rate was recently found in a large series of 195 children with severe aplastic anemia undergoing unrelated donor transplants: in that series, when the donor and recipient were 8/8 matched at the allelic level, mortality was 43%, whereas when the matching was less than 8/8, mortality was 61%.4 One of the problems is rejection of the graft, which has always been a peculiar complication in patients with severe aplastic anemia undergoing allogeneic transplants, also from HLA identical siblings.5 The risk of graft rejection has declined over the years,6 although it is difficult to reduce it below 10% (European Group for Blood and Marrow Transplantation 2008, unpublished data). Currently, it may be possible to predict graft rejection by monitoring donor chimerism, using short tandem repeat polymerase chain reaction analysis: patients with complete donor chimerism do not experience graft rejection, whereas patients with progressive mixed chimerism, and increasing levels of autologous cells, are at high risk of graft rejection, especially when cyclosporine is withdrawn.7
In a previous report, the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) described a TBI-free regimen, consisting of fludarabine, cyclophosphamide and antithymocyte globulin (FCA), for patients with severe aplastic anemia undergoing an unrelated donor transplant.8 The overall graft rejection rate in patients given this conditioning regimen was 18%, with the rate being higher (32%) in patients older than 14 years (median age) and lower in children younger than 14 (5%).8 Of interest, the dynamics of rejection were somewhat different from those of classic, straightforward rejection: indeed a significant proportion of patients with graft rejection could be rescued with a second transplant, or experienced autologous recovery.8 Nevertheless, due to the high rejection rate in adults, and in keeping with results regarding TBI-containing programs in the USA and in Japan,2,3 some centers in Europe started adding low dose TBI (2 Gy) to the FCA program for patients above the age of 14 undergoing an unrelated donor transplant. The FCA program, without TBI, was still offered to children under the age of 14 years.
Here we report on 100 patients, identified from the EBMT database, who received a transplant from an unrelated donor or a one HLA antigen-mismatched family donor and were treated with these two conditioning regimens: 52 patients were given the FCA regimen and the other 48 were given the FCA-TBI regimen.
Design and Methods
Patients
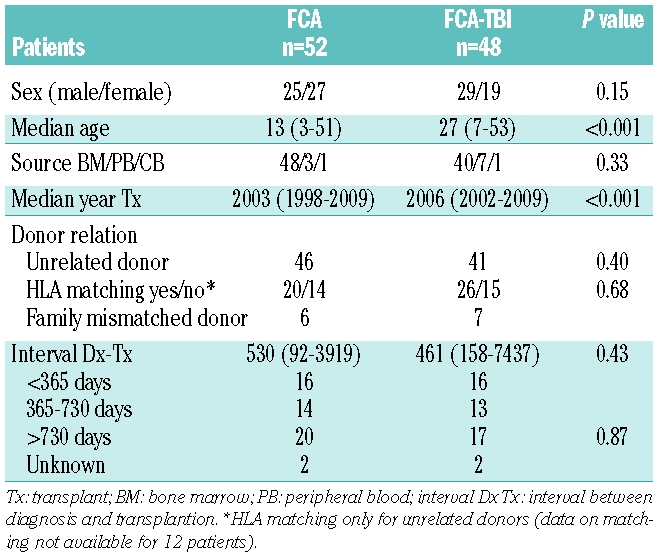
The clinical characteristics of the patients are outlined in Table 1. Patients were selected from the EBMT database on the basis of their having acquired aplastic anemia and having received a transplant from an alternative donor (see below) following conditioning with either the FCA or FCA-TBI regimen. Transplants were performed between 26.3.1998 and 07.10.08 in 22 Transplant Centers (see appendix). The transplant protocols described in this study were approved by local ethical committees of the participating centers. All patients signed informed consent before the allogeneic transplant and were treated in compliance with local guidelines.
Table 1.
Clinical data of the 100 transplanted patients with SAA.
All patients were transplanted after one or more courses of antithymocyte globulin therapy had failed and were dependent on transfusions at the time of transplantation.
Bone marrow donors
The donor was an unrelated volunteer for 87 patients and an HLA-mismatched family member for the other 13. Of the unrelated donors 46 were reported to be matched at the allelic level at A, B, C, DRB1, whereas 29 were mismatched at one or more loci; matching data were not available for 12 patients. The 13 related donors were mismatched at one class I (n=10) or class II (n=3) antigen.
Stem cell source
The stem cell source was unmanipulated bone marrow (n=88), unmanipulated peripheral blood (n=10), or cord blood (n=2).
The fludarabine, cyclophosphamide and antithymocyte conditioning regimen
The FCA regimen consisted of fludarabine 30 mg/m2 on days -6, -5, -4, and -3, cyclophosphamide 300 mg/m2 on days -6, -5, -4, and -3, and antithymocyte globulin (Thymoglobulin, Genzyme, USA), 3.75 mg/kg on days -6, -5, -4, and -3. On day 0 an unmanipulated graft was infused. Prophylaxis and monitoring of viral and fungal infections and post-transplant monitoring for cytomegalovirus, Epstein-Barr virus and Aspergillus spp. were carried out according to the institutional protocols in the different Centers. The outcome of 38 of the 52 patients who received the FCA regimen have already been reported.6
The total body irradiation-containing conditioning regimen
The FCA-TBI regimen consisted of fludarabine 30 mg/m2 on days -6, -5, -4, and -3, cyclophosphamide 300 mg/m2 on days -6, -5, -4, and -3, and antithymocyte globulin (Thymoglobulin, Genzyme, USA) 3.75 mg/kg/on days -4 and -3 plus TBI 2 Gy. The TBI was administered on day -1, from a linear accelerator, in antero-posterior position, as a single fraction at the dose rate of 15 rads/min. On day 0 an unmanipulated graft was infused. Prophylaxis and monitoring of viral and fungal infections and post-transplant monitoring for cytomegalovirus, Epstein-Barr virus and Aspergillus spp. were carried out according to the institutional protocols in the different Centers.
Graft-versus-host disease
All patients received graft-versus-host disease (GvHD) prophylaxis with a combination of cyclosporine A and methotrexate (days +1, +3, and +6) according to institutional protocols. The usual dose of cyclosporine was 2 mg/kg and methotrexate was given at the dose of 10 mg/m2 on day+1, then 8 mg/m2 on days +3 and +6.
Graft failure
Graft failure was classified as follows: (i) primary non-engraftment (failure to reach a neutrophil count of 0.5×109/L after transplantation); (ii) rejection (decrease in neutrophil counts to less than 0.5×109/L after having achieving a neutrophil count of 0.5×109/L); (iii) late graft failure, (decrease of blood counts beyond day +100, to less than 1×109/L neutrophils and less than 30×109/L platelets).
Chimerism studies
Chimerism studies were performed on unfractionated bone marrow cells, using a short tandem repeat polymerase chain reaction technique, when available in participating Centers. Results are stratified according to time after transplant: less than or more than 100 days after transplantation. Chimerism data were available for 58 patients.
Statistical analysis
The Number Cruncher Statistical System package was used to analyze data. The probability of survival was estimated by the Kaplan-Meier method and the log-rank test (Mantel-Cox) was used to assess differences between survival curves. The cumulative incidences of graft failure and GvHD are reported. A multivariate Cox analysis was run to assess factors predicting survival: the variables considered were the patient’s age (continuous), interval between diagnosis and transplant (2 years or less versus more than 2 years), type of donor (unrelated versus family mismatched), and conditioning regimen (FCA versus FCA-TBI).
Results
Graft failure
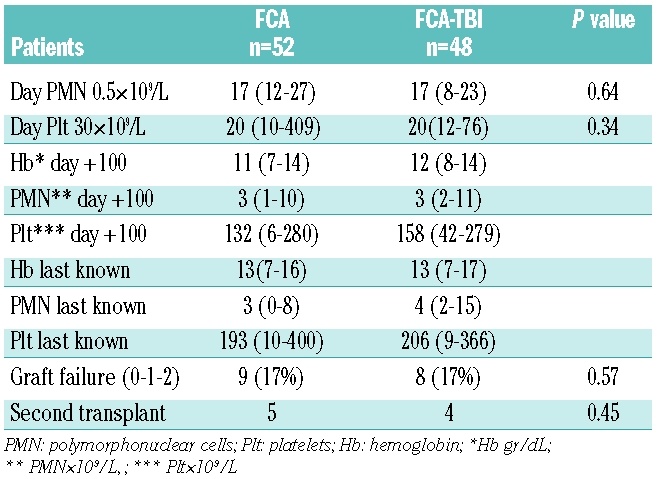
Table 2 summarizes the engraftment data. Graft failure occurred in 17 patients and was the primary cause of death in 7: the overall cumulative incidence in patients receiving FCA or FCA-TBI was 17%. Graft failure could be further subdivided into primary non-engraftment (n=2), rejection (n=12) and late graft failure (n=3). Among the 17 patients with graft failure, nine received a second transplant and five survive; eight did not receive a second transplant: three had autologous recovery and are alive and well, one is waiting for a second transplant, and four died. Overall, of the 17 patients, nine are long-term survivors. The actuarial survival of patients with and without graft failure is 53% and 80%, respectively (P<0.001). As regards predictors of graft failure, patients in whom the interval between diagnosis and transplantation was more than 2 years tended to have a higher risk of graft failure (22%) than those who received their transplant within 1 year (12%) or between 1–2 years after diagnosis (14%) (P=0.3). We could not identify any other clinical data discriminating patients who rejected their grafts from patients in whom engraftment was successful. The median number of infused nucleated cells was 4.5×108/kg versus 4.24×108/kg for patients with or without sustained engraftment. Graft failure was seen in one of the ten (10%) peripheral blood transplants, 15/88 (17%) bone marrow transplants and one of the two cord blood grafts.
Table 2.
Engraftment data.
Chimerism data
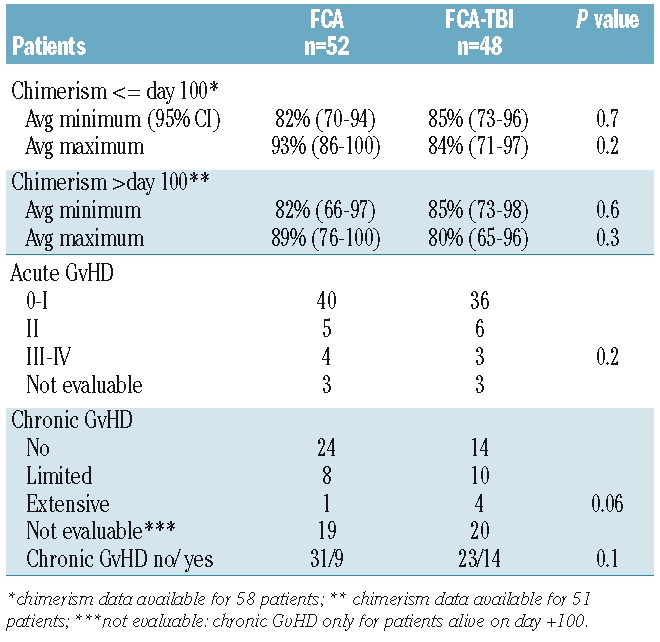
Table 3 outlines the average minimum and maximum donor chimerism less than and more than 100 days after transplantation (with 95% confidence intervals), in the 58 patients for whom chimerism data are available. Within 100 days after bone marrow transplantation, the average minimum donor chimerism was 82% and 85% in the FCA and FCA-TBI groups, respectively, whereas the corresponding average maximum was 93% and 84%, respectively. Beyond day +100, the average minimum chimerism was 82% and 85% in the FCA and FCA-TBI groups, respectively, and the average maximum was 89% and 80%, respectively.
Table 3.
Chimerism data and graft-versus-host disease.
Graft-versus-host disease
The overall cumulative incidence of acute GvHD grades II–IV and III–IV was 18% and 7% respectively, with no difference between the two regimens (Table 3). Chronic GvHD was recorded in 27% of the FCA group and 50% of the FCA-TBI group (P=0.06). Extensive chronic GvHD was recorded in one patient conditioned with the FCA regimen and in four patients given the FCA-TBI conditioning regimen. GvHD was the primary cause of death in two patients in each group.
Survival
The actuarial 5-year overall survival for all patients is 75%, being 73% and 79% for the FCA and FCA-TBI groups, respectively (Figure 1).
Figure 1.
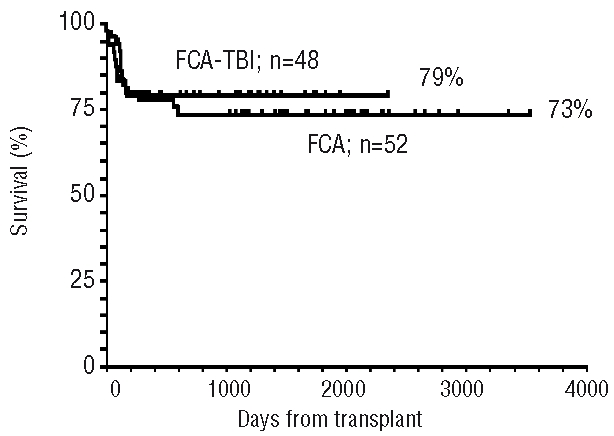
Actuarial survival of 52 SAA patients receiving FCA and 48 SAA patients receiving FCA + 2Gy TBI.
Age
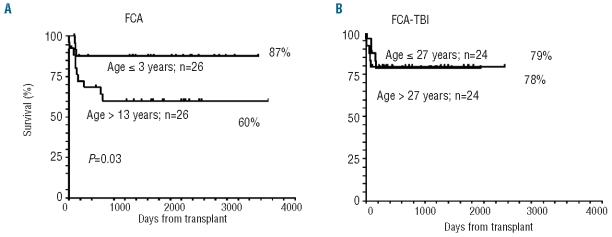
There was a strong effect of age on overall survival in the FCA group, with the 5-year overall survival being 87% among 26 children 13 years of age (median) or younger, compared with 60% among the 26 patients over the age of 13 (P=0.03) (Figure 2A). In contrast, there was no age effect in patients receiving FCA-TBI divided into two groups according to the median age of 27 years. The overall survival rate was 79% in those older than the median and 78% in those younger (Figure 2B).
Figure 2.
(A) Effect of age in patients receiving FCA (left panel), stratified by the median age of 13 years. A significant disadvantage can be seen for patients greater than 13 years of age. (B) The right panel shows no age effect in patients receiving FCA-TBI, stratified by the median age of 27 years.
Interval between diagnosis and transplantation
There was an overall strong influence of the interval between diagnosis and transplantation: patients grafted within 1 year of diagnosis (n=32), between 1–2 years after diagnosis (n=27) or more than 2 years of diagnosis (n=37) had actuarial survival rates of 87%, 86%, and 55%, respectively. The actuarial survival rates of patients divided according to whether their transplant was performed less than or more than 2 years after diagnosis are 87% and 55%, respectively (P=0.0004) (Figure 3). This favorable effect of early transplantation was seen in both the FCA and FCA-TBI groups.
Figure 3.
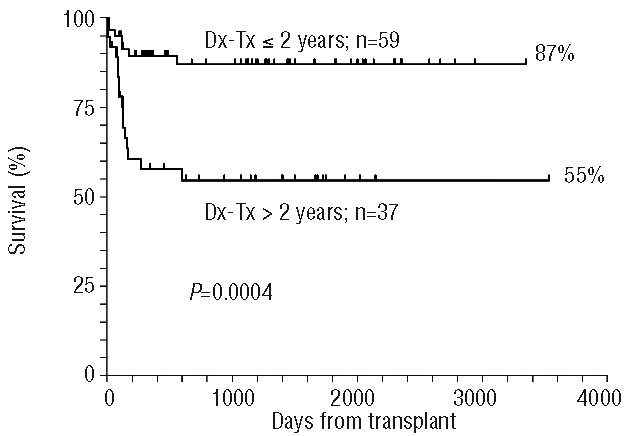
Actuarial survival of patients grafted within 2 years of diagnosis or later: the difference is highly significant (P=0.0004). The diagnosis-transplantation (Dx-Tx) interval is not known for four patients.
HLA matching
Data on HLA matching were available for 88 patients. Patients transplanted from an HLA disparate family donor (n=13) were excluded from this analysis. We studied the outcomes of transplants from 75 unrelated donor: 46 were classified as HLA-matched (reported as 8/8 or 10/10 allele-matched) and 29 were mismatched, the donor and recipient having a mismatch for one or more allele. The crude mortality of HLA-matched and HLA-mismatched transplant recipients was 17% versus 34%, respectively (P=0.1). However, the difference in mortality was mainly seen in the group of patients conditioned with the FCA regimen (n= 34) (15% versus 40%, P=0.07) rather than in the FCA-TBI group (n=41) (22% versus 19%, P=0.7). Interestingly patients grafted within 2 years of diagnosis (n=45) had low mortality, whether HLA-matched or HLA-mismatched (10% matched versus 7% mismatched, P=0.7), whereas patients grafted more than 2 years after diagnosis (n=30) had a higher mortality, and more so when HLA-mismatched (33% versus 53%, P=0.2). The crude mortality of the 12 patients for whom data on HLA matching were not available was 16%.
Donor type
The actuarial 5-year survival is 77% for 87 patients grafted from unrelated donors and 66% for patients grafted from HLA-disparate family donors (P=0.5). When stratified according to conditioning regimen, the crude mortality in the FCA group for recipients of grafts from unrelated donors (n=46) or family mismatched donors (n=6) was 21% versus 50% (P=0.1), whereas in the FCA-TBI group, mortality was 22% among unrelated graft recipients (n=41) and 14% among those who were grafted from family mismatched donors (n=7) (P=0.6).
Stem cell source
The majority of patients received stem cells derived from bone marrow (n=88), and their mortality is 22%; the mortality rate among the patients receiving peripheral blood stem cells (n=10) is 30%, while one of the two cord blood recipients has died.
Year of transplant
The median year of transplant was 2004, and the actuarial survival of patients grafted before or in 2004 is 68%, while it is 83% among those grafted after 2004 (P=0.06), suggesting a trend for improvement over time (Figure 4). In the most recent period (after 2004) the actuarial 5-year survival for patients grafted within 2 years of diagnosis (n=24; median age, 23 years; range, 6–53 years) is 92%.
Figure 4.
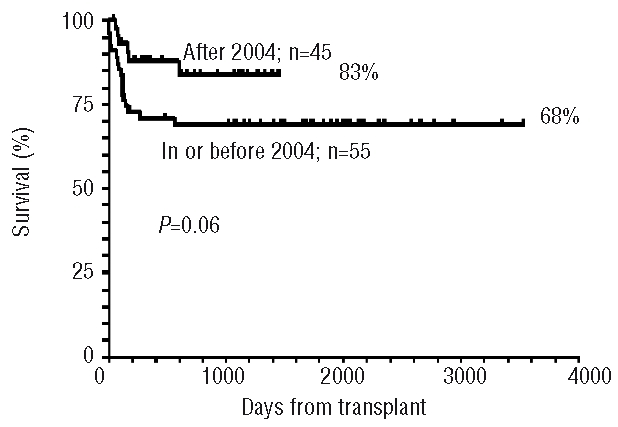
The effect of transplant era: patients are stratified by the median year of transplant (2004): a trend for improved survival is seen for transplants performed after 2004.
Multivariate analysis
There were two predictors of survival in multivariate analysis: (i) the interval between diagnosis and transplantation (P=0.001), with a relative risk (RR) of 4.4 for patients grafted more than 2 years after diagnosis as compared to patients grafted within 2 years, and (ii) transplantation after 2004 (P=0.056, RR 0.33). The multivariate analysis showed no effect of HLA match, age, or conditioning regimen.
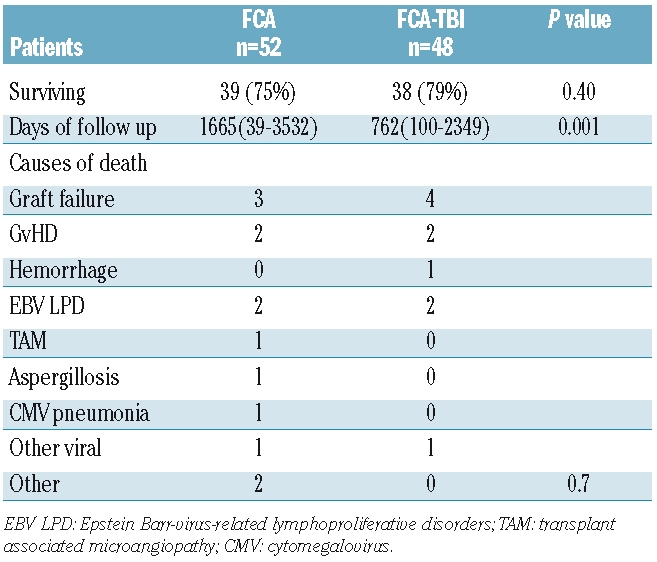
Causes of death
Twenty-three patients died, 13/52 in the FCA group and 10/48 in the FCA-TBI group (Table 4). The primary cause of death was rejection in seven patients, GvHD in four patients and Epstein-Barr virus lymphoproliferative disease in four other patients. The median interval between transplantation and death was 127 days for the FCA group (range, 6–601 days) and 80 days for the FCA-TBI group (range, 7–176 days) (P=0.6).
Table 4.
Outcome and causes of death.
Discussion
We have shown in this study that patients with severe aplastic anemia grafted from alternative donors, following a conditioning regimen of FCA, with or without low-dose TBI, have an encouraging 5-year survival of 75%. The FCA regimen is associated with a good outcome in young children grafted from matched donors, whereas the FCA-TBI extends the benefit of transplantation also to adults, with equally good results. There is a strong negative effect when the transplant is delayed beyond 2 years from diagnosis.
As regards the first result, an overall 5-year actuarial survival rate of 75% brings the outcome of alternative donor transplants almost to the level of that of HLA identical sibling transplants. Although this is a Registry-based study, it is also a multi-institutional one, suggesting that this outcome can be achieved in several different Centers. A Japanese study on 154 patients, with a similar median age (17 years), recorded an overall actuarial 5-year survival of 56%.3 This inferior outcome may be due to the period of transplantation of the patients in the Japanese study (1993–2000), which was almost a decade earlier than that in the present study; more recent transplants are associated with better donor/recipient matching and better supportive care.17 A second reason for the difference may be related to the introduction of fludarabine combined with cyclophosphamide and antithymocyte globulin. This FCA combination has been widely used in recent years as a conditioning regimen for patients with acquired aplastic anemia, contributing to excellent survival in both HLA-identical sibling and alternative donor transplants:8–14 interestingly the studies showing these good results were performed in a variety of countries worldwide. In keeping with these results, the EBMT has recently shown that FCA may be preferable to the conventional high dose cyclophosphamide (200 mg/kg) in patients with severe aplastic anemia above the age of 30 years receiving an HLA-identical graft.15 Therefore, in transplant settings other than young patients with an HLA-identical donor, the FCA combination is a valid option and is being adopted in many Centers worldwide.
In this report we have analyzed patients receiving FCA with or without 2 Gy TBI: the overall survival was quite comparable for the two regimens, though significant differences were found following more detailed analysis of subgroups. The median age of the FCA group was 13 years, whereas it was significantly older (27 years) for the FCA-TBI group. When looking for an age effect, stratifying patients according the median age of the group, this was rather strong in the FCA group and not significant in the FCA-TBI group. The same can be said for donor/recipient matching: the effect of better matching was evident in the FCA group (15% versus 40%, for matched versus mismatched, P=0.07), but not in the FCA-TBI group (22% versus 19%, P=0.7). Therefore, the FCA conditioning regimen seems suitable for very young patients with well-matched donors; in other settings the addition of TBI 2 Gy to the FCA regimen seems to offer a better chance of cure, in keeping with results of other recent studies.9,10
The most relevant predictor of outcome was the interval between diagnosis and transplantation, with the relative risk of death being 4.4 if the transplant was delayed beyond 2 years: this was due to more graft failure and more GvHD in patients grafted more than 2 years after diagnosis. All of the seven rejection-related deaths and three of the four GvHD-related deaths were in patients grafted more than 2 years after their diagnosis of severe aplastic anemia. The effect of delaying the transplant was evident in both the FCA and FCA-TBI groups and in young and older patients. A similar detrimental effect of delayed transplantation was seen in a recent study,4 with a relative risk of 2.9 for children grafted more than 4 years after diagnosis.
For patients grafted within 2 years of diagnosis, the overall actuarial 5-year survival rate is 87%, being 92% among those patients (n=24) grafted after 2004: these results could change our current strategies of treatment of severe aplastic anemia. One could ask whether this is also true for adults above the age of 30 or 40: the crude survival rates for patients receiving FCA-TBI conditioning and transplanted within 2 years of diagnosis in the age groups 0–20 (n=7), 21–30 (n=12), 31–40 (n=6), and 41–53 (n=4) are 85%, 100%, 83%, and 75%, respectively; these numbers of patients are small, but the results are consistent in the various age groups. Older age was predictive of outcome in patients receiving FCA, but not in patients receiving FCA-TBI.
Problems still remain: graft failure/rejection was seen in 17% of patients of the whole group, and was the first cause of death (7%): this is in keeping with the results of another EBMT study, suggesting a persistent risk of graft failure above 10%,17 and indeed some patients had mixed chimerism in both groups. One possible way to overcome graft failure would be to increase the dose of cyclophosphamide from the current 1200 mg/m2 to the more conventional 120 mg/kg. Increasing the dose of TBI may not be advisable, especially because aplastic anemia is not a malignant disorder, and radiation is known to cause infertility and to increase the risk of secondary tumors.16 It should be noted that these reported effects refer to total lymphoid, thoracoabdominal or total body radiation in doses ranging from 6 to 10 Gy:16 a dose of 2 Gy, as currently given in the conditioning regimen for transplantation in patients with severe aplastic anemia, may have different effects although this remains to be demonstrated with longer follow-up. With the current dose of 2 Gy TBI, we have not seen – as yet – any cases of second tumors in these 100 patients. The use of peripheral blood to overcome graft failure may not be the best choice because of the higher risk of chronic GvHD with this source of stem cells.18
A second problem is Epstein-Barr virus-associated lymphoproliferative disorders: four patients died of this complication. Some studies have suggested that prophylactic rituximab, given on day +5 after transplantation, may considerably reduce the risk of Epstein-Barr virus-associated lymphoproliferative disorders,19 and some Centers have, therefore, introduced prophylactic rituximab on day +5 into the management of patients with aplastic anemia undergoing an unrelated donor transplant. The use of rituximab may also lower the risk of GvHD,20 thus allowing for a reduction in the dose of antithymocyte globulin.
Another way to reduce infections may be to reduce the total dose of antithymocyte globulin administered, especially since the incidence of acute GvHD grades II–IV was low in our cohort (18%) compared with the over 40% in other studies.17 Chronic GvHD was more frequent, as expected, when TBI 2 Gy was added to FCA (50% versus 27%): this was particularly true when peripheral blood was used as a source of stem cells. Indeed, the risk of extensive chronic GvHD was 3% for bone marrow recipients and 20% for peripheral blood recipients, in keeping with the findings of the EBMT in a study of HLA-identical sibling transplants.18 For this reason the use of peripheral blood should be discouraged whether the donor is a sibling or unrelated.
In conclusion, alternative donor transplants for patients with acquired aplastic anemia can now be considered a safe procedure with an acceptable risk of mortality, especially if the transplant is performed within the first 2 years of diagnosis. Further refinement of the transplant protocol aimed at reducing major complications, such as graft failure and infections, may further improve current results.
Appendix
Centers reporting patients to the EBMT registry and selected for this study with the number of patients contributed in brackets. CIC 202 Basel (6); CIC 207 Paris St. Louis (13); CIC 217 Genova S Martino (21); CIC 232 Roma Sapienza (1); CIC 248 Pescara (1); CIC 252Creteil (4); CIC 265 Milano Universita’ (1); CIC 274 Genova Gaslini (9); CIC 285 Padova Pediatria (3); CIC 286 Pavia Ematologia (1); CIC 287 Roma S Camillo (5); CIC 289 Gotenburg (3) CIC 304 Firenze Careggi (2); CIC 305 Torino Pediatria (1); CIC 539 London St. George (3); CIC 557 Pavia Pediatria (7); CIC 590 Berlin Free University (1); CIC 658 Bergamo Ematologia (2); CIC 754 Tel Hashomer (4); CIC 766 Napoli (1); CIC 790 Bologna Pediatria (8); Minnesota (1).
Footnotes
Funding: this work was partly supported by the European Group for Blood and Marrow Transplantation (EBMT), Fondazione CARIGE Genova, and Associazione Italiana Ricerca contro il Cancro (A.I.R.C.) Milano.
Authorship and Disclosures
All authors contributed equally to writing this manuscript. The authors reported no potential conflicts of interest.
References
- 1.Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K, et al. French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92(5):589–96. doi: 10.3324/haematol.10899. [DOI] [PubMed] [Google Scholar]
- 2.Deeg HJ, Amylon ID, Harris RE, Collins R, Beatty PG, Feig S, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7(4):208–15. doi: 10.1053/bbmt.2001.v7.pm11349807. [DOI] [PubMed] [Google Scholar]
- 3.Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A, et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood. 2002;100(3):799–803. doi: 10.1182/blood.v100.3.799. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Albuerne ED, Eapen M, Klein J, Gross TJ, Lipton JM, Baker KS, et al. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. Br J Haematol. 2008;141(2):216–23. doi: 10.1111/j.1365-2141.2008.07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storb R, Leisenring W, Anasetti C, Appelbaum FR, Buckner CD, Bensinger WI, et al. Long-term follow-up of allogeneic marrow transplants in patients with aplastic anemia conditioned by cyclophosphamide combined with antitymocyte globulin. Blood. 1997;89(10):3890–1. [PubMed] [Google Scholar]
- 6.McCann SR, Bacigalupo A, Gluckman E, Hinterberger W, Hows J, Ljungman P, et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant. 1994;13(3):233–7. [PubMed] [Google Scholar]
- 7.Lawler M, McCann SR, Marsh JC, Ljungman P, Hows J, Vandenberghe E, et al. Severe Aplastic Anaemia Working Party of the European Blood and Marrow Transplant Group. Serial chimerism analyses indicate that mixed haemopoietic chimerism influences the probability of graft rejection and disease recurrence following allogeneic stem cell transplantation (SCT) for severe aplastic anaemia (SAA): indication for routine assessment of chimerism post SCT for SAA. Br J Haematol. 2009;144(6):933–45. doi: 10.1111/j.1365-2141.2008.07533.x. [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socié G, Maury S, et al. Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36(11):947–50. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 9.Okuda S, Terasako K, Oshima K, Sato M, Nakasone H, Kako S, et al. Fludarabine, cyclophosphamide, anti-thymocyteglobulin, and low-dose total body irradiation conditioning enables 1-HLA-locus-mismatched hematopoietic stem cell transplantation for very severe aplastic anemia without affecting ovarian function. Am J Hematol. 2009;84(3):167–9. doi: 10.1002/ajh.21355. [DOI] [PubMed] [Google Scholar]
- 10.Koh LP, Koh MB, Ng HY, Hwang WY, Goh YT, Linn YC, et al. Allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia following non-myeloablative conditioning using 200-cGy total body irradiation and fludarabine. Biol Blood Marrow Transplant. 2006;12(8):887–90. doi: 10.1016/j.bbmt.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Prem S, Mahapatra M, Seth T, Chowdhary DR, Mishra P, et al. Fludarabine, cyclophosphamide and horse antithymocyte globulin conditioning regimen for allogeneic peripheral blood stem cell transplantation performed in non-HEPA filter rooms for multiply transfused patients with severe aplastic anemia. Bone Marrow Transplant. 2006;37(8):745–9. doi: 10.1038/sj.bmt.1705321. [DOI] [PubMed] [Google Scholar]
- 12.George B, Methews V, Viswabandya A, Kavitha ML, Srivastava A, Chandy M. Fludarabine and cyclophosphamide based reduced intensity conditioning (RIC) regimens reduce rejection and improve outcome in Indian patients undergoing allogeneic stem cell transplantation for severe aplastic anemia. Bone Marrow Transplant. 2007;40(1):13–8. doi: 10.1038/sj.bmt.1705669. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133 (3):305. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 14.Resnick IB, Aker M, Shapira MY, Tsirigotis PD, Bitan M, Abdul-Hai A, et al. Allogeneic stem cell transplantation for severe acquired aplastic anaemia using a fludarabine-based preparative regimen. Br J Haematol. 2006;133 (6):649. doi: 10.1111/j.1365-2141.2006.06084.x. [DOI] [PubMed] [Google Scholar]
- 15.Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socié G, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94(9):1312–5. doi: 10.3324/haematol.2009.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierga JY, Socie G, Gluckman E, Devergie A, Henry-Amar M, Bridier A, et al. Secondary solid malignant tumors occurring after bone marrow transplantation for severe aplastic anemia given thoraco-abdominal irradiation. Radiother Oncol. 1994;30(1):55–8. doi: 10.1016/0167-8140(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 17.Viollier R, Socié G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41(1):45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 18.Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110(4):1397–400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacigalupo A, Dominietto A, Soracco M, Raiola AM, van Lint MT, Lamparelli T, et al. Rituximab prophylaxis of EBV reactivation after alternative donor transplants following anti-thymocyte globulin-based conditioning regimens. Blood. 2008;112 abstr 2232. [Google Scholar]
- 20.van Dorp S, Pietersma Fì, Wölfl M, Verdonck LF, Petersen EJ, Lokhorst HM, et al. Rituximab treatment before reduced-intensity conditioning transplantation associates with a decreased incidence of extensive chronic GVHD. Biol Blood Marrow Transplant. 2009;15(6):671–8. doi: 10.1016/j.bbmt.2009.02.005. [DOI] [PubMed] [Google Scholar]