Abstract
The actin cytoskeleton regulates exocytosis in all secretory cells. In neutrophils, Rac2 GTPase has been shown to control primary (azurophilic) granule exocytosis. Here, we propose that Rac2 is required for actin cytoskeletal remodeling to promote primary granule exocytosis. Treatment of neutrophils with low doses (≤ 10 μM) of the actin depolymerizing drugs, latrunculin B (Lat B) or cytochalasin B (CB), enhanced both formyl peptide receptor and Ca2+ ionophore stimulated exocytosis. Higher concentrations of CB or Lat B, or stabilization of F-actin with jasplakinolide (JP) inhibited primary granule exocytosis measured as myeloperoxidase release, but did not affect secondary granule exocytosis determined by lactoferrin release. These results suggest an obligatory role for F-actin disassembly prior to primary granule exocytosis. However, lysates from secretagogue-stimulated neutrophils showed enhanced actin polymerization activity in vitro. Microscopic analysis showed that resting neutrophils contain significant cortical F-actin which was redistributed to sites of primary granule translocation when stimulated. Exocytosis and actin remodelling was highly polarized when cells were primed with CB, however, polarization was reduced by Lat B preincubation, and both polarization and exocytosis was blocked when F-actin was stabilized with JP. Treatment of cells with the small molecule Rac inhibitor, NSC23766, also inhibited actin remodelling and primary granule exocytosis induced by Lat B/fMLF or CB/fMLF, but not Ca2+ ionophore. Therefore, we propose a role for F-actin depolymerization at the cell cortex coupled with Rac-dependent F-actin polymerization in the cell cytoplasm to promote primary granule exocytosis.
Keywords: Rac GTPase, actin, latrunculin, cytochalasin, jasplakinolide, NSC23766
Human neutrophils are prominent white blood cells that play an important role in defense against microbial infection. Intense activation by bacterial or inflammatory stimuli trigger neutrophil degranulation, releasing a range of antibacterial factors which can exert collateral tissue damage. Degranulation involves granule translocation to the plasma membrane followed by exocytosis, a tightly regulated process (33). Excessive degranulation from neutrophils is a central feature in numerous inflammatory disorders such as severe asthma, emphysema, and rheumatoid arthritis (43).
Neutrophils contain four different granule subtypes: primary (azurophilic), secondary (specific)and tertiary (gelatinase) granules, as well as secretory vesicles. The signaling pathways that control exocytosis of granules appear to be unique to each subtype. Granules are released in a hierarchical fashion in response to secretagogues by first releasing secretory vesicles, followed by tertiary, secondary, and finally primary granules (42). Granule subtypes contain overlapping as well as unique luminal contents. Primary granules contain some of the most potent cytolytic enzymes that aid in digestion of pathogens, such as elastase and myeloperoxidase (MPO), which also significantly contribute to host tissue damage (23, 26), which emphasizes the importance of studying the regulation of their exocytosis mechanism.
Rab and Rho GTPases have been shown to regulate exocytosis in a variety of secretory cells including mast cells (5, 29, 39, 40), cytotoxic T lymphocytes (3, 20, 46) and eosinophils (31, 35). Recent studies have defined the need for these two classes of small monomeric GTPases in regulating exocytosis of primary granules in neutrophils. Both Rab27a- and Rac2-deficient mice show impaired secretion of the primary granule enzyme MPO (1, 37). Rab27a, via its effector protein JFC1/Slp1, may act to discriminate between primary granules destined for exocytosis from those which preferentially fuse intracellularly with phagosomes (37). The precise role that Rac2 plays in exocytosis remains unclear. In many cell types, Rho GTPases such as Rac are known to be key regulators of cytoskeletal remodeling. This was demonstrated in their ability to activate cytoskeletal remodeling and contribute to cell motility and chemotaxis (39, 41). Importantly, neutrophils deficient in Rac2 have defects in filamentous (F-) actin assembly which prevents cell migration, as distinct from Rac1 deficiency leading to inhibition of cell spreading (14, 19). Rac2 is also the predominant Rac protein expressed in neutrophils, and is the main GTPase required for activation of the superoxide-generating NADPH oxidase complex (14, 22).
Myeloid cells contain an F-actin-rich cortical region that is proposed to act as a barrier against granule docking and fusion at the plasma membrane. A proteomic analysis of neutrophil granule subtypes revealed that actin associates with all granule populations (28). Indeed, exocytosis of all granules is enhanced by pre-incubation of neutrophils with the actin depolymerization drug, cytochalasin B, which favors the actin-barrier hypothesis (7, 28). However, other studies have shown that actin depolymerization inhibits exocytosis, suggesting that F-actin formation instead facilitates exocytosis (13, 16, 27, 34, 36). This is plausible since neutrophil chemotaxis is triggered by polarized F-actin assembly, which could similarly drive polarized mobilization of granules on this actin network (18, 39). Therefore, a role for both actin depolymerization and polymerization during exocytosis is feasible.
In this study, we propose that Rac-mediated actin polymerization is necessary for directing granules in the cell cytoplasm to the plasma membrane, while actin depolymerization must occur concurrently at the cell cortex to allow exocytosis. We found that drugs which promote actin depolymerization stimulated receptor-mediated exocytosis at low dosage. Stabilization of F-actin specifically inhibited primary granule exocytosis, but had little effect on secondary granule exocytosis. Microscopic analyses of neutrophils stimulated with the actin drug, cytochalasin B together with f-Met-Leu-Phe, showed that cortical actin was remodelled to a polarized state with primary granule marker co-localization. The actin drugs, latrunculin and jasplakinolide affected both granule distribution and the polarization of actin remodelling, however, only jasplakinolide blocked exocytosis. A recently discovered small molecule inhibitor of Rac (15) was also found to block actin remodelling and primary granule exocytosis. Surface exposure of the primary granule membrane marker CD63 was used to further quantify the effects of actin and Rac-directed drugs. Our findings suggest that Rac-mediated F-actin formation is necessary for primary granule movement to the cell membrane, while concurrent actin depolymerization at the cell cortex stimulates granule exocytosis in general.
METHODS
Isolation of human peripheral blood neutrophils
Human polymorphonuclear neutrophils were isolated from healthy donors in accordance with the University of Alberta Health Research Ethics Board. 50–100 ml of whole blood was drawn from donors, mixed with 6% dextran in RPMI 1640 (Invitrogen, Burlington, ON), and incubated at room temperature for 30 min to allow sedimentation of red blood cells. The upper leukocyte-rich phase was layered onto 15 ml of 5.7% Ficoll and centrifuged at room temperature at 400g for 30 min to separate leukocytes and monocytes from granulocytes. The granulocyte pellet was exposed to 1.5 ml of sterile deionized water for 20 s to lyse any remaining red blood cells and then quickly placed into excess buffer A (RPMI-1640 and 5 mM EDTA) and centrifuged at room temperature at 300g for 5 min. Following centrifugation, the cell pellet was resuspended in buffer B (RPMI-1640, 5 mM EDTA and 2% FBS). Cells were then allowed to rest on ice for 1 h before experiments.
Secretion assays
Secretion assays were performed by analyzing levels of granule proteins secreted into cell supernatants and by analyzing surface expression of CD63 via flow cytometry (1, 28). Resting cells were resuspended at 1 × 106 cells/ml in phenol red–free RPMI 1640. For biochemical analysis of granule marker exocytosis, 50 μl of cell suspension was added to each well of a 96 v-well plate containing actin drugs and stimuli in RPMI 1640 to a final volume of 250 μl Following stimulation, microplates were centrifuged at 300g at 4°C for 6 min, and the levels of myeloperoxidase (MPO) and lactoferrin (LTF) in supernatants were determined as a measurement of primary and secondary granule exocytosis, respectively. MPO was assayed using tetramethylbenzidine (TMB) and LTF by quantitative immunoblot analysis. For flow cytometry analysis 1 ml of cell suspension was aliquoted into microfuge tubes containing actin drugs and stimuli in RPMI. After stimulation cells were pelleted, fixed in 5% formalin, blocked in PBS containing 5% non-fat milk, and stained with FITC conjugated anti-CD63 (Serotec, Raleigh, NC). Cytochalasin B (CB; destabilizes F-actin; Sigma-Aldrich, Mississauga, ON), latrunculin B (Lat B; destabilizes F-actin; Calbiochem, San Diego, CA), jasplakinolide (JP; stabilizes F-actin; Calbiochem), and the small molecule Rac inhibitor NSC23766 (Calbiochem) were prepared as 10 mM stock solutions in DMSO and diluted before use. Neutrophils were pre-treated with these drugs for 5 – 15 min at 37°C prior to stimulation with 2.5 μM Ca2+ ionophore (A23187) or 5 μM f-Met-Leu-Phe (fMLF) (Sigma-Aldrich) for 15 min at 37°C to induce degranulation. In some cases, neutrophils were preincubated with 10 μM CB for 5 min prior to addition of 5 μM fMLF for 15 min (CB/fMLF--typical secretagogue). Cells showed > 95% viability as determined by trypan blue exclusion at the end of all incubations.
Rac activation assays
Activated (GTP-bound) Rac1 and Rac2 were affinity precipitated from neutrophil lysates using GST-PBD (2). Lysates were prepared from 8 × 106 cells by sonication in 400 μl of H-buffer (20 mM HEPES-KOH, pH 7.5, 1 mM DTT, 5 mM MgCl2, 60 mM NaCl, 1% Triton X-100 + protease inhibitor cocktail (PIC): 1 μg/ml leupeptin, pepstatin, antipain and aprotinin each, 1 mM phenylmethylsulfonyl fluoride). Cell debris was removed by centrifugation and 300 μg of lysate was incubated with 30 μg of immobilized GST-PBD in 400 μl H-buffer for 30 min at 4°C. The beads were recovered, washed four times in H-buffer and resuspended in 45 μl of Laemmli sample buffer. 15 μl of each sample was analyzed by immunoblot using antibodies specific for Rac1 (ARC03; Cytoskeleton Inc., Denver, CO) or Rac2 (07-604; Upstate, Waltham, MA). Immuno-reactive bands were detected using IRDye800 secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA) and an Odyssey image analysis system (LiCor, Seattle, WA).
Measurement of O2− release from neutrophils
Generation of extracellular O2− from cells in suspension was measured as previously described (32). Briefly, cells (1–2 × 106) were suspended in 1-ml microcuvettes containing supplemented PBS (PBS+; PBS, pH 7.4, with 1.2 mM MgCl2, 5 mM KCl, 0.5 mM CaCl2, 5 mM glucose, and 0.1% BSA) and 50 μM ferricytochrome c at 25°C. The mixture was blanked at 550 nm in a Beckman DU 640 spectrophotometer (Beckman Instruments, Mississauga, ON) before adding 16 nM PMA (Calbiochem) or 5 μM fMLF. To test the effects of NSC23766 on O2− production, 160 μM of NSC23766 was added to 2 × 107 cells/ml in RPMI 1640 and incubated at 37°C for 15 min before treatment with PMA or fMLF.
Confocal microscopy
Stimulated cells were fixed in freshly prepared 2% paraformaldehyde in 0.25 M sucrose/PBS while still in suspension. Fixed cells were adhered to poly-L-lysine-coated glass slides, permeabilized with 0.5 % Triton X-100 in PBS and stained with 10 μg/ml anti-CD63 (Serotec) conjugated to Alexa Fluor 488 (Invitrogen) to detect primary granules and 0.3 μM rhodamine-phalloidin (Invitrogen) to detect F-actin. Images were acquired on an Olympus FV1000 confocal laser scanning microscope (Olympus Canada, Markham, ON), with a 63X/1.4 N.A. plan apochromat objective and processed using Olympus Fluoview software.
Electron microscopy
Stimulated cells were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate, pH 7.2 at 4°C and stained with DAB solution for 5 min (2.5 mM diaminobenzidine and 0.02% hydrogen peroxide in 0.1 M cacodylate, pH 7.2) to enhance the electron density of peroxidase-containing vesicles. Cell pellets were washed three times in 0.1 M cacodylate, pH 7.2, embedded in 1% ultrapure agarose, and post-fixed in 0.2% aqueous osmium tetroxide for 1 h at 4°C. Samples were embedded in Epon resin after serial dehydration; ultrathin sections were cut and mounted on copper coated grids and post-stained with saturated uranyl acetate and lead citrate. Sections were viewed on a Philips 410 transmission electron microscope (Philips Electron Optics, Eindhoven, Holland) and images were acquired using an SIS Megaview III CCD digital camera and AnalySIS and software (Olympus).
Actin polymerization assay
To assay cellular activity inducing actin polymerization, we used an established pyrene-actin polymerization assay (9, 25). Briefly, 12 μg neutrophil lysate in 70 μl lysis buffer (5 mM Tris-Cl, pH 8, 50 mM KCl, 0.2 mM CaCl2, 0.2 mM ATP, 0.17% NP-40, 0.35 mM MgCl2 + PIC) was mixed with 50 μl of actin polymerization stock mixture containing 35% pyrene-labeled actin (12 μM) in G-buffer (5 mM Tris-Cl, pH 8, 0.2 mM CaCl2, 0.2 mM ATP) (Cytoskeleton Inc.). Fluorescence intensity readings were taken every 18 s using a QM-4SE spectrofluorometer (Ex 360 nm/Em 407 nm, 10 nm bandwidth, 2 s integration), with a four-position heated sample holder set to 30°C (Photon Technologies Inc., London, ON). The pyrene-actin stock mixture was equilibrated for 10 min, then test samples were added and fluorescence measurements taken for 30 min. Actin polymerization activity (A.P.A.) was calculated from polymerization curves by determining the average rate of fluorescence intensity increase for 30 min of reaction time, divided by the number of micrograms of test sample protein (ΔFI/μg). A. P. A. values were normalized to untreated resting cells for each experiment with the A.P.A. of lysis buffer subtracted.
Calculations and statistical analysis
Data was analyzed by one-way statistical analysis of variance (ANOVA) and post-hoc analysis was determined by Tukey’s post test. The data are depicted in figures as means plus or minus the standard error of the mean (± SEM).
RESULTS
Effect of actin drugs on primary granule exocytosis
Bacterially-derived N-formyl methionyl derivatives (fMLF) are potent stimuli for neutrophil activation. Isolated neutrophils can degranulate in response to fMLF stimulation in vitro, with modest secretion of most granule types except for primary granules which fail to undergo exocytosis (1). However, primary granule exocytosis may be induced in vitro by “priming” via pre-treatment with the actin depolymerizing drug CB. This drug blocks the barbed ends of actin filaments leading to cortical actin meshwork disassembly, and enhancing fMLF-induced primary granule release (21, 44). This well-known effect of CB in neutrophils suggests that F-actin depolymerization from the cell cortex is specifically required for primary granule exocytosis.
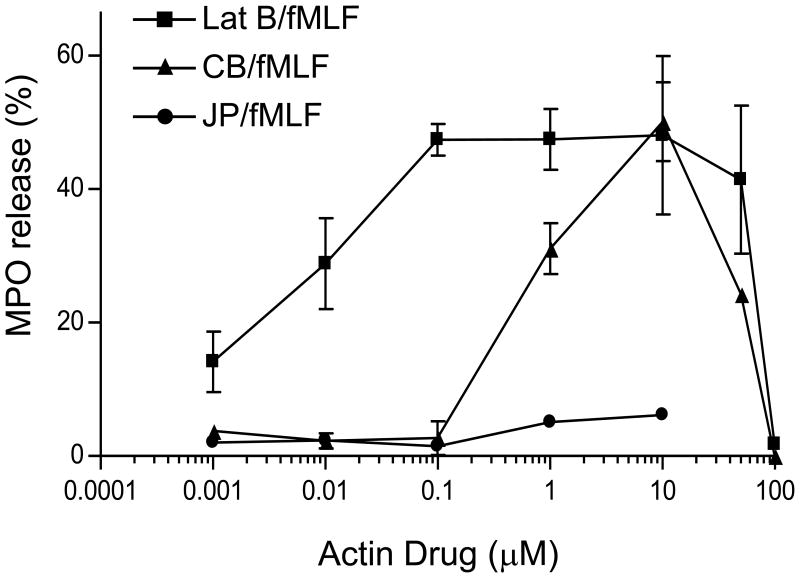
To explore the role of actin remodeling in the regulation of human neutrophil primary granule exocytosis, we tested several actin drugs alone or in combination with fMLF-stimulation. Incubation of neutrophils with the actin depolymerizing drugs CB or latrunculin B (Lat B) alone did not stimulate primary granule exocytosis, as measured by MPO secretion, a luminal marker of primary granules (data not shown). However, both CB and Lat B induced a dose-dependent increase in MPO secretion in response to fMLF at concentrations ≤ 10 μM, while higher doses inhibited primary granule exocytosis (Fig. 1). Lat B, which is more specific for actin than CB, and promotes actin depolymerization by sequestering actin monomers (45), enhanced fMLF-induced secretion at doses 100-fold lower than CB. Jasplakinolide (JP), which binds to and stabilizes F-actin (6), did not stimulate exocytosis in response to fMLF (Fig. 1). These results suggest that partial actin depolymerization is needed to promote exocytosis, while complete F-actin depolymerization is inhibitory.
Figure 1.
Effects of actin drugs on fMLF-stimulated primary granule exocytosis. Neutrophils were preincubated with increasing concentrations of either CB, Lat B, or JP for 5 min followed by stimulation with 5 μM fMLF for 15 min at 37°C. Extracellular supernatants were collected and assayed for MPO activity. Released MPO was calculated as a percentage of total activity (± SEM) from at least 3 independent experiments (except n = 1 for JP). p < 0.01 for all repeated experiments compared to fMLF alone.
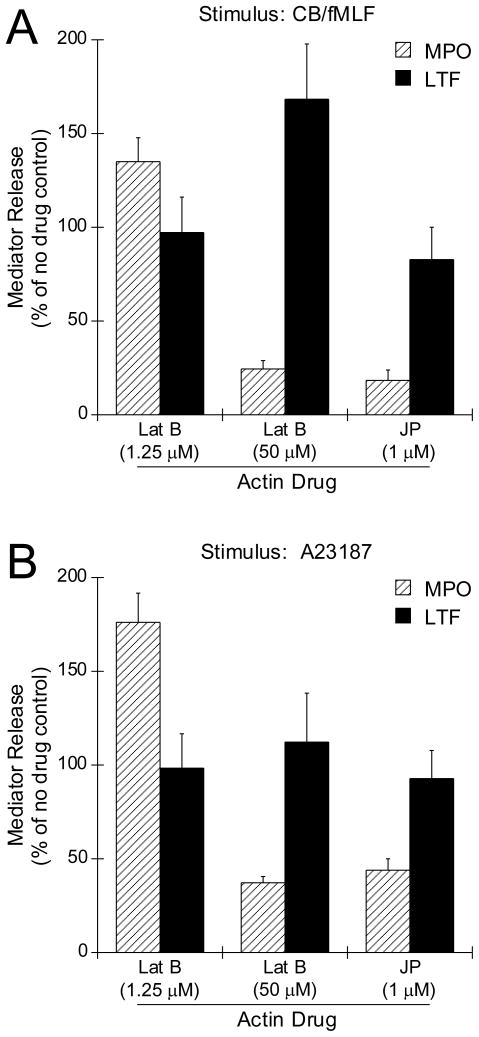
We next determined whether JP affected granule exocytosis when neutrophils were stimulated with the typical secretagogues CB/fMLF and A23187 (Fig. 2, A and B, respectively). For both conditions, JP inhibited primary granule exocytosis (Fig. 2, MPO), but had little effect on secondary granule exocytosis (Fig. 2, LTF). This confirms an obligatory requirement for actin depolymerization in initiating exocytosis specifically for primary granules.
Figure 2.
Effects of actin drugs on CB/fMLF- and A23187-induced primary and secondary granule exocytosis. Neutrophils were preincubated 1.25 μM Lat B, 50 μM Lat B or 1 μM JP for 5 min followed by stimulation with 10 μM CB/5 μM fMLF (A) or 2.5 μM A23187 (B) for 15 min at 37°C. Supernatants were collected from each condition and assayed for MPO or LTF. Enzyme released was calculated as a percentage of total (± SEM) of lysed cells, normalized to stimulus alone, for each of at least three independent experiments, p < 0.001 for all MPO measurements except, p < 0.01 for A, Lat B at 50 μM; B, Lat B 1.25 μM compared to stimulus alone. There was no statistical difference for LTF measurements except, p < 0.05 for A, Lat B at 50 μM.
Since Lat B is a more potent actin depolymerization drug than CB (45), we hypothesized that addition of Lat B to CB/fMLF- or A23187-stimulated cells might enhance exocytosis. However, we found that Lat B had a bimodal effect which was specific for primary granule exocytosis. At a low dosage (1.25 μM), Lat B enhanced both CB/fMLF- and A23187-stimulated primary granule exocytosis, but had little effect on secondary granule exocytosis (Fig. 2, A and B, MPO and LTF, respectively). Higher doses of Lat B (50 μM) inhibited primary granule exocytosis, while secondary granule exocytosis was slightly enhanced under these conditions. High doses of Lat B did not result in loss of cell viability as determined by Trypan blue exclusion. This suggests that the mode of action which Lat B enhanced exocytosis was by facilitating moderate actin depolymerization,. This regulatory mechanism was not apparent for secondary granule exocytosis and therefore further studies focused on primary granule exocytosis.
F-actin and primary granule distribution in stimulated neutrophils
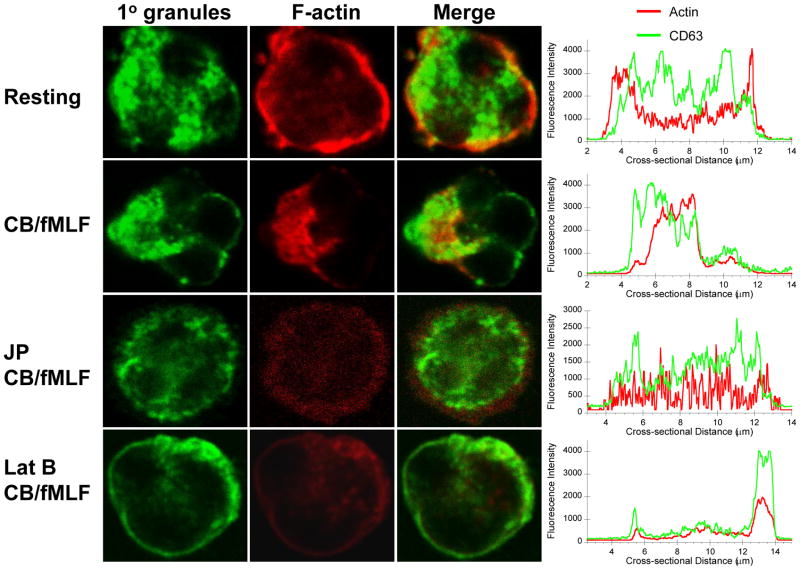
To confirm our biochemical findings and to visualize the effects of actin-altering drugs, we examined human neutrophils by confocal microscopy. Neutrophil primary granules were labelled with anti-CD63 antibodies conjugated to Alexa Fluor 488 and F-actin with rhodamine-phalloidin. Cells were treated similar to those in secretion assays, then fixed while in suspension and adhered to poly-L-lysine-coated glass slides for staining. Resting neutrophils exhibited diffuse primary granule staining and cortical actin staining in a ring-like structure (Fig. 3, Resting). Intensity profiling (Fig. 3, right column) showed sharp peaks at the cell periphery for rhodamine-phalloidin, corresponding to the actin ring, but relatively even distribution of the primary granule marker CD63. Upon stimulation with CB/fMLF, F-actin polarization to the cell’s edge occurred along with a redistribution of primary granules to the same sites; this is evident from colocalizing peaks on one side of the intensity profile. CB or fMLF alone did not induce these rearrangements (Fig. S1, supplementary data). Pre-treatment with JP followed by CB/fMLF stimulation resulted in primary granule and F-actin staining throughout the cell with diffuse cortical staining (Fig. 3, JP CB/fMLF). Interestingly, Lat B increased primary granule translocation to the cell periphery; however, it also reduced cytoplasmic F-actin to a diffuse, nonpolarized state (Fig. 3, Lat B CB/fMLF). These findings confirm biochemical results showing Lat B enhances CB/fMLF-induced primary granule exocytosis, although it disrupts the polarization mechanism.
Figure 3.
Distribution of primary granules and F-actin in stimulated neutrophils pre-treated with actin drugs. Neutrophils were preincubated with vehicle, 1 μM JP, 1.25 μM Lat B (Lat B) or for 15 min, followed by stimulation with 10 μM CB/5 μM fMLF for 15 min at 37°C. Cells were fixed while in suspension and mounted on poly-L-lysine coated slides for confocal microscopy. F-actin was stained with rhodamine-phalloidin (red) and primary granules were stained with Alexa Fluor 488-conjugated CD63 antibodies (green). Cross-sectional intensity profiles for F-actin (red line) and primary granules (green line) are shown on the right. Scale: each panel is 12 μm × 12 μm with cross-sections taken from the middle left to right. CB-only and fMLF-only conditions are shown in supplementary data (Fig. S1).
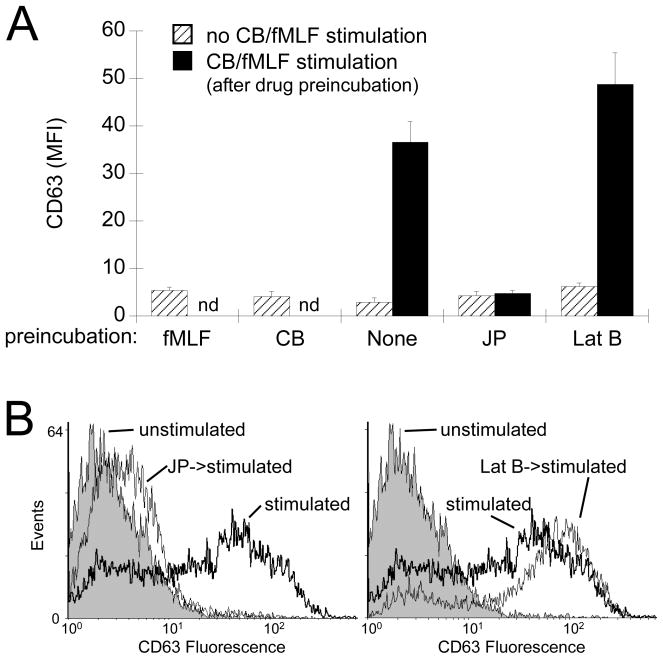
Although confocal microscopy showed that CD63 accumulated at more peripheral regions of the cell following stimulation, the resolution of this technique did not allow for quantification of exocytosis. We used flow cytometry to quantitatively analyze surface upregulation of CD63, which occurs following primary granule exocytosis. Unstimulated neutrophils or incubation with either CB or fMLF alone showed little surface CD63 labelling (Fig. 4A, hatched bars). Stimulation with CB/fMLF triggered elevated levels of CD63 at the plasma membrane which could be further enhanced by 30% when pre-incubated with Lat B (Fig. 4A, black bars). JP effectively blocked exocytosis to resting cell levels (Fig. 4A, JP).
Figure 4.
Analysis of exocytosis by flow cytometry. Neutrophils were preincubated with actin drugs or with vehicle (None) as indicated on the x-axis. Cells were then stimulated with CB/fMLF (black bars) or vehicle for control samples (hatched bars). Exocytosis of primary granules was determined by measuring the mean fluorescence intensity (MFI) of fixed cells stained with CD63 antibodies followed by FITC-conjugated secondary antibody. (A) The average MFI (± SEM) from at least three independent experiments. (B) Representative histograms from flow analysis. Left panel: Unstimulated (grey area), CB/fMLF stimulated (thick black line), JP preincubated--CB/fMLF stimulated (thin black line) cells. Right panel: Unstimulated (grey area), CB/fMLF stimulated (thick black line), Lat B preincubated-- CB/fMLF stimulated (thin black line) cells. Drug concentrations were: 5 μM, fMLF; 10 μM CB; 1 μM JP; 1.25 μM Lat B. p < 0.001 for None and Lat B compared to their respective unstimulated controls.
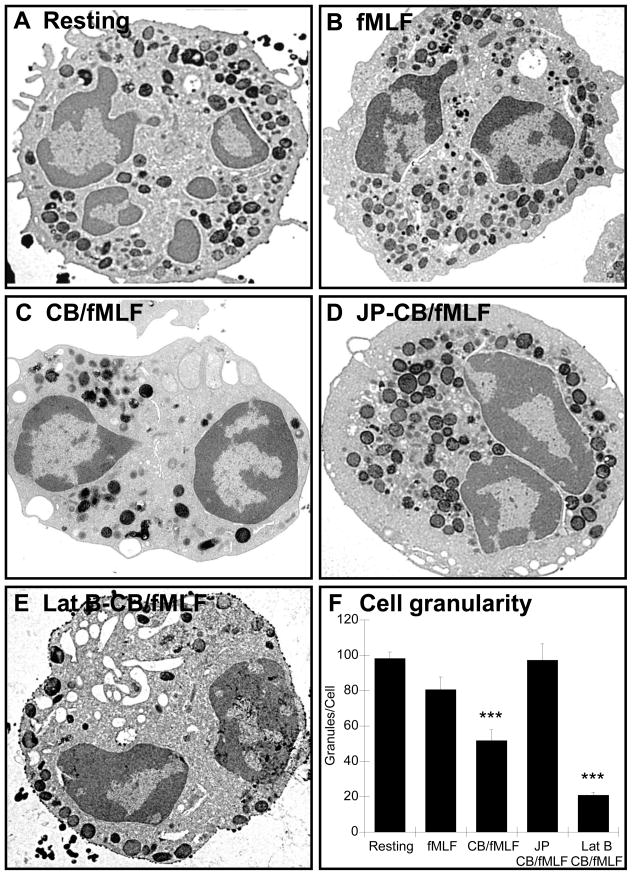
Morphological analysis of neutrophils by electron microscopy
We used electron microscopy to obtain high resolution images of neutrophils and further characterize neutrophil morphology after treatment with actin drugs. Resting or fMLF-treated cells showed numerous electron-dense granules evenly distributed throughout the cytoplasm, while CB/fMLF stimulated cells showed significantly fewer vesicles (Fig. 5, compare Resting, fMLF and CB/fMLF). Pre-treatment with JP prior to CB/fMLF stimulation resulted in clustering of primary granules in the centre of the cell (Fig. 5D) with similar granule count as resting cells (Fig. 5F). Interestingly, pre-treatment with Lat B prior to CB/fMLF decreased the number of primary granules within the neutrophil, and those that remained were at the cell periphery (Fig. 5E). These findings confirm our initial conclusions that moderate actin depolymerization is needed for primary granule exocytosis.
Figure 5.
Morphological analysis of neutrophils by electron microscopy. Neutrophils were treated as described in Figure 3, fixed with 2.5% glutaraldehyde and peroxidase-containing granules stained with DAB. Scale: each panel is 8 μm × 8 μm, 9100× magnification. Neutrophil granularity was determined by granule counts after exposure to actin drugs (panel F). The total number of DAB-stained granules were counted from least three EM sections cut from three independent experiments. Bars represent the average number of granules per cell (± SEM). *** p < 0.001.
Rac1 and Rac2 activation in stimulated human neutrophils
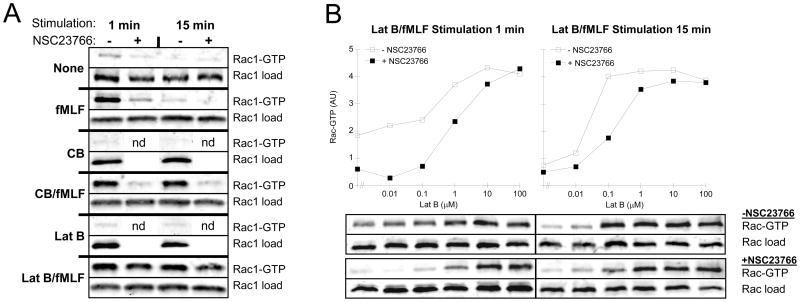
We have recently shown that primary granule exocytosis was reduced in bone marrow neutrophils isolated from rac2−/− mice, which was associated with a lack of primary granule translocation to the cell membrane during stimulation (1). Therefore, we investigated whether Rac regulates primary granule exocytosis by altering actin cytoskeleton dynamics in human neutrophils. To do this we used the small molecule Rac inhibitor, NSC23766, which has been shown to inhibit GDP/GTP exchange for both Rac1 and Rac2; a requirement for small G-protein activation and signaling (8, 15). NSC23766 blocks Rac function by binding to Trp56 and specifically inhibiting the binding of GEFs Trio and Tiam1 (15). However, it has yet to be shown to inhibit GTP binding to Rac1 and Rac2 in human neutrophils. Therefore, we examined the effects of this inhibitor on stimulated neutrophils using a Rac activation assay (2). This assay uses GST conjugated to the Rac-binding domain of p21 activated kinase (PAK) to affinity isolate GTP-bound Rac. Stimulation with fMLF showed high levels of GTP-bound Rac1 (Fig. 6A) and Rac2 (Fig. S2) within 1 min of treatment, which was reduced to basal levels by 15 min. Interestingly, the addition of actin drugs resulted in sustained levels of activated Rac (Fig. 6A and S2, compare 1 min and 15 min). These observations show that CB and Lat B prolong the activation of Rac1 and Rac2 initiated by fMLF. Stimulated with actin drugs alone (CB or Lat B) showed no activation of Rac.
Figure 6.
Detection of activated Rac in stimulated neutrophils. (A) Neutrophils were preincubated with 50 μM NSC 23766 (Rac inhibitor) or vehicle for 15 min followed by stimulation for 1 min (left columns) or 15 min (right columns) with 5 μM fMLF, 10 μM CB, 10 μM Lat B or combinations of CB/fMLF and Lat B/fMLF (10 μM/5 μM). (B) Neutrophils, preincubated with 50 μM NSC23766 or vehicle, were stimulated with 5 μM fMLF and increasing concentrations of Lat B. Quantification of activation was determined by band densitometry (arbitrary units). Activated Rac1-GTP (A and B) or Rac2-GTP (supplementary data, Fig. S2) was precipitated from 300 μg of lysate by incubation with 30 μg of GST-PBD beads (in 500 μl) and immunoblotting for Rac1 and Rac2 in the bound fraction. Loads = 30 μg of lysate from each sample.
We next tested the effects of the small molecule Rac inhibitor, NSC23766, on Rac in pulldown assays. Preincubation of neutrophils with NSC23766 effectively inhibited fMLF and CB/fMLF triggered Rac activation (Fig. 6A and S2, compare NSC23766 + to − lanes). The inhibitory effect of NSC23766 was less evident on Lat B/fMLF-stimulated neutrophils (see Discussion). We investigated this further, via titration of Lat B, to define the critical concentration of Lat B at which the inhibitory effect of NSC23766 on Rac activation could be overcome. Cells stimulated for 1 min required Lat B concentrations above 0.1 μM to enhance exocytosis as well as initiate the reversal of NSC23766 inhibition, however, complete reversal was not obtained until 100 μM Lat B was used (Fig. 6B, left panel). At 15 min of stimulation, 0.1 μM Lat B was adequate to initiate reversal of NSC23766 inhibition and complete reversal of NSC23766 inhibition was observed between 1 and 10 μM Lat B (Fig 6B, right panel). Since the action of Lat B is well defined (binds to actin monomers) we conclude that the effects of Lat B on enhancing Rac activation may be due to a secondary cellular response to the shortage of F-actin.
NSC23766 inhibits primary granule exocytosis and actin polymerization
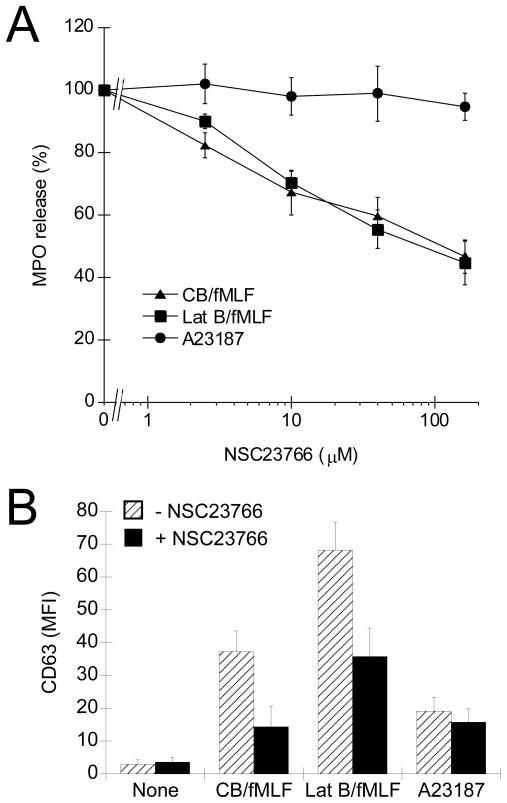
To link Rac activation to the exocytosis mechanism in human neutrophils we examined the affects of NSC23766 on primary granule secretion. Human neutrophils were pre-treated with NSC23766 for 15 min and then stimulated with CB/fMLF. This brief pre-treatment significantly reduced the secretion of primary granule MPO (Fig. 7A) and upregulation of CD63 as determined by flow cytometry (Fig. 7B), which correlates with the inhibition of Rac activation (Fig. 6). Inhibition was evident at 10 μM NSC23766 and maximal at > 40 μM. Secretion of MPO and upregulation of CD63 in response to A23187 was unaffected by NSC23766, even at doses up to 160 μM. Lat B/fMLF-stimulated cells also showed reduced primary granule exocytosis when pre-treated with NSC23766 (Fig. 7), even though Rac remained activated (GTP-bound) under these conditions (10 μM Lat B, see Fig. 6).
Figure 7.
Effect of Rac inhibition, via NSC23766 pre-treatment, on neutrophil exocytosis. (A) Analysis of exocytosis by MPO secretion assay. Neutrophils were preincubated with increasing concentration of NSC23766 for 15 min followed by stimulation with CB/fMLF, Lat B/fMLF, or A23187 for 15 min at 37°C. Supernatants were collected from each condition and assayed for MPO activity. Released MPO was calculated as a percentage of total activity (± SEM) of lysed cells normalized to stimulus alone, for at least three independent experiments, p < 0.001 for all measurements except, p < 0.01 for Lat B/fMLF at 40 μM NSC23766 and CB/fMLF at 10 μM NSC23766, compared to stimulus alone. (B) Analysis of exocytosis by flow cytometry. Neutrophils were preincubated with 50 μM NSC23766 (black bars), or vehicle (None) for control samples (hatched bars), for 15 min followed by stimulation with CB/fMLF, Lat B/fMLF, or A23187 for 15 min at 37°C. Exocytosis of primary granules was determined by measuring the mean fluorescence intensity (MFI) via flow cytometry of fixed cells stained with CD63 antibodies followed by FITC-conjugated secondary antibody. Shown is the average MFI (± SEM) from at least three independent experiments, p < 0.05 for CB/fMLF and Lat B/fMLF compared to their respective untreated controls. Drug concentration were: 5 μM, fMLF; 10 μM CB; 10 μM Lat B; 2.5 μM A23187.
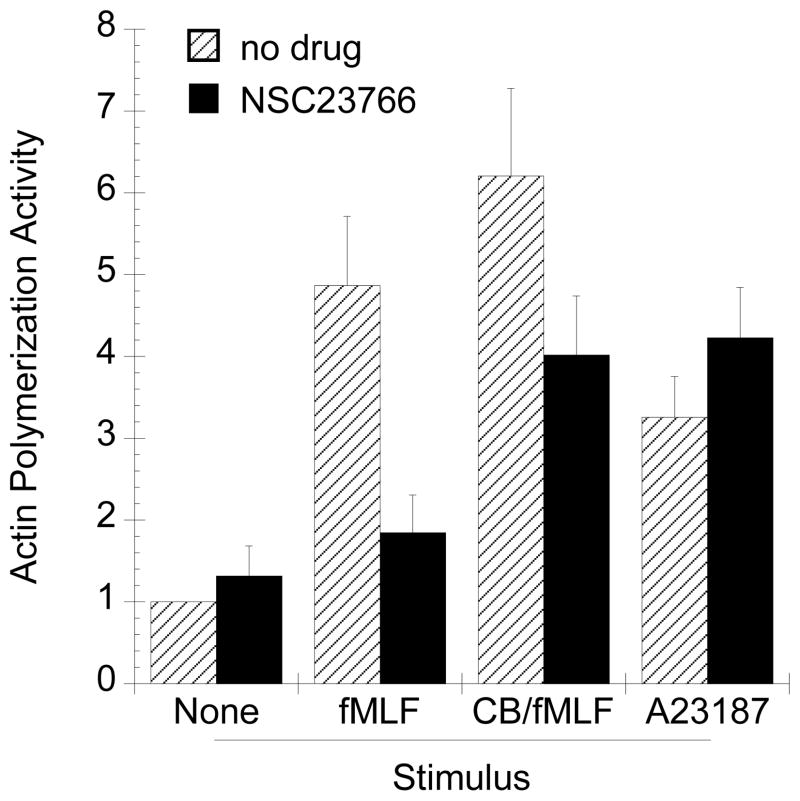
To link the role of Rac to F-actin formation, we investigated whether inhibition of Rac by NSC23766 would affect F-actin. We used an in vitro actin polymerization assay that measures the capacity of a sample to stimulate the polymerization of exogenously added pyrene-actin. Pyrene-actin undergoes a fluorescence intensity increase when incorporated into F-actin (9). Lysates prepared from fMLF- or CB/fMLF-stimulated cells exposed to NSC23766 showed reduced capacity for F-actin formation as compared to those unexposed (Figs. 8 and S3, supplementary data). Interestingly, there were negligible differences in lysates prepared from cells that were stimulated with A23187.
Figure 8.
Determination of actin polymerization activity of neutrophil lysates. Actin polymerization stimulated by neutrophil lysates was determined by pyrene-actin polymerization assay as described in Methods. Polymerization reactions contained 5 μM pyrene-actin and 0.1 mg/ml neutrophil lysate prepared from resting cells, fMLF, CB/fMLF, or A23187 stimulated cells. Lysates prepared from fMLF or CB/fMLF stimulated neutrophils showed enhanced polymerization activity (grey bars), which was reduced when cells were preincubated with 50 μM NSC23766 (black bars). Shown are the average activities (± SEM) calculated from at least three experiments normalized to unstimulated samples (Resting). Drug concentration were: 5 μM, fMLF; 10 μM CB; 10 μM Lat B; 2.5 μM A23187. Typical polymerization curves (Fig. S3) and actual A.P.A. (Fig. S4) are shown in supplementary data.
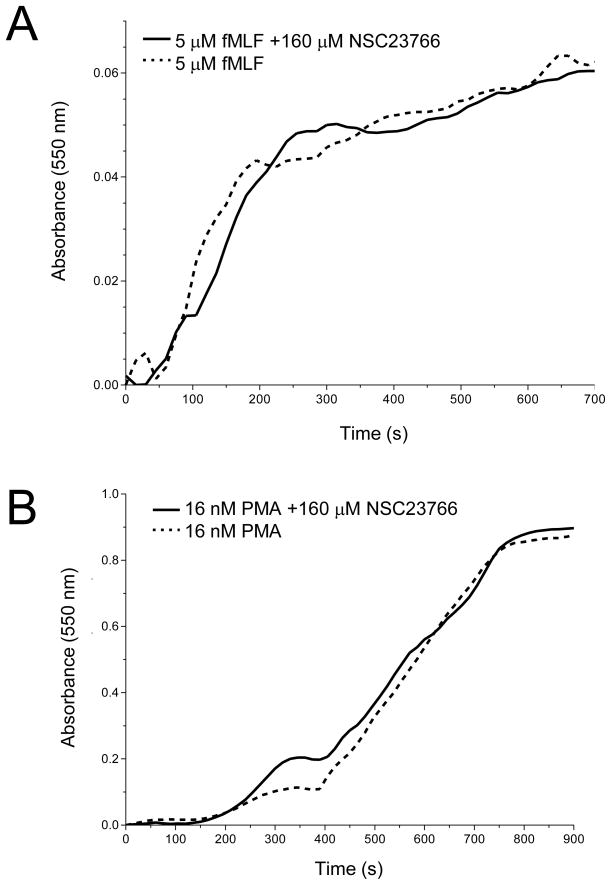
We also examined the effects of NSC23766 on respiratory burst induced by PMA or fMLF. This was done to determine the specificity of NSC23766 on Rac-mediated functions in neutrophils. Rac2 is essential for O2− release from neutrophils (17). Pre-treatment of neutrophils with NSC23766 for 15 min had no effect on high levels of O2− release from neutrophils stimulated with PMA (Fig. 9A) or the much reduced fMLF-stimulated levels (Fig. 9B). Although it has been proposed that activated Rac regulates respiratory burst (reviewed in 11), our observation, combined with Rac activation results (Fig. 6), correlates with previous findings in which Rac-GTP formation was not required for activation of the NADPH oxidase system in human neutrophils (17) or macrophages (4). Stimuli that activate G protein-coupled receptors, such as fMLF, have been shown to activate the GEFs Vav1 and P-Rex1, leading to Rac activation required for respiratory burst (30, 50). Since NSC23766 was unable to block O2− release from PMA stimulated neutrophils, this suggests that a different GEF, such as Trio or Tiam1, may be responsible for activating Rac-mediated exocytosis of primary granules. Taken together, these results show that the Rac inhibitor, NSC23766, specifically targets Rac-regulated actin remodeling and exocytosis in response to CB/fMLF and Lat B/fMLF.
Figure 9.
Effect of NSC23766 on respiratory burst. Neutrophils (2 × 107 cells/ml) were preincubated with 160 μM NSC23766 (Rac inhibitor) or vehicle for 15 min at 37°C. After preincubation, 2 × 106 of NSC23766 or vehicle treated neutrophils were placed in 1-ml microcuvettes containing PBS+ and 50 μM ferricytochrome c at 25°C. The mixture was blanked at 550 nm and the stimulation of respiratory burst was examined after addition of 5 μM fMLF (A), or 16 nM PMA (B). Readings were taken every 15 s for a total run time of 15 min. Shown are typical results from three independent experiments.
Morphological analysis of NSC23766-treated neutrophils
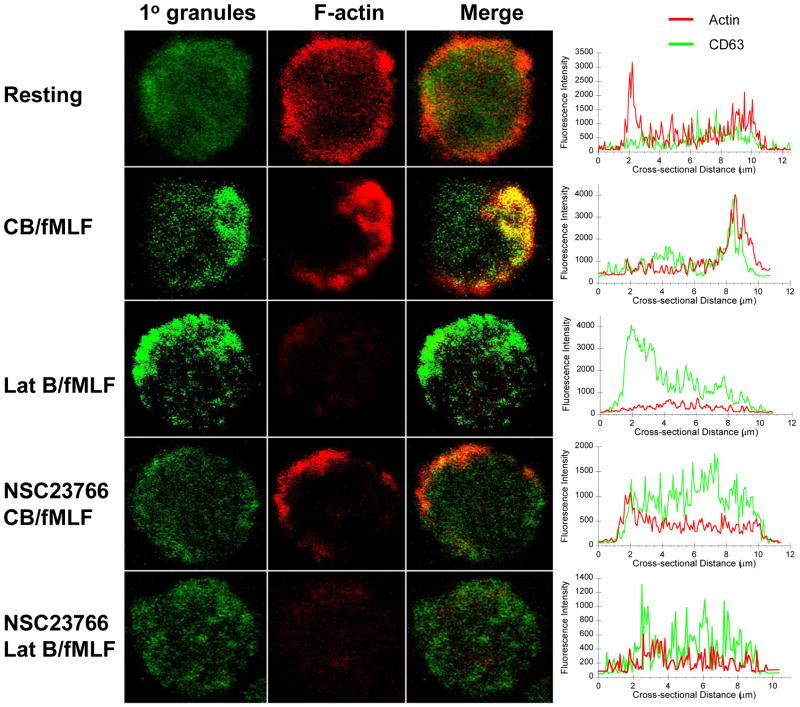
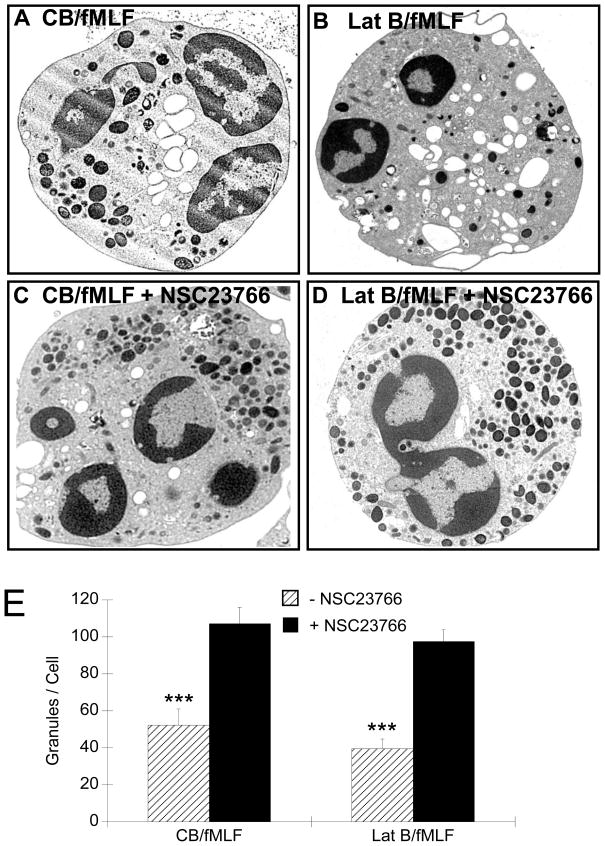
Our findings indicate that primary granule exocytosis was inhibited by NSC23766 in a dose-dependent fashion when stimulated with CB/fMLF or Lat B/fMLF. To confirmed these results we examined NSC23766-treated neutrophils via confocal and electron microscopy. Stimulation of neutrophils with CB/fMLF or Lat B/fMLF, resulted in primary granule translocation to the cell periphery (Fig. 10 and 11), which was also observed in intensity profiles as large peaks located at the peripheries (Fig. 10, right column). Pre-treatment of cells with NSC23766 prior to stimulation with CB/fMLF modestly reduced F-actin polarization at cell edges (Fig. 10), inhibited primary granule translocation to the cell membrane (Fig. 11), and inhibited exocytosis as determined by granule counts of EM sections (Fig. 11E). Similar results were obtained for NSC23766 pre-treated Lat B/fMLF-stimulated neutrophils. These results suggest that NSC23766 inhibited primary granule movement to the cell membrane in cells in suspension. Taken together, our results suggest that Rac plays a vital role in primary granule exocytosis via stimulation of actin polymerization to aid in granule mobilization to the cell periphery.
Figure 10.
Distribution of primary granules and F-actin in stimulated neutrophils pre-treated with Rac inhibitor. Neutrophils were preincubated with 50 μM of NSC23766 (Rac inhibitor) for 15 min, followed by stimulation with CB/fMLF or Lat B/fMLF for 15 min at 37°C Cells were fixed while still in suspension and mounted on poly-L-lysine coated slides for confocal microscopy. F-actin was stained with rhodamine-phalloidin (red) and primary granules were stained with Alexa Fluor 488-conjugated CD63 antibodies (green). Cross-sectional intensity profiles for F-actin (red line) and primary granules (green line) are shown on the right. Scale: each panel is 12 μm × 12 μm with cross-sections taken from top left to bottom right. Drug concentration were: 5 μM, fMLF; 10 μM CB; 10 μM LatB.
Figure 11.
Morphological analysis of NSC23766-treated neutrophils by electron microscopy. Neutrophils were preincubated for 15 min with 50 μM of the Rac inhibitor, NSC 23766 (C, D), or vehicle (A, B) and then 15 min with CB/fMLF, or Lat B/fMLF as indicated. After stimulation, cells were fixed with 2.5% glutaraldehyde and peroxidase-containing granules stained with DAB. Scale: each panel is 8 μm × 8 μm, 9100× magnification. (E) Neutrophil granularity was determined by granule counts after exposure to actin drugs (± NSC23766). The total number of DAB-stained granules were counted from least three EM sections cut from three independent experiments. Bars represent the average number of granules per cell (± SEM). Drug concentration were: 5 μM, fMLF; 10 μM CB; 10 μM LatB. ***p< 0.001.
DISCUSSION
Role of actin remodeling during exocytosis
Actin remodeling is considered to be integral to exocytosis of neutrophil granules (7, 28, 48), although the specific mechanism has not been fully elucidated. Here we show a requirement for Rac-mediated actin polymerization as well as cortical actin depolymerization in primary granule exocytosis. In resting cells, we observed an F-actin cortical ring, similar to that observed in resting mast cells (38), which dispersed after stimulation with CB/fMLF and Lat B/fMLF. This is in agreement with the concept of an F-actin barrier preventing inadvertent granule docking and fusion in resting cells. However, the presence of the F-actin ring in resting cells is distinct from previous studies which showed diffuse F-actin in resting neutrophils that assembled into a cortical ring upon stimulation by fMLF (12, 14). This discrepancy may be due to differences in neutrophil isolation techniques. In earlier studies, human neutrophils were prepared using dextran sedimentation and discontinuous plasma-Percoll gradients, while we used Ficoll to separate neutrophils from dextran-generated plasma, and then rested the cells in EDTA-containing medium on ice to lower neutrophil activity (i.e., to achieve “resting cells”) prior to experiments.
We also examined neutrophil morphology by EM to obtain high resolution images. These images showed a clear difference in the effects of actin drugs (Fig. 5D vs 5E). Stabilization of actin with JP resulted in the central accumulation of granules, while depolymerization allowed for significant plasma membrane localization, especially in the case of Lat B (Fig. 5E). Biochemical assays confirm that low concentrations of Lat B increase granule translocation and exocytosis, indicating a requirement for limited actin depolymerization, which is similar to that reported for exocytosis in neuroendocrine cells (16, 36).
Signaling for actin remodeling during exocytosis could be linked to other neutrophil functions. For example, when neutrophils encounter a chemoattractant, they undergo actin remodeling for chemotaxis (14, 47, 51), a mechanism clearly linked to Rho protein function (41), and it is likely that this may also facilitate polarized granule translocation and exocytosis. This apparently conflicts with the need for depolymerization of F-actin during primary granule exocytosis, since depolymerizing agents are required to stimulate exocytosis in vitro (1, 21, 44). Results from confocal analysis of resting neutrophils suggest that the cortical actin ring-like structure acts as a physical barrier to prevent uncontrolled granule docking and fusion as previously shown in mast cells (38, 39).
Our results show that upon stimulation with CB/fMLF there is a polarized reorganization of the F-actin to sites of primary granule translocation (Fig. 3 and 10, CB/fMLF panels). This is seemingly incompatible with the uniform application of stimulus in our experiments. However, it has been previously shown that neutrophils contain an inherent “sidedness” and produce a polarized response, even when placed in an environment of uniform stimuli (51, 52). We reproducibly observed similar polarized responses in all image analyses performed. Polarized actin was not as evident in Lat B/fMLF-stimulated neutrophils, therefore Lat B treatment likely disrupts the polarized exocytosis mechanism, but not exocytosis itself.
Role of Rac signaling during exocytosis
Rho proteins such as Rac control actin remodeling during neutrophil response to chemoattractant (41, 51). Rac is also activated in response to the secretagogue fMLF (ref. 8, Fig. 6), however, fMLF alone is not sufficient to stimulate primary granule exocytosis (28). Recent studies have defined the need for the Rab27a small GTPases in regulating exocytosis of primary granules in immune cells and secretory granules in neuronal cells (10, 37). Furthermore, these studies also link Rab function to actin-based transport of secretory vesicles. It is interesting to speculate these two GTPases act co-ordinately in their regulation of exocytosis since Rab GTPases have been shown to tether vesicles to actin-based motors while Rac may act to remodel actin filaments (33).
We showed that combinations of CB/fMLF or Lat B/fMLF are potent secretion stimuli, and interestingly these combinations also caused sustained Rac activation for up to 15 min (Fig. 6). This finding suggests that actin depolymerization by CB or Lat B feeds back into the Rac activation pathway to maintain a predominantly GTP-bound form. Studies of sustained activation of small GTPases including Rab27a have been previously observed to enhance exocytosis (10). For example, studies using GTPγS as a secretagogue have concluded that sustained activation of GTP-binding proteins may be a sufficient stimulus for membrane fusion, however, the GTPase class involved has not been confirm (i.e. Arf, Rab or Rho) (5, 40, 49). Our result would suggest that Rac may be a primary target of sustained activation by GTPγS in secretion. This is further supported by studies of the small molecule Rac inhibitor NSC23766. We show for the first time that NSC23766 inhibits primary granule exocytosis in response to CB/fMLF or Lat B/fMLF stimulation. This effect was specific for primary granules since LTF secretion, a marker for secondary granule exocytosis, was not inhibited by NSC23766 (data not shown). We were unable to distinguish whether NSC23766 mainly inhibited Rac1 or its closely related homolog Rac2 in neutrophil primary granule exocytosis. However, neutrophils mainly express Rac2, and Rac2 is essential for primary granule release (1). Therefore, these results support a role for Rac2 in F-actin-mediated human neutrophil primary granule exocytosis.
Biochemical analysis showed that NSC23766 effectively blocked fMLF- and CB/fMLF-stimulated Rac-GTP formation, but not that of Lat B/fMLF (Fig. 6). These results suggest that the more potent actin depolymerization reagent (Lat B over CB) may act to sustain Rac signaling via a feedback loop, similar to that observed with CB/fMLF at 15 min of stimulation, but that additionally overrides the inhibitory effects of NSC23766. This could be explained by the existence of a molecular sensor of increased G-actin that promotes continued activation of Rac via a GEF that is not inhibited by NSC23766. Currently, the association of two Rac GEFs, Trio and Tiam1, are known to be blocked by NSC23766, while this inhibitor has no effect on Vav1 association (15).
Our findings from imaging and biochemical analyses of granule exocytosis indicated that NSC23766 inhibited cytoplasmic F-actin polymerizing activity, as well as primary granule translocation for subsequent exocytosis, in response to CB/fMLF or Lat B/fMLF. Flow cytometry experiments confirmed that fMLF or CB/fMLF stimulation caused little if any increase in F-actin, and similarly, NSC23766 pre-treatment does not affect overall levels of F-actin (data not shown). In addition, NSC23766 had no effect on actin depolymerization by CB or Lat B combined with fMLF (Fig. 10). This suggest that inhibition of Rac does not result in actin depolymerization, and instead defines a role for Rac as a mediator of F-actin remodeling to facilitate granule translocation to the plasma membrane. It has recently been shown that Rac controls actin remodeling by generating free barbed ends and new filament assembly, which is in accord with our theory (47).
In Ca2+ ionophore-induced exocytosis, NSC23766 showed no effect, suggesting that Ca2+ acts downstream of Rac to induce granule translocation and secretion. It has been shown in RBL-2H3 cells, that Ca2+ signaling is highly important in granule exocytosis, and that Ca2+ levels were reduced when cells are exposed to dominant-negative constructs of Rac proteins, and restored when exposed to constitutively active forms of Rac (24). Therefore, Rac-mediated signaling in neutrophils may exploit Ca2+ signaling as a mode of primary granule translocation and exocytosis.
Taken together, our results illustrate a possible mechanism by which Rac regulates exocytosis of primary granules, via facilitating F-actin formation necessary for granule translocation to sites of exocytosis. Depolymerization of actin at the cell cortex must occur concurrently with cytoplasmic F-actin formation for exocytosis of primary granules. These results contribute to our understanding of neutrophil biology, with relevance to their role in degranulation and release of cytotoxic mediators in disease.
Supplementary Material
Acknowledgments
The authors would like to thank Honey Chan and Emily MacLean for their expert technical assistance. P. Lacy is a Canadian Lung Association/CIHR New Investigator and G. Eitzen is a CIHR New Investigator and AHFMR Scholar.
This work was supported by grants from Allergen NCE, the Canadian Lung Association and the Canadian Institutes of Health Research.
References
- 1.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 2.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Bio Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 3.Billadeau DD, Brumbaugh KM, Dick CJ, Schoon RA, Bustelo XR, Leibson PJ. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberg Y, Shani E, Joseph G, Gorzalczany Y, Sperling O, Pick E. The GDP-bound form of the small G protein Rac1 p21 is a potent activator of the superoxide-forming NADPH oxidase of macrophages. J Biol Chem. 1994;269:7055–7058. [PubMed] [Google Scholar]
- 5.Brown AM, O’Sullivan AJ, Gomperts BD. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubb MR, Senderwoicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 7.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 8.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JA, Pollard TD. Methods to measure actin polymerization. Methods Enzymol. 1982;85:182–210. doi: 10.1016/0076-6879(82)85021-0. [DOI] [PubMed] [Google Scholar]
- 10.Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Ménasché G, de Saint Basile G, Petit C, Cribier S, Henry JP, Darchen F. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diebold BA, Bokoch GM. Rho GTPases and the control of the oxidative burst in polymorphonuclear leukocytes. Curr Top Microbiol Immunol. 2005;291:91–111. doi: 10.1007/3-540-27511-8_6. [DOI] [PubMed] [Google Scholar]
- 12.Downey GP, Elson EL, Schwab B, Erzurum SC, Young SK, Worthen GS. Biophysical properties and microfilament assembly in neutrophils: modulation by cyclic AMP. J Cell Biol. 1991;114:1179–1190. doi: 10.1083/jcb.114.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eitzen G, Wang L, Thorngren N, Wickner W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J Cell Biol. 2002;158:669–679. doi: 10.1083/jcb.200204089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U SA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijsen N, van Delft S, Raaijmakers JA, Lammers JW, Collard JG, Koenderman L, Coffer PJ. Regulation of p21rac activation in human neutrophils. Blood. 1999;94:1121–1130. [PubMed] [Google Scholar]
- 18.Glogauer M, Hartwig J, Stossel T. Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. J Cell Biol. 2000;150:785–796. doi: 10.1083/jcb.150.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 20.Haddad EK, Wu X, Hammer JA, 3rd, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henson PM, Zanolari B, Schwartzman NA, Hong SR. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978;121:851–855. [PubMed] [Google Scholar]
- 22.Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J Biol Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 23.Hogg JC. Neutrophil kinetics and lung injury. Physiol Rev. 1987;67:1249–1295. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- 24.Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. Activated Cdc42/Rac reconstitutes FcεRI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc Natl Acad Sci U S A. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isgandarova S, Jones L, Forsberg D, Loncar A, Dawson J, Tedrick K, Eitzen G. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J Biol Chem. 2007;282:30466–30475. doi: 10.1074/jbc.M704117200. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 27.Jahraus A, Egeberg M, Hinner B, Habermann A, Sackman E, Pralle A, Faulstich H, Rybin V, Defacque H, Griffiths G. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 2001;12:155–170. doi: 10.1091/mbc.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:1690–1700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 29.Khurana D, Leibson PJ. Regulation of lymphocyte-mediated killing by GTP-binding proteins. J Leukoc Biol. 2003;73:333–338. doi: 10.1189/jlb.0802385. [DOI] [PubMed] [Google Scholar]
- 30.Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 31.Lacy P, Thompson N, Tian M, Solari R, Hide I, Newman TM, Gomperts BD. A survey of GTP-binding proteins and other potential key regulators of exocytotic secretion in eosinophils. Apparent absence of rab3 and vesicle fusion protein homologues. J Cell Sci. 1995;108(Pt 11):3547–56. doi: 10.1242/jcs.108.11.3547. [DOI] [PubMed] [Google Scholar]
- 32.Lacy P, Mahmudi-Azer S, Bablitz B, Gilchrist M, Fitzharris P, Cheng D, Man SF, Bokoch GM, Moqbel R. Expression and translocation of Rac2 in eosinophils during superoxide generation. Immunology. 1999;98:244–252. doi: 10.1046/j.1365-2567.1999.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacy P, Eitzen G. Control of granule exocytosis in neutrophils. Front Biosci. 2008;13:5559–5570. doi: 10.2741/3099. [DOI] [PubMed] [Google Scholar]
- 34.Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol. 2003;111:923–932. [PubMed] [Google Scholar]
- 36.Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763:1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Munafó DB, Johnson JL, Ellis BA, Rutschmann S, Beutler B, Catz SD. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem J. 2007;402:229–239. doi: 10.1042/BJ20060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norman JC, Price LS, Ridley AJ, Hall A, Koffer A. Actin filament organization in activated mast cells is regulated by heterotrimeric and small GTP-binding proteins. J Cell Biol. 1994;126:1005–1015. doi: 10.1083/jcb.126.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman JC, Price LS, Ridley AJ, Koffer A. The small GTP-binding proteins, Rac and Rho, regulate cytoskeletal organization and exocytosis in mast cells by parallel pathways. Mol Biol Cell. 1996;7:1429–1442. doi: 10.1091/mbc.7.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberhauser AF, Monck JR, Balch WE, Fernandez JM. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992;360:270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- 41.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sengeløv H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N. Subcellular localization and dynamics of Mac-1 (αmβ2) in human neutrophils. J Clin Invest. 2003;92:1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skubitz KM. Neutrophilic leukocytes. In: Greer JP, Foerster J, Lukens J, Rodgers G, Paraskevas F, Glader B, editors. Wintrobe’s Clinical Hematology. 11. Philadelphia: Williams & Wilkins; 2004. pp. 267–310. [Google Scholar]
- 44.Showell HJ, Freer RJ, Zigmond SH, Schiffmann E, Aswanikumar S, Corcoran B, Becker EL. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976;143:1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 46.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun CX, Magalhães MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179:239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizoli SB, Rotstein OD, Parodo J, Phillips MJ, Kapus A. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol. 2000;279:619–633. doi: 10.1152/ajpcell.2000.279.3.C619. [DOI] [PubMed] [Google Scholar]
- 49.Rosales JL, Ernst JD. GTP-dependent permeabilized neutrophil secretion requires a freely diffusible cytosolic protein. J Cell Biochem. 2000;80:37–45. doi: 10.1002/1097-4644(20010101)80:1<37::aid-jcb40>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 50.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867–1873. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Wang F, van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sigimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;115:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 52.Zigmond SH, Levistsky HI, Krell BJ. Cell polarity: an examination of its behavioural expression and its consequences for polymorphonuclear leukocyte chemotaxis. J Cell Biol. 1981;89:585–592. doi: 10.1083/jcb.89.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.