Abstract
Introduction
Cannabinoids produce a spectrum of effects in humans including euphoria, cognitive impairments, psychotomimetic effects, and perceptual alterations. The extent to which dopaminergic systems contribute to the effects of Δ-9-tetrahydrocannabinol (Δ-9-THC) remains unclear. This study evaluated whether pretreatment with a dopamine receptor antagonist altered the effects of Δ-9-THC in humans.
Materials and methods
In a 2-test-day double-blind study, 28 subjects including healthy subjects (n=17) and frequent users of cannabis (n=11) were administered active (0.057 mg/kg) or placebo oral haloperidol in random order followed 90 and 215 min later by fixed order intravenous administration of placebo (vehicle) and active (0.0286 mg/kg) Δ-9-THC, respectively.
Results
Consistent with previous reports, intravenous Δ-9-THC produced psychotomimetic effects, perceptual alterations, and subjective effects including “high.” Δ-9-THC also impaired verbal recall and attention. Haloperidol pretreatment did not reduce any of the behavioral effects of Δ-9-THC. Haloperidol worsened the immediate free and delayed free and cued recall deficits produced by Δ-9-THC. Haloperidol and Δ-9-THC worsened distractibility and vigilance. Neither drug impaired performance on a motor screening task, the Stockings of Cambridge task, or the delayed match to sample task. Frequent users had lower baseline plasma prolactin levels and blunted Δ-9-THC induced memory impairments.
Conclusions
The deleterious effects of haloperidol pretreatment on the cognitive effects of Δ-9-THC are consistent with the preclinical literature in suggesting crosstalk between DAergic and CBergic systems. However, it is unlikely that DA D2 receptor mechanisms play a major role in mediating the psychotomimetic and perceptual altering effects of Δ-9-THC. Further investigation is warranted to understand the basis of the psychotomimetic effects of Δ-9-THC and to better understand the crosstalk between DAergic and CBergic systems.
Keywords: Schizophrenia, Cannabinoids, Dopamine, Antipsychotic, Cognition, Memory, Addiction, Attention, Haloperidol, Endocrine
Introduction
Cannabis is one of the most widely used illicit substances (Compton et al. 2004; SAMHSA 2004). The primary active cannabinoid in cannabis is Δ-9-tetrahydrocannabinol (Δ-9-THC), but cannabis also contains more than 60 other cannabinoids, some of which may contribute either directly or indirectly to the effects of Δ-9-THC. The spectrum of effects produced by cannabis includes euphoria, relaxation, anxiety, perceptual alterations, paranoia, and impairments in attention and memory. Laboratory experiments have demonstrated that synthetic and natural cannabinoids can induce transient psychotomimetic effects in healthy human subjects (D’Souza et al. 2004; Henquet et al. 2006; Leweke et al. 1999) and exacerbate psychosis in individuals with schizophrenia (D’Souza et al. 2005). Finally, a growing body of epidemiological studies (reviewed in Fergusson et al. 2006; Henquet et al. 2005) have drawn attention to the link between cannabinoids and psychosis (D’Souza 2007).
Converging preclinical evidence suggests interactions between cannabinoid (CB1) and dopamine (DA) systems (reviewed in Gardner 2005; Laviolette and Grace 2006a). CB1 and D2 receptors are coexpressed in several brain regions (Hermann et al. 2002), and there is signal transduction convergence in these regions (Meschler and Howlett 2001). Δ-9-THC activates DAergic mesolimbic neurons (French 1997; French et al. 1997; Gardner 2005; Gessa et al. 1998) and induces DA release in the striatum (Chen et al. 1990a; Tanda et al. 1997a) in animals. CB1 agonists induce c-fos in the nucleus accumbens (Miyamoto et al. 1996) and A10 DArgic neurons within the ventral tegmentum (Patel and Hillard 2003). At a molecular level, DA D2 receptors are believed to have a significant modulatory role in determining the G-protein coupling specificity of CB1 receptors (Jarrahian et al. 2004). Haloperidol and Δ-9-THC produce more catalepsy in rats than haloperidol alone (Marchese et al. 2003), haloperidol reverses Δ-9-THC reductions in prepulse inhibition and startle response in mice (Nagai et al. 2006); however, haloperidol has no influence on Δ-9-THC-induced catalepsy in mice (Kinoshita et al. 1994). Given the preclinical evidence of crosstalk between CB and DA systems, one logical question is to what extent, if any, are DA receptor mechanisms involved in the psychotomimetic and rewarding effects of cannabinoids in humans?
Haloperidol was hypothesized to reduce the psychotomimetic, perceptual altering, euphoric, and cognitive effects of Δ-9-THC in humans and that the two drugs would have additive cataleptic effects. Further, there are long-term changes associated with exposure to cannabinoids (reviewed in D’Souza et al. 2008: In review; Kolb et al. 2006; Lichtman and Martin 2005; Ranganathan et al. 2007), including regional changes in DA transmission (Jentsch et al. 1998; Verrico et al. 2003). Therefore, individuals who frequently used cannabis were hypothesized to differ in their response to the interactive effects of haloperidol and Δ-9-THC.
Materials and methods
The study was conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System, West Haven, CT) with the approval of the Institutional Review Boards of VA Connecticut Healthcare System and Yale University School of Medicine and in accordance with the Helsinki Declaration of 1975. Subjects were informed about the side effects of both haloperidol (sedation, akathisia, dystonia, etc.) and Δ-9-THC (euphoria, anxiety, paranoia, etc.). Subjects were recruited by public advertisement and compensated for their research participation. Confidentiality of study data was protected.
Participants
The sample consisted of two groups, frequent users of cannabis and healthy subjects. Frequent users were defined as (1) lifetime cannabis exposure greater than or equal to 100 times, (2) last exposure to cannabis within the past week, (3) recent cannabis exposure greater than ten times per month as quantified by Time Line Follow Back (Sobell and Sobell 1992), and (4) positive urine toxicological test for cannabis at screening. These subjects also met criteria for Diagnostic and Statistical Manual of Mental Disorders (DSM) IV cannabis abuse disorder, while none of the healthy subjects did.
In addition to obtaining written informed consent, subjects had to successfully complete a questionnaire about the key risks and benefits of the study. Subjects (18–55 years) underwent a structured psychiatric interview for DSM (First et al. 2002; Spitzer 1990) and were carefully screened for any DSM axes I or II lifetime psychiatric or substance use disorder (except for cannabis in the case of frequent users) and family history of major axis I disorder. All subjects were asked to estimate their lifetime cannabis exposure (number of times), heaviest exposure, and last exposure to cannabis. Subjects were excluded for recent abuse (3 months) or dependence (1 year) to alcohol or any substances, other than nicotine in both groups. Cannabis-naïve individuals were excluded to minimize any risk of promoting future cannabis use/abuse. The history provided by subjects was confirmed by a telephone interview conducted with an individual (spouse or family member) identified by the subject before screening. A general physical and neurological examination, electrocardiogram, and laboratory tests (serum electrolytes, liver function tests, complete blood count with differential, and urine toxicology) were also conducted. Subjects were instructed to refrain from alcohol, illicit drugs, or prescription drugs not approved by the research team for 2 weeks before the study and throughout study participation. Healthy subjects were required to have a negative urine toxicological test at screening. Frequent users were permitted to use cannabis until 24 h before each test day, to minimize cannabis withdrawal, a syndrome that has been described by several groups (Budney et al. 2003; Haney 2005; Haney et al. 1999a, b). Abstinence from cannabis in the 24 h before each test day was confirmed by subject interview on each test day. Self-reported cannabis use is a valid method of assessing cannabis use (Martin et al. 1988).
Experimental design
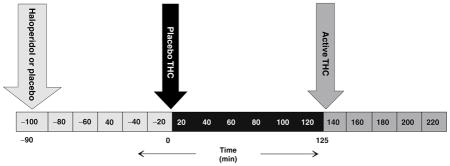
Subjects completed 2 test days during which they received placebo or active (0.057 mg/kg) haloperidol in random counterbalanced order, followed 90 min later by placebo Δ-9-THC (vehicle) and 215 min later by active Δ-9-THC (0.0286 mg/kg) administered intravenously (i.v.) over 20 min in a fixed order (Table 1).
Table 1.
Schedule of procedures
 | |
|---|---|
| Time (Min) | Procedures |
| −90 | Screening: Urine drug screen and urine pregnancy test. Confirmation of abstinence from caffeine, alcohol, drugs and prohibited medications. |
| Vital Signs (blood pressure, heart rate, temperature), standard light breakfast and placement of intravenous lines | |
| Baseline Serum Sampling for (1) prolactin (2) cortisol | |
| Behavioral Assessments: Positive and Negative Syndrome Scale (PANSS), Clinician Administered Dissociative Symptoms Scale (CADSS), Visual Analog Scale (VAS) for “high,” “calm and relaxed,” “anxious,” “tired” and “panic,” and Timeline Follow-Back (Day 1). | |
| Motor Assessments: Simpson Angus Scale for Parkinsonism (SAS), Barnes Akathisia Scale (BAS) | |
| Haloperidol or Placebo PO | |
| −30 | Behavioral ratings: PANSS, CADSS, VAS. |
| Motor ratings: SAS, BAS | |
| Vital Signs | |
| −10 | Bloods Levels: (1) prolactin (2) cortisol levels |
| Vital Signs | |
| 0 | Placebo Intravenous delta-9-THC over 20 min |
| Vital Signs Q 2 min. | |
| +15 | Vital Signs Q 5 min. |
| Behavioral ratings: PANSS, CADSS, VAS. | |
| Motor ratings: SAS, BAS | |
| +35 | Vital Signs Q 10 min. |
| Cognitive battery: Hopkins Verbal Learning Test Immediate and Delayed (30 min) recall (HVLT), Continuous Performance Task (CPT), Motor Screening (MS), Spatial Working Memory Task (SWMT), Stockings of Cambridge (SOC), Delayed Match to Sample (DMS) | |
| +65 | Vital Signs |
| Behavioral ratings: PANSS, CADSS, VAS, Drug Liking Scale, Similarity to Cannabis Scale. | |
| Bloods Levels: (1) prolactin (2) cortisol levels | |
| +85 | Vital Signs |
| +125 | Active Intravenous delta-9-THC (0.0286 mg/kg) over 20 min |
| Vital Signs Q 2 min. | |
| +140 | Vital Signs Q 5 min. |
| Behavioral ratings: PANSS, CADSS, VAS. | |
| Motor ratings: SAS, BAS | |
| +160 | Vital Signs: Monitoring set to q10 min. Switch to manual during cognitive battery. |
| Cognitive battery: HVLT, CPT, MS, SWMT, SOC, DMTS | |
| +190 | Vital Signs |
| Behavioral ratings: PANSS, CADSS, VAS, Drug Liking Scale, Similarity to Cannabis Scale. | |
| Bloods Levels: (1) prolactin (2) cortisol levels | |
| +210 | Vital Signs |
| End of each day | Safety Assessment: Field sobriety test, Minimental State Examination, vital signs, physician evaluation. |
| Subject provided with Benadryl PRN for EPS and contact information for On-Call Research Psychiatrist. | |
| Last day | Exit interview |
| Months 1, 3, 6 | Safety Follow up: 1. Assessment of cannabis use, desire, craving, 2. Assessment for emergence of new psychiatric or medical problems. |
Drugs
The dose and rate of administration of Δ-9-THC was chosen to mimic the dose range of recreational cannabis use and to be equivalent to about 0.5–1.5 of a standard National Institute of Drug Abuse cannabis cigarette. Further, in pilot studies, because subjects were unable to tolerate higher doses, Δ-9-THC (0.05, 0.035 mg/kg) was given as a 2-min bolus after receiving haloperidol. A 20-min infusion was selected to mimic the time frame of recreational cannabis consumption. The rationale for the route of Δ-9-THC administration and preparation of both Δ-9-THC and placebo is described previously (D’Souza et al. 2004, 2005). The psychoactive effects of cannabis in humans are due primarily to Δ-9-THC (Wachtel et al. 2002). Δ-9-THC of 99.6% purity was dissolved in 95% ethanol (Agurell et al. 1986) to yield a concentration of 2 mg/ml stock solution, which was then passed through a 0.22 μm polymer filter, subjected to sterility and pyrogenicity testing, assayed by gas chromatography–mass spectrometry to confirm its concentration, and stored at −20°C for future use. For the control condition, an equivalent volume (≅x2 ml) of ethanol (vehicle) was used, which would amount to a concentration of 0.0004% in an adult with average blood volume (4–5 l). Postinjection blood sampling at multiple time points failed to detect ethanol.
The DA receptor antagonist haloperidol was chosen because of its relative specificity for D2 receptors. In pilot studies, 0.05 mg/kg haloperidol (3.5 mg in a 70 kg individual) produced no appreciable effects, while at 0.071 mg/kg (5 mg in a 70 kg individual), it was associated with a high rate of dropouts related to side effects (D’Souza, unpublished observations). Therefore, an inter-mediate dose of haloperidol (0.057 mg/kg), equivalent to 4 mg in a 70-kg individual, was chosen. This dose was expected to produce antipsychotic effects without producing significant extrapyramidal side effects (Farde et al. 1992; Kapur et al. 1997, 2000) and is within the dose range recommended by the British Association of Psychopharmacology consensus conference for the use of haloperidol in healthy volunteers (King 1997). Further, at this dose, haloperidol is a relatively selective antagonist at DA D2 receptors (Schotte et al. 1996) compared to actions at other receptors (e.g., muscarinic M1 or histaminergic H1) that might modulate Δ-9-THC effects.
Test days were separated by at least 1 week (greater than three times the elimination half life of Δ-9-THC) to minimize carryover effects (Wall et al. 1976). To keep both subjects and raters blind to study conditions and the order of drug administration, both the subjects and raters were told of a possibility of a third “very low” dose of both haloperidol and Δ-9-THC, deliberately described in ambiguous terms. In actuality, subjects never received the “very low” dose of both haloperidol and Δ-9-THC.
Schedule of testing
Urine toxicology was conducted on the morning of each test day to rule out recent illicit drug use. A positive urine drug screen resulted in exclusion from the study except when positive for cannabis in frequent users. Subjects fasted overnight, reported to the test facility around 8 A.M., and were provided a standard breakfast. A positive urine pregnancy test also resulted in exclusion.
The detailed schedule of test procedures is described in Table 1. Subjects were attended to by a research psychiatrist, a research nurse, and a research coordinator. Clear “stopping rules” were determined a priori, and rescue medications were available during testing (lorazepam and benztropine) if necessary and also at discharge (diphenhydramine). Subjects were recontacted at 1, 3, and 6 months post-study for safety follow-up.
Outcome measures
Behavioral and subjective ratings were conducted before and after haloperidol (active or placebo) and after placebo and active Δ-9-THC administration (Table 1). Positive, negative, and general symptoms associated with psychosis were assessed using relevant subscales of the Positive and Negative Syndrome Scale (PANSS; Kay et al. 1989), perceptual alterations were measured using the Clinician-administered Dissociative Symptoms Scale (CADSS; Bremner et al. 1998), and feeling states associated with cannabis intoxication were measured using a self-reported visual analog scale of five items (“high,” “calm and relaxed,” “tired,” “panic,” and “anxious”; Haertzen 1965, 1966). Subjects were also asked to rate on a 0–100 scale (0=not at all to 100=most of all) (1) the similarity of the experience compared to recreational cannabis use and (2) how much they enjoyed the experience. Inter-rater reliability sessions were conducted every 1–2 months, and, for example, intraclass correlation coefficient for the PANSS were consistently greater than 0.85.
A cognitive test battery was administered 30 min after receiving both placebo and active Δ-9-THC infusions. Learning and recall were measured using the Hopkins Verbal Learning Test (Brandt et al. 1992; Bylsma et al. 1991). This test consists of three consecutive trials of immediate free recall of a 12-item, semantically categorized list, followed 30 min later by testing of delayed free, cued, and recognition recall. Different but equivalent versions of the test were administered within and across test days. Vigilance and distractibility to visual stimuli were measured using a continuous performance task (Gordon 1986) in which subjects attended to numbers presented sequentially on a screen. The subject pushed a button to signal when a ‘1’ was followed by a ‘9.’ The distractibility task was identical to the vigilance task with the exception that numbers were presented sequentially in three contiguous columns. Subjects were instructed to attend to the middle column and ignore the outer two columns.
Executive function (Stockings of Cambridge: SOC), spatial working memory (SWM), visual recognition memory (delayed match to sample: DMTS), and motor screening were tested using the Cambridge Neuroscience Test Battery (CANTAB; http://www.camcog.com; Sahakian and Owen 1992). Intelligence quotient was remeasured as part of the CANTAB using the National Adult Reading Test (NART). A motor-screening task (MOT) was administered to ensure that subjects could point accurately and also to measure speed and accuracy, both of which are indices of motor skill. A series of crosses were shown in different locations on the screen. After a demonstration, the subject had to point to the crosses in turn. The time taken for the subject to touch the cross after it appeared was measured as MOT latency. The arithmetic mean was calculated from the latencies of the ten crosses presented, which were correctly responded to. The DMTS tests visual memory in a four-choice delayed recognition memory paradigm. Subjects were shown a complex visual pattern (the sample). After a variable delay (of 0, 4, or 12 s), three distractor patterns and the target pattern were shown (Robbins et al. 1994). Subjects were instructed to choose the target pattern. The number of occasions upon which the subject selected the correct targets in trials was calculated as a percentage (DMTS percent correct score). In the SWM task, subjects had to find individually hidden “blue tokens” without returning to a box where one had previously been found (Owen et al. 1990). Errors included selecting boxes that had previously been found to be empty (within errors) and revisiting boxes that had already been found to contain a token (between errors). The SOC test is a spatial-planning task based upon the “Tower of London” test. Subjects had to move a set of balls to replicate a reference pattern. The number of occasions that a subject successfully replicated a reference pattern in the minimum possible number of moves was measured. CANTAB tests have satisfactory levels of test–retest reliability, with some outcome measures reaching correlations of better than 0.9. All the CANTAB tests used had parallel versions to facilitate repeated testing, and further, the tests tapped nonstrategic cognitive functions, which are less likely to be subject to significant practice effects.
Akathisia and drug-induced Parkinsonism were evaluated using the Barnes akathisia scale (Barnes 1989) and the Simpson Angus Scale (Simpson and Angus 1970), respectively. However, as subjects were not allowed to ambulate during testing because of safety concerns, the latter was modified to include only the items for tremor and a composite rigidity score (shoulders, elbows, wrist, head rotation) as described elsewhere (D’Souza et al. 2005).
Vital signs were recorded periodically, and blood was sampled for prolactin and cortisol from the intravenous line opposite to the one used for administering the study drug, to provide a behaviorally independent measure of cannabinoid effects. Immediately after collection, blood samples were placed on ice and centrifuged, and the extracted plasma was aliquoted into vials for storage at −70°C until the time of the assay. Prolactin and cortisol assays were run in duplicate pairs using radioimmunoassay kits to determine prolactin (Serono Diagnostics) and cortisol (Baxter Travenol Diagnostics) levels.
Statistical analyses
Initially, data were examined descriptively using means, standard deviations, and graphs. Each outcome was tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. Some, but not other, outcomes were approximately normal. Some of the skewed data were successfully normalized by log transformation. However, some skewed data could not be normalized by log transformation. These non-normal outcomes were analyzed using the nonparametric approach for repeated measures data (Brunner et al. 2002), where the data were first ranked and then fitted using a mixed-effects model with an unstructured variance–covariance matrix and p values adjusted for analysis of variance-type statistics (ATS). Linear mixed models were used to analyze normal or normalized (log-transformed) data. If data required normalization or could not be normalized, it is specified in “Results.” For all the CANTAB data, vigilance and distractibility data, and the verbal recall models, each outcome, in turn, represented the dependent variable, while haloperidol (placebo vs active) and THC (placebo vs active) were included as within-subject explanatory factors, and group (frequent users vs healthy subjects) was included as a between-subjects factor. However, because no group differences were found, group was eliminated from the models for most of the outcomes except for endocrine, immediate recall, and SWM data. In addition, all CANTAB models were controlled for NART-IQ and motor function (latency). For verbal immediate recall, trial (1–3) was included as an additional within-subject factor. These models allowed for testing of all main and interaction effects of haloperidol and THC. When appropriate, post-hoc comparisons were performed. In the above models, subject was used as the clustering factor. Similar models as above were used to evaluate CADSS, PANSS, and VAS outcomes, except that the THC factor was replaced by time (−90, −30, 15, 65, 140, 190) as a within-subject factor. Data on the similarity to cannabis and enjoyment of the experience were analyzed similarly except that measurements were taken only at time 65 and 190. Because there was little or no variance at earlier time points, analyses of the akathisia and Parkinsonism data were restricted to time 140 only; these models included only the within-subject factor haloperidol. Data were analyzed using SAS, version 9.1 (SAS Institute, Cary, NC). All results were considered statistically significant at P<0.05. Bonferroni correction was applied within but not across hypotheses. Thus, for the positive symptoms subscale of the PANSS, a cutoff alpha level of 0.05/2=0.025 was used to declare effects significant for PANSS positive.
Results
Of the 54 subjects who were screened, a total of 28 subjects (17 healthy subjects and 11 frequent users of cannabis) initiated the study (Table 2). Frequent users and healthy subjects were not significantly different across several measures except for years of education (Table 2) and cannabis use histories (Table 3). However, because there were no group differences found across most outcome measures, unless otherwise specified, the results are presented below for the combined sample. Eight subjects (five healthy subjects and three frequent users) did not complete the study because they disliked the psychotomimetic and anxiogenic effects of Δ-9-THC (n=2), scheduling difficulties (n=1), and/or nonstudy issues (n=5). One subject who did not disclose a history of migraine at screening experienced a headache on the placebo condition. No serious adverse events occurred during the study or during the follow-up period, as determined by face-to-face contact and telephone interview.
Table 2.
Demographics
| Mean (SD) | ||
|---|---|---|
| Total (n=28) | Healthy subjects | 17 |
| Frequent users | 11 | |
| Age (SD) years | 24.89 (±6.98) | |
| National Adult Reading Test (NART) IQ | 113 (±7.3) | |
| Years of education | All | 15.78 (±1.9)** |
| Healthy subjects | 16.35 (±2.23) | |
| Frequent users | 14.91 (±0.94) | |
| Race | Caucasian | 19 |
| Native American | 0 | |
| African American | 3 | |
| Hispanic | 2 | |
| Asian | 4 | |
| Weight | 159.35 (±28.59) | |
t test; p=0.054
Table 3.
Cannabis use histories
| Healthy subjects | Frequent users | |
|---|---|---|
| Estimated self-reported lifetime cannabis exposure | ||
| Less than 5 times | 2 | 0 |
| 5–10 times | 4 | 0 |
| 11–20 times | 2 | 0 |
| 21–50 times | 4 | 0 |
| 51–100 times | 3 | 0 |
| >100 times | 2 | 11 |
| Group difference (Wilcoxon signed rank test) | P<0.0001 | |
| Last exposure to cannabis | ||
| Past week | 0 | 10 |
| 1 week–1 month | 5 | 1 |
| 1–6 months | 3 | 0 |
| 6months–1 year | 3 | 0 |
| 1–5 years | 5 | 0 |
| 5–10 years | 1 | 0 |
| >10 years | 0 | 0 |
| Group difference (Wilcoxon signed rank test) | P<0.0001 | |
Psychotomimetic effects
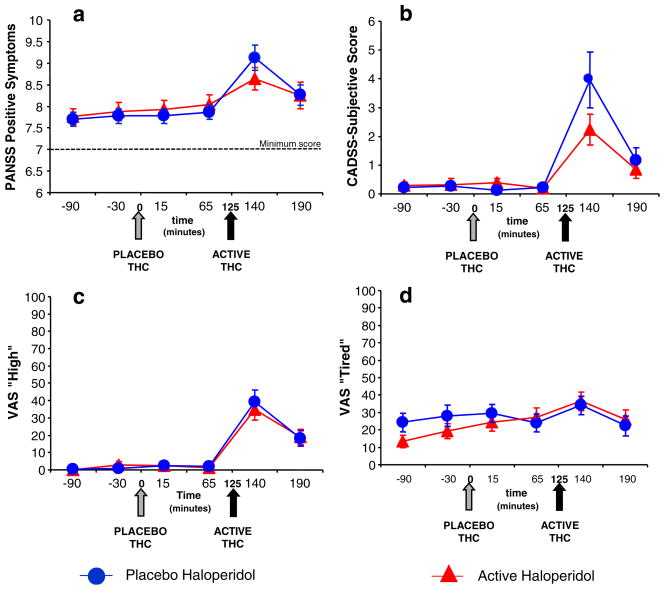
PANSS and CADSS data were highly skewed. Δ-9-THC produced significant increases in PANSS Total (ATS=24.7, num df=2.9, unadjusted P<0.0001), PANSS positive symptoms subscale (ATS=10.1, num df=2.8, adjusted P< 0.0001), CADSS subjective [ATS=15.9, num df=3.5, unadjusted P<0.0001), and CADSS objective (ATS=20.8, num df=2.9, P<0.0001) scores. There were no significant effects of haloperidol or haloperidol by Δ-9-THC-interactive effects on any of these outcomes (Fig. 1a and b).
Fig. 1.
a Positive symptoms of psychosis measured by the Positive Symptoms subscale of the Positive and Negative Syndrome Scale (PANSS). b Perceptual Alterations measured by the Subject rated subscale of the Clinician Administered Dissociative Symptoms Scale (CADSS). c “High” measured by the visual analog scale (VAS). d “Tired” measured by the visual analog scale (VAS; T bars indicate SEMs)
Visual analog scale feeling states
VAS “high,” “anxious,” and “panic” data were highly skewed, while “calm and relaxed” and “tired” data were approximately normal. Δ-9-THC produced significant increases in VAS “high” (ATS=44.6, num df=3.2, P<0.0001) and “tired” (F[5, 27]=5.01, P=0.0022) but did not significantly change any other feeling states. There were no significant effects of haloperidol or haloperidol by Δ-9-THC-interactive effects on any of these outcomes (Fig. 1c and d).
Similarity to cannabis and enjoyment of the experience
Data on these measures were skewed (Table 4). Δ-9-THC produced significant increases in ratings of the similarity of the experience to cannabis (ATS=116.5, num df=1, P< 0.0001) and enjoyment of the experience (ATS=41.7, num df=1, P<0.0001). Haloperidol reduced ratings of enjoyment of the experience (ATS=8.0, num df=1, P=0.005). There were no significant interactive effects of haloperidol and Δ-9-THC.
Table 4.
Behavioral, subjective, and motor results (means [±SD])
| Outcome | Placebo haloperidol |
Active haloperidol |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −90 | −30 | 15 | 65 | 140 | 190 | −90 | −30 | 15 | 65 | 140 | 190 | ||
| Pre THC | Placebo THC | Active THC | Pre THC | Placebo THC | Active THC | ||||||||
| PANSS | Positive | 7.7 (0.76) | 7.78 (0.8) | 7.78 (0.8) | 7.87 (0.81) | 9.13 (1.39) | 8.26 (1.14) | 7.76 (0.93) | 7.88 (1.05) | 7.92 (1.08) | 8.04 (1.17) | 8.64 (1.29) | 8.25 (1.54) |
| Total | 30.7 (3.53) | 30.83 (2.72) | 31.04 (3.08) | 30.83 (2.44) | 35.5 (3.6) | 31.39 (2.25) | 30.44 (2.65) | 30.6 (2.6) | 30.96 (2.79) | 31.36 (3.05) | 34.58 (2.89) | 32.21 (3.27) | |
| CADSS | Subject rated | 0.22 (0.6) | 0.26 (0.69) | 0.13 (0.46) | 0.22 (0.6) | 3.96 (4.64) | 1.17 (2.12) | 0.28 (0.68) | 0.32 (1.07) | 0.38 (0.77) | 0.2 (0.58) | 2.24 (2.68) | 0.84 (1.57) |
| Clinician rated | 0.09 (0.29) | 0.14 (0.35) | 0.17 (0.65) | 0.17 (0.65) | 2.43 (2.74) | 0.52 (1.08) | 0.04 (0.2) | 0 (0) | 0.24 (0.6) | 0.28 (0.89) | 1.96 (2.07) | 0.92 (1.41) | |
| VAS | Anxious | 5.52 (13.5) | 3.65 (10.45) | 1.7 (4.19) | 2.65 (6.11) | 4.13 (8.15) | 1.91 (5.2) | 8.8 (13.3) | 4.48 (9.24) | 2.92 (5.28) | 1.4 (2.74) | 4.28 (10.44) | 6.96 (19.77) |
| Calm | 76.91 (21.9) | 78.96 (20.5) | 76.74 (25.65) | 76.74 (23.86) | 80.13 (25) | 80.43 (19.82) | 69.76 (27.16) | 74.2 (25.31) | 77.76 (18.82) | 72.96 (23.36) | 73.6 (30.7) | 70.67 (25.65) | |
| High | 0.48 (0.99) | 0.7 (1.11) | 2.52 (5.23) | 2.04 (4.57) | 39.32 (31.15) | 18.13 (20.9) | 0.12 (0.33) | 2.72 (10.06) | 2.4 (5.63) | 1.13 (4.08) | 34.96 (30.71) | 18.88 (21.88) | |
| Panic | 0.65 (1.23) | 0.65 (1.19) | 0.57 (0.95) | 0.61 (1.12) | 0.74 (1.18) | 0.91 (1.44) | 0.28 (0.61) | 0.32 (0.69) | 0.48 (1.05) | 0.4 (0.87) | 1.4 (3.11) | 1.29 (2.58) | |
| Tired | 24.26 (25.5) | 28.13 (28.88) | 29.52 (24.26) | 24 (25.39) | 34.09 (25.91) | 22.3 (27.26) | 13.4 (18.31) | 19.28 (22.47) | 24.4 (25.7) | 27.12 (27.22) | 36.56 (26.42) | 26 (26.36) | |
| Similarity to cannabis | 4.13 (10.99) | 59.17 (30.64) | 5.52 (15.58) | 55.24 (29.65) | |||||||||
| Enjoyment of experience | 31.43 (25.97) | 61.04 (26.64) | 19.88 (22.98) | 47 (26.24) | |||||||||
PANSS Positive and Negative Syndrome Scale, CADSS Clinician Administered Dissociative Symptoms Scale, VAS visual analog scale
Verbal learning and recall
Immediate recall data were approximately normal while all other outcomes were highly skewed.
No significant practice effects both within and across test days were detected on verbal recall or any of the other cognitive measures (Table 4).
Immediate recall
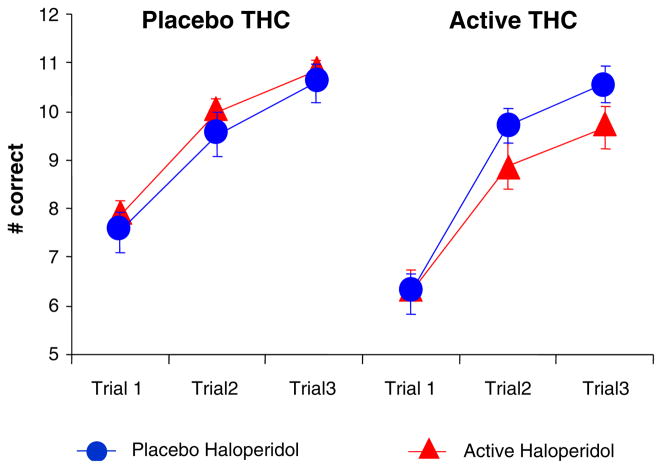
Δ-9-THC significantly impaired immediate total recall (F[1, 27]=12.49, P=0.0015). Recall improved significantly across trials (learning) (F[2, 54]=145.9, P=0.0001). There was a trend toward a significant interaction between haloperidol and Δ-9-THC (F[1, 19]=3.4, P=0.08) with the haloperidol further worsening Δ-9-THC-induced recall deficits. There was a significant three-way interaction between haloperidol, Δ-9-THC, and group (F[1, 18]=6.0, P=0.025); this was a result of the combined effect of haloperidol and Δ-9-THC in producing significant recall deficits in healthy subjects (F[1, 18]=10.43, P=0.0046) but not in frequent users (F[1, 18]=2.66, P=0.12; Fig. 2).
Fig. 2.
Immediate verbal recall measured by the Hopkins Verbal Learning Task (T bars indicate SEMs). Δ-9-THC impaired immediate recall and haloperidol worsened Δ-9-THC induced immediate recall impairments
Delayed free, cued, and recognition recall
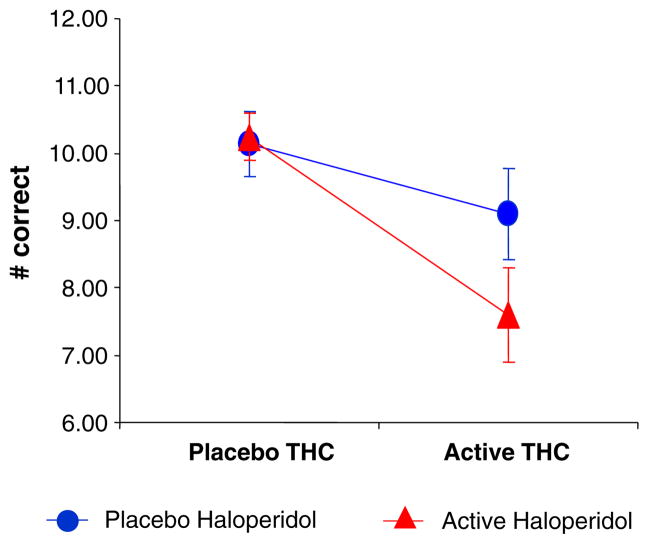
Δ-9-THC (ATS=8.9, num df=1, P=0.0028) and haloperidol (ATS=3.6, num df=1, P=0.056) impaired delayed free recall. Δ-9-THC but not haloperidol impaired delayed cued recall (ATS=5.6, num df=1, P=0.0185). The interaction between haloperidol and Δ-9-THC was significant for delayed free recall (ATS=4.2, num df=1, P=0.04) and delayed cued recall (ATS=8.9, num df=1, P=0.003) with both effects explained by haloperidol further worsening Δ-9-THC-induced recall impairments. Neither Δ-9-THC nor haloperidol impaired delayed recognition recall (Fig. 3).
Fig. 3.
Delayed verbal recall measured by the Hopkins Verbal Learning Task (T bars indicate SEMs). Δ-9-THC impaired delayed free recall and haloperidol worsened Δ-9-THC induced delayed free recall impairments
Errors
Δ-9-THC significantly increased the number of intrusions (ATS=7.9, num df=1, P=0.005) and false-positive (ATS= 22.1, num df=1, P<0.0001) responses but not perseverations. The interaction between haloperidol and Δ-9-THC trended to increase false-positive responses (ATS=3.5, num df=1, P=0.06) with the effects explained by haloperidol further increasing Δ-9-THC false-positive responses.
Attention
Both vigilance and distractibility data were highly skewed (Table 4).
Vigilance
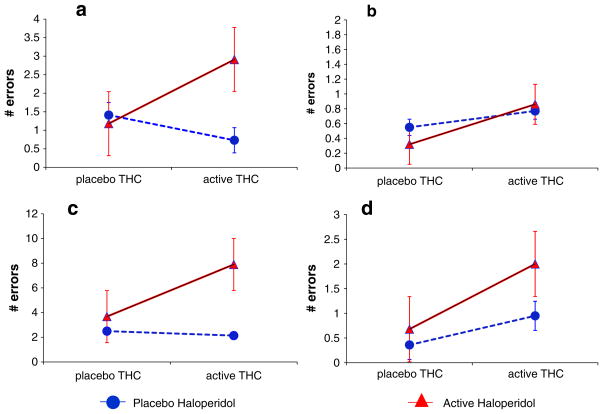
Haloperidol (ATS=4.4, num df=1, P=0.037) and the interaction of Δ-9-THC and haloperidol (ATS=5.4, num df=1, P= 0.02) but not Δ-9-THC alone significantly increased omission errors. There were no significant effects of Δ-9-THC, haloperidol, or group on commission errors (Fig. 4a and b).
Fig. 4.
a, b Vigilance omission and commission errors measured by the Gordon Box Continuous Performance Task (CPT). Haldol alone and in combination with Δ-9-THC increased omission errors. c, d Distractibility omission and commission errors measured by the Gordon Box CPT. Δ-9-THC alone and haloperidol alone increased omission and commission errors. T bars indicate SEMs
Distractibility
Δ-9-THC (ATS=10.0, num df=1, P=0.0016), haloperidol (ATS=25.6, num df=1, P<0.0001), and the interaction of Δ-9-THC and haloperidol (ATS=7.0, num df=1, P=0.008) significantly increased omission errors. The latter was driven by the effects of haloperidol. Haloperidol significantly (ATS=6.1, num df=1, P=0.014) and Δ-9-THC trended (ATS=3.5, num df=1, P=0.06) to increase commission errors, but there were no significant interactive effects between the two (Fig. 4c and d).
CANTAB
Motor screening
Δ-9-THC (F[1, 64]=6.4, P=0.01) but not haloperidol or the interactions between the two significantly reduced mean latency motor performance (Table 4).
Spatial working memory
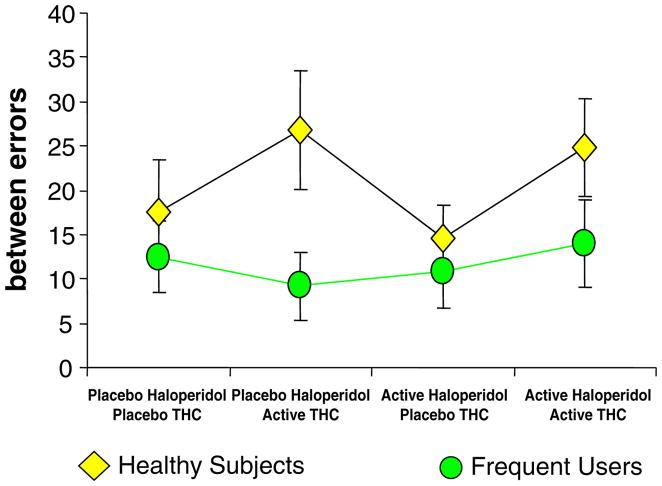
Between-errors data were normalized by log transformation. Δ-9-THC increased between (F[1, 26]=5.2, P=0.03) but not within errors, while haloperidol had no effects on either type of error. This was one of the few measures where there was a significant interactive effect of group and Δ-9-THC on between errors (F[1, 25]=4.2, P=0.05); this was due to healthy subjects having higher Δ-9-THC induced between errors (F[1, 25]=9.88, P=0.004; Fig. 5).
Fig. 5.
Spatial working memory measured by the CANTAB (Cambridge Neuroscience Battery; T bars indicate SEMs). Δ-9-THC and the combination of Δ-9-THC increased between errors only in healthy subjects
Stockings of Cambridge
There were no significant effects of Δ-9-THC, haloperidol, group, or any interactions between the two on the number of occasions that subjects successfully replicated a reference pattern in the minimum possible number of moves.
Delayed match to sample task
Haloperidol significantly reduced percent correct performance (F[1, 62]=6.0, P=0.017). There were no effects of Δ-9-THC, group, or any interactions between Δ-9-THC, group, or haloperidol on any of the measures.
Akathisia, rigidity, and tremor
All outcomes were highly skewed. Δ-9-THC (ATS=5.1, num df=1.6, P<0.0098) but not haloperidol increased Barnes Global Akathisia scores. Neither Δ-9-THC nor haloperidol had any effect on tremor or rigidity (see Table S1 in Electronic supplementary material). There were no significant effects of haloperidol or group or the interactions between haloperidol, Δ-9-THC, and group on any of the other motor outcomes.
Neuroendocrine effects
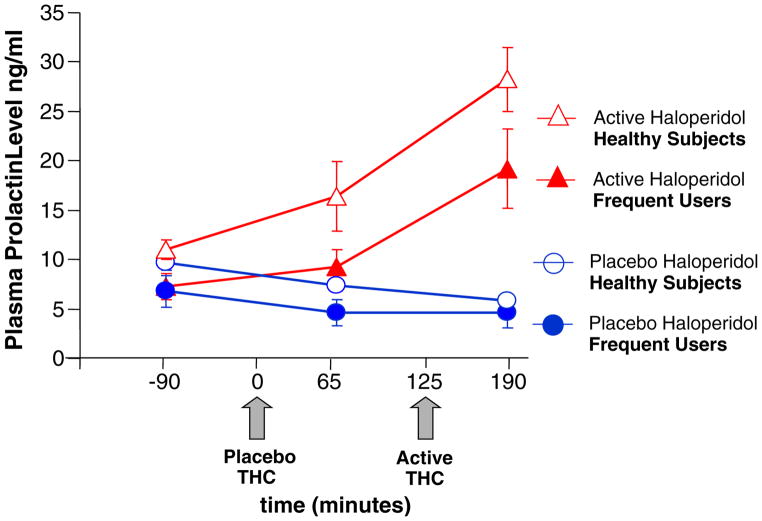
Prolactin and cortisol levels were approximately normally distributed after log transformation. Serum cortisol levels decreased over time (F[2, 107]=51.05, P<0.0001), but there were no significant effects of haloperidol or interactions between haloperidol and Δ-9-THC (Fig. 6).
Fig. 6.
Plasma prolactin levels (T bars indicate SEMs). Lower baseline prolactin levels in frequent users. Haloperidol but not Δ-9-THC increases prolactin levels
Haloperidol increased (F[1, 107]=77.7, P<0.0001) and time/Δ-9-THC decreased (F[2, 107]=10.8, P≤0.0001) serum prolactin levels. The interaction between haloperidol and time/Δ-9-THC was significant (F[2, 107]=56.8, P< 0.0001) where prolactin levels on the active haloperidol condition were significantly greater at 65 (F[1, 107]=15.8, P=0.0001) and 190 min (F[1, 107]=144.5, P<0.0001) but not at baseline (not significant). Of note, serum prolactin levels were lower in frequent users of cannabis (F[1, 102]=7.1, P=0.009).
Discussion
This is the first published report to our knowledge on the interactions between haloperidol and Δ-9-THC in humans. The principal finding of this study is that haloperidol, at a dose that produced effects consistent, e.g., with prolactin elevation with acute antagonism of DA D2 receptors, failed to reduce any Δ-9-THC effects. Instead, haloperidol worsened some of the cognitive effects of Δ-9-THC.
Effects of haloperidol alone
Haloperidol worsened performance on some cognitive tasks similar to other reports (Saeedi et al. 2006). As expected, haloperidol increased serum prolactin levels. In addition, the observation that haloperidol did not produce any obvious behavioral or motor effects confirms the appropriateness of the dose that was selected. At this dose, most of haloperidol’s effects would be expected to be related to its antagonism of DA D2 receptors. Its affinity for histaminic H1 and muscarinic M1 receptors is greater than 600 and greater than 2,000 times less than its affinity for DA D2 receptors. Similarly, any interactions between Δ-9-THC and haloperidol most likely converge through DAergic systems.
Effects of Δ-9-THC alone
The dose and rate of Δ-9-THC administration in this study were chosen in an attempt to mimic recreational cannabis use. The observation that subjects reported that the experience was similar to cannabis and that they enjoyed it supports the generalizability of the findings to recreational cannabis use.
The effects of intravenous Δ-9-THC observed in this study were similar to but of smaller magnitude than the effects produced by higher doses of intravenous Δ-9-THC (2.5 and 5 mg over 2 min; D’Souza et al. 2004, 2005, 2006). For example, the Δ-9-THC dose (2 mg over 20 min in a 70-kg individual) in this study produced peak self-rated perceptual alteration CADSS score (3.96) nearly four times lower than the effects (14.95) of a higher Δ-9-THC dose (5 mg over 2 min) (D’Souza et al. 2004). Nevertheless, the peak increases in PANSS positive symptoms scores induced by this Δ-9-THC dosing paradigm were comparable to the peak increases in positive symptom scores induced by (1) amphetamine 0.25 mg/kg administered i.v. over 1 min (Krystal et al. 2005), (2) low-dose ketamine (bolus 0.081 mg/kg over 10 min followed by an infusion of 0.4 mg kg−1 h−1; Krystal et al. 2006), and m-chlorphenyl-piperazine (D’Souza et al. 2006). As discussed earlier, we attempted a shorter infusion and higher dose of Δ-9-THC in combination with haloperidol, but this was poorly tolerated.
Some of the cognitive effects of Δ-9-THC that have been well described in animals but not in humans were measured in this study. Cannabinoids impair spatial memory in animals (Aigner 1988; Carlini et al. 1970a, b; Winsauer et al. 1999), and we now show for the first time in humans that Δ-9-THC impairs SWM as evidenced by an increase in between but not within errors. This profile of effects suggests that while subjects were able to perform the basic task, the longer they had to maintain information online, the worse they performed. Further, the observation that Δ-9-THC did not impair performance in frequent users of cannabis (Fig. 5) is consistent with the preclinical observations of tolerance to the effects of cannabinoids on SWM (Lichtman et al. 1995). Further, the absence of an effect in frequent users of cannabis is important to consider in interpreting the cannabis literature, as most studies have included frequent or heavy users of cannabis. Finally, the observation of group differences only on the SWM task suggests that the latter may be most sensitive in detecting differences between frequent users and healthy subjects.
In animals, cannabinoids impair performance on the DMTS, a visual recognition memory task, only when the delay is long (Heyser et al. 1993; Winsauer et al. 1999). Δ-9-THC did not impair DMTS in this study even when the delay was long. Other studies of Δ-9-THC on DMTS performance have had mixed results (Heishman et al. 1997; Lane et al. 2005). Perhaps, differences in the delay periods, other task parameters, Δ-9-THC doses, and degree of cannabinoid tolerance across samples might account for the disparate results. Consistent with a wealth of clinical data (reviewed in Ranganathan and D’Souza 2006), Δ-9-THC impaired several aspects of verbal recall. The fact that Δ-9-THC impaired free verbal recall but not visual and verbal recognition recall suggests that Δ-9-THC does not impair encoding but rather impairs retrieval and/or consolidation.
While Δ-9-THC reduced the number of problems solved in minimum moves on the SOC task (Table 5), these effects were not statistically significant. Of note, Ramaekers et al. (2006) showed that higher doses of Δ-9-THC impair performance on a task analogous to the SOC, the Tower of London task. They went on to argue that Δ-9-THC effects on executive function may be relatively small at low doses, similar to those used in this study, but may become “very substantial” at higher doses.
Table 5.
Cognitive measures results (mean [±SD])
| Outcome | Placebo haloperidol |
Active haloperidol |
||||
|---|---|---|---|---|---|---|
| Placebo THC | Active THC | Placebo THC | Active THC | |||
| Verbal recall (HVLT) | Total immediate recall | 27.61 (5.25) | 26.52 (4.53) | 28.64 (3.39) | 24.84 (6.27) | |
| Delayed recall | Free | 10.13 (2.32) | 9.09 (3.25) | 10.24 (1.74) | 7.60 (3.46) | |
| Cued | 10.00 (2.22) | 10.30 (1.58) | 10.40 (1.78) | 8.72 (2.41) | ||
| Recognition | 11.57 (0.66) | 11.65 (0.65) | 11.80 (0.50) | 11.32 (1.22) | ||
| False positives | 0.57 (0.90) | 1.22 (1.68) | 0.36 (0.76) | 1.68 (1.97) | ||
| Vigilance | Omissions | 1.41 (2.89) | 0.68 (1.21) | 1.18 (2.22) | 2.91 (4.23) | |
| Commissions | 0.55 (0.91) | 0.77 (1.23) | 0.32 (0.57) | 0.86 (1.58) | ||
| Distractibility | Omissions | 2.50 (4.70) | 2.14 (2.98) | 3.68 (4.36) | 7.90 (6.66) | |
| Commissions | 0.36 (0.58) | 0.95 (1.81) | 0.68 (1.25) | 2.00 (2.66) | ||
| CANTAB | Motor function (MOT) | Latency | 773.05 (198.3) | 747.95 (224.6) | 790 (155.09) | 717.84 (165.04) |
| Planning and execution (SOC) | Minimum moves | 9.3 (1.69) | 9.22 (1.98) | 9.04 (1.9) | 8.33 (2.01) | |
| Spatial working memory | Between errors | 15.52 (18.74) | 19.91 (22.3) | 13.17 (13.48) | 20.29 (19.08) | |
| Within errors | 1.43 (2.86) | 1.74 (3.79) | 1.71 (4.37) | 0.75 (1.07) | ||
| Total errors | 16.17 (19.38) | 20.48 (23.24) | 14.29 (14.25) | 20.63 (19.34) | ||
| Visual recognition memory (DMTS) | Percent correct | 87.54 (8.6) | 86.67 (10.05) | 82.13 (12.28) | 80.83 (12.17) | |
HVLT Hopkins Verbal Recall Test, MOT motor screening task, SOC Stockings of Cambridge, DMTS delayed match to sample
Δ-9-THC also reduced motor latency. Others have reported that Δ-9-THC produces an increase in the speed of responding along with worse performance suggesting that under the influence of cannabinoids, individuals trade speed for accuracy (Curran et al. 2002).
Interactive effects of haloperidol and Δ-9-THC
Behavioral and subjective outcomes
While both PANSS positive symptoms (Fig. 1a) and CADSS perceptual alterations (Fig. 1b) scores induced by Δ-9-THC were lower on the active vs the placebo haloperidol condition (see Table 4), these effects were not statistically significant. These results are not consistent with the report that “cannabis-induced psychosis” is responsive to treatment with DA D2 receptor antagonists (Berk et al. 1999). In contrast, the current results are consistent with the observation that Δ-9-THC increased psychotomimetic symptoms in schizophrenic patients despite chronic treatment with DA D2 receptor antagonists (D’Souza et al. 2005). Perhaps, the effects of Δ-9-THC better model those positive symptoms that are resistant to DA D2 receptor antagonist antipsychotic drugs. Further, whereas Δ-9-THC produces positive and negative symptoms and cognitive deficits (D’Souza et al. 2004, 2005), amphetamine produces predominantly positive symptoms (Angrist et al. 1974b; Angrist and Gershon 1970; Griffith et al. 1972). Finally, haloperidol reverses psychosis induced by amphetamine (Angrist et al. 1974a). However, haloperidol pretreatment did not reduce the psychosis induced by ketamine (Krystal et al. 1999), lysergic acid diethylamide, and, in the current study, Δ-9-THC. Taken collectively, this suggests that DA D2 mechanisms may not play a major role in the pathophysiology of Δ-9-THC-induced psychotomimetic effects. Perhaps, the use of a single and relatively low dose of haloperidol combined with the small increases in Δ-9-THC-induced psychotomimetic symptoms may explain the lack of a haloperidol effect in this study. Alternatively, the failure of haloperidol to block Δ-9-THC-induced psychotomimetic effects reflects a limitation in the relevance of the cannabinoid “model” of psychosis.
Similarly, the lack of any effect of haloperidol on Δ-9-THC-induced VAS measured subjective effects (“high,” “tired,” etc.) is consistent with our observations that chronic antipsychotic treatment did not blunt the euphoric effects of Δ-9-THC in schizophrenic patients (D’Souza et al. 2005). The failure of haloperidol to block the euphoric effects of Δ-9-THC was surprising given that the rewarding properties of cannabinoids, like other drugs of abuse, have been associated with an increased activity of mesolimbic DA transmission (Chen et al. 1990b, 1991; Fadda et al. 2006; French 1997; French et al. 1997; Ng Cheong Ton et al. 1988; Patel and Hillard 2003; Tanda et al. 1997a, b). Relevant to both the euphoric and psychotomimetic effects of Δ-9-THC, the DA D2 receptor antagonist sulpiride failed to block Δ-9-THC-induced c-fos expression in both the striatum and nucleus accumbens (Miyamoto et al. 1996). Regardless, the current findings suggest that acute DA D2 receptor blockade antagonists may not be useful in cannabis addiction or psychosis, but whether repeated dosing is useful remains unclear.
Cognitive outcomes
Together, haloperidol and Δ-9-THC impaired sustained attention (increased omission errors on both vigilance and distractibility tasks) and immediate and delayed verbal recall. Further, while the interactive effects of haloperidol and Δ-9-THC on visual recognition memory, SWM, and planning and execution did not reach statistical significance, the effects were in the direction of the combination of the two drugs worsening performance to a greater extent than either drug alone. The observed findings were not predicted and at present can only be speculated upon. Attention, execution function, visual recognition memory, SWM, verbal learning, and recall all require varying contributions of and complex interactions between the prefrontal cortex (PFC) and hippocampus. For instance, cannabinoids could alter PFC and hippocampal function via their effects on the release of DA, acetylcholine, glutamate, or norepinephrine. While it is out of the scope of this paper to discuss all these possibilities, we briefly discuss some of the preclinical evidence of crosstalk between CB1 and DA systems in the PFC, which might explain some of the interactive cognitive effects of haloperidol and Δ-9-THC.
Systemically administered cannabinoids can modulate the activity of DAergic pathways in the PFC either directly or indirectly, by influencing the activity of DAergic neurons through either post- or presynaptic mechanisms (Egerton et al. 2006; Laviolette and Grace 2006b; Pistis et al. 2002). CB-induced DAergic hyperactivity in the PFC may contribute to working memory deficits associated with CB exposure (Diana et al. 1998; Jentsch et al. 1997). Acute administration of haloperidol may result in acute DA D2 blockade but also compensatory DA release in the medial PFC and nucleus accumbens (Liegeois et al. 2002; Moghaddam and Bunney 1990; Moghaddam et al. 1990). Thus, the combination of Δ-9-THC and haloperidol may result in additive DA release in the PFC. Given that either too high or low DAergic activity in the PFC can lead to impairments in PFC-related cognitive functions (Goldman-Rakic 1996; Murphy et al. 1996; Zahrt et al. 1997), this may explain the observations that the combination of haloperidol and Δ-9-THC worsened cognition.
Prolactin
Δ-9-THC produces an early and brief increase followed by a predominantly inhibitory effect on prolactin release (Harclerode 1984; Murphy et al. 1998) that is believed to be mediated by CB-1R activation of tuberoinfundibular (TIDA) DA neurons (Rodriguez De Fonseca et al. 1992). Of note, frequent users of cannabis had lower prolactin levels. Prolactin release is under tonic DA control (Selmanoff 1981), and prolactin controls its own release by altering DA release (Gregerson and Selmanoff 1988). Thus, the lower-baseline prolactin levels in frequent users may reflect increased DA tone in the TIDA pathway of frequent users that is related to either residual cannabis effects or long-term adaptive changes in DA function in response to chronic cannabis exposure. In a previous study, 5 mg but not 2.5 mg Δ-9-THC increased prolactin levels in healthy human subjects (D’Souza et al. 2004). Perhaps, the lack of a significant acute effect of Δ-9-THC on plasma prolactin in this study may be explained by the small dose (2 mg) and limited sampling.
Limitations
There were several limitations to this study. First, the use of multiple doses of haloperidol and Δ-9-THC may have been more informative than the current design but was not feasible, as discussed earlier. Second, because haloperidol has affinity for other receptors (D1 and α1), any interactions of Δ-9-THC and haloperidol cannot be attributed solely to interactions with D2 receptors. Third, the smaller magnitude of Δ-9-THC induced behavioral and cognitive effects in this study compared to previous studies, which used higher doses and a faster infusion (D’Souza et al. 2004, 2005), may have reduced the likelihood of detecting a beneficial effect of haloperidol. Fourth, the limited sample size may, in part, contribute to the lack of significant findings. For example, the difference in Δ-9-THC-induced increase in PANSS positive symptom score between the placebo and active haloperidol test days was 0.65 (SD 1.56). This translates into a small to medium effect size (d=0.42) for the reduction in Δ-9-THC-induced positive symptoms, which, with 28 subjects, would not be adequately powered to detect. However, with 28 subjects, we still had 80% statistical power to detect medium/large effects size (d= 0.55) with 80% statistical power for any given outcome. Fifth, a random order of Δ-9-THC (placebo or active) administration would have been preferred. Sixth, in contrast to the cognitive assessments, the behavioral and subjective effects were measured repeatedly during each Δ-9-THC session. This should be noted in interpreting the effects of haloperidol pretreatment on Δ-9-THC effects.
Conclusions
The current study replicates a growing body of research characterizing the cognitive effects, perceptual altering, psychotomimetic, and euphoric of Δ-9-THC. The failure of haloperidol pretreatment to antagonize the psychotomimetic effects of Δ-9-THC suggests that these effects of Δ-9-THC are not likely mediated by DA D2 receptor mechanisms and that novel pharmacologic approaches will be needed to block these effects. Given the growing interest in the link between cannabinoids and psychosis, future research should be directed toward understanding the precise mechanisms underlying the psychotomimetic symptoms produced by Δ-9-THC. While admittedly speculative, the search for drugs that block the psychotomimetic symptoms produced by Δ-9-THC may lead to novel approaches to the treatment of psychotic disorders. The deleterious effect of haloperidol pretreatment on the cognitive effects of Δ-9-THC is consistent with the preclinical literature in suggesting crosstalk between DAergic and CBergic systems. However, further work is necessary to fully understand the crosstalk between the two systems in humans.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the critical clinical research contributions of the Biological Studies Unit, VA Connecticut Healthcare System, including Elizabeth O’Donell, R.N; Angelina Genovese, R.N.; Sonah Yoo, R.Ph.; Robert Sturwold, R.Ph., and Mr. Willie Ford. This study was supported by the National Institute of Drug Abuse (DA12382-01 to DCD). In addition, the authors acknowledge support from the (1) Department of Veterans Affairs Schizophrenia Biological Research Center (John Krystal), (2) National Institute of Mental Health (MH61019-02 to DCD), (3) National Institute of Alcohol Abuse and Alcoholism (R03 AA11413-02 to DCD), (4) Stanley Medical Research Institute (DCD), and (5) Donaghue Foundation (DCD).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-007-1042-2) contains supplementary material, which is available to authorized users.
Disclosure/Conflict of interest None of the authors have any potential conflict/s of interest relating to the subject of the report.
Contributor Information
Deepak Cyril D’Souza, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA; Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, CT, USA; Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Psychiatry Service, 116A, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, CT 06516, USA, deepak.dsouza@yale.edu.
Gabriel Braley, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA; Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Rebecca Blaise, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA; Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Michael Vendetti, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA; Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Stephen Oliver, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA; Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Brian Pittman, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, CT, USA.
Mohini Ranganathan, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Savita Bhakta, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Zoran Zimolo, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA.
Thomas Cooper, Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Edward Perry, Schizophrenia Biological Research Center, VA Connecticut Healthcare System, West Haven, CT, USA.
References
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- Aigner TG. Delta-9-tetrahydrocannabinol impairs visual recognition memory but not discrimination learning in rhesus monkeys. Psychopharmacology. 1988;95:507–511. doi: 10.1007/BF00172964. [DOI] [PubMed] [Google Scholar]
- Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis–preliminary observations. Biol Psychiatry. 1970;2:95–107. [PubMed] [Google Scholar]
- Angrist B, Lee HK, Gershon S. The antagonism of amphetamine-induced symptomatology by a neuroleptic. Am J Psychiatry. 1974a;131:817–819. doi: 10.1176/ajp.131.7.817. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974b;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Berk M, Brook S, Trandafir AI. A comparison of olanzapine with haloperidol in cannabis-induced psychotic disorder: a double-blind randomized controlled trial. Int Clin Psychopharmacol. 1999;14:177–1780. [PubMed] [Google Scholar]
- Brandt J, Corwin J, Krafft L. Is verbal recognition memory really different in Huntington’s and Alzheimer’s disease. J Clin Exp Neuropsychol. 1992;14:773–784. doi: 10.1080/01688639208402862. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. Wiley; New York: 2002. [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Bylsma FW, Rebok GW, Brandt J. Long-term retention of implicit learning in Huntington’s disease. Neuropsychologia. 1991;29:1213–1221. doi: 10.1016/0028-3932(91)90035-7. [DOI] [PubMed] [Google Scholar]
- Carlini EA, Hamaoui A, Bieniek D, Korte F. Effects of (−) delta-9-trans-tetrahydrocannabinol and a synthetic derivative on maze performance of rats. Pharmacology. 1970a;4:359–368. doi: 10.1159/000136165. [DOI] [PubMed] [Google Scholar]
- Carlini EA, Santos M, Claussen U, Bieniek D, Korte F. Structure activity relationship of four tetrahydrocannabinols and the pharmacological activity of five semi-purified extracts of Cannabis sativa. Psychopharmacologia. 1970b;18:82–93. doi: 10.1007/BF00402387. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990a;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990b;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL. Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett. 1991;129:136–180. doi: 10.1016/0304-3940(91)90739-g. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Jama. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- D’Souza DC. Cannabinoids and psychosis. Int Rev Neurobiol. 2007;78:289–326. doi: 10.1016/S0074-7742(06)78010-2. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Gil RB, Zuzarte E, MacDougall LM, Donahue L, Ebersole JS, Boutros NN, Cooper T, Seibyl J, Krystal JH. Gamma-aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Biol Psychiatry. 2006;59:128–137. doi: 10.1016/j.biopsych.2005.06.020. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted Psychotomimetic and Amnestic Effects of Delta-9-tetrahydrocannabinol in Frequent Users of Cannabis. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301643. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10:2825–2830. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. Bmj. 2006;332:172–175. doi: 10.1136/bmj.332.7534.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders—non-patient edition. American Psychiatric Association; Washington, DC: 2002. [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Microprocessor-based assessment of attention deficit disorders (ADD) Psychopharmacol Bull. 1986;22:288–290. [PubMed] [Google Scholar]
- Gregerson KA, Selmanoff M. Selective effects of hyper-prolactinemia on in vitro dopamine release from median eminence synaptosomes. J Neurosci. 1988;8:2477–2484. doi: 10.1523/JNEUROSCI.08-07-02477.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Cavanaugh J, Held J, Oates JA. Dextroamphetamine. Evaluation of psychomimetic properties in man. Arch Gen Psychiatry. 1972;26:97–100. doi: 10.1001/archpsyc.1972.01750200001001. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Addiction Research Center Inventory (ARCI): development of a general drug estimation scale. J Nerv Ment Dis. 1965;141:300–307. doi: 10.1097/00005053-196509000-00006. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Harclerode J. Endocrine effects of marijuana in the male: preclinical studies. In: Braude MC, Ludford JP, editors. NIDA Research Monograph Series. National Institute on Drug Abuse; Rockville, MD: 1984. pp. 45–114. [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of Cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M, Ramaekers JG, van Os J. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Jarrahian A, Watts VJ, Barker EL. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. J Pharmacol Exp Ther. 2004;308:880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr, Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Verrico CD, Le D, Roth RH. Repeated exposure to delta 9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neurosci Lett. 1998;246:169–172. doi: 10.1016/s0304-3940(98)00254-7. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Roy P, Jones C, Remington G, Reed K, Houle S. The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berl) 1997;131:148–152. doi: 10.1007/s002130050277. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatr Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- King DJ. Guidelines for the use of antipsychotic drug studies in healthy volunteers. The BAP Consensus Group. J Psychopharmacol. 1997;11:201–209. doi: 10.1177/026988119701100302. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Hasegawa T, Katsumata Y, Kameyama T, Yamamoto I, Nabeshima T. Effect of dizocilpine (MK-801) on the catalepsy induced by delta 9-tetrahydrocannabinol in mice. J Neural Transm Gen Sect. 1994;95:137–143. doi: 10.1007/BF01276432. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, Belger A, D’Souza DC. Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism. Neuropsychopharmacology. 2006;31:1793–1800. doi: 10.1038/sj.npp.1300994. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, Tcheremissine OV. Marijuana effects on human forgetting functions. J Exp Anal Behav. 2005;83:67–83. doi: 10.1901/jeab.2005.22-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006a;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006b;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Thies M, Munte TF, Emrich HM. Effects of synthetic delta9-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology (Berl) 1999;142:230–235. doi: 10.1007/s002130050884. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intra-hippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Liegeois JF, Ichikawa J, Meltzer HY. 5-HT(2A) receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Res. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- Marchese G, Casti P, Ruiu S, Saba P, Sanna A, Casu G, Pani L. Haloperidol, but not clozapine, produces dramatic catalepsy in delta9-THC-treated rats: possible clinical implications. Br J Pharmacol. 2003;140:520–526. doi: 10.1038/sj.bjp.0705478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GW, Wilkinson DA, Kapur BM. Validation of self-reported Cannabis use by urine analysis. Addict Behav. 1988;13:147–150. doi: 10.1016/0306-4603(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Yamamoto T, Ohno M, Watanabe S, Tanaka H, Morimoto S, Shoyama Y. Roles of dopamine D1 receptors in delta 9-tetrahydrocannabinol-induced expression of Fos protein in the rat brain. Brain Res. 1996;710:234–240. doi: 10.1016/0006-8993(95)01352-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Roth RH, Bunney BS. Characterization of dopamine release in the rat medial prefrontal cortex as assessed by in vivo microdialysis: comparison to the striatum. Neuroscience. 1990;36:669–676. doi: 10.1016/0306-4522(90)90009-s. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LL, Munoz RM, Adrian BA, Villanua MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- Nagai H, Egashira N, Sano K, Ogata A, Mizuki A, Mishima K, Iwasaki K, Shoyama Y, Nishimura R, Fujiwara M. Antipsychotics improve Delta(9)-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacol Biochem Behav. 2006;84:330–336. doi: 10.1016/j.pbb.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid-induced Fos expression within A10 dopaminergic neurons. Brain Res. 2003;963:15–25. doi: 10.1016/s0006-8993(02)03797-6. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza D. the acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Perry E, Braley G, Cooper T, Gueorguieva R, Krystal J, D’Souza DC. Blunted “negative” but not “positive” effects of thc in cannabis abusers: implications for cannabis use in schizophrenia. In: Schulz CTaC., editor. Schizophrenia Bulletin; International Congress on Schizophrenia Research; Colorado Springs; 2007. p. 477. [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Fernandez-Ruiz JJ, Murphy LL, Cebeira M, Steger RW, Bartke A, Ramos JA. Acute effects of delta-9-tetrahydrocannabinol on dopaminergic activity in several rat brain areas. Pharmacol Biochem Behav. 1992;42:269–275. doi: 10.1016/0091-3057(92)90526-l. [DOI] [PubMed] [Google Scholar]
- Saeedi H, Remington G, Christensen BK. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr Res. 2006;85:222–231. doi: 10.1016/j.schres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration. Rockville, MD: 2004. Results from the 2003 National Survey on Drug Use and Health: national findings. [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Selmanoff M. The lateral and medial median eminence: distribution of dopamine, norepinephrine, and luteinizing hormone-releasing hormone and the effect of prolactin on catecholamine turnover. Endocrinology. 1981;108:1716–1722. doi: 10.1210/endo-108-5-1716. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Humana; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R-patient edition (SCID-P, version 1.0) American Psychiatric; Washington, DC: 1990. [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997a;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism [comment] Science. 1997b;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003;49:61–66. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wall ME, Brine DR, Perez-Reyes M. Metabolism of cannabinoids in man. In: Braude MC, Szara S, editors. Pharmacology of marihuana. Vol. 1. Raven; New York: 1976. p. 536. [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.