Abstract
We consider the long lived pool of B and T cells that recirculate through blood, tissues and the lymphatic system of an animal with body mass M. We derive scaling rules (allometric relations) for: (1) the rate of production of mature lymphocytes; (2) the accumulation of lymphocytes in the tissues; (3) the flux of lymphocytes through the lymphatic system; (4) the number of lymph nodes, (5) the number of lymphocytes per clone within a lymph node, and (6) the total number of lymphocytes within a lymph node. Mass-dependent aspects of immune learning and of the immunological self are shown to be not very significant. Our treatment is somewhat heuristic and aims at a combination of immunological data with recent progress in biological scaling.
1. Introduction
The long-lived pool of B and T cells that recirculate throughout the body in the blood and lymph have been studied for over 30 years. In an early review, G.I. Bell summarized what was then known about these traffic patterns (Bell, 1978). He derived numerical values for some of the important parameters and noticed that “the source of lymphocytes from proliferation in the bone marrow far exceeds that required to maintain the recirculating pool”. In the present paper we revisit the issues Bell considered, especially in view of the progress made in the understanding of the scaling properties of organisms, i.e., the way in which basic features of a living organism depend on its mass (Peters, 1983; Schmidt-Nielsen, 1984; Calder, 1996; Brown & West, 2000; West & Brown, 2005; Bonner, 2006). Here we use new ideas on biological scaling to predict the body mass-dependence of certain properties of circulating lymphocytes and of the lymphatic system.
The motivation of this work is two-fold. First, the scaling approach to biology, as developed by West, Brown and Enquist (1997), often leads to a unified view of what otherwise would have been a glut of raw experimental data. Second, most immunological data has been collected for small mammals, like mice and rats, whereas one would like to know the corresponding numbers for humans. This, of course, necessitates a reliable scaling theory of the immune system.
In our analysis we shall take for granted all the main features of the West, Brown and Enquist (WBE) model, as expounded in (West, Brown & Enquist, 1997; Brown & West, 2000). We shall also use results from our earlier paper on the scaling properties of the immune system (Wiegel & Perelson, 2004). For simplicity, we shall consider the immune systems of mammals, although most of our predictions are expected to be far more general. The mass of an animal will be denoted by M. An allometric (scaling) relation for some physiological quantity A will be written as A ∼ Mα. This means an approximate, quantitative relation
| (1.1) |
where α is the scaling exponent and a denotes a constant. The exponent α has no dimension: it keeps the same value when the units in which one measures A and M are changed. The constant a has a value that does depend on these units; its dimension, denoted [a], is obviously given by [a] = [A] [M]-α.
Our treatment will be somewhat heuristic in the following way. Most of the predictions of the original WBE model for blood circulation and respiration are well confirmed by the biological data. This holds especially for the scaling law for the total metabolic rate B:
| (1.2) |
cf. West, Woodruff and Brown (2002), where it is shown to hold over 27 orders of magnitude! It was already demonstrated in Wiegel and Perelson (2004) that these predictions imply certain global scaling properties for the immune system. The special features of the pool of recirculating, long lived lymphocytes enable us to extend our predictions to various other properties. In those cases where experimental data are available we shall compare them with our predictions; occasionally the data will inspire a specific choice between various theoretical alternatives. Hence this paper's main aim is to stimulate more experiments on the scaling properties of mammalian immune systems, and to take another step on the road towards an adequate mathematical theory of the immune response.
The paper is organized around the events that occur during the life cycle of a circulating lymphocyte. For most of the stages of this cycle there is enough information to derive – or at least to conjecture – the existence of a scaling law. Hence we discuss in order (i) the generation of lymphocytes in the bone marrow, (ii) their transport in blood, (iii) their diffusion in tissues, (iv) their transport in the lymphatic system, and finally their stay inside a lymph node. We also address immune learning and use experimental data to calculate two constants that determine the mass-dependence of clonal diversity.
2. Rate of lymphocyte production in the bone marrow
Lymphocytes, like all blood cells, are generated in the bone marrow. B cells mature there; whereas T cells mature in the thymus. A subpopulation of the mature cells comprises a pool of long-lived lymphocytes that circulate from blood, through the tissues, to lymph and back again to blood.
For a 200 g rat, the rate of lymphocyte generation in the bone marrow is approximately 2×107/hr, and the total number of long-lived recirculating lymphocytes is approximately 1.6×109 (Bell, 1978). For sheep (M=30 kg) the number of recirculating lymphocytes is approximately 1011, which comprises about 10% of the entire lymphocyte population of 1012, and is about a factor of 10 larger than the number of lymphocytes found in the blood at any time (1010) (Young & Hay, 1995).
At this early stage of our analysis a scaling law already suggests itself as follows. Assume the bone marrow occupies a fixed fraction of bone volume and that the bone marrow has the same average metabolic rate as the entire organism. The metabolic rate of an animal's bone marrow can be estimated as the product (volume of the bones) × (metabolic rate per unit volume). The scaling properties of the two factors can easily be established. The metabolic rate for the whole animal is given by eqn. (1.2). As mass and volume are proportional, the metabolic rate per unit volume will scale ∼M¾/M=M-¼.
The total bone volume of a land animal also obeys a simple scaling law. Biewener (2000) gives an approximate allometric relation for the bone cross-sectional area for terrestrial mammals (Table 1, pg. 54) as
As the length of a bone will scale as the animal's length (M1/3) and as the number of bones is approximately constant, bone volume should scale as
This is consistent with the relationship reported by Schmidt-Nielsen (1984) that for mammals from mouse to elephant the skeletal mass ∼ M1.04 with a 95% confidence interval of 0.04 for the scaling exponent.
Using this relationship, the metabolic rate of the animal's bone marrow is expected to scale as
As it should take a fixed amount of energy to build one lymphocyte, this implies the scaling law
| (2.1) |
If a fixed fraction of the body's metabolic rate is devoted to the production of lymphocytes, then the rate at which lymphocytes are generated should be given by a number that scales ∼M3/4, whereas above we calculated that the rate with which new lymphocytes are formed is proportional to M0.8. Whether this discrepancy of 0.05 in the scaling exponent is accurate is unclear, but if the true rate does scale as M0.8, it suggests that lymphocyte production may take a slightly higher fraction of metabolic energy in larger animals. This may reflect the fact that the majority of the newborn lymphocytes are rapidly eliminated through positive and negative selection processes that remove clones with defective receptors and self-reactive clones that could lead to autoimmune disease.
3. The accumulation of lymphocytes in a service volume
One of the novel features of the WBE theory of scaling was the introduction of the idea of a “service volume”. In the WBE theory the body is divided into a certain number of small volumes, each of which is served by a single capillary to supply oxygen and remove waste products. In Wiegel and Perelson (2004) we hypothesized that the service volumes for the circulatory system are the same as the service volumes for antigen surveillance, i.e., the volume of the tissue that a lymphocyte might explore before returning to the circulation. Within a service volume, consider those B-cells or T-cells that belong to a specific clone. Their number, N(t), will vary in time due to the following two processes:
An influx of these cells due to the blood supply to the service volume. The WBE model (West, Brown & Enquist, 1997; Brown & West, 2000) assumes that the rate of blood delivery is the same for all service volumes and for all animals. As the lymphocytes under consideration are mixed uniformly with the other blood cells, their rate of entry will have a constant value (υo) which to a good approximation should be the same for all service volumes and for all animals. There are exceptions in that there are places in the body where lymphocyte entry is more selective, e.g. moving across the blood brain barrier, getting into the cornea of the eye or into particular regions of lymphoid tissue where entry may be selective for certain types of lymphocytes.
-
It was shown in Wiegel and Perelson (2004) that the time (ts) which a lymphocyte spends randomly moving inside a service volume – from the region where it is released from a blood capillary to the region where it exits into a lymph capillary – scales with mass as
(3.1) provided the moving cell is characterized by a “diffusion coefficient” that is independent of body mass. This latter assumption is discussed in detail in Wiegel and Perelson (2004).
Equation (3.1) implies a relation for the mass-dependence of the rate (υe) that lymphocytes exit a service volume. Assuming that the exit rate υe is constant, the probability density that a lymphocyte that entered the service volume at time 0 exits the service volume at time t is given by the exponential distribution, i.e., υe exp(-υet). This means that the average time until the lymphocyte exits the service volume
Hence,
| (3.2) |
If one combines these two processes a simple dynamical equation for N(t) is found:
| (3.3) |
In the stationary state the solution (to be denoted by Neq) is
| (3.4) |
This scaling law has exactly the same form that was shown in Wiegel and Perelson (2004) to be necessary in order to guarantee timely contact between a lymphocyte and a new antigen that has appeared in the service volume (compare case 1,1 of Table 1 in Wiegel and Perelson (2004) and the discussion in section 3 of that paper).
According to the WBE theory the volume of a service volume scales as M1/4. Since the number of lymphocytes per service volume, Neq, also scales as M1/4, this implies that the lymphocyte concentration in tissue is independent of animal size. Thus, one would not be able to use information about lymphocyte density in a tissue to tell the size of an animal. However, since the residence time for a lymphocyte in a service volume does scale as M1/4, the ability of a lymphocyte to detect antigen will depend on animal size. This issue is discussed more fully in Wiegel and Perelson (2004). Also, Perelson et al. (2006) mention that adult blood lymphocyte concentrations among a diverse set of 138 mammalian species scale as M-0.07, suggesting that there might be a small discrepancy from the predicted M0 law suggested by the WBE theory.
4. The lymphatic system
The pool of mature recirculating lymphocytes as well as dendritic cells (DC) continuously scan the body for the possible presence of any disease-causing entity (antigen) and to report this presence as soon as possible to other parts of the immune system (lymph nodes etc.) where appropriate action can be taken. This “scanning” function occurs when such a lymphocyte or DC, after leaving the circulatory system through a blood capillary, moves through the tissues of a service volume, until it is absorbed into the lymphatic system (Catron et al., 2004). Multiphoton imaging techniques have allowed one to visualize the movement of T cells and dendritic cells in vivo (cf., von Adrian & Mempel, 2003; Miller et al., 2003; Halin et al., 2005), and this has lead to the development of models characterizing T cell movement (Meyer-Hermann & Maini, 2005; Preston et al. 2006; Beauchemin et al., 2007). Recent experimental work has focused on the molecular signals that control trafficking, such as chemokines (Sallusto & Baggiolini, 2008; Bromley et al., 2008), the lipid G protein-coupled receptor agonist sphingosine 1-phosphate (S1P) that coordinates lymphocyte egress from lymphoid organs (Mandala et al., 2002; Schwab & Cyster, 2007), and environmental cues that influence trafficking patterns (Sigmundsdottir & Butcher, 2008). Here however, we take a more macroscopic approach and ignore the molecular details underlying lymphocyte movement and trafficking.
The lymphatic system consists (apart from the lymph nodes, which will be discussed in section 5) of lymphatic vessels, the three main types of which are: (a) lymph capillaries; (b) lymphatics; (c) lymph ducts. The lymphatic system is a drainage system which collects interstitial fluid and returns it to the blood. In humans, at rest, the volume of lymph which is transported back to the blood in this way is approximately 120 ml per hour (Guyton, 1976). For a review of the literature on the lymphatic vessels, and the flow of lymph, the reader might want to consult Schmid-Schoőnbein (1990).
The walls of a lymph capillary are lined with one-way gaps which are (probably) opened as a result of excess pressure in the interstitial fluid in the tissues which surround the capillary. Moreover, the system of lymphatic vessels is full of one-way valves, which guarantee that lymph flows in one direction only.
These features of the lymphatic system strongly suggest the fact that the ongoing transport of lymph is a form of “passive” transport, which is driven by the periodic contraction and relaxation of the muscles and other surrounding tissues. The four main mechanisms seem to be as follows:
Periodic action of the lungs
Periodic action of the heart
Peristaltic movements of the intestines
Locomotion of the body.
The first three forms of movement occur continuously throughout the whole lifespan but the last activity will occur only during some fraction of the time. These considerations suggest two scaling laws, which will be derived below.
First, consider case (i). Each time the lungs perform a cycle of respiration, the adjacent tissues will be squeezed and relaxed. Let us assume that a fraction ξ1 of these adjacent tissues will consist of lymph squeezed into the lymphatic capillaries. As the volume of these tissues will scale ∼ M one finds that the amount of lymph that enters the lymphatic capillaries during each respiratory cycle will be proportional to ξ1M, and hence ∼ M, because ξ1 can be expected to be independent of the size of the animal. According to the WBE theory, the period of the respiratory cycle scales ∼M¼ (West, Brown & Enquist, 1997; Brown & West, 2000). Hence the lymph flow rate (volume per unit of time) due to mechanism (i) will scale with the animal's mass as ∼M¾.
Exactly the same reasoning holds for the flow of lymph due to mechanisms (ii) and (iii); of course with different proportionality constants.
The fourth mechanism involving muscle contraction during locomotion for the transport of lymph is of a somewhat different type and leads to some interesting observations. For simplicity, we shall sketch the argument for land animals. If the animal has a body with a linear size L then its period of walking will be proportional to (Schmidt-Nielsen, 1984), where g denotes the acceleration due to gravity. Since L∼M1/3 the period of walking ∼L1/2∼M1/6. This calculation is based on a simple model viewing a land animal's leg as a rigid pendulum. Nonetheless, Schmidt-Nielsen (1984; p.173) notes the measured period of locomotion for animals of mass 15 kg to 300 kg scales as M0.17, and that the scaling exponent is the same for walking, trotting and cantering. More recent data reported by Bejan & Marden (2006) is consistent with this and shows that the stride frequency for animals running on soft ground ∼ M-1/6 over a span of about 10 orders of magnitude in mass. Assuming the volume of muscle and hence the amount of lymph transport is proportional to M, i.e., an amount ξ4M will enter into the lymphatic system during each time interval ∼ L1/2∼ M1/6, where ξ4 is another constant fraction. This mechanism would give a flux of lymph ∼ ξ4M5/6 which would give a scaling law ∼M5/6.
In addition to walking and running other muscular motions could also drive lymph flow. The totality of these motions would be limited by the metabolic rate and hence scale as M3/4 as are the motions of types (i) – (iii).
The previous considerations combine to give an expression for the total flow rate (Qℓ)) of lymph through the entire lymphatic system
| (4.1) |
where a′ and a″ are constants. The first term is due to mechanisms (i) – (iii) and the second term comes from mechanism (iv). This expression should be compared to the total flow rate (Qb) of blood through the arterial system, as predicted by the WBE theory:
| (4.2) |
where b′ is another constant.
Before comparing prediction (4.1) with the few available experimental data, we note that a land animal will not be continuously in a state of locomotion, but will move around only during some fraction γ of the time. Hence it should be more accurate to write (4.1) in the form
| (4.3) |
where 0< γ <1. As this fraction might depend on the mass of the animal we conclude that there is no simple (i.e. single-exponent) scaling law for the lymph flow rate, unless:
| (4.4) |
in which case one has
| (4.5) |
If this latter prediction would turn out to be experimentally correct one would have learned that the fraction of time a (land) animal moves around, scales down with its mass ∼M-1/12. This prediction (“large animals are somewhat lazier than small animals”) seems to be in agreement with everyday observations, but is contradicted by observations on the length of time various mammals sleep, with mice sleeping ∼ 14 hours per day relative to the ∼ 3.5 hours per day that elephants sleep (Savage & West, 2007).
As one of the reviewers pointed out to us, if lymph flow did scale as M3/4 this would be an intriguing result, since the passive lymph system would exhibit the same scaling as the active blood flow system. This might be natural since blood flow ultimately is the source of lymph flow, as it is the fluid that escapes from the circulatory system that forms the lymph. Some organisms, such as amphibians, reptiles and flightless birds, have lymph hearts and lymph is actively circulated. But if lymph flow is maintained in lock step with the circulation then one could wonder why these organisms need active lymph pumping. The alternative, of course, is that lymph circulation does not scale simply as M3/4.
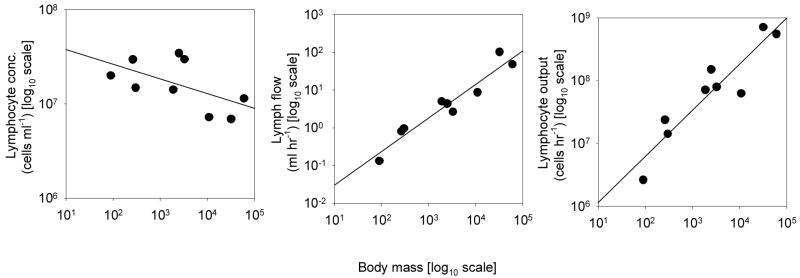
Experimental data on the total flow of lymph in the thoracic duct have been collected from Altman and Dittmer (1974) by our collaborator Jason Bragg. The data cover nine mammalian species, with body mass ranging from 0.09 kg (hamster) to 70 kg (human). They can approximately be represented by the scaling law (Fig. 1)
Figure 1.
Allometric scaling of a) concentration of lymphocytes in lymph ∼ M-0.16 (r2=0.33, p=0.10), b) rate of flow of lymph ∼ M0.89 (r2=0.92,p< 0.001), and c) total lymphocyte output from the thoracic duct ∼ M0.73 (r2=0.86,p<0.001), for 9 mammalian species. Body mass expressed in grams. Data taken from Altman & Dittmer (1974).
| (4.6) |
where M is measured in kg and Qℓ in ml (hr)-1 (Perelson et al., 2006). This empirical finding is more suggestive of an M5/6 = M0.83 scaling than an M3/4 power law.
The lymphocyte content of the lymph has also been measured (Altman and Dittmer, 1974). Calling this quantity nℓ, the data can be approximated by the scaling law (Fig. 1.)
| (4.7) |
where nℓ is measured in units of 106 lymphocytes per ml (Perelson et al., 2006).
In conclusion, while simple scaling laws appear to summarize the experimental data, which hold only for a restricted range of masses; our prediction (4.3) suggests that for a larger range of masses no single-exponent scaling law might be found to adequately represent the data.
A striking feature of this data is that they also imply an experimental result for the total number of lymphocytes that pass through the thoracic duct (in units of 106 lymphocytes per hour): multiply (4.6) with (4.7) and find (Fig. 1)
| (4.8) |
although there is some danger in multiplying empirical scaling laws as errors also multiply. The exponent is near to the ¾ exponent that we calculated in Wiegel and Perelson (2004). There we showed that ∼Mℓ n(cM), where c is a constant, lymphocytes recirculate through the animal's body with a total recirculation time, Tc, that scales ∼M1/4. The factor ℓn(cM) would, of course, be virtually undetectable in any experimental context. Thus, one would expect the flow rate of lymphocytes to scale as M/M1/4 = M3/4.
For a rat (M = 200 g) the recirculation time has been measured by injecting radioactively labeled lymphocytes into the blood and monitoring their subsequent appearance in the thoracic duct (Stekel et al., 1997; Stekel, 1998). The number of labeled lymphocytes in the thoracic duct reaches a maximum between 12 and 20 hrs later. The majority, if not all, of the lymphocytes in the thoracic duct are recirculating, and thus we assume that the recirculation time, Tc, for the rat can be as short as 12 hrs. For other mammals the recirculation time can be calculated from the Tc∼M1/4 scaling relation; the results are listed in Table I.
Table I.
Average time needed for a long-lived lymphocyte to complete a full cycle through blood, tissues and the lymphatic system. The second column assumes the average recirculation time, Tc, in the rat is 12 hours; while the third column assumes Tc in the rat is 16 hours. The average circulation times for other mammals are computed using the scaling relationship Tc∼M1/4. The estimates of Tc in the rat are based upon the data quoted in Stekel et al. (1997) and Stekel (1998).
| M | Tc | Tc | |
|---|---|---|---|
| Mouse | 10 g | 5.7 hrs | 7.6 hrs |
| Rat | 200 g | 12 hrs | 16 hrs |
| Human | 70 kg | 52 hrs | 69 hrs |
| Elephant | 1600 kg | 113 hrs | 151 hrs |
The results of this section suggest a simplified model, in which lymphocytes are transported through the lymphatic system in a time that scales ∼M1/4.
5. The lymph node
Turning our attention to lymph nodes, we try to predict the scaling properties of their number and size.
The metabolic rate of lymph nodes has been studied experimentally by several authors, c.f. Ottaway (1988, p. 176) for a review of this literature. The data for the fraction of the cardiac output that is delivered to one gram of lymph node (LN) can be fitted to the scaling relation (Ottaway, 1988)
| (5.1) |
where the animal's mass is measured in gram. For the total cardiac output (in ml min-1) the data can be represented by (Ottaway, 1988)
| (5.2) |
Multiplication of these formulae shows that the amount of blood which is delivered to one gram of a lymph node, equals about 0.48 ml min-1, independent of the mass of the animal. Ottaway (1988) quotes direct measurements of this quantity for rabbit, giving 0.46 ml min-1, and for cat, giving numbers in the range 0.4 to 0.6 ml min-1.
Because oxygen and nutrients are supplied to the node by the blood, these data, although limited, suggest that lymph nodes might have “privileged” metabolism, i.e. a metabolic rate per unit of lymph node mass that is the same for all animals. This might also shed light on the power law (4.6) for the total lymph flow, which shows an exponent of 0.89, considerably higher than the ¾ power law for the total metabolic rate. However, this exponent is surprisingly close to the scaling exponent of 0.87 measured for the maximum metabolic rate (MMR) by Weibel et al. (2004), consistent with the idea that lymph nodes may be operating with a privileged metabolism. Also, the MMR is attained during vigorous exercise, i.e., vigorous muscle contraction, so MMR could be related to lymph flow. In fact, Painter (2005) suggests that MMR is limited by the pulmonary capillary pressures that produce edema, i.e., maximum fluid transport out of capillaries.
Estimates for the number of lymph nodes in dogs, humans, horses and cattle are available (Altman & Dittmer, 1974). For humans the number is 465 (Altman & Dittmer, 1974). While there is some suggestion of an increase in the number of lymph nodes with mass (Perelson, Bragg & Wiegel, 2006), it is difficult to define a scaling relationship reliably with data for so few species, and where body masses and lymph node counts were not available for the same individuals.
Size of a lymph node
From a theoretical point of view one should ask the question what determines the size of a single lymph node, and whether this quantity might depend on the mass of the animal through a single-exponent scaling law. We can envision a number of possible scenarios. First, as the lymphatic system is a structural element of the body that develops in similar ways in all mammals, one might expect that the number of lymph nodes is constant, independent of mass. Further, if the fraction of body mass devoted to lymph nodes is constant, then the mass of a lymph node, mL, will be proportional to M. Hence larger animals would have larger lymph nodes. A second possibility is that lymph nodes are designed to drain a constant volume of tissue and thus could have size independent of mass, in which case their number would increase with body volume, i.e., be proportional to M. Clearly, another possibility is that both lymph node size and number increase with M. Lastly, since lymph nodes are important in initiating immune responses, following Bell (1978), one might envision that a single lymph node should be large enough to initiate a humoral immune response to any possible antigen and as rapidly as possible.
The limited available data suggest that the first scenario is not strictly valid as the number of lymph nodes in dog, humans, cattle and horses have been reported as approximately 60, 465, 300, and 8000, respectively (Altman & Dittmer, 1974), suggesting that lymph node number is not constant and increases with body mass. Nonetheless, as predicted by this first scenario, lymph node size does appear to increase with body mass as lymph nodes in mice are typically order of one mm (Halin et al., 2005), while those in humans are typically weigh a gram or less, i.e., are of order one cm (Parham, 2000). Data on the number and size of lymph nodes in different animals is hard to find. Further data collection on this issue is clearly needed, but the data at hand suggest that both size and number of lymph nodes increase with M.
The last scenario, which requires that a single lymph node should be large enough to always initiate an immune response, is tantamount to the restriction that a complete lymphocyte repertoire be represented by sessile cells in the node. Alternatively, transport between nearby nodes could be fast enough that a collection of nodes house a complete repertoire. Since the time for a lymphocyte to recirculate through the entire lymphatic system may vary from hours to days [Table 1], transport through the entire system could limit the speed at which an immune response is generated. There is some data to support this view. For example, immune responses to influenza or lymphocytic choriomeningitis virus infection in mice occur more rapidly than responses to viral infections in humans or monkeys. In mice, T cell expansion is observed to start within one to two days of infection (DeBoer et al., 2001), while in rhesus macaques antigen specific T cell expansion may not occur until one to two weeks after infection (Davenport et al., 2004; Davenport et al., 2005). However, the reasons for this are unclear. Slower recirculation might also imply slower transport of antigen to the draining lymph node to initiate a response rather than a limitation in the ability of the cells within a single node to recognize the antigen. In fact, the shape-space theory of clonal selection (Perelson & Oster, 1979) suggests that immune recognition is somewhat “sloppy”, and that antigens can be recognized by many different lymphocyte clones. In a mouse, with a repertoire of about 107, one can estimate that only 5 × 105 different clones are needed to recognize any antigen (Perelson & Oster, 1979). Thus the full repertoire in an animal need not be present in a node, only enough of the repertoire so that one or more clones recognize the antigen. This property of having sufficient diversity to recognize any antigen is called repertoire completeness. A lymphocyte has a diameter of about 10 μm. Thus a 1 mm diameter lymph node could contain about 106 lymphocytes, suggesting that in mice a single lymph node or a few rapidly communicating nodes could contain a complete repertoire. In larger animals, such as humans with 1 cm lymph nodes this is even more likely to be the case. Here we shall ignore the possibility that a few communicating nodes are required to contain the repertoire as it will hardly affect scaling results, and we shall examine the implications of assuming that a single lymph node is large enough to contain a complete repertoire.
In Wiegel and Perelson (2004) we argued that the diversity of B cell clones should scale ∼ ln (cM) cells, where c is a constant that has units of 1/mass. Consequently, the minimum size of a lymph node should correspond to the case in which the node contains ∼ ln (cM) cells each clone being represented by a single cell.
The second requirement, that the immune response shall be initiated in a sufficiently short time in order to provide protection from the effects of infection, means that a recirculating B cell should make contact in the node, with the corresponding sessile T cell, in a sufficiently short time. (and similarly for a circulating T cell contacting a sessile antigen presenting cell). Clearly, the body is faced here with the problem of finding an optimal balance between the following two competing design criteria:
-
The time which a B cell needs to make contact, inside the lymph node, with a sessile T cell which belongs to the correct (complementary) clone of T cells, will be as short as possible if all the T cells of that clone are placed inside the node. In Wiegel and Perelson (2004) we showed that clone size scales ∼M, so this design criterion suggests that the body builds only a single lymph node, in which all clones are represented, each clone with ∼M copies. In general the design criteria that the search for the complementary T cell will be as short as possible, and that this complementary T cell will be represented by a sufficiently large number of copies inside the lymph node, leads to the criterion: Number of lymphocytes per clone per lymph node is maximal. This can also be written as
(5.3a) -
It is also desirable that the drainage region of the lymph node should be as small as possible. This is the case because the humoral immune response, initiated in the node, will be most effective if the original infection is located as accurately as possible. This second design criterion suggests that the body builds ∼M lymph nodes, each of which contains only a single representative of every clone in the repertoire! In a more general way this design criterion can be written in the form
(5.3b)
In the absence of any further information on energetic, material or structural features that would constrain achieving both optima, we assign arbitrary weights, A and B, to achieving these two optima. Then, equating the weighted criteria to obtain an optimal balance between the two design criteria we find
which results in the rule
| (5.3c) |
If we assume A and B to be approximately independent of body mass, and that evolution has converged towards this optimum, we find the scaling laws:
| (5.4) |
| (5.5) |
| (5.6) |
Of course, these predictions also imply scaling laws for the mass (volume) of the typical lymph node:
| (5.7) |
| (5.8) |
From eqs. (5.3-5.8) one can try to estimate the average time (to) it takes for a (circulating) B cell, once it has entered into the interior of a lymph node, to make first contact with a (sessile) T cell of the complementary clone. This estimate will necessarily be very inaccurate in view of the intricate structure of lymph nodes (Nossal & Ada, 1971). The actual movement of the B cell inside the node might show features of flow as well as features of random search and chemotaxis. In the case in which the movement can be represented by a random walk, the average time (t1) that a B cell needs to make first contact with a particular T cell of the complementary clone scales like
| (5.9a) |
cf. Perelson and Wiegel (1999) and Wiegel and Perelson (2004). The right-hand side is proportional to the mass of the lymph node. Using (5.7) one finds
| (5.9b) |
From eq. (5.6) the number of copies of the complementary T cell clone that are expected to be present ∼ M1/2. Hence the average time which the B cell needs to make first contact with some T cell of the complementary clone should scale like
| (5.10) |
Hence this time is practically independent of the mass of the animal because the logarithmic dependence should be practically unobservable. The conclusion expressed by (5.10) holds as long as the dynamics of the search mechanism is independent of the animal's mass. This latter assumption is plausible in view of the privileged metabolism of the lymph nodes which we discussed earlier in this section.
6. Are there scaling aspects of immune learning?
In sections 2-5 we examined the pool of long-lived recirculating lymphocytes, which form the backbone of the immune system's surveillance function. Once an antigen has been detected in the drainage region of a lymph node it is transported to that node where both B and T cell clonal expansion will occur, as well as an intense “learning” process, in which the recognizing B cells will hypermutate their antigen-binding receptors selecting those that fit the antigen better for retention in the memory pool. In an earlier paper, we gave a mathematical description of this learning process (Perelson & Wiegel, 1999). Below, we try to identify those aspects of clonal expansion and immune learning that are sensitive to the mass of the animal.
The first relevant observation is the fact, discussed in section 5, that the information-processing activities of a lymph node proceed at a privileged metabolic rate. This implies that the rate of proliferation of the antigen specific B and T cells during immune responses (one cell division in every 6 to 7 hours) should be essentially the same in all mammals.
A second observation is that most life forms – and certainly all mammals – share very similar molecules. This means that their immune systems are faced with the tasks of recognizing very similar antigens, and avoiding cross-reactivity with very similar self-antigens.
These two observations combine to conclude that the overall processes of clonal expansion and immune learning by somatic hypermutation will be essentially the same in all higher animals, although the genetic and biochemical mechanisms of performing somatic hypermutation and selection may be different. The only effect of body mass is due to the increase in life span with size. We previously showed (Wiegel & Perelson, 2004) that this means that clonal variety should be proportional to ln (cM), where c is a constant.
During an immune response resting B cells become activated and generate a clone of antibody-producing cells. The number of antibody producing cells in that clone will be proportional to Mβ, with some exponent β ≥ 0, which our model cannot predict. Hence the number (h) of cell divisions of the original B cell follows from
| (6.1a) |
The result
| (6.1b) |
shows that the time that is needed to transform the B cell into a sufficiently large clone of antibody forming cells, is only weakly dependent on the animal's mass through the term (ln M). Similarly, during viral infections when the T cell arm of the immune system is activated, resting T cells generate a clone of effector T cells of size 2h, which is proportional to Mβ′, with some exponent β′ ≥ 0, which may be different than β. Thus, both the B cell and T cell responses are expected to be only weakly dependent on body mass.
What are the implications of this weak dependence on body mass? Notably, it suggests that immune responses in small and large animals should have features in common. However, what this implies for true protection from disease and lifespan is more complicated than the analysis given here. Pathogens exploit their hosts in a variety of ways and thus Cable et al. (2007) suggest that rates of pathogenesis should be tied to the host's metabolism and hence scale as the mass specific metabolic rate, M-1/4. This further implies that times associated with pathogenesis, e.g. the time to onset of symptoms or time to death, should scale as M1/4. They showed that for the small set of pathogens they examined these predictions are roughly met (Cable et al., 2007). For fast growing pathogens adaptive immune responses are not necessarily protective during the first encounter with the pathogen and it is only on subsequent encounters that the faster memory response provides true protection. Thus, in certain circumstances metabolic effects rather than features of the immune response may set the time scale for the onset of symptoms and death.
7. Scaling of clonal diversity
It has been remarked several times that the clonal variety of, for example, T cells was predicted in Wiegel and Perelson (2004) to have the form
| (7.1) |
where k and c are two unknown constants (for B cell clonal diversity one expects the same formula). They could be calculated as soon as the clonal diversity is determined experimentally for two organisms. Fortunately, this is the case: the data of Arstila et al. (1999) give for the T cell clonal diversity of a 20 to 30 year old human the approximate value n1≅2.5×107. The T cell clonal diversity of mice has been measured by Casrouge et al. (2000) with the result n2≅2×106. If we estimate M1=80 kg and M2=10 g then we have to solve the two equations
| (7.2a) |
| (7.2b) |
for the two constants k and c. Take the ratio of these equations, and call n1/n2=β. The solution is
| (7.3) |
as can be verified by substitution. With the explicit values of n1 and n2 and choices of body masses given above one finds c≅0.22 g-1. For the dimensionless constant k one finds k ≅ 2.6 × 106.
In view of the similarity of the molecules and biochemical processes used by all mammals one would expect the variety of self-antigens to be practically the same for all animals. This means that this aspect of self-nonself discrimination should not show any scaling behavior. On the other hand, the clonal variety of B and T cells, i.e. the immune repertoire increases weakly with the mass, proportional to ln (cM). Hence one would expect a slight increase with M of the probability that a lymphocyte clone erroneously initiates an immune response to a molecular shape that belongs to self, thereby causing an autoimmune disorder.
8. Discussion
We have tried to present the outlines of a scaling theory of lymphocyte trafficking. Data upon which to rigorously build such a theory is still largely lacking. We have made various assumptions in the paper that need to be tested experimentally and thus our conclusions about scaling laws should not be interpreted as facts but rather as our best guesses. It is our hope that theoretical discussions, such as the one presented here, will stimulate greater collection of quantitative data about the immune system in diverse animal species.
Although at present the truth is still hidden, from the work presented here one can perhaps dimly discern the outlines of a scaling theory of lymphocyte trafficking.
Acknowledgments
We thank Geoffrey West and Jason Bragg for valuable conversations about this work. We also thank the reviewers for many helpful comments and references. J. Bragg also supplied Fig. 1. Much of this work was inspired by G. I. Bell, a colleague, friend and collaborator for many years who died in May, 2000. Portions of this work were done under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396 and supported by NIH grants AI28433 and RR06555. This work was also facilitated by interactions at the Santa Fe Institute and supported at the Santa Fe Institute by research grant RPG10/2004 from the Human Frontiers Science Program.
References
- Altman PL, Dittmer DS. Biology Data Book. 2nd. Vol. 3. Bethesda, MD: Federation of American Societies for Experimental Biology; 1974. [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human αβ T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Beauchemin C, Dixit NM, Perelson AS. Characterizing T cell movement within lymph nodes in the absence of antigen. J Immunol. 2007;178:5505–5512. doi: 10.4049/jimmunol.178.9.5505. [DOI] [PubMed] [Google Scholar]
- Bejan A, Marden JH. Unifying constructal theory for scale effects in running, swimming and flying. J Exp Biol. 2006;209:238–248. doi: 10.1242/jeb.01974. [DOI] [PubMed] [Google Scholar]
- Bell GI. Lymphocyte traffic patterns and cell-cell interactions. In: Bell GI, Perelson AS, Pimbley GH, editors. Theoretical Immunology. Marcel Dekker; New York: 1978. pp. 341–378. [Google Scholar]
- Biewener AA. Scaling of terrestrial support. In: Brown JH, West GB, editors. Scaling in Biology. Oxford University Press; Oxford: 2000. pp. 51–66. [Google Scholar]
- Beltman JB, Marée AFM, Lynch JN, Miller MJ, de Boer RJ. Lymph node topology dictates T cell migration behavior. J Exp Med. 2007;204:771–780. doi: 10.1084/jem.20061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JT. Why Size Matters. Princeton University Press; Princeton, NJ: 2006. [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- Brown JH, West GB. Scaling in Biology. Oxford University Press; Oxford: 2000. [Google Scholar]
- Cable JM, Enquits BJ, Moses ME. The allometry of host-pathogen interactions. PloS One. 2007;2(11):e1130. doi: 10.1371/journal.pone.0001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder WA. Size, Function, and Life History. Dover; New York: 1996. [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the αβ TCR repertoire of naïve mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 2004;21:341–347. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Davenport MP, Ribeiro RM, Perelson AS. Kinetics of virus specific CD8+ T cells and the control of HIV infection. J Virol. 2004;78:10096–10103. doi: 10.1128/JVI.78.18.10096-10103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MP, Zhang L, Bagchi A, Fridman A, Fu TM, Schleif W, Shiver JW, Ribeiro RM, Perelson AS. High potency HIV vaccination leads to delayed and reduced CD8+ T cell expansion, but nevertheless improved viral control. J Virol. 2005;79:10059–10062. doi: 10.1128/JVI.79.15.10059-10062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer RJ, Oprea M, Antia R, Murali-Krishna K, Ahmed R, Perelson AS. Recruitment times, proliferation, and apoptosis rates during the CD8+ T cell response to LCMV. J Virol. 2001;75:10663–10669. doi: 10.1128/JVI.75.22.10663-10669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, St-Pierre Y, Beauchemin C, Desrosiers M, Potworowski EF. The fate of thymocytes labeled in vivo with CFSE. Exp Cell Res. 1998;240:75–85. doi: 10.1006/excr.1997.3900. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Textbook of Medical Physiology. 5th. Vol. 398 Saunders; Philadelphia: 1976. [Google Scholar]
- Halin C, Rodrigo Mora J, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Ann Rev Cell Devel Biol. 2005;21:581–603. doi: 10.1146/annurev.cellbio.21.122303.133159. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane CA, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by spingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Meyer-Hermann ME, Maini PK. Interpreting two-photon imaging data of lymphocyte motility. Phys Rev E. 2005;71:061912. doi: 10.1103/PhysRevE.71.061912. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal GJV, Ada GL. Antigens, Lymphoid Cells and the Immune Response. Academic Press; New York: 1971. pp. 69–79. [Google Scholar]
- Ottaway CA. Dynamic aspects of lymphoid cell migration. In: Husband AJ, editor. Migration and Homing of Lymphoid Cells. CRC Press; Boca Raton: 1988. pp. 167–194. [Google Scholar]
- Parham P. The Immune System. Vol. 13 Garland; New York: 2000. [Google Scholar]
- Painter P. Allometric scaling of the maximum metabolic rate of mammals: oxygen transport from the lungs to the heart is a limiting step. Theoret Biol Med Modeling. 2005;2:31. doi: 10.1186/1742-4682-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Bragg JG, Wiegel FW. The complexity of the immune system: Scaling laws. In: Deisboeck TS, Yasha Kresh J, editors. Complex Systems Science in Biomedicine. Springer; New York: 2006. pp. 451–459. [Google Scholar]
- Perelson AS, Oster G. Theoretical studies of clonal selection. J Theoret Biol. 1979;81:645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Wiegel FW. Some design principles for immune system recognition. Complexity. 1999;4:29–37. [Google Scholar]
- Peters RH. The Ecological Implications of Body Size. Cambridge Univ Press; Cambridge: 1983. [Google Scholar]
- Preston SP, Waters SL, Jensen OE, Heaton PR, Pritchard DI. T-cell motility in the early stages of the immune response modeled as a random walk amongst targets. Phys Rev E. 2006;74:011910. doi: 10.1103/PhysRevE.74.011910. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Naure Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- Savage VM, West GB. A quantitative, theoretical framework for understanding mammalian sleep. Proc Natl Acad Sci USA. 2007;104:1051–1056. doi: 10.1073/pnas.0610080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Scaling: Why is Animal Size so Important? Cambridge University Press; Cambridge: 1984. [Google Scholar]
- Schmid-Schoőnbein GW. Microlymphatics and lymph flow. Physiological Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekel DJ. The simulation of density-dependent effects in the recirculation of T-lymphocytes. Scand J Immunol. 1998;47:426–430. doi: 10.1046/j.1365-3083.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- Stekel DJ, Parker CE, Nowak MA. A model of lymphocyte recirculation. Immunology Today. 1997;18:216–221. doi: 10.1016/s0167-5699(97)01036-0. [DOI] [PubMed] [Google Scholar]
- von Adrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir Physiol Neurobiol. 2004;140:115–132. doi: 10.1016/j.resp.2004.01.006. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci USA. 2002;99:2473–2478. doi: 10.1073/pnas.012579799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel FW, Perelson AS. Some scaling principles for the immune system. Immunol Cell Biol. 2004;82:127–131. doi: 10.1046/j.0818-9641.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- Young AJ, Hay JB. Rapid turnover of the recirculating lymphocyte pool in vivo. Intl Immunol. 1995;7:1607–1615. doi: 10.1093/intimm/7.10.1607. [DOI] [PubMed] [Google Scholar]