Abstract
Stress and renewed contact with drug (a “slip”) have been linked to persisting relapse of methamphetamine abuse. Human brain microglial activation has been linked with methamphetamine abuse, and inhibitors of glial cell activation, certain phosphodiesterase (PDE) inhibitors, and glial cell derived neurotrophic factor (GDNF) have been reported to modulate drug abuse effects. Our objective was to determine whether the glial cell attenuator, 3-isobutyryl-2-isopropylpyrazolo-[1,5-a]pyridine (AV411, ibudilast), a non-selective PDE inhibitor and promoter of GDNF, could reduce stress- and methamphetamine prime-induced reinstatement of methamphetamine-seeking behavior. Male Long-Evans hooded rats were trained to lever press reinforced with 0.1 mg/kg i.v. methamphetamine infusion according to fixed-ratio 1 (FR1) reinforcement schedules during daily, 2-h experimental sessions. After performance had stabilized, lever pressing was extinguished for 12 consecutive sessions and doses of 0 (vehicle), 2.5 and 7.5 mg/kg AV411 were then administered intraperitoneally b.i.d. on the last two days of extinction and then once on the testday to separate groups of 12 rats. During testing, the rats were given 15 min of intermittent footshock or a 1 mg/kg i.p. methamphetamine prime followed by a 2-h reinstatement test session. AV411 significantly reduced response levels of footshock-induced (2.5 and 7.5 mg/kg) and prime-induced (7.5 mg/kg) reinstatement of extinguished methamphetamine-maintained responding. AV411 has properties consistent with the ability to attenuate relapse precipitated by stress and methamphetamine “slips” during abstinence. These results thus reinforce interest in atypical neurobiological mechanisms which could be exploited for developing novel medications for treating drug abuse disorders.
Keywords: AV411, ibudilast, methamphetamine, glial, relapse, self-administration
1. Introduction
Glial cells (astrocytes, microglia and oligodendrocytes) constitute the majority of cells in the central nervous system (CNS), with the remaining cells being neurons. The glia contain receptors (e.g., Fumagalli et al., 2003; Khan et al., 2001; Kukley et al., 2001; Parpura et al., 1994), secrete neurotransmitters, neurotrophic and neuroinflammatory factors (Benz et al., 2004; Bezzi et al., 1998; Kang et al., 1998; Parpura et al., 1994; Watkins et al., 2007), control clearance of neurotransmitters from synaptic clefts (Camacho and Massieu, 2006), and are involved with synaptic plasticity (e.g., Ullian et al., 2004). Given these multiple modes of controlling neurological functions, it is not surprising that glia and their secretions have been reported to modulate the effects of drugs of abuse, or these drugs have been reported to modulate glial function (e.g., Fantegrossi et al., 2004; LaVoie et al., 2004; Narita et al., 2006; Song and Zhao, 2001; Suzuki et al., 2007; Thomas et al., 2004b; Watkins et al., 2005; Watkins et al., 2007). Particularly provocative have been reports that glial cell line-derived neurotrophic factor (GDNF), which glial cells release and for which they also serve as a target, have been reported to block conditioned place preference (CPP) induced by methamphetamine (Niwa et al., 2007c) and cocaine (Messer et al., 2000), and sensitization to methamphetamine's effects (Niwa et al., 2007c).
Phosphodiesterase (PDE) activity, which modulates cAMP levels within CNS cells including glia, can also influence the effects of abused drugs. For instance, the selective inhibitor of PDE 4, rolipram, attenuates many of methamphetamine's effects, as well as the discriminative stimulus and hyperlocomotor effects of morphine (Iyo et al., 1996; Iyo et al., 1995; Mori et al., 2000; Yan et al., 2006).
AV411 (aka, ibudilast) (3-isobutyryl-2-isopropylpyrazolo-[1,5-a]pyridine) is a non-selective PDE inhibitor preferentially targeting PDE's 3, 4, 10, and 11 (Gibson et al., 2006). AV411 modulates the activity of microglia and astroglia, suppressing the lypopolysaccharide (LPS) and interferon-gamma (IFN-γ) production of inflammatory tumor necrosis factor-alpha (TNF-α), interleukins IL-1β and IL-6, and nitric oxide (NO), while increasing the productions of nerve growth factor, GDNF, neurotrophin-4 and anti-inflammatory cytokine IL-10 (Kawanokuchi et al., 2004; Mizuno et al., 2004; Suzumura et al., 1999). Administration of AV411 attenuates several of morphine's effects including its activation of brain microglia and astrocytes, and its induction of elevated dopamine levels, CPP, tolerance and dependence (Hutchinson et al., 2007; Hutchinson et al., 2009; Ledeboer et al., 2007). Because AV411 does share in these biochemical effects reported to modulate the pharmacology of methamphetamine (e.g., Iyo et al., 1996; Iyo et al., 1995; Mori et al., 2000; Narita et al., 2006; Niwa et al., 2007a; Yan et al., 2006; Yan et al., 2007), as well as its already reported ability to modulate the effects of another drug of abuse, morphine (Hutchinson et al., 2007; Hutchinson et al., 2009; Ledeboer et al., 2007), we tested whether AV411 could attenuate prime- and stress-induced reinstatement of extinguished responding in rats previously reinforced with methamphetamine.
2. Material and methods
2.1 Subjects
Adult male Long-Evans hooded rats (Harlan, Indianapolis, IN) weighing 275-300 g at the start of studies were acclimated to the vivarium for at least one week prior to catheter implantation. When not in testing, rats were individually housed in standard plastic rodent cages in a temperature-controlled (22° C), in an American Association of Animal Laboratory Care-accredited facility in which they had ad libitum access to water. The rats were allowed ad libitum rat chow for at least one week prior to commencement of training, after which they were maintained at 320 g by controlled feedings. The rats were maintained on a reversed, 12 hr/12 hr light-dark cycle (0600-1800 lights off) for the duration of the experiment and they were trained and tested during the dark segment of this cycle.
Studies were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University and conformed with NIH Guidelines for Care and Use of Laboratory Animals.
2.1.1 Infusion assembly system
Catheters were constructed from polyurethane tubing (Access Technologies, Skokie, IL; 0.044″ outer diameter × 0.025″ inner diameter). The proximal 3.2 cm of the catheter was tapered by stretching following immersion in hot sesame oil. The catheters were prepared with a retaining cuff approximately 3 cm from the proximal end of the catheter. A second larger retaining cuff was positioned approximately 3.4 cm from the proximal end of the catheter. Mid-scapula cannula/connectors were obtained from Plastics One (Roanoke, VA). The cannula/connectors consisted of a threaded plastic post through which passed an “L” shaped section of 22 gauge stainless steel needle tubing. The lower surface of the plastic post was affixed to a 2 cm diameter disc of Dacron mesh. During sessions the exposed threaded portion of the infusion cannula was connected to an infusion tether consisting of a 35 cm length of 0.40 mm inner diameter polypropylene tubing encased within a 30 cm stainless steel spring to prevent damage. The upper portion of the 0.40 polypropylene tubing was connected to a fluid swivel (Lomir Biomedical, Inc, Quebec, Canada) that was, in turn, attached via 0.40 polypropylene tubing to the infusion syringe.
2.1.2 Surgical procedure
Following acclimation to the laboratory environment, indwelling venous catheters were implanted into the right external jugular vein. Surgical anesthesia was induced with a combination of 50 mg/kg ketamine (KetaThesia, Butler Animal Health Supply, Dublin, OH) and 8.7 mg/kg xylazine (X-Ject E, Butler Animal Health Supply, Dublin, OH). Rats were additionally administered 8 mg/kg oral enrofloxacin (Baytril, Bio-Serv, Frenchtown, NJ) for three days post-surgery. The ventral neck area and back of the rat were shaved and wiped with povidone-iodine, 7.5% (Betadine, Purdue Products L.P., Stamford, CT) and isopropyl alcohol. The rat was placed ventral side down on the surgical table and a 3 cm incision was made 1 cm lateral from mid-scapula. A second 0.5 cm incision was then made mid-scapula. The rat was then placed dorsal side down on the operating table and a 2.5 cm incision was made longitudinally through the skin above the jugular area. The underlying fascia was bluntly dissected and the right external jugular vein isolated and ligated. A small cut was made into the vein using an iris scissors and the catheter was introduced into the vein and inserted up to the level of the larger retaining cuff. The vein encircling the catheter between the two cuffs was then tied with silk suture. A second suture was then used to anchor the catheter to surrounding fascia. The distal end of the catheter was passed subcutaneously and attached to the cannula/connector that was then inserted subcutaneously through the larger incision. The upper post portion of the connector/cannula exited through the smaller mid-scapula incision. All incisions were then sprayed with a gentamicin sulfate/betamethasone valerate topical antibiotic (Betagen, Med-Pharmex, Inc., Pomona, CA) and the incisions were closed with Michel wound clips.
Rats were allowed to recover from surgery for at least 5 days before self-administration training began. Periodically throughout training, methohexital (1.5 mg/kg) or ketamine (5 mg/kg) (KetaThesia, Butler Animal Health Supply, Dublin, OH) was infused through the catheters to determine patency as inferred when immediate anesthesia was induced. Between sessions the catheters were flushed and filled with 0.1 ml of a 25% glycerol (Acros, New Jersey)/75% sterile saline locking solution containing: 250 units/ml heparin (Abraxis Pharmaceutical Products, Schaumburg, IL) and 250 mg/ml ticarcillin/9 mg/ml clavulanic acid (Timentin, GlaxoSmithKline, Research Triangle Park, NC). If during the experiment a catheter was determined to be in-patent, the left external jugular was then catheterized and the rat was returned to testing. During extinction and reinstatement testing, infusions through catheters did not occur, and these catheter maintenance procedures were not employed.
2.2 Apparatus
Commercially-obtained test chambers equipped with two retractable levers, a 5-w house light, and a Sonalert® tone generator (MED Associates, Inc., St. Albans, VT) were used. Positioned above each lever was a white cue light. The grid floors of the chambers were connected to a shock-generating device that was able to deliver 0.63 mA-scrambled foot-shock. A syringe pump (Model PHS-100; MED Associates, Inc., St. Albans, VT) when activated, delivered a 6-sec, 0.2 ml infusion. Recording of lever presses and activation of lights, shockers, pumps, and Sonalerts were accomplished by a microcomputer, interface, and associated software (MED-PC® IV, MED Associates, Inc., St. Albans, VT).
2.3 Self-administration and extinction procedures
Methamphetamine self-administration training sessions were conducted five days per week (M–F) for 2 h daily. Each response (fixed ratio 1, FR1) on the right-side lever resulted in delivery of a 0.1 mg/kg methamphetamine infusion (0.2 ml/6 sec) followed by a 14-s timeout period. At the start of an infusion the house light was extinguished, the Sonalert® was sounded, and the cue lights above each lever flashed at 3 Hz. The Sonalert® and cue lights remained activated during the 6 s infusion. Twenty seconds following the onset of the infusion the house light was re-illuminated, and the opportunity to self-administer methamphetamine was again made available (i.e., each methamphetamine infusion initiated a 20 s period during which lever presses were recorded but were without scheduled consequences and further infusions could not be obtained). Active (right-side) lever presses during the infusions as well as all inactive (left-side) lever presses were recorded but were without scheduled consequences.
Self-administration training continued until three criteria had been met: 1) at least 12 self-administration sessions had occurred; 2) at least 15 methamphetamine infusions had occurred during each of the last four sessions; and, 3) at least 125 lifetime methamphetamine infusions had been obtained, after which extinction training began. Subsequently, twelve, two-hour daily (Mon-Sun) extinction sessions were conducted. During extinction sessions, methamphetamine infusions were not delivered. Other conditions during extinction were identical to those during self-administration. That is, during extinction sessions both levers were extended, the houselight was activated, and Sonalert and cue lamp activations occurred as a result of responding according to FR1 schedules. For rats scheduled for stress-reinstatement testing, each extinction session was also preceded by a 15-minute period in the operant chamber during which levers were retracted and the house light was not illuminated to parallel the 15-min footshock period during the reinstatement test sessions. For rats scheduled for prime-reinstatement testing, an injection of saline (the vehicle for methamphetamine prime) was administered i.p. 30 min pre-session before the last four extinction sessions to habituate the rats to eventual prime injections. AV411 or its vehicle was administered on the last two days of extinction, 60 min pre-session and again at approximately 1600 hrs depending upon the eventual test condition for a rat. Rats were considered to be eligible for reinstatement testing provided that the overall mean number of active-lever presses during the last 3 sessions of extinction was lower than the overall mean number of active-lever presses during the first 3 sessions of extinction. Rats that did not meet this extinction criterion were excluded from subsequent testing. Six rats failed to meet this criterion during stress-induced reinstatement testing, and two did so during prime-reinstatement testing.
2.3.1 Testing the effectiveness of AV411 in preventing footshock-induced reinstatement
Reinstatement testing followed extinction training. Conditions during reinstatement testing were identical to those during extinction except that 15 min of intermittent footshock (administered at 0.63 mA, with a 0.5s activation time and an average inter-activation interval of 40s) was administered immediately prior to the start of the test session. Rats were administered a dose of AV411 or its vehicle i.p. 60 min prior to the start of the two-hour test session (45 min prior to the start of footshock administration). Twelve rats were assigned to each of three reinstatement test conditions: 1) footshock + AV411 vehicle; 2) footshock + 2.5 mg/kg AV411; and 3) footshock + 7.5 mg/kg AV411. Doses of 2.5 and 7.5 mg/kg were selected and were administered i.p. in the indicated regimen because these doses produce blood levels in the rat following i.p. administration which bracket clinically-relevant Cmax and AUC levels (Rolan et al., 2008; Rolan et al., 2009).
2.3.2 Testing the effectiveness of AV411 in preventing prime-induced reinstatement
Reinstatement testing followed extinction training. Conditions during reinstatement testing were identical to those during self-administration conditions except that 1 mg/kg i.p. methamphetamine was administered 30 min pre-session (i.e., methamphetamine prime), AV411 test doses or vehicle were administered 60 min pre-session, and methamphetamine self-administered infusions did not occur. Doses of 0 (vehicle), 2.5 and 7.5 mg/kg i.p. of AV411 were tested using separate groups of 12 rats each.
2.4 Drugs
(+)-Methamphetamine hydrochloride (#M8750; Sigma-Aldrich, Inc., St. Louis, MO) was prepared in sterile 0.9% saline. Methamphetamine stock solutions were sterilized by filtration through 0.2 μm filtration disks. Heparin (5 units/ml) was additionally added to methamphetamine and saline infusates. AV411 (molecular weight=230.31) was supplied by the National Institute on Drug Abuse (Rockville, MD) and was dissolved in a 35% PEG400, 10% Cremophor RH40 aqueous vehicle. AV411 was administered i.p. in 1 ml/kg body weight volume.
2.5 Data Analysis
Initially, reinstatement testday data were analyzed using the Grubbs test for outliers (Extreme Studentized Deviate) and a rat's data were excluded from subsequent analyses if P<0.05 for its results (GraphPad QuickCalcs Web site: http://www.graphpad.com/quickcalcs/Grubbs1.cfm, accessed April 2008). Numbers of active-lever presses (i.e., the right-side lever, the presses of which were previously-reinforced with methamphetamine) in the vehicle-treated group were compared to those of each AV411 dosage group using Dunnett's one-tailed post-tests (Prism 5 for Macintosh, GraphPad Software, Inc., San Diego, CA and (Sheskin, 2007)). Numbers of active-lever presses occurring during the last session of self-administration and during the last-session of extinction amongst groups within the prime and stress reinstatement conditions were compared using an ANOVA (Prism 5 for Macintosh, GraphPad Software, Inc., San Diego, CA). If results with the ANOVA were found significant (P<0.05), comparisons between all groups were conducted using Tukey-Kramer tests (Prism 5 for Macintosh, GraphPad Software, Inc., San Diego, CA). A paired, one-tailed t-test was conducted comparing levels of active-lever presses during the last extinction session with those during the reinstatement test session of the vehicle groups to determine if the methamphetamine prime and stress conditions used were capable of reinstating responding. All types of comparisons were considered statistically significant if P<0.05.
3. Results
3.1 Stress Reinstatement Tests
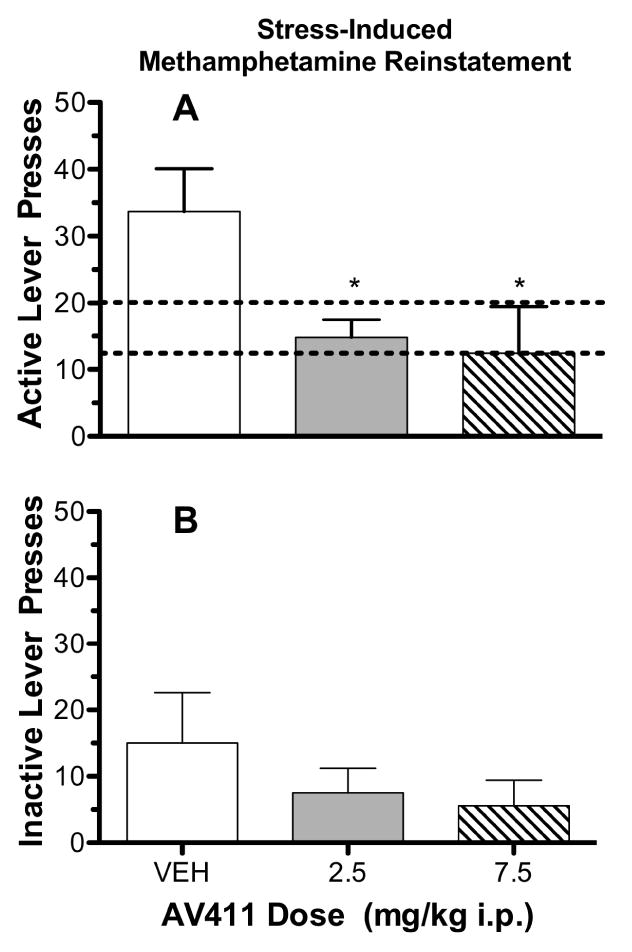
Grubb's Test analyses identified one rat in the 7.5 mg/kg AV411 stress-reinstatement group as an outlier (z= 2.93) and its data were excluded from subsequent analyses. The mean numbers (±S.E.M.) of active lever presses during the last day of self-administration for the vehicle, 2.5 mg/kg AV411 and 7.5 mg/kg AV411 groups were 47.00 (±7.65), 48.00 (±5.39) and 54.17 (±7.85), respectively, and were non-significantly different amongst one another, indicating that the rats had been trained to self-administer methamphetamine to similar levels prior to extinction training [F(2,32)=0.0356; P=0.5275]. Mean (±S.E.M.) active lever presses during the last session of extinction were 20.08 (±3.10), 17.75 (±3.39) and 12.45 (±2.54) in the vehicle, 2.5 mg/kg and 7.5 mg/kg AV411 test groups, respectively. The numbers of active lever presses during the last day of extinction, were not significantly different [F(2,33)=1.591; P=0.2194] amongst the test groups. Mean (±S.E.M.) number of active lever presses during the last session of extinction emitted by the vehicle treatment group was 20.08 (±3.10), and increased to 33.67 (±6.43) during the reinstatement test session which was a statistically significant increase (t=1.851, df=11, P=0.0456) indicating that footshock was able to effectively reinstate responding under the present conditions (see Fig. 1A).
Fig. 1.
Upper panel, “A”: Mean number of active lever presses during the footshock-induced reinstatement test session as a function of AV411 dose. Brackets through the bars indicate ±S.E.M. “VEH” = results of the vehicle-treatment group. Dashed horizontal lines indicate the range of the means of active lever presses across dosage groups occurring during the last session of extinction. Asterisks (*) indicate significantly different (P<0.05) from vehicle. Lower panel, “B”: Mean number of inactive lever presses during the footshock-induced reinstatement test session as a function of AV411 dose. Other details as in the upper panel.
Fig. 1A shows mean numbers of active lever presses emitted during the reinstatement test session for each of the test groups. Pretreatment with 2.5 (q=2.401) and 7.5 mg/kg (q=2.645) AV411 significantly reduced (P<0.05, one-tailed comparisons) footshock-induced reinstatement relative to vehicle pretreatment (VEH). Inactive-lever presses (Fig. 1B) were uniformly low for all test groups and nonsignificantly different [F(2,32)=0.836; P=0.4428] during the reinstatement test session from one another.
3.2 Prime Reinstatement Tests
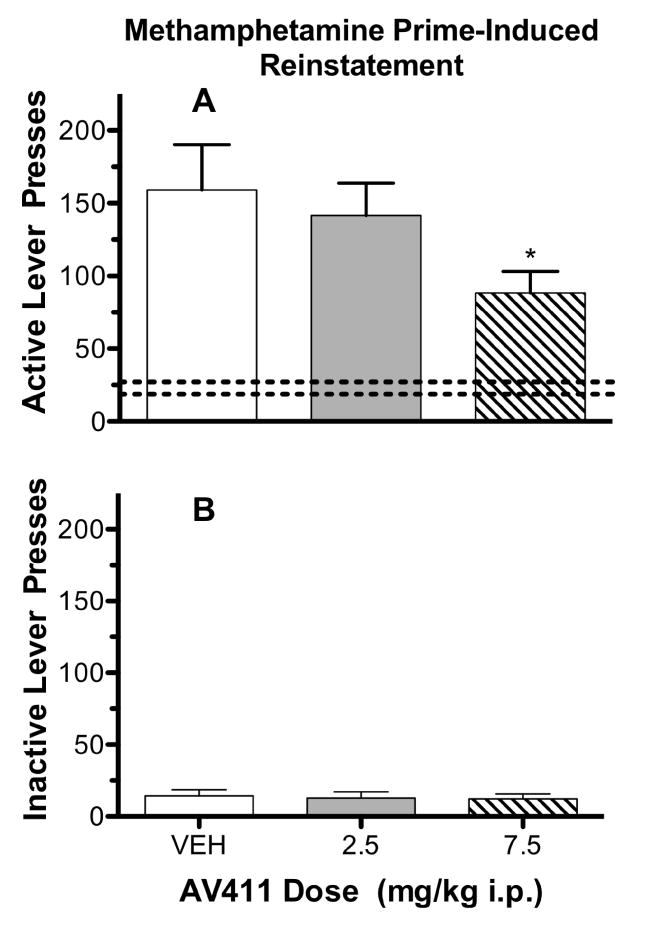
The mean numbers (±S.E.M.) of active lever presses during the last day of self-administration for the vehicle, 2.5 mg/kg AV411 and 7.5 mg/kg AV411 groups were 46.92 (±3.15), 64.83 (±10.73) and 58.33 (±15.61), respectively, and were non-significantly different amongst one another, indicating that the rats had been trained to self-administer methamphetamine to similar levels prior to extinction training [F(2,33)=0.6691; P=0.5190]. Mean (±S.E.M.) active lever presses during the last session of extinction were 27.08 (±4.67) 20.92 (±5.57) and 18.67 (±13.05), in the vehicle, 2.5 and 7.5 mg/kg AV411 test groups, respectively. The numbers of active lever presses during the last session of extinction, were not significantly different [F(2,33)=0.8500; P=0.4366] amongst the test groups. Mean (±S.E.M.) number of active lever presses during the last session of extinction emitted by the vehicle treatment group was 27.08 (±4.67), and increased to 159.1 (±31.19) during the reinstatement test session which was a statistically significant increase (t=4.36, df=11, P=0.0006) indicating methamphetamine primes were able to effectively reinstate responding under the present conditions (see Fig. 2A).
Fig. 2.
Upper panel, “A”: Mean number of active lever presses during the methamphetamine-prime reinstatement test session as a function of AV411 dose. Brackets through the bars indicate ±S.E.M. “VEH” = results of the vehicle-treatment group. Dashed horizontal lines indicate the range of the means of active lever presses across test groups occurring during the last session of extinction. Asterisk (*) indicates significantly different from vehicle (P<0.05). Lower panel, “B”: Mean number of inactive lever presses during the methamphetamine-prime reinstatement test session as a function of AV411 dose. Other details as in the upper panel.
Fig. 2A shows mean numbers of active lever presses emitted during the reinstatement testday for each of the test groups. Pretreatment with AV411 resulted in dose-dependent decreases in active lever-presses, and were significantly lower (P<0.05, one-tailed comparison) in the 7.5 mg/kg treatment group (q=2.111) relative to the vehicle-treatment group. Inactive-lever presses (Fig. 1B) were uniformly low for all test groups and non-significantly different [F(2,33)=0.061; P=0.9406] from one another.
4. Discussion
The footshock conditions used in this study effectively reinstated responding previously reinforced with methamphetamine in vehicle-treated rats. To our knowledge, there has only been one other published report in which footshock has been used to reinstate responding previously reinforced with methamphetamine, and that was by Shepard and colleagues (Shepard et al., 2004). The conditions used in that study were different in many ways compared to the present study, although the strain of rat (Long-Evans hooded), self-administered methamphetamine dose (0.1 mg/kg/injection) and shock intensity (one shock intensity tested in the Shepard study was 0.6 mA) were similar.
The methamphetamine priming conditions used in the present study also effectively reinstated responding and were similar to those we have previously reported (Shelton and Beardsley, 2008). The observations that the vehicle-treated rats in both the footshock and prime conditions emitted significantly more lever presses on the testday relative to their corresponding last day of extinction indicates that the experimental conditions used were appropriate for evaluating treatments which could reduce levels of reinstatement. When AV411 was tested it reduced levels of stress-induced reinstatement at 2.5 and 7.5 mg/kg, and of prime-induced reinstatement at 7.5 mg/kg. This is the first report of a drug attenuating stress-induced methamphetamine responding, and adds to the few other published studies which have found a drug effective in attenuating methamphetamine-prime induced reinstatement (e.g., Anggadiredja et al., 2004; Davidson et al., 2007; Hiranita et al., 2004; Hiranita et al., 2006; Moffett and Goeders, 2007; Qi et al., 2009).
AV411 is a non-selective PDE inhibitor, it attenuates the activation of microglia and astroglia, and increases the production of various anti-inflammatory and nerve growth factors including IL-10 and GDNF (Kawanokuchi et al., 2004; Mizuno et al., 2004; Suzumura et al., 1999). Each of these mechanisms has been reported to individually attenuate some of the effects of drugs of abuse, including methamphetamine's. For instance, in mice the PDE4 inhibitor, rolipram, reduced the level of CPP induced by cocaine and morphine when it was coadministered with these drugs (Thompson et al., 2004), and suppressed methamphetamine- and morphine-induced hyperlocomotion (Mori et al., 2000). In rats, rolipram dose-dependently inhibited locomotor hyperactivity and rearing induced by methamphetamine (Iyo et al., 1995), reduced behavioral sensitization to methamphetamine (Iyo et al., 1996), and attenuated the discriminative stimulus effects of methamphetamine and morphine (Yan et al., 2006). The attenuation of glial activity has also been separately identified as a modulator of the drugs abuse, although inhibition of PDE and attenuation of the activation of microglia and astroglia may be intimately linked. Particular attention has been focused in this regard on methamphetamine's effects, which induces microglial activation (e.g., Escubedo et al., 1998; Guilarte et al., 2003; LaVoie et al., 2004; Pubill et al., 2003; Pubill et al., 2002; Thomas et al., 2004a; Thomas et al., 2004b). For instance, the inhibitor of microglial activation, minocycline, a tetracycline-type antibiotic, has been reported to attenuate locomotion and striatal extracellular dopamine levels, and to reduce striatal dopamine transporter levels induced by methamphetamine (Zhang et al., 2006), and to ameliorate the methamphetamine-induced impairment of recognition memory and the development of methamphetamine-induced behavioral sensitization (Mizoguchi et al., 2008). In addition, the methylxanthine-type PDE inhibitor and glial-cell modulator, propentofylline, was reported to reduce the level of CPP induced by both methamphetamine and morphine (Narita et al., 2006). Another effect of AV411 noted above, that of increasing the production of anti-inflammatory and nerve growth factors like IL10 or GDNF, respectively, has also been identified to reduce the effects of drugs of abuse. For example, Leu-Ile, a hydrophobic dipeptide which induces GDNF synthesis (Nitta et al., 2004), attenuates the expression of methamphetamine- and morphine-induced CPP and its behavioral sensitization (Niwa et al., 2007b; Niwa et al., 2007c). Importantly, Yan and colleagues (Yan et al., 2007) observed that partial reduction in the expression of GDNF (through the use of GDNF (+/-) vs wild-type mice) potentiated methamphetamine self-administration, enhanced the motivation to self-administer methamphetamine as determine by break points using progressive ratio schedules, increased vulnerability to drug-primed reinstatement, and prolonged cue-induced reinstatement of extinguished methamphetamine-seeking behavior (Yan et al., 2007). Administration of GDNF into the ventral tegmental area of rats reduced levels of preference induced by cocaine in a CPP procedure, and blocked morphine-induced increases of tyrosine hydroxylase (Messer et al., 2000). Several other studies have reported that GDNF ameliorates methamphetamine-induced neurotoxicity and its reduction exacerbates it (e.g., Boger et al., 2007; Cass, 1996; Cass et al., 2000; Cass et al., 2006; Cass et al., 1999; Melega et al., 2000).
Thus, any of AV411's multimodal means of neuroregulation (e.g., its ability to inhibit PDE, to attenuate the activation of glia, or to increase the production of GDNF), or their combination, could be mechanisms through which it reduced both footshock- and prime-induced methamphetamine reinstatement in the present study. Other than these neuropharmacological mechanisms, it is unlikely that other known actions of AV411 could have mediated the observed effects. AV411 has been shown to be relatively inactive against a broad range of receptor systems. No significant activity (inhibition or stimulation) was demonstrated by AV411 when tested at 10 μM (2.3 μg/ml) against various (human) target families, including dopamine (D1, D2S, D3, D4.2), opioid (δ, κ, μ), sigma (σ1, σ2), serotonin (5-HT1A, 5-HT3), and cannabinoid receptors (CB1, CB2), and the dopamine and serotonin transporters (DAT, SERT) (Ledeboer et al., 2007). In a separate study, receptor binding of AV411 was evaluated in rat glioma C6 cells expressing the μ- or δ-opioid receptor, and Chinese hamster ovary cells expressing the κ- receptor. AV411 had no affinity at the μ, δ, or κ-opioid receptors (all Ki values >10 μM). At least given our current understanding of AV411's neuropharmacology, it appears it blunted footshock- and prime-induced reinstatement in the present study because of its ability to inhibit PDE, produce GDNF, or to modulate glial functioning, or some combination of these effects.
During the two days immediately prior to the reinstatement testday, AV411 or its vehicle was administered. Multiple administrations of AV411 were given because a minimum period of time (2.5 d) was perceived for it to obtain steady state drug levels in various tissue compartments and to enable minimally sufficient glial attenuation which, in other preclinical procedures had correlated with the onset of efficacy (Hutchinson et al., 2009; Ledeboer et al., 2007; Ledeboer et al., 2006). On the day immediately preceding the reinstatement testday, active lever presses were nonsignificantly lower in AV411 treated prime and footshock groups, relative to respective vehicle groups. Several variables could have been responsible for these declining rates during extinction with increasing AV411 dose. For instance, AV411 may have been ameliorating effects of methamphetamine abstinence which was supporting continued drug-seeking. Consistent with this possibility are reports that AV411 (Hutchinson et al., 2009; Ledeboer et al., 2007), as well as drugs which overlap its pharmacology (Hamdy et al., 2001; Mamiya et al., 2001), can attenuate withdrawal effects of the opiates. Another possibility is that AV411 nonspecifically suppressed responding. If this were so, it would be likely that pressing of the inactive lever would have also been systematically suppressed with increasing dose, and which would be evident on the day of extinction preceding the testday, but this was not the case (It is interesting to note, however, that a dose-related trend associated with levels of inactive lever pressing was observed on the testday when footshock or methamphetamine primes had been administered; see Figs. 1B and 2B). In other studies, AV411 was reported to produce slight transient sedation and decreased reactivity to touch in the Irwin test at i.p. doses ≥7.5 mg/kg for durations under 1 h (Ledeboer et al., 2006), and to reduce rates of lever pressing by rats previously reinforced with food delivery (R. L. Vann, personnel communication). It is important to note that AV411 plasma exposures associated with the dose regimens utilized in these rat studies were at or below those recently reported in clinical trials wherein AV411 was well-tolerated without sedating or related CNS side effects (Johnson et al., unpublished data and Rolan et al., 2008). Overall, the nature of the results lend support to a specific pharmacological action of AV411 to attenuate footshock- or prime-induced methamphetamine relapse, although the exact mechanism(s) are unknown at present.
The present report is the first to describe data in which AV411 was able to modulate effects of methamphetamine or the repercussions of its use. There have been several complimentary reports, of AV411's ability to modulate the effects of the opiates. Concurrent administration of AV411 with morphine augments its analgesic effects and attenuates the development of tolerance and dependence (Hutchinson et al., 2009; Ledeboer et al., 2007) as well as reduces morphine conditioned place preference (Hutchinson et al., 2007). AV411 also is able to attenuate spontaneous morphine withdrawal once dependence had been established (Hutchinson et al., 2009). Because of the multiple ways AV411 can modulate the analgesic, reinforcing and physical dependence effects of the opiates, it is being clinically examined for therapeutic applications in treating neuropathic pain and in the treatment of opiate-dependent patients (Anonymous, 2009).
5. Conclusions
In the current studies, AV411 was shown to effectively attenuate stress-induced and prime-induced reinstatement of extinguished responding previously reinforced with methamphetamine. These results are consistent with a growing literature which indicates that PDE inhibitors and glial cell modulators, including those which increase GDNF levels, can modulate the effects of the drugs of abuse. In tandem with recent studies with opioids, the current results reinforce interest in under-recognized neurobiological mechanisms which could be exploited for developing novel medications for treating drug abuse disorders.
Acknowledgments
The authors wish to thank Dr. Frank Vocci of Friends Research Institute and Drs. David McCann and Jane Acri of the National Institute on Drug Abuse for their substantive scientific input regarding the execution of these studies.
Footnotes
Disclosure Statement: Research was conducted under support by the National Institute on Drug Abuse contract N01DA-4-8848 to P. M. B. Throughout all testing, laboratory personnel were blinded to test compounds and to their nature. Subsequent to the completion of testing, P. M. B. has served as a consultant to Avigen Inc. K. W. J. is an employee of Avigen, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Anonymous. GlobeNewswire. GlobeNewswire, Inc.; Los Angeles, CA: 2009. Avigen announces positive data for AV411 in pain relief and drug addiction. [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37:11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cass WA. GDNF selectively protects dopamine neurons over serotonin neurons against the neurotoxic effects of methamphetamine. J Neurosci. 1996;16:8132–8139. doi: 10.1523/JNEUROSCI.16-24-08132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Manning MW, Bailey SL. Restorative effects of GDNF on striatal dopamine release in rats treated with neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2000;914:127–136. doi: 10.1111/j.1749-6632.2000.tb05190.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Harned ME, Seroogy KB. Protection by GDNF and other trophic factors against the dopamine-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:272–281. doi: 10.1196/annals.1369.024. [DOI] [PubMed] [Google Scholar]
- Cass WA, Walker DJ, Manning MW. Augmented methamphetamine-induced overflow of striatal dopamine 1 day after GDNF administration. Brain Res. 1999;827:104–112. doi: 10.1016/s0006-8993(99)01314-1. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gopalan R, Ahn C, Chen Q, Mannelli P, Patkar AA, Weese GD, Lee TH, Ellinwood EH. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol. 2007;565:113–118. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jimenez A, Pubill D, Pallas M, Camins A, Camarasa J. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D'Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43:218–203. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hamdy MM, Mamiya T, Noda Y, Sayed M, Assi AA, Gomaa A, Yamada K, Nabeshima T. A selective phosphodiesterase IV inhibitor, rolipram blocks both withdrawal behavioral manifestations, and c-Fos protein expression in morphine dependent mice. Behav Brain Res. 2001;118:85–93. doi: 10.1016/s0166-4328(00)00315-6. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Anggadiredja K, Fujisaki C, Watanabe S, Yamamoto T. Nicotine attenuates relapse to methamphetamine-seeking behavior (craving) in rats. Ann N Y Acad Sci. 2004;1025:504–507. doi: 10.1196/annals.1316.062. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S. Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. Eur J Pharmacol. 1996;312:163–170. doi: 10.1016/0014-2999(96)00479-7. [DOI] [PubMed] [Google Scholar]
- Iyo M, Maeda Y, Inada T, Kitao Y, Sasaki H, Fukui S. The effects of a selective cAMP phosphodiesterase inhibitor, rolipram, on methamphetamine-induced behavior. Neuropsychopharmacology. 1995;13:33–39. doi: 10.1016/0893-133X(94)00133-K. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Kato H, Mitsuma N, Suzumura A. Effects of interferon-beta on microglial functions as inflammatory and antigen presenting cells in the central nervous system. Neuropharmacology. 2004;46:734–742. doi: 10.1016/j.neuropharm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci U S A. 2001;98:1964–1969. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Barden JA, Steinhauser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Ren X, Hamdy M, Furukawa S, Kameyama T, Yamada K, Nabeshima T. Involvement of cyclic AMP systems in morphine physical dependence in mice: prevention of development of morphine dependence by rolipram, a phosphodiesterase 4 inhibitor. Br J Pharmacol. 2001;132:1111–1117. doi: 10.1038/sj.bjp.0703912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Lacan G, Desalles AA, Phelps ME. Long-term methamphetamine-induced decreases of [(11)C]WIN 35,428 binding in striatum are reduced by GDNF: PET studies in the vervet monkey. Synapse. 2000;35:243–249. doi: 10.1002/(SICI)1098-2396(20000315)35:4<243::AID-SYN1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2008;196:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue- and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Nitta A, Nishioka H, Fukumitsu H, Furukawa Y, Sugiura H, Shen L, Furukawa S. Hydrophobic dipeptide Leu-Ile protects against neuronal death by inducing brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis. J Neurosci Res. 2004;78:250–258. doi: 10.1002/jnr.20258. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Shen L, Noda Y, Nabeshima T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav Brain Res. 2007a;179:167–171. doi: 10.1016/j.bbr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Noda Y, Nabeshima T. Tumor necrosis factor-alpha and its inducer inhibit morphine-induced rewarding effects and sensitization. Biol Psychiatry. 2007b;62:658–668. doi: 10.1016/j.biopsych.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Shen L, Noda Y, Furukawa S, Nabeshima T. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007c;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Pubill D, Verdaguer E, Sureda FX, Camins A, Pallas M, Camarasa J, Escubedo E. Carnosine prevents methamphetamine-induced gliosis but not dopamine terminal loss in rats. Eur J Pharmacol. 2002;448:165–168. doi: 10.1016/s0014-2999(02)01949-0. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Rolan P, Gibbons JA, He L, Chang E, Jones D, Gross MI, Davidson JB, Sanftner LM, Johnson KW. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66:792–801. doi: 10.1111/j.1365-2125.2008.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Effect of drug-paired exteroceptive stimulus presentations on methamphetamine reinstatement in rats. Pharmacol Biochem Behav. 2008;90:434–440. doi: 10.1016/j.pbb.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sheskin D. Handbook of parametric and nonparametric statistical procedures. 4th. Chapman & Hall/CRC; Boca Raton: 2007. [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shindo K, Miyatake M, Kurokawa K, Higashiyama K, Suzuki M, Narita M. Lack of development of behavioral sensitization to methylphenidate in mice: correlation with reversible astrocytic activation. Eur J Pharmacol. 2007;574:39–48. doi: 10.1016/j.ejphar.2007.06.062. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837:203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004a;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine Neurotoxicity in Dopamine Nerve Endings of the Striatum Is Associated with Microglial Activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The Type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur J Neurosci. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizuno T, Nakajima A, Yamada K, Nabeshima T. Discriminative-stimulus effects of methamphetamine and morphine in rats are attenuated by cAMP-related compounds. Behav Brain Res. 2006;173:39–46. doi: 10.1016/j.bbr.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]