Abstract
Recent studies in rodents have suggested a role for the central endocannabinoid system in the regulation of mood and alcohol related behaviors. Alcohol use disorder is often associated with suicidal behavior. In the present study, we examined whether abnormalities in the endocannabinoid system in the ventral striatum are associated with alcohol dependence and suicide. The levels of CB1 receptors, receptor-mediated G-protein signaling, and activity and level of the fatty acid amide hydrolase (FAAH) were analyzed postmortem in the ventral striatum of alcohol-dependent nonsuicides (CA, n=9), alcohol-dependent suicides (AS, n=9) and nonpsychiatric controls (C, n=9). All subjects underwent a psychological autopsy, and toxicological and neuropathological examinations. The levels of the CB1 receptors and the CB1 receptor-mediated G-protein signaling were significantly lower in the ventral striatum of CA compared to the control group. However, these parameters were elevated in AS when compared to CA group. The activity of FAAH enzyme was lower in CA compared to the control group while it was found to be significantly higher in AS compared with CA group. These findings suggest that alcohol dependence is associated with the downregulation of the CB1 receptors, while suicide is linked to the upregulation of these receptors in the ventral striatum. Alteration in the activity of FAAH enzyme that regulates the anandamide (AEA) content might in turn explain differences in the CB1 receptor function in alcohol dependence and suicide. These findings may have etiological and therapeutic implications for the treatment of alcohol addiction and suicidal behavior.
Keywords: Alcoholism, Suicide, Striatum, Cannabinoid, Anandamide, G-protein
1. Introduction
Research carried out during the past decade has demonstrated existence of an endogenous cannabinoid (endocannabinoid) system in the CNS, which consists of G-protein coupled receptors (GPCR), CB1 and CB2, endocannabinoid agonists and proteins that are involved in the metabolism of the endocannabinoids. This novel system modulates functions in several neurobiological processes (Howlett AC). The CB1 receptors, localized primarily in the neural tissue, are coupled to adenylyl cyclase (AC) via Gi-protein and to several other effector molecules (Howlett, 2005). These receptors are one of the most abundant neuromodulatory GPCRs in the mammalian brain and are expressed densely in the cerebral cortex, hippocampus, cerebellum and striatum (Herkenham et al, 1991; Glass et al., 1997; Childers and Breivogel 1998; Howlett 2005). The endocannabinoids, N-arachidonoyl ethanolamide (AEA/anandamide) and 2-arachidonoyl glycerol (2-AG) are present in the CNS and in non-neural organs (Devane et al., 1992; Di Marzo et al., 1999). Several other lipid molecules have been identified recently as putative endocannabinoids and their pharmacological and physiological functions are under investigation. The endocannabinoids are synthesized within neurons and released into the synaptic cleft by stimulus-dependent cleavage of membrane phospholipids (Wilson and Nicoll 2001; Kreitzer and Regehr 2001). A membrane bound serine hydrolase, a fatty acid amide hydrolase (FAAH) is involved in the degradation of AEA (Di Marzo et al., 1999; Deutsch et al., 2001) while 2-AG is hydrolyzed mainly by monoglyceride lipase (MGL) (Di Marzo et al., 1999).
The dysfunction in the monoamine neurotransmitter systems has long been implicated in the pathophysiology of alcohol dependence and suicidal behavior (Callado et al., 1998; Arango et al., 2002; Gonzalez-Maeso et al., 2002; Pandey et al., 2002, Mann 2003; Underwood et al., 2008). In addition, recent studies have revealed a critical role for the endocannabinoid system in alcohol addiction and mood disorders (Schmidt et al., 2003; Wang et al., 2003; Vinod et al., 2005; Steiner et al., 2008). Moreover, alcoholism is related to more aggression and impulsivity, which are part of the diathesis for suicide (Rich et al., 1998; Potash et al., 2000; Ray et al., 2000; Koller et al., 2002; Preuss et al., 2002; Makhija and Sher 2007). A dysfunction of the frontocortico-striatal circuit has been postulated in the pathophysiology of drug addiction and mood disorders (Eisch et al., 2003), raising the question of whether the endocannabinoid system in the ventral striatum plays a role in alcohol addiction and suicide. In the present study we sought to determine the levels of CB1 receptors, CB1 receptor-mediated G-protein signaling, and levels and activity of the FAAH enzyme in the ventral striatum of alcohol-dependent nonsuicides (CA) and alcohol-dependent suicides (AS), compared to psychiatrically normal controls (C). This study was focused on the CB1 receptor as it is highly expressed in the CNS compared to the CB2 receptor and it regulates mood and drug reward (Childers and Breivogel 1998; Howlett, 2005; Steiner et al., 2008). In addition, we selected the ventral striatum because of its critical role in drug reward and impulsivity; both are risk factors for alcohol addiction and suicide. Using three comparison groups enabled us to identify the biochemical alterations associated with alcoholism and suicide with a greater specificity.
2. Methods and Materials
2.1. Subjects
Brain tissue from the right hemisphere was obtained from the brain tissue collection of the Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, USA. All samples used in this study were collected at the Allegheny County Coroner Office in accordance with protocols approved by the Institutional Review Board of the University of Pittsburgh. Consent to use brain tissue was obtained from the next-of-kin. The ventral striatum from CA (n=9) and AS (n=9) were studied along with normal controls (n=9). All groups were matched for sex, and as closely as possible, for age, postmortem interval (PMI), brain pH and race. The brain pH was within neutral range. Peripheral toxicology was carried out by the Coroner’s office in urine, blood or vitreal samples. Brain pH determination and toxicological analyses (over 30 drugs) were performed on cerebellar tissue, ruling out the presence of psychoactive substances (except alcohol in the alcohol-dependent groups). The demographic variables and clinical characteristics of subjects are summarized in Table 1. Multiple regions of the left hemisphere were fixed and examined for neuropathology and only cases free of neuropathology were included in the study. All subjects had data from a psychological autopsy including previously validated structured clinical interview for Axis I and Axis II diagnoses based on DSM-IV criteria (Kelly and Mann 1996). These interviews were carried out with family members and/or close friends and confirmed that all the subjects in the alcohol groups (CA and AS) had a diagnosis of alcohol dependence, while subjects in the control group were free of psychopathology. The brain samples were coded to mask the investigators to the diagnostic groups.
Table 1.
Demographic and clinical characteristics of subjects
| Group | Age | Sex | PMI | Brain pH |
Cause of death | Axis I diagnosis |
Toxicology |
|---|---|---|---|---|---|---|---|
| C | 15 | M | 16.0 | 6.71 | Homicide (GSW) | None | Lido |
| C | 25 | M | 9.5 | 6.87 | Accident (Stabbing) | None | CO |
| C | 25 | M | 17.5 | 6.52 | Accident (Industrial) | None | CO |
| C | 39 | M | 13.5 | 6.49 | Accident (MVA) | None | Lido, Caffeine |
| C | 41 | M | 8.0 | 6.12 | Natural (Cardiovascular) | None | Lido |
| C | 47 | M | 14.5 | 6.72 | Natural (Cardiovascular) | None | Clear |
| C | 48 | M | 7.5 | 6.65 | Natural (Cardiovascular) | None | Lido |
| C | 54 | M | 9.0 | 6.63 | Natural (Cardiovascular) | None | Caffeine |
| C | 66 | M | 8.0 | 6.56 | Natural (Cardiovascular) | None | Clear |
| CA | 17 | M | 12.0 | 6.87 | Accident (MVA) | EtOH | Clear |
| CA | 22 | M | 16.0 | 6.69 | Accident (MVA) | EtOH | Clear |
| CA | 28 | M | 14.5 | 6.43 | Accident (MVA) | EtOH | Clear |
| CA | 36 | M | 16.5 | 6.92 | Natural (Cardiovascular) | EtOH | Clear |
| CA | 42 | M | 15.0 | 6.78 | Natural (Cardiovascular) | EtOH | Lido |
| CA | 47 | M | 12.0 | 6.55 | Natural (Cardiovascular) | EtOH | Lido |
| CA | 48 | M | 7.5 | 6.87 | Natural (Cardiovascular) | EtOH | Clear |
| CA | 59 | M | 16.0 | 6.63 | Natural (Cardiovascular) | EtOH | Caffeine |
| CA | 67 | M | 9.0 | 6.90 | Natural (Cancer) | EtOH | Caffeine |
| AS | 20 | M | 24.0 | 6.50 | Suicide (GSW) | EtOH | Caffeine |
| AS | 26 | M | 9.0 | 6.59 | Suicide (GSW) | EtOH, MDD | Caffeine |
| AS | 29 | M | 22.0 | 6.45 | Suicide (GSW) | EtOH | Caffeine |
| AS | 36 | M | 15.0 | 6.56 | Suicide (GSW) | EtOH, MDD | Lido |
| AS | 37 | M | 8.0 | 6.42 | Suicide (GSW) | EtOH, MDD | ACE,Caffeine |
| AS | 42 | M | 12.5 | 6.56 | Suicide (Jump from height) | EtOH | Clear |
| AS | 48 | M | 11.5 | 6.98 | Suicide (Hanging) | EtOH, SCZ | Clear |
| AS | 51 | M | 17.5 | 6.64 | Suicide (GSW) | EtOH, SCZ | Clear |
| AS | 61 | M | 17.0 | 6.43 | Suicide (GSW) | EtOH | Caffeine |
C: normal control; CA: alcohol-dependent nonsuicide; AS: alcohol-dependent suicide; PMI: postmortem interval; EtOH: alcohol dependence; MDD: major depressive disorder: SCZ: schizophrenia; MVA: motor vehicle accident; GSW: gun shot wound; CO: carbon monoxide; Lido: lidocaine: ACE: acetaminophen. There were no statistical significant differences in age (p<0.97), PMI (p<0.22) and brain pH (p<0.13) across the groups.
2.2. Brain dissection
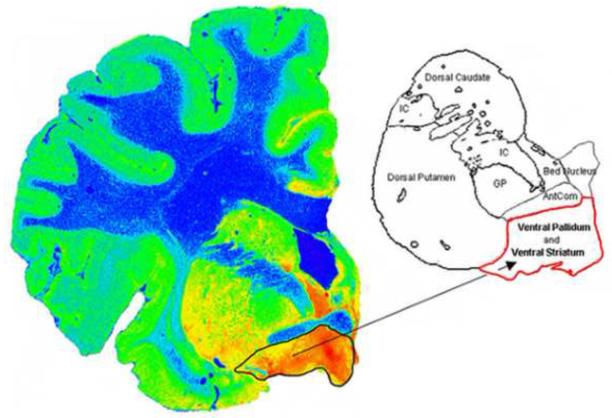
The right hemisphere was sectioned fresh into 10-12 coronal slabs, rapidly frozen in Freon and stored at −80°C. Frozen sections (20μm) were taken from the coronal slab containing the anterior, precommissural and commissural striatum and the nucleus accumbens and stained for Nissl. In order to aid in the identification of the boundaries of the ventral striatum, autoradiography to the serotonin transporter was carried out using [3H]Cyanoimipramine (Figure 1). Tissue from the ventral striatum was then carefully dissected from the coronal slab and frozen at −80°C until assay.
Figure 1.
The autoradiogram of [3H]Cyanoimipramine shows the distribution of serotonin transporters in the human postmortem brain. The serotonin transporters are highly expressed in the ventral striatum compared to dorsal striatum. This assisted in delineating the ventral striatum from the dorsal striatum.
2.3. [3H]Cyanoimipramine autoradiography
Briefly, the brain sections were pre-incubated in Tris-NaCl buffer (pH 7.4) for 30 minutes at 23°C followed by incubation with Tris-NaCl buffer containing 0.4 nM [3H]cyanoimipramine for 24 hour at 4°C. Non-specific binding was determined in the presence of 10 μM sertraline HCl. Sections were then washed for 60 min (3 × 20) in Tris-NaCl buffer at 4°C followed with a dip in cold distilled water to remove the buffer salts. Sections were then dried under a stream of desiccated cold air, and then exposed to Kodak Biomax MS film for 17-18 weeks.
2.4. Sample preparation
The brain tissue (~1 g) was homogenized in 20 vol. of ice-cold TME buffer (50 mM Tris-HCl, 3 mM MgCl2 and 1 mM EDTA, pH 7.4) containing 0.32 M sucrose, protease and phosphatase inhibitors. The homogenate was centrifuged at 1,000g for 10 min at 4°C and resulting supernatant was then centrifuged at 22,000g for 20 min. The membrane pellet was dissolved in TME buffer and re-centrifuged at 22,000g for 20 min. Aliquots of supernatant (for the analysis of FAAH) and membrane fractions (for the analysis of CB1 receptor and G-protein activation) were made and stored at −80°C until further assay. The protein contents of isolated fractions were determined by Lowry’s method (Lowry et al 1951).
2.5. Immunoblot analysis of the CB1 receptor and the FAAH enzyme
Aliquots (30μg protein) of crude synaptic membrane and post-synaptic membrane supernatant of all the samples were separated on 12% polyacrylamide gel, and electrophoretically transferred (wet method) to nitrocellulose membrane (Idea Scientific Company, Minneapolis, MN). All the samples were loaded in the same gel to avoid the gel and lane inter-assay variability. The membranes were treated with blocking buffer (TTBS [10 mM Tris, 0.9% NaCl; 0.1% Tween 20 containing 5% milk powder] of pH 7.4) for 1 hr at room temperature. The membranes were incubated either with the CB1 receptor antibody (1:1000, Biosource International, Camarillo, CA) or FAAH antibody (1:1000, Abnova, Walnut, CA) overnight at 4°C. The blots were washed three times with TTBS and then incubated with HRP conjugated IgG antibody (1:5000, Santa Cruz Biotech, Santa Cruz, CA) for 1 hr at room temperature. After washing the blot for three times with TTBS, the immunoreactive bands were visualized using ECL chemiluminescence reagent (GE Healthcare, Piscataway, NJ). The blots were stripped and then reprobed with α-tubulin antibody (1:1000, Santa Cruz Biotech, Santa Cruz, CA) to ensure equal protein loading.
2.6. Agonist-stimulated [35S]GTP S binding assay
The CB1 receptor-mediated [35S]GTPγS binding assay was performed as described previously with minor modifications (Vinod et al., 2005). Briefly, an aliquot of membrane (50 μg protein) was pre-incubated in assay buffer (TME buffer, 0.1% fatty acid free BSA and 100 mM NaCl) containing GDP (30 μM) in silicone-treated test tubes for 15 min. Later [35S]GTP S (0.05 nM) was added to the reaction mixture and incubated for 1 hr at 37°C. The CB1 receptor agonist, CP-55,940 (1 μM) was used to examine the CB1 receptor-mediated [35S]GTPγS binding. The basal activity was estimated in absence of CP-55,940. The non-specific binding of the radioligand was determined in presence of 10 μM GTPγS. The reaction was terminated by addition of ice-cold Tris-HCl buffer containing 0.1% BSA followed by rapid filtration. The radioactivity was then measured using liquid scintillation spectroscopy.
2.7. Measurement of the FAAH activity
The FAAH activity was determined as described previously with minor modification (Vinod et al., 2006). Briefly, post-synaptic membrane supernatant (30 μg protein) was incubated with a reaction mixture (500 μl volume) containing 50 mM Tris-HCl (pH 8.0), 0.05% fatty acid free BSA, 5 nM [3H]AEA and 5 μM of cold AEA for 30 min at 37°C. The reaction was then terminated and samples were extracted with 2 vol. of chloroform/methanol (1:1 v/v). The radioactivity of hydrolyzed product, [3H]ethanolamine in the aqueous layer was measured using liquid scintillation spectroscopy.
2.8. Statistical analyses
The differences in covariates (Age, PMI and Brain pH) among subject groups were analyzed using one-way ANOVA (GraphPad, San Diego, CA). The dependent variables (CB1 receptor, G-protein activation, activity and level of FAAH) with the main effect, subject groups and covariates were analyzed in a multiple analysis of covariance (MANCOVA) model using SAS program (version 9.1). In the presence of a significant MANCOVA, univariate analysis of covariance (ANCOVA) was performed for each dependent variable. Immunoblots were analyzed using the ImageJ software program (NIH, Bethesda, USA). Data are presented as mean ± SEM and are considered to be statistically significant at p<0.05.
3. Results
The demographic and clinical characteristics of control, CA and AS subjects are provided in table 1. One-way ANOVA revealed no significant differences in age (p<0.97), PMI (p<0.22) and brain pH (p<0.13) across the groups. MANCOVA showed an effect of age on the dependent variables across the groups. The covariates, brain pH and PMI were not significant in the MANCOVA and in any of the ANCOVAs and were excluded in the reported analyses.
3.1. Quantification of CB1 receptor and G-protein activation
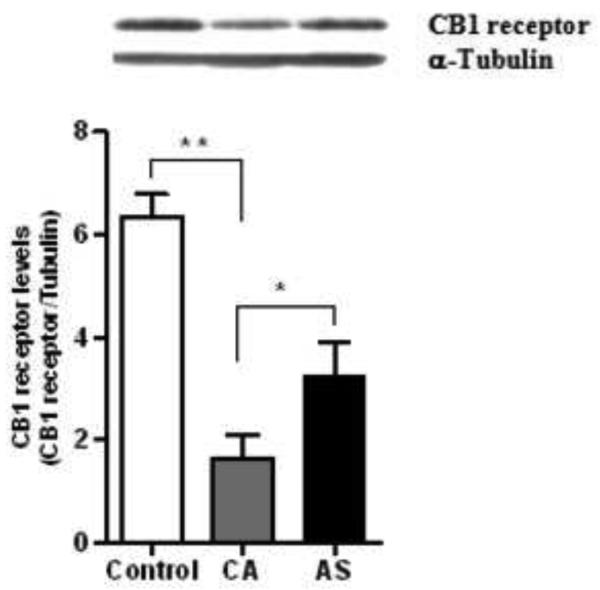
The western blot analysis demonstrated a single immunoreactive band corresponding to the relative molecular weight (~58 kDa) of the CB1 receptor. There was a significant group effect on the levels of CB1 receptor (F=21.07; df=2,23; p<0.0001) and G-protein activation (F=10.05; df=2,23; p=0.0007). However, there was no overall effect of age on the levels of CB1 receptor (F=1.77; df=1,23; p=0.197) and G-protein activation (F=3.10; df=1,23; p=0.092) among the subject groups. Multiple comparison analysis revealed a significant lower level of the CB1 receptors in the ventral striatum of CA (74%, p<0.0001) and AS (48%, p=0.0003) compared to normal controls (Figure 2). However, a marked higher level of the CB1 receptors was evident in AS (98%, p=0.045) compared to CA group (Figure 2). A representative immunoblot of the CB1 receptor is provided in figure 2 (Upper panel).
Figure 2.
The CB1 receptor levels were found to be lower in the ventral striatum of CA (74, p<0.0001) and AS (48%, p<0.001) compared to normal controls (Figure 1). However, a marked increase in level of the CB1 receptors was evident in the AS (98%, p<0.05) compared with CA. A representative immunoblot of the CB1 receptor is provided in the upper panel.
The CB1 receptor agonist-stimulated [35S]GTP S binding assay was conducted in membranes isolated from the ventral striatum to determine the functional coupling between the CB1 receptor and the Gi/o-protein. The CB1 receptor-mediated [35S]GTPγS binding was significantly lower in CA (35%, p=0.008) compared to normal controls (Figure 3). However, G-protein activation was found to be higher in AS (32%, p=0.0002) compared to CA group (Figure 3). There was no significant difference in the G-protein activation between As and normal control (p=0.143).
Figure 3.
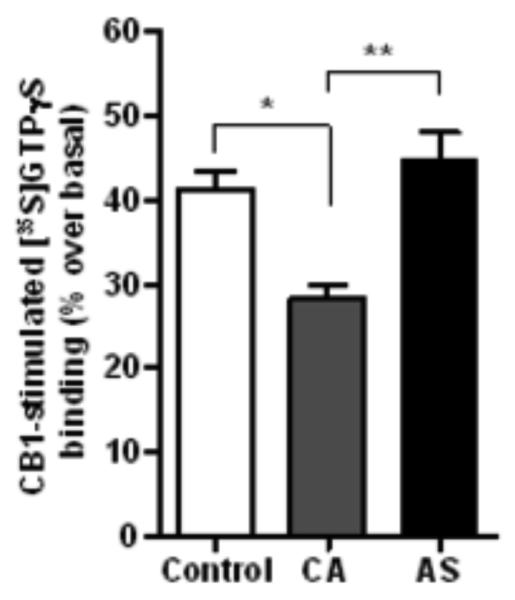
The CB1 receptor-mediated [35S]GTP S binding was significantly lower in the membranes isolated from the ventral striatum of CA (35%, p<0.01) compared to normal controls. However, G-protein activation was higher in AS (32%, p<0.001) compared to CA group. Data is presented as percentage of stimulation over the basal binding.
3.2. Activity and level of FAAH enzyme
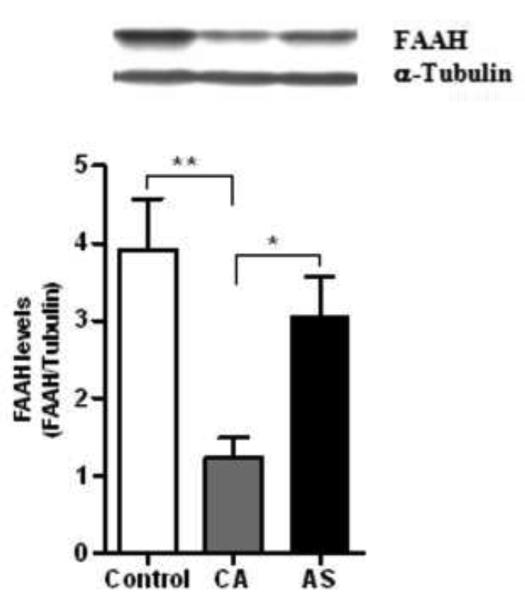
The western blot analysis demonstrated a single immunoreactive band corresponding to a relative molecular weight (~64 kDa) of the FAAH. Age showed an effect on the FAAH activity (F=8.98; df=1,23; p=0.006) and FAAH levels (F=7.37; df=1,23; p=0.012) across the subject groups. There was a significant group effect on the FAAH activity (F=22.10; df=2,23; p<0.0001) and FAAH levels (F=8.86; df=2,23; p=0.001) after controlling for the effect of age. Multiple comparison analysis revealed a lower activity of the FAAH in the ventral striatum of CA (50%, p<0.0001) and AS (23%, p=0.005) compared to normal controls (Figure 4). However, the FAAH activity was found to be higher in AS (56%, p=0.0017) than CA group (Figure 4). Similarly, a marked lower level of the FAAH immunoreactivity was evident in CA (68%, p=0.0004) compared to normal controls, whereas it was found to be higher in AS (51%, p=0.013) compared to CA group (Figure 5). A representative immunoblot of the FAAH is shown in the figure 5 (Upper panel).
Figure 4.
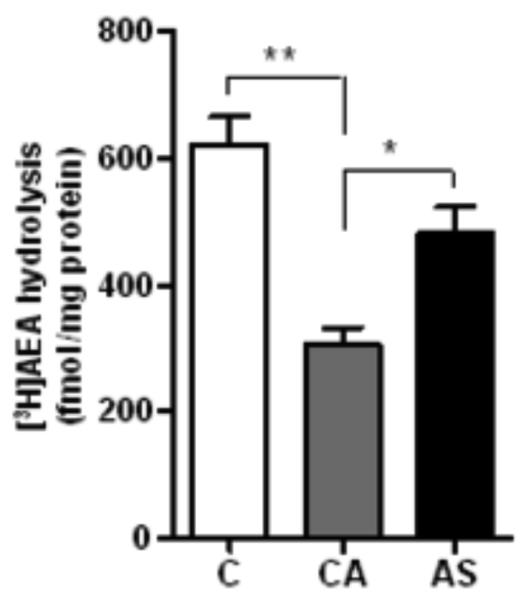
The activity of FAAH was markedly reduced in the ventral striatum of CA (50%, p<0.0001) and AS (23%, p<0.01) compared to normal controls. However, there was a significant higher activity of the FAAH in AS (56%, p<0.01) compared to CA group.
Figure 5.
A marked reduction in the level of FAAH was observed in CA (68%, p<0.001) compared to normal controls, whereas the FAAH immunoreactivity was found to be higher in AS (51%, p<0.05) than CA group. A representative immunoblot of the FAAH is shown in the upper panel.
4. Discussion
The present study revealed a lower level of the CB1 receptors in the ventral striatum of alcohol dependents while they found to be higher in alcohol-dependent subjects who died by suicide. This is the first study to report alterations in the endocannabinoid system in the ventral striatum of alcoholics who died by suicide and other means. The CB1 receptor-mediated G-protein signaling was lower in the CA compared with normal controls whereas it was greater in AS compared with CA. These alterations were consistent with changes in the level of CB1 receptors in both CA and AS. The consequence of alterations in the CB1 receptor levels observed in this study is unknown. The lower level of CB1 receptors in CA is in agreement with the previous observations that chronic alcohol exposure leads to downregulation of the CB1 receptor and G-protein activation in the brain of rodents (Basavarajappa et al., 1998; Ortiz et al., 2004; Vinod et al., 2006; Matrirattanakul et al., 2007).
The FAAH activity was lower in the ventral striatum of CA compared to normal controls. Since FAAH is a major degrading enzyme of the AEA, decrease in its activity could increase the level of AEA in CA and vice-versa. Indeed, animal studies have reported increase in the AEA content in the striatum and “limbic” brain by chronic alcohol exposure (Gonzalez et al., 2002; Gonzalez et al., 2002; Vinod et al., 2006) by reducing the FAAH activity (Vinod et al., 2006). Thus, repeated alcohol consumption could desensitize the CB1 receptor function in the ventral striatum of alcoholic dependent patients as a consequence of a compensatory response to increased AEA. Most notably, an association of impaired FAAH activity with alcohol reward is supported by an increased alcohol self-administration in rats, which were given intra-PFC injection of URB597 (Hansson et al., 2007). The genetic deletion and pharmacological inhibition of FAAH have also been shown to enhance alcohol drinking behavior in mice (Blednov et al., 2007; Vinod et al., 2008). In addition, an increased vulnerability to drug and alcohol abuse in humans has recently been suggested to be due to polymorphism in the FAAH gene and reduced FAAH expression and activity (Sipe et al., 2002; Chiang et al., 2004). An administration of AEA alone or URB597 is further shown to increase the DA levels in NAc shell suggesting a critical role for the AEA in producing a reward effect through the activation of the mesolimbic dopaminergic system (Solinas et al., 2006). An association between polymorphism in the CB1 receptor gene and alcohol dependence has been also reported (Schmidt et al., 2003; Zuo et al., 2007). It remains to be established whether this polymorphism is related to the changes in the level or function of the CB1 receptor. Importantly, alcohol related behaviors have been shown to be mediated through the CB1 receptor. For example, blockade of the CB1 receptor is shown to reduce alcohol drinking behavior (Hungund et al., 2003; Wang et al., 2003; Malinen and Hyytia 2008; Vinod et al., 2008) and to inhibit alcohol-induced DA release in the nucleus accumbens (Hungund et al., 2003; Cheer et al., 2007) indicating an important role for the nucleus accumbal endocannabinoid system in alcohol addiction.
In AS, the upregulation of the CB1 receptor and the CB1 receptor-mediated G-protein signaling in the ventral striatum compared to CA appears to be due to feedback mechanism in response to reduced level of the AEA. This hypothesis is based on the observed higher expression and activity of the FAAH in AS compared to CA. Our previous study, however, showed elevated levels of both the CB1 receptor and endocannabinoids (AEA and 2-AG) in the prefrontal cortex of AS (Vinod et al., 2005), indicating the possibility of higher level of endocannabinoids in the ventral striatum of AS. The question is how does elevation in the CB1 receptors be related to suicide when both CA and AS groups show decreased levels compared to normal controls? We believe that increase in CB1 receptor levels is associated with suicide since AS show markedly higher CB1 receptors compared to CA (alcohol abuse is a common factor in both groups). As discussed previously, chronic alcohol exposure significantly decreases the levels of central CB1 receptor binding sites (Basavarajappa et al., 1998; Ortiz et al., 2004; Vinod et al., 2006; Matrirattanakul et al., 2007), which might be partly associated with decreased levels of CB1 receptors in both CA and AS groups compared to normal controls.
Alteration in the CB1 function seems to be region-specific because the previous study revealed elevation in the CB1 receptor-mediated G-protein signaling in the prefrontal cortex but not in occipital cortex of AS (Vinod et al., 2005). Whether the present findings are directly associated with suicidal behavior or they are part of neuroadaptative changes in response to alteration in other neuronal substrate/s remain uncertain. Nevertheless, the exogenous cannabinoid, THC, that exerts its effect mainly through the CB1 receptor, appears to modulate impulsive behavior (McDonald et al., 2003; Vinod and Hungund 2006; Pattij and Vanderschuren 2008). The AEA is also shown to exert THC-like discriminative and neurochemical effects (Solinas et al., 2007). Therefore, the impulsive behavior that is one of the contributing factors for suicidal behavior (Mann et al., 1999; Koller et al., 2002) might be associated with the dysfunction of the striatal CB1 receptor signaling. The ventral striatum has been shown to mediate anhedonia and impulsive behavior (Eisch et al., 2003; Tremblay et al., 2005; Juckel et al., 2006; Kumar et al., 2008). Considering the reported abnormalities in the CB1 receptor function in the frontal cortex of suicide victims (Hungund et al., 2004; Vinod et al., 2005), the findings of this study suggest that the dysfunction of the endocannabinoid system in the frontocortico-striatal circuitry is likely to produce behavioral deficits associated with suicidal behavior.
The comorbidity of suicidal behavior with several psychiatric disorders and drugs of abuse (Suominen et al., 1996; Rich et al., 1998; Kessler et al., 1999; Potash et al., 2000) might also be associated with observed alterations. However, there were no psychoactive drugs (other than alcohol) detected postmortem in CA and AS subjects who had a diagnosis of alcohol dependence and all controls had clear toxicology. The observed changes in the endocannabinoid system in CA subjects, which are consistent with previous animal studies, appear to be mainly associated with alcohol dependence. Although the mood of AS subjects at the time of death is unknown, the alterations in the CB1 receptor function might also be due to stress or anxiety related disorders (Kamprath et al., 2009; Mangieri and Piomelli, 2007; Patel et al., 2004; Patel et al., 2008; Steiner et al., 2008a; Vinod and Hungund, 2006). Indeed, there were five AS subjects who had comorbid Axis I psychiatric disorders; major depressive disorder in three and schizophrenia in two cases. In addition, there were five CA and four AS subjects who had comorbid Axis II personality disorders. Although this study includes a modest sample size, future studies in larger samples are needed to confirm these observations. It is also necessary to examine other components of the endocannabinoid system (CB2 receptor, endocannabinoids, MGL etc) to better understand the pathophysiology of alcohol addiction and suicide.
Whether the endocannabinoid system directly associated with the pathophysiology of alcohol dependence and suicide or does so via modulating the functions of neurotransmitter systems remain to be elucidated. Importantly, the abnormalities in many neurotransmitter systems and other neuronal substrates might also underlie these psychiatric disorders. For instance, previous studies have suggested that dysfunction in the activity of hypothalamic-pituitary-adrenal (HPA) axis is associated with the development of alcohol addiction, depression and suicidal behavior (Sher 2007; Richardson et al., 2008; Steiner et al., 2008b). In addition, the serotonergic, glutamatergic and dopaminergic systems, which are involved in the regulation of mood, fear, reward and impulsive behaviors, functionally interact with the endocannabinoid system (Patel et al., 2003; Vinod and Hungund 2006; Mangieri and Piomelli 2007; Kamprath et al., 2009). Importantly, the animal studies have shown the modulation of the function of HPA axis by the endocannabinoid system (Patel et al., 2004; Vinod and Hungund 2006; Steiner et al., 2008b; Kamprath et al., 2009). Therefore, an abnormal interaction of the endocannabinoid system with the HPA axis and other neurotransmitter systems might constitute at least in part the underlying mechanism of alcohol addiction and suicidal behavior. In addition, the cAMP-CREB pathway is a target for several monoamine and neuromodulatory systems, and has been shown to play a pivotal role in neuronal plasticity associated with stress, drug addiction and suicidal behavior (Self and Nestler 1998; Reiach et al., 1999; Dwivedi et al., 2002; Dwivedi et al., 2003). The dysfunction of CB1 receptors in the ventral striatum of CA and AS might lead to alterations in the cAMP content as CB1 receptors are coupled to adenylyl cyclase. This impact on the cAMP pathway and gene regulation may in turn affect the normal physiology and behavior. The pharmacological agents that modulate the endocannabinoid tone or CB1 receptor function might have therapeutic potential in the treatment of alcohol addiction and prevention of suicidal behavior.
Acknowledgements
Funding for this study was provided by the American Foundation for Suicide Prevention Young Investigator Grant (KVY) and PHS grants MH40210 (VA), AA09004 (VA) and MH62185 (JJM). We wish to thank Mihran J. Bakalian and Virginia L. Johnson for their assistance with the analysis of receptor autoradiograms and Joseph Wanderling for his assistance with the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Progress Brain Research. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- 2.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Research. 1998;79:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 3.Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- 4.Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, Garcia-Sevilla JA. Selective increase of alpha2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. Journal of Neurochemistry. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheer JF, Wassum KM, Sombers LA, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. Journal of Neuroscience. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human Molecular Genetics. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 7.Childers SR, Breivogel CS. The functional neuroanatomy of brain cannabinoid receptors. Neurobiology of Disease. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, et al. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. Journal of Biological Chemistry. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- 9.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 10.Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: an historical overview and some recent developments. Lipids. 1999;34:319–325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- 11.Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [3H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. American Journal of Psychiatry. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Dwivedi Y, Rao JS, Rizavi HS, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subject. Archives of General Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 13.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biological Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Research. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez S, Valenti M, de Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, et al. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. British Journal of Pharmacology. 2004;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. Neurotransmitter receptor-mediated activation of G-proteins in the brains of suicide victims with mood disorders: selective supersensitivity of α2A-adrenoceptors. Molecular Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- 18.Hansson AC, Bermudez-Silva FJ, Malinen H, et al. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- 19.Herkenham M, Lynn AB, Johnson MR, Melvin LS, deCost BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain; a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;16:8057–8066. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howlett AC. Cannabinoid receptor signaling. Handbook of Experimental Pharmacology. 2005;68:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 21.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. Journal of Neurochemistry. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 22.Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPγS binding in the prefrontal cortex of depressed suicide victims. Molecular Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- 23.Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wüstenberg T, Villringer A, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 24.Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B, Wotjak CT. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behavior. 2009;8:203–11. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: A comparison with antemortem diagnosis. Acta Psychiatrica Scandinavica. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC, Borges G, Walters EE. Prevalence of risk factors for lifetime suicide attempts in the National Comorbidity Survey. Archives of General Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 27.Koller G, Preuss UW, Bottlender M, Wenzel K, Soyka M. Impulsivity and aggression as predictors of suicide attempts in alcoholics. European. Archives of Psychiatry and Clinical Neuroscience. 2002;252:155–160. doi: 10.1007/s00406-002-0362-9. [DOI] [PubMed] [Google Scholar]
- 28.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AG, Randall RJ. Protein measurement with folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Makhija NJ, Sher L. Preventing suicide in adolescents with alcohol use disorders. International Journal of Adolescence and Medical Health. 2007;19:53–59. doi: 10.1515/ijamh.2007.19.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clinical and Experimental Research. 2008;32:1976–1983. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 33.Mangieri RA, Piomelli D. Enhancement of endocannabinoid signaling and the pharmacotherapy of depression. Pharmacological Research. 2007;56:360–366. doi: 10.1016/j.phrs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 35.Mann JJ. Neurobiology of suicidal behaviour. Nature Review Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 36.McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 37.Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, et al. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clinical and Experimental Research. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 38.Ortiz S, Oliva JM, Pérez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- 39.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. American Journal of Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 40.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. Journal of Pharmacology and Experimental Therapeutics. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- 41.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 42.Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. European Journal of Neuroscience. 2008;27:2821–9. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends in Pharmacological Sciences. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Potash JB, Kane HS, Chiu YF, Simpson SG, McKinnon DF, et al. Attempted suicide and alcoholism in bipolar disorder: clinical and familial relationships. American Journal of Psychiatry. 2000;157:2048–2050. doi: 10.1176/appi.ajp.157.12.2048. [DOI] [PubMed] [Google Scholar]
- 45.Preuss UW, Schuckit MA, Smith TL, Danko GR, Dasher AC, Hesselbrock MN, et al. A comparison of alcohol-induced and independent depression in alcoholics with histories of suicide attempts. Journal of Studies on Alcohol. 2002;63:498–502. doi: 10.15288/jsa.2002.63.498. [DOI] [PubMed] [Google Scholar]
- 46.Reiach JS, Li PP, Warsh JJ, Kish SJ, Young LT. Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. Affective Disorder. 1999;56:141–151. doi: 10.1016/s0165-0327(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 47.Rich CL, Dhossche DM, Ghani S, Isacsson G. Suicide methods and presence of intoxicating abusable substances: some clinical and public health implications. Annals Clinical Psychiatry. 1998;10:169–175. doi: 10.1023/a:1022346129659. [DOI] [PubMed] [Google Scholar]
- 48.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy A. Relation of family history of suicide to suicide attempts in alcoholics. American Journal of Psychiatry. 2000;157:2050–2051. doi: 10.1176/appi.ajp.157.12.2050. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, et al. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Dependence. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- 51.Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol and Dependence. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 52.Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. International Journal of Adolescence and Medical Health. 2007;19:3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- 53.Solinas M, Justinova J, Goldberg SR, Tanda GJ. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rat. Journal of Neurochemistry. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 54.Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. Journal of Pharmacology and Experimental Therapeutics. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- 55.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of Notational Academy of Sciences. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steiner MA, Wanisch K, Monory K, Marsicano G, Borroni E, Bächli H, Holsboer F, Lutz B, Wotjak CT. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharmacogenomics Journal. 2008;8:196–208. doi: 10.1038/sj.tpj.6500466. [DOI] [PubMed] [Google Scholar]
- 57.Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suominen K, Henriksson M, Suokas J, Isometsa E, Ostamo A, Lonnqvist J. Mental disorders and comorbidity in attempted suicide. Acta Psychiatrica Scandinavica. 1996;94:234–240. doi: 10.1111/j.1600-0447.1996.tb09855.x. [DOI] [PubMed] [Google Scholar]
- 59.Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 60.Underwood MD, Mann JJ, Huang YY, Arango V. Family history of alcoholism is associated with lower 5-HT2A receptor binding in the prefrontal cortex. Alcohol Clinical Experimental Research. 2008;32:593–599. doi: 10.1111/j.1530-0277.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 61.Vinod KY, Arango V, Kassir SA, Cooper TB, Mann JJ, Hungund BL. Elevated levels of endocannabinoids, CB1 receptors and agonist-mediated G-protein signaling in the prefrontal cortex of alcoholic suicide victims. Biological Psychiatry. 2005;57:480–486. doi: 10.1016/j.biopsych.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 62.Vinod KY, Hungund BL. The role of endocannabinoid system in depression and suicide. Trends in Pharmacological Sciences. 2006;27:539–545. doi: 10.1016/j.tips.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. Journal of Neurochemistry. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- 64.Vinod KY, Yalamanchili R, Xie S, Ramalhete R, Michaelides M, Thanos PK, et al. Genetic and pharmacological manipulations of the CB1 receptor alter ethanol consumption and dependence in ethanol preferring and non-preferring mice. Synapse. 2008;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinod KY, Yalamanchilli R, Xie S, Cooper R, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochemistry International. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proceedings of Notational Academy of Sciences. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 68.Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biological Psychiatry. 2007;62:616–26. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]