Abstract
Background
Information is scarce regarding the effect of dietary protein type, with specific focus on the lysine to arginine (Lys:Arg) ratio, on cardiovascular risk factors and vascular reactivity in humans.
Objective
Determine effect of dietary Lys:Arg ratio on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults.
Design
Randomized cross-over design of two 35-day diet phases; thirty adults (21 females and 9 males, ≥50 y, LDL cholesterol ≥120 mg/dL). Diets had 20% energy (E) protein, 30%E fat, 50%E carbohydrate and were designed to have low (0.7) or high (1.4) Lys:Arg ratio. Measures included fasting and postprandial lipid, lipoprotein, apolipoprotein concentrations; fasting high sensitivity C-reactive protein (hsCRP), small dense LDL (sdLDL)-cholesterol, remnant lipoprotein cholesterol (RemLC), glycated albumin, adiponectin and immunoreactive insulin concentrations, endogenous cholesteryl ester transfer protein (CETP) and lecithin:cholesterol acyl transferase (LCAT) activities; cholesterol fractional synthesis rate (FSR); and flow mediated dilation (FMD) and peripheral artery tonometry (PAT).
Results
No differences were observed in fasting and/or postprandial total, LDL, HDL and sdLDL cholesterol, RemLC, Lp(a) or apo B concentrations, LCAT and CETP activities, FSR, glycated albumin, immunoreactive insulin, FMD or PAT. The low, relative to the high, Lys:Arg ratio diet resulted in lower postprandial VLDL cholesterol (−24%, P=0.001) and triglycerides (−23%, P=0.001), and small but significant differences in fasting (−3%, P=0.003) and postprandial (−3%, P=0.018) apo AI, and fasting adiponectin concentrations (+7%, P=0.035). Fasting and postprandial hsCRP concentrations were 23% lower after the low Lys:Arg ratio diet (P=0.020 for both).
Conclusions
Diets differing in Lys:Arg ratios had no or small effects on cardiovascular risk factors and vascular reactivity.
Keywords: lysine:arginine ratio, lipoproteins, small dense LDL (sdLDL)-cholesterol, remnant lipoprotein cholesterol (RemLC), cholesterol fractional synthesis rate (FSR), flow mediated dilation (FMD), peripheral artery tonometry (PAT)
Introduction
Early studies in a variety of experimental animal models of atherosclerosis suggest that proteins from vegetable sources are less hypercholesterolemic and atherogenic than proteins from animal sources [1–4]. In humans, observational and interventional studies suggest a cardioprotective effect of plant-based diets relative to those containing animal based products [5]. Comparative studies have documented lower blood pressure and concentrations of total cholesterol, LDL-cholesterol, triglycerides, high sensitivity C-reactive protein (hsCRP), and glucose among individuals consuming a vegetarian diet relative to omnivores [6–9]. The majority of intervention studies in humans conducted to assess the cholesterolemic effect of dietary protein were carried out using soy protein and appeared to confirm the findings in animals [10]. For the most part, clinical trials have compared soy protein to casein; only a few studies have evaluated the effects relative to other protein sources [11–14]. However, recent well controlled intervention trials comparing soy protein to an animal protein, independent of the fatty acid profile of the diet, have not supported the original observations [15]. The effects have either been modest [16–22] or null [23–30].
Throughout the mid and latter part of the 20th century, there was sporadic interest in the effect of dietary amino acid profile, independent of protein source or associated fatty acids, on cardiovascular risk factors. The primary focus was on the lysine-to-arginine (Lys:Arg) ratio. Rats or rabbits fed diets with a higher Lys:Arg ratio were reported to have higher total, LDL, and HDL cholesterol concentrations than animals fed a lower Lys:Arg ratio [3, 4, 31–38]. Moreover, addition of lysine to diets containing soy or cottonseed protein, such that the resulting Lys:Arg ratio was comparable to that of casein, increased atherogenicity, whereas the addition of arginine to casein-containing diets reduced the atherogenicity [4, 31, 37, 38]. These data suggested that dietary lysine was a hypercholesterolemic amino acid whereas arginine had the opposite effect. In addition, altering the L-Arg- nitric oxide (NO) pathway has b een reported to alter flow mediated dilatation (FMD), a surrogate measure of endothelial function [39]. An acute oral dose of L-Arg was found to increase FMD in patients with coronary artery disease [40, 41], hypercholesterolemia [42, 43] and hypertension [44]. Nonetheless, these findings have not been observed consistently and few other cardiovascular risk factors were reported in these studies [45–47]. Primary sources of dietary arginine include nuts and legumes, whereas most grains have limited quantities of this amino acid. The main dietary sources of lysine are foods of animal origin, including dairy products, fish, eggs, poultry, and beef.
The aim of the study was to determine the effect of the dietary Lys:Arg ratio, rather than focusing on single amino acid supplementation or type of protein, on a range of CVD risk factors, including plasma lipids, lipoproteins, apolipoproteins and lipoprotein particle concentrations, inflammatory factors, and endothelial function in moderately hypercholesterolemic adults. Our hypothesis was that, within the context of similar dietary fatty acid profiles, a low Lys:Arg ratio diet would result in a risk factor profile consistent with a lower cardiovascular disease risk, relative to a high Lys:Arg ratio diet.
Methods
Subjects
Thirty-nine study participants were recruited from the greater Boston area. Inclusion criteria included >50 years, LDL cholesterol >120 mg/dL, free of apparent chronic disease, and for women, postmenopausal status. Exclusion criteria included use of medications or dietary supplements known to affect lipid metabolism; abnormal kidney, liver, thyroid, or cardiac function; abnormal fasting glucose concentration; irritable bowel syndrome; chronic use of anti-inflammatory medications; smoking; alcohol consumption >7 drinks per week; unwillingness to maintain body weight throughout the study; hypertriglyceridemia (TG >400 mg/dL); and BMI >35 kg/m2 . All study participants gave written consent. The study protocol was approved by the Human Investigation Review Committee of Tufts University and Tufts Medical Center and was registered in the ClinicalTrials.gov registry (Identifier #NCT00175084). Thirty participants, 21 postmenopausal women and nine men, completed the study. Nine participants initially recruited did not complete the study, six dropped out during phase 1 and three during phase 2, citing the following reasons: scheduling conflict (phase 1, n=1); skin cancer diagnosis (phase 1, n=1); change in medical status (phase 2, n=1); dislike of study food (n=1 during phase 1, n=1 during phase 2); intolerance to protocol diet (phase 1, n=1) and no longer interested (n=2 during phase 1, n=1 during phase 2). Five of these participants terminated participation within the first week of their start date. Data from participants who did not complete the study were excluded from the statistical analysis. Staggered enrollment allowed for the replacement of participants who terminated participation prematurely so that the target of 30 participants was achieved. There were no significant differences in baseline characteristics between female and male participants (Table 1).
Table 1.
Baseline characteristics of participants1
| Variable | All participants N=30 |
Females N=21 |
Males N=9 |
P value2 |
|---|---|---|---|---|
| Age (y) | 61.8 ± 6.5 (51–76) |
61.8 ± 6.0 (51–76) |
62.0 ± 7.9 (51–75) |
0.935 |
| Body mass index (kg/m2) | 26.7 ± 3.2 (21.3–32.9) |
26.9 ± 3.29 (22.0–32.9) |
26.2 ± 3.2 (21.3–31.7) |
0.581 |
| Weight (kg) | 74.5 ± 11.5 (56–111) |
72.4 ± 9.3 (56–88) |
79.2 ± 15.0 (61–111) |
0.138 |
| Systolic BP (mm Hg) | 126 ± 16 (95–160) |
125 ± 15 (95–150) |
127 ± 18 (103–160) |
0.726 |
| Diastolic BP (mm Hg) | 74 ± 9 (55–89) |
72 ± 10 (55–89) |
77 ± 9 (63–88) |
0.271 |
| Plasma lipids, lipoproteins and apoproteins | ||||
| TC (mg/dL) | 223 ± 25 (182–300) |
228 ± 24 (196–300) |
211 ± 23 (182–258) |
0.095 |
| LDL-C (mg/dL) | 145 ± 17 (118–203) |
148 ± 19 (126–203) |
138 ± 12 (118–149) |
0.132 |
| VLDL-C (mg/dL) | 23 ± 10 (8–45) |
23 ± 11 (8–45) |
22 ± 10 (14–42) |
0.828 |
| HDL-C (mg/dL) | 55 ± 13 (35–96) |
56 ± 14 (35–96) |
51 ± 12 (36–68) |
0.333 |
| TG (mg/dL) | 117 ± 52 (41–228) |
119 ± 54 (41–228) |
114 ± 48 (70–210) |
0.816 |
| TC/HDL-C | 4.26 ± 0.97 (2.48–6.64) |
4.25 ± 1.05 (2.48–6.64) |
4.27 ± 0.81 (3.31–6.12) |
0.956 |
Values are expressed as mean±SD (range). BP: blood pressure; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; TC: total cholesterol; TG: triglycerides; VLDL-C: VLDL cholesterol.
An independent samples t-test was carried out for the male: female comparison.
Experimental Design
This was a randomized cross-over design study consisting of two 35-day diet phases separated by a break period that lasted 14 to 21 days. Laboratory personnel were blinded with regard to the identification of the study samples. Participants visited our Metabolic Research Unit three times per week for a review of changes in exclusion criteria or medical condition, consumption of one meal, and food pickup. Blood pressure and body weight were monitored at each visit. All foods and beverages were provided to the participants in quantities sufficient to maintain initial body weight. Energy requirements were calculated using the Harris-Benedict equation [48], and amounts of food were provided to study participants in increments of 250 kcal to the closest amount to their energy needs for weight maintenance. Participants were instructed to consume the meals entirely, not to substitute any food or beverage item, and not to add any calorie- or monosodium glutamate-containing condiments. Caloric intake was adjusted, when necessary, to maintain a stable body weight throughout the study period (± 1.0 kg from initial weight). Body weight and blood pressure values from the three test days (see below) were used for this report. BMI was calculated as kg/m2. Study participants were encouraged to maintain their usual level of physical activity and report any changes in approved medication use.
On three separate days after day 28, and within the last week of each diet phase, blood samples were collected after a 12-h fast into evacuated tubes for measurement of serum lipids or EDTA-containing tubes for the other biochemical or enzymatic assays. Twenty-four hours prior to one of these blood collections participants were asked to consume 0.48 g deuterated water (D2O)/kg estimated body water (60 percent body weight) to measure fractional cholesterol synthesis rate (FSR). Consistent with humans spending a greater amount of time in a postprandial than fasting state, biochemical measures were evaluated both fasting and four hours after consumption of the mid-day meal of a composition consistent with the appropriate diet phase. Fasted and postprandial lipids, lipoproteins, apoproteins and hsCRP concentrations were measured. During one of the three blood collection days, waist and hip circumference was measured. Prior data from our laboratory has indicated that under the specified study conditions plasma lipid concentrations were stable after day 28 of a feeding protocol [49]. Serum and plasma were separated from red blood cells by centrifugation at 1100 × g at 4°C, and each component was aliquoted and stored at −80°C for subsequent analysis.
Diets
Diets were designed to contain 20% of energy from protein and to have either a low Lys:Arg ratio (Lys:Arg = 0.7) or a high Lys:Arg ratio (Lys:Arg = 1.4) using commonly available foods. No amino acid supplements were used. Most of the protein sources for the low Lys:Arg ratio diet were from vegetable origin, including nuts and legumes. No attempt was made to avoid animal protein. Most of the protein sources for the high Lys:Arg ratio diet was from animal sources, including dairy products, fish, eggs, poultry, and beef. Diets were matched to the extent possible for polyunsaturated to saturated fat ratio, trans fatty acids, fiber, cholesterol, and micronutrients. The macronutrient and amino acid composition of the diets was confirmed by chemical analysis (Covance Laboratories, Madison, WI, USA) (Table 2). Micronutrients were calculated using the Nutrition Data System for Research software version 4.05 (2004), developed by the Nutrition Coordinating Center, University of Minnesota (Minneapolis, MN) [50]. Both diets provided adequate amounts of micronutrients (Table 6; Online appendix).
Table 2.
Composition of experimental diets1
| Constituent | Low Lys:Arg | High Lys:Arg |
|---|---|---|
| Percent of daily energy intake | ||
| Protein | 19.8 | 21.8 |
| Carbohydrate | 47.9 | 52.7 |
| Fat | 31.8 | 25.1 |
| Saturated fatty acids | 6.6 | 5.5 |
| Monounsaturated fatty acids | 11.3 | 8.0 |
| Polyunsaturated fatty acids | 13.0 | 10.6 |
| Trans fatty acids | 0.4 | 0.3 |
| ω-6 fatty acids2 | 12.1 | 10.5 |
| ω-3 fatty acids3 | 1.4 | 0.6 |
| Amount per 1000 Kcal | ||
| Cholesterol (mg) | 69 | 75 |
| Fiber (g) | 17.8 | 21.5 |
| Amino acids (mg/100g) | ||
| Asp | 540 | 470 |
| Thr | 180 | 210 |
| Ser | 270 | 240 |
| Glu | 1120 | 970 |
| Pro | 300 | 330 |
| Gly | 220 | 180 |
| Ala | 220 | 240 |
| Cys | 60 | 60 |
| Val | 230 | 260 |
| Met | 70 | 120 |
| Ile | 220 | 230 |
| Leu | 390 | 420 |
| Tyr | 170 | 180 |
| Phe | 260 | 230 |
| His | 130 | 150 |
| Lys | 280 | 380 |
| Arg | 400 | 270 |
| Trp | 60 | 60 |
| Lys:Arg ratio | 0.70 | 1.41 |
Macronutrients, fiber, cholesterol, fatty acids and amino acids were determined by chemical analysis of food.
ω-6 fatty acids: linoleic acid + arachidonic acid
ω-3 fatty acids: linolenic acid + eicosapentaenoic acid + docosahexaenoic acid
Biochemical measurements
Serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride concentrations were measured using an Olympus AU400 with enzymatic reagents and calibrators (Olympus America Inc., Melville, NY). VLDL cholesterol concentrations were calculated as the difference between total cholesterol and LDL cholesterol plus HDL cholesterol. Plasma apoprotein (apo) A-I and apo B (KAMIYA Biomedical Company, Seattle, WA) and Lp(a) concentrations (Wako Chemicals USA, Inc., Richmond, VA) were measured using an Olympus AU400 immunoturbidimetrically. Plasma hsCRP was measured using a Roche Cobas Fara centrifugal clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) immunoturbidimetrically (DiaSorin, Inc., Stillwater, MN). Proficiency testing for these procedures was done through the College of American Pathologists (CAP) Interlaboratory Comparison and Survey Proficiency Program (Northfield, IL). Linearity studies for all procedures were done through the Verichem Laboratories Linear Testing Program (Providence, RI).
Measurement of triglyceride-rich remnant lipoprotein cholesterol (RemLC) was performed as described by Miyauchi et al. [51] using a homogenous assay that allows for measurement of cholesterol in chylomicron remnants, VLDL remnants, and intermediate density lipoproteins (IDL). Plasma small, dense LDL (sdLDL) cholesterol was measured using a simple heparin-magnesium precipitation method as previously described [52]. Plasma glycated albumin was measured enzymatically as previously reported [53]. A latex particle-enhanced turbidimetric immunoassay was used for the measurement of plasma adiponectin [54]. Plasma immunoreactive insulin was measured with a latex immunoassay [55]. Endogenous cholesteryl ester transfer protein (CETP) and lecithin:cholesterol acyl transferase (LCAT) activities were measured in plasma as previously reported [56].
Cholesterol Fractional Synthesis
Free cholesterol from approximately 0.5g of red blood cells was extracted and analyzed by gas chromatography-thermal conversion-isotope ratio mass spectrometry (GC-TC-IRMS; Delta V Plus, Thermo Electron Corporation) to determine the 2H/1H ratio versus VSMOW [57]. The cholesterol precursor pool was taken as the mean plasma water deuterium enrichment which was determined by TCEA-IRMS from plasma prepared by membrane filtration and centrifugation removing proteins > 5 kilodaltons [58]. Cholesterol FSR rates were calculated using the following equation:
Where δ is deuterium enrichment of cholesterol or plasma water above baseline and time refers to the 2h deuterium incorporation period. The factor 0.478 is the fraction of hydrogen atoms per cholesterol molecule possibly labeled by a deuterium [58].
Endothelial function measurement
At the end of each dietary period, participants underwent a fasting brachial artery reactivity test (BART) to assess endothelium-dependent flow mediated dilatation (FMD). The diameter of the brachial artery was measured as described by Celermajer et al. [59] by high-resolution external vascular ultrasound during two conditions: baseline (after a 10-min supine rest) and in response to the inducement of hyperemia by inflation to 250 mm Hg and subsequent deflation of a sphygmomanometer cuff around the forearm to occlude arterial flow for 5 minutes. Peripheral vascular endothelial function was assessed simultaneously to the BART by peripheral arterial tonometry (PAT) as previously described [60].
Statistical analyses
Prior to the analysis, descriptive statistics and graphs (PROC UNIVARIATE and PROC MEANS) (SAS v 9.1 for Windows, Cary, N.C.) were used to summarize the overall effects of diets and distributions of the outcome measures. Data were tested for normality; when basic testing assumptions were violated, log10- transformations of the data were conducted to achieve normality prior to analysis and are so indicated in the tables. Data were analyzed using a paired t-test. When no transformation was appropriate, a nonparametric signed-rank test was used to compare means. Untransformed data are presented in text and tables as mean ± SD. Differences were considered significant at the 0.05 alpha level.
Results
The mean energy intake (mean ± SD) of the participants was 2617 ± 468 kcal when consuming the low Lys:Arg ratio diet and 2662 ± 490 kcal when consuming the high Lys:Arg ratio diet (n.s.; transformed to rank data before statistical analysis); energy intake for males was ~700 kcal greater than for women during both experimental diet phases (PSex=0.001). There were no significant differences in body weight, BMI, waist:hip ratio, or systolic and diastolic blood pressure at the end of the two diet phases (Table 3).
Table 3.
Anthropometric characteristics and blood pressure at end of experimental diet phases
| Low Lys:Arg | High Lys:Arg | P value | |
|---|---|---|---|
| Body weight (kg) | 73.4 ± 11.5 | 73.6 ± 11.6 | 0.358 |
| Body mass index (kg/m2) | 26.3 ± 3.3 | 26.3 ± 3.3 | 0.375 |
| Waist:Hip ratio | 0.861 ± 0.078 | 0.865 ± 0.073 | 0.675 |
| Systolic blood pressure (mm Hg) |
113 ± 12 | 111 ± 10 | 0.101 |
| Diastolic blood pressure (mm Hg) |
70 ± 7 | 69 ± 7 | 0.291 |
A paired t-test was carried out for each variable.
Transformed to log10 values before statistical analysis.
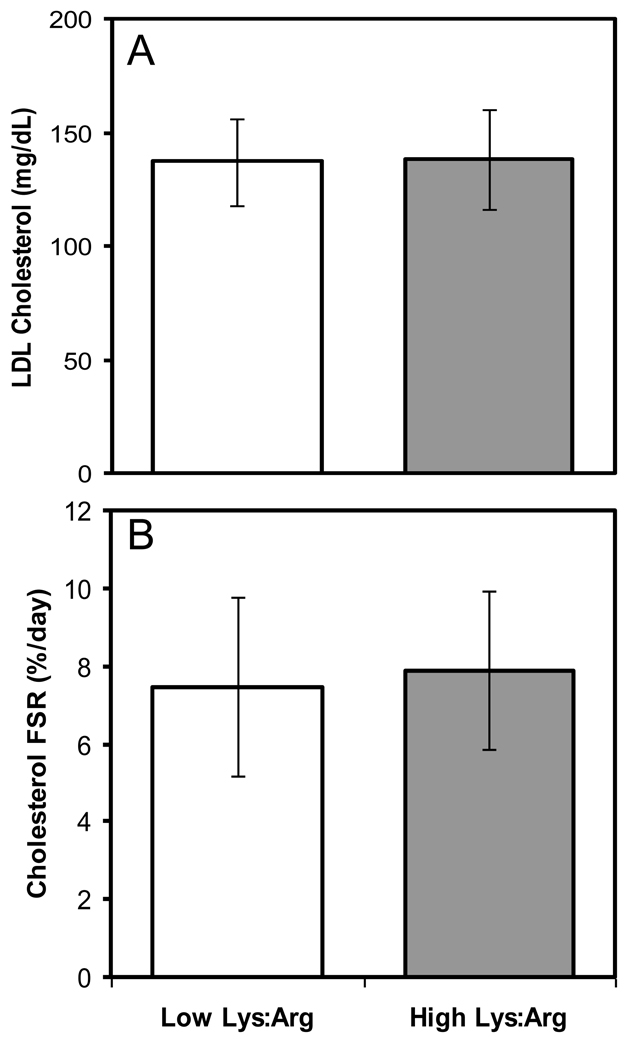
Consumption of the two experimental diets resulted in no significant differences in fasting total, LDL, VLDL, HDL and sdLDL cholesterol, RemLC or triglyceride concentrations and the total cholesterol:HDL cholesterol ratio (Table 4). At the end of the two diet phases there were no significant differences in Lp(a) or apo B concentrations. Compared to the high Lys:Arg ratio diet, the low Lys:Arg ratio diet resulted in a modestly lower apo AI (3%, P=0.003) and higher adiponectin concentration (7%; P=0.035). There were no significant differences in fasting concentrations of glycated albumin or immunoreactive insulin at the end of the two experimental diet phases. Relative to the high Lys:Arg ratio diet, the low Lys:Arg ratio diet resulted in 23% lower fasting hsCRP concentrations (P=0.020). The mean values for both were within the normal range. Endogenous LCAT and CETP activities were not significantly different at the end of the two diet phases. Similarly, cholesterol FSR was not significantly altered by the Lys:Arg ratio of the diet (Figure 1)
Table 4.
Fasting lipoprotein-related parameters and hsCRP concentration at the end of experimental diet phases.
| Low Lys:Arg | High Lys:Arg | P value | |
|---|---|---|---|
| Total cholesterol (mg/dL) | 207 ± 24 | 210 ± 27 | 0.236 |
| LDL cholesterol (mg/dL) | 137 ± 19 | 138 ± 22 | 0.593 |
| VLDL cholesterol (mg/dL)# | 15 ± 9 | 16 ± 10 | 0.189 |
| HDL cholesterol (mg/dL) | 55 ± 13 | 57 ± 13 | 0.133 |
| Triglyceride (mg/dL)* | 104 ± 35 | 109 ± 42 | 0.317 |
| Total cholesterol:HDL cholesterol# | 3.89 ± 0.85 | 3.86 ± 0.86 | 0.741 |
| Lipoprotein (a) (mg/dL) #; | 35.6 ± 25.3 | 35.6 ± 26.3 | 0.63 |
| Apoprotein AI (mg/dL) | 148.7 ± 22.8 | 152.7 ± 22.3 | 0.003 |
| Apoprotein B (mg/dL) | 101.5 ± 14.5 | 103.0 ± 15.8 | 0.267 |
| sdLDL cholesterol (mg/dL) | 36.1 ± 10.2 | 34.1 ± 9.3 | 0.152 |
| RemLC (mg/dL) | 5.99 ± 3.36 | 5.77 ± 2.60 | 0.601 |
| hsCRP (mg/dL) | 1.87 ± 2.45 | 2.44 ± 3.14 | 0.020 |
| Glycated Albumin (%)# | 13.6 ± 0.95 | 13.6 ± 0.9 | 0.863 |
| Adiponectin (µg/mL)* | 12.4 ± 6.58 | 11.6 ± 5.4 | 0.035 |
| Immunoreactive Insulin (µU/mL)* | 9.90 ± 3.96 | 10.8 ± 5.9 | 0.076 |
| LCAT (µmol chol • L−1 • h−1)* | 48.0 ± 15.5 | 49.2 ± 13.9 | 0.647 |
| CETP (µmol chol • L−1 • h−1) | 33.2 ± 19.4 | 32.6 ± 17.6 | 0.931 |
| Cholesterol FSR (%/day) | 7.48 ± 2.28 | 7.89 ± 2.04 | 0.406 |
A paired t-test was carried out for each variable.
Transformed to log10 values before statistical analysis.
Transformed to rank data before statistical analysis.
Figure 1.
Fasting LDL cholesterol concentrations (mg/dL, panel A) and cholesterol fractional synthesis rate (FSR; %/day; panel B) at the end of the two diet phases. Results at the end of the low and high Lys:Arg ratio experimental diet phases are shown in clear and dark bars, respectively. A paired t-test was carried out for each variable.
In the postprandial state, consumption of the two experimental diets resulted in no significant differences in total, LDL, and HDL cholesterol concentrations (Table 5) although the total cholesterol:HDL cholesterol ratio was marginally lower after participants consumed the low Lys:Arg ratio diet compared to the high Lys:Arg ratio diet (−1.5%; P=0.05). Interestingly, compared to the high Lys:Arg ratio diet phase, postprandial serum VLDL cholesterol and triglyceride concentrations were 24% (P=0.001) and 23% (P=0.001) lower, respectively, following the low Lys:Arg ratio diet phase. This difference in triglyceride concentrations between the two diets was not reflected in HDL cholesterol concentrations. There were no significant differences in Lp(a) or apo B concentrations at the end of the two diet phases. As observed in the fasting state, compared to the high Lys:Arg ratio diet, the low Lys:Arg ratio diet resulted in modestly lower postprandial apo AI (3%, P=0.018) and lower hsCRP concentrations (29%, P=0.020).
Table 5.
Postprandial lipids, lipoproteins, apoproteins and hsCRP at the end of test phases
| Low Lys:Arg | High Lys:Arg | P value | |
|---|---|---|---|
| Total cholesterol (mg/dL) | 202 ± 24 | 207 ± 28 | 0.073 |
| LDL cholesterol (mg/dL)# | 128 ± 18 | 126 ± 20 | 0.904 |
| VLDL cholesterol (mg/dL)* | 22 ± 11 | 29 ± 16 | 0.001 |
| HDL cholesterol (mg/dL) | 52 ± 12 | 53 ± 12 | 0.594 |
| Triglyceride (mg/dL)* | 137 ± 52 | 178 ± 80 | 0.001 |
| Total cholesterol:HDL cholesterol# |
4.03 ± 0.89 | 4.09 ± 0.93 | 0.050 |
| Lipoprotein (a) (mg/dL)# | 33.8 ± 25.0 | 34.3 ± 26.3 | 0.057 |
| Apoprotein AI (mg/dL) | 147.0 ± 21.9 | 151.4 ± 21.9 | 0.018 |
| Apoprotein B (mg/dL)# | 98.5 ± 13.6 | 100.3 ± 15.7 | 0.136 |
| hsCRP (mg/dL) | 1.81 ± 2.30 | 2.34 ± 2.89 | 0.020 |
A paired t-test was carried out for each variable.
Transformed to log10 values before statistical analysis.
Transformed to rank data before statistical analysis.
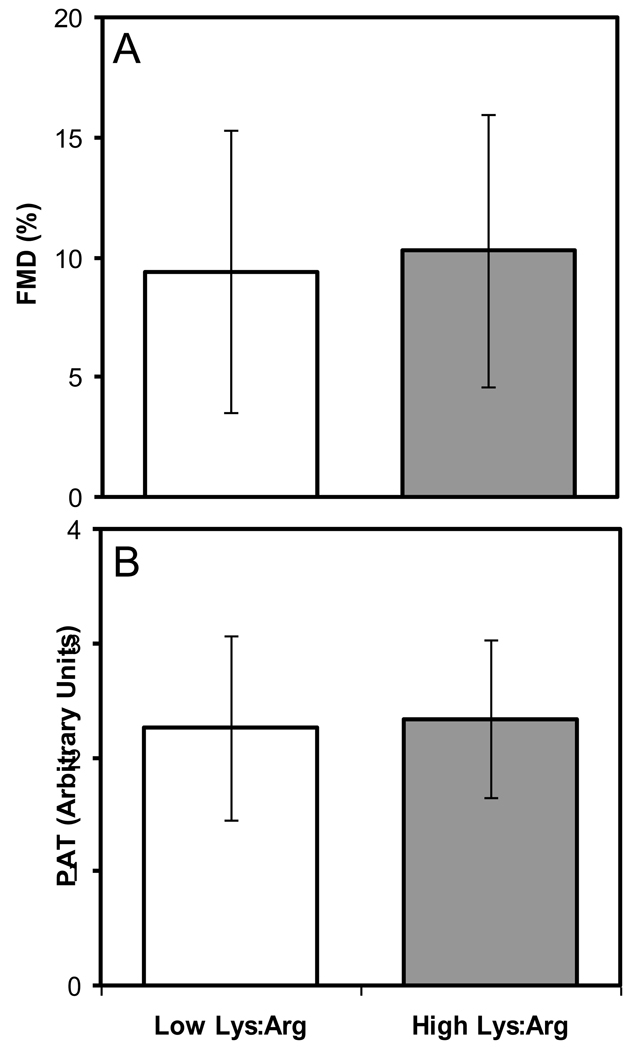
Vascular reactivity was not significantly different at the end of the two experimental diet phases as measured by flow mediated dilation or peripheral artery tonometry (Figure 2).
Figure 2.
Vascular reactivity at the end of the two experimental phases was assessed by measuring flow mediated dilation (FMD; %; panel A) and peripheral artery tonometry (PAT; Arbitrary Units; panel B). Results at the end of the low and high Lys:Arg ratio experimental diet phases are shown in clear and dark bars, respectively. A paired t-test was carried out for each variable. Flow mediated dilation data were transformed to rank data before statistical analysis.
Discussion
Previous work had suggested that a shift in the diet to lower Lys:Arg ratios had a favorable effect on cardiovascular risk factors in animal models and humans. By design, the intent of this study was to evaluate diets with different amino acid profiles, rather than focusing on a single amino acid or type of protein. This end was achieved by using commercially available food products and without amino acid supplements. Taking this approach the Lys:Arg ratio was shifted from 0.7 to 1.4. Nuts and legumes displaced foods containing animal protein from the diet to decrease the Lys:Arg ratio. By substituting nuts and legumes for animal protein the Arg content of the low Lys:Arg diet was 1.5-times higher than in the high Lys:Arg ratio diet. For moderately hypercholesterolemic participants, the difference in Lys:Arg ratio resulted in a null or small effect on cardiovascular disease risk factors. When differences were observed they were, for most part, in the non-fasting state.
From observational data, daily Arg intake has been estimated to range between 4.3 and 13.8 g/d, dictated by different regional dietary habits [61, 62]. The Arg intake of our participants ranged from 8–12 g/day and 5–8 g/day, when consuming the low Lys:Arg and high Lys:Arg ratio diets, respectively, depending on energy requirements needed to maintain a stable body weight. These values are well within the range from observational studies.
The difference in Lys:Arg ratio between the two diets was large in terms of the ratio and what could be reasonably achieved using habitually consumed foods alone (0.7 vs. 1.4), yet less than that used in animal studies. Differences in Lys:Arg ratios among studies involving animal models range from 0.3 to 2.2. In contrast to the current work, these animal studies reported a significant effect of Lys:Arg ratio on lipoprotein concentrations. For example, Sprague-Dawley rats fed a high Lys:Arg ratio (1.58 ratio) diet had the highest, whereas those fed a low Lys:Arg ratio (0.36 ratio) had the lowest total and HDL-cholesterol concentrations [31]. Similar results were observed in male albino rats (low Lys:Arg ratio, 0.67; high Lys:Arg ratio, 2.0) [32] and rabbits (high Lys:Arg ratio, 2.2 ratio; low Lys:Arg ratios, 0.9 and 0.3) [33]. In all these cases, the fatty acid profile of the diets did not differ as a result of manipulating the amino acid profile of the diets. Notably, these studies were conducted using animal species that lack analogies with human cholesterol and lipoprotein metabolism [63, 64].
Several studies using rats and rabbits also focused on the effects of other amino acids and suggested that the sulfur-containing amino acids, methionine (Met) and cysteine (Cys), were also hypercholesterolemic, whereas glycine (Gly) was hypocholesterolemic [35, 65–67]. Similar to the low Lys:Arg ratio, proteins having a low Met:Gly ratio were purported to elicit a hypocholesterolemic effect [67]. Of note, in the present study the low Lys:Arg ratio diet had a Met:Gly ratio of 0.32, whereas the high Lys:Arg ratio diet had a Met:Gly ratio of 0.67. These values are comparable to those reported for soy protein and casein (Met:Gly ratio 0.32 and 0.64, respectively).
Postprandial triglyceride and apo AI concentrations were significantly lower at the end of the low Lys:Arg ratio diet phase. However, this difference in triglyceride concentrations was not reflected in higher HDL cholesterol concentrations, as is frequently observed [68]. The small absolute difference in apo AI concentration is of questionable clinical significance. Furthermore, no significant differences were observed in LCAT or CETP activities at the end of diet phases. There is no obvious explanation for this observation. We cannot rule out the possibility that different sources of carbohydrates and fiber in the two diets may have contributed to the observed postprandial triglyceride concentrations. Interestingly, the effect on triglyceride concentrations was not observed in the fasting state, which suggests an effect on chylomicron-triglyceride, rather than VLDL-triglyceride clearance. It is possible that consumption of the low Lys:Arg ratio diet may have induced greater postprandial insulin secretion relative to the high Lys:Arg ratio. Moreover, the concentration of adiponectin, known to affect triglyceride concentrations and increase insulin sensitivity [69], was significantly higher, albeit modestly, at the end of the low Lys:Arg ratio diet phase compared to the high Lys:Arg ratio diet phase. It is possible that the higher adiponectin concentrations resulting from the low Lys:Arg ratio diet may have contributed to the difference in insulin sensitivity and enhanced triglyceride clearance despite the lack of differences in fasting insulin concentrations.
The consumption of diets with different Lys:Arg ratios or absolute amounts of Arg had no significant effect on endothelial function assessed by measuring FMD or PAT. Reports suggesting that endothelial function was improved in humans by supplementary L-Arg were at intake levels for the amino acids that far exceeded the amount that could be achieved by manipulating dietary sources of protein as attained for this study. These observations have been made in control participants (21 g L-Arg/d for 4 weeks) [70], coronary heart disease patients (21 g L-Arg/d for 3 days) [41], and hypertensive patients (single dose of 6 g L-Arg) [44]. Notably, studies reporting an effect of supplementary L-Arg on FMD were of much shorter duration than the present intervention, and more likely represent acute effects of L-Arg supplementation. Conversely, more modest doses of supplemental L-Arg failed to have a significant effect on endothelial function in peripheral arterial disease patients (3 g L-Arg/d for 6 months) [71] and hypercholesterolemic patients (2 bar per day with 3.3 g L-Arg/bar for 1 week) [72] with the exception of one study involving hypercholesterolemic individuals (2 bar per day with 3.3 g L-Arg/bar for 1 week) in which a significant improvement was reported [73]. In contrast to the current work, studies in which L-Arg was observed to have an effect on FMD, supplementation resulted in an Arg intake greater than what is consumed through dietary protein alone and the potential effect of dietary lysine was not taken into consideration. Moreover, it has been suggested that the amount of dietary arginine that enters the NO synthesis pathway is relatively low and therefore is unlikely to affect endothelial function [74].
In the current study, both fasting and postprandial hsCRP concentrations were significantly lower after the low Lys:Arg ratio diet phase. Consistent with this finding, observational data from the Third National Health Nutrition and Examination Survey (NHANES III) suggested an association between higher consumption of Arg-rich foods and lower serum hsCRP concentrations [75]. Despite the statistically significant difference observed in the current study, both mean hsCRP concentrations were within the normal range at the end of each diet phase. At this time there is a dearth of data on the effect of dietary amino acids or protein types on inflammatory markers. Additional data are needed prior to adequately interpret these findings.
We cannot rule out the possibility that factors associated with different dietary protein sources or phytochemicals were responsible to the differences in cardiovascular risk factors previously reported. Likewise, we cannot eliminate the possibility that undetected differences in the fatty acid profile of the diets in prior work had a significant effect on the outcome measures. In the current study we attempted to minimize differences between the two diets beyond that of the Lys:Arg ratios. However, due to constraints in the sources of amino acids and the intent to manipulate the Lys:Arg ratio with commonly consumed foods rather than amino acid supplements, we may have impacted study outcomes in unrecognized ways. Despite efforts towards maintaining comparable macronutrient and fiber contents between both diets, the lower Lys:Arg ratio diet had more dietary fat (31.8% energy vs. 25.1% energy) and less dietary fiber (17.8 g/1000 kcal vs. 21.5 g/1000 kcal) than the higher Lys:Arg ratio diet. We cannot rule out the possibility that changes due to these differences may have masked the absolute effect of differences in the Lys:Arg ratio. Lastly, whereas Lys is an essential amino acid, one that humans cannot synthesize adequate amounts to meet requirements, Arg is not. We were unable to determine the potential contribution of endogenous Arg synthesis to the total pool and how this may have affected the Lys:Arg ratio.
In conclusion, the amino acid profile of the diet, characterized by its Lys:Arg ratio, had no effect on fasting cardiovascular risk factors, vascular reactivity, or fractional cholesterol synthesis rate in mildly hypercholesterolemic adults. Overall, no convincing evidence was found to support the positive or negative modulation of cardiovascular disease risk factors based on characterizing dietary proteins by the Lys:Arg ratio.
Supplementary Material
Acknowledgments
The authors acknowledge Susan M. Jalbert, Blanche Ip and Dr. Alice Dillard-Hirschel for their helpful contributions and the study participants for their cooperation. AHL and SV-L were involved with intervention design and execution, data interpretation and manuscript preparation. NRM was involved with intervention execution and statistical analysis. LMA performed the statistical analysis and data interpretation. SVH assisted with data interpretation and manuscript preparation, and carried out IRMS analysis with assistance from TCR and supervision from PJHJ. AF designed the diet menus. MA and SO performed sample analysis for sdLDL, RemLC, glycated albumin, adiponectin and immunoreactive insulin; EJS supervised these analyses. JTK was involved in FMD and PAT measurements. None of the authors had any advisory board affiliations or financial interests in any organization sponsoring the research.
This work was supported by grant NIH/HL 58008 e and the USDA agreement No. 58-1950-4-401.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
References
- 1.Carroll KK, Hamilton MG. Effects of dietary protein and carbohydrate on plasma cholesterol levels in relation to atherosclerosis. Journal of Food Science. 1975;40:18–23. [Google Scholar]
- 2.Kritchevsky D. Dietary protein, cholesterol and atherosclerosis: a review of the early history. J. Nutr. 1995;125:589S–593S. doi: 10.1093/jn/125.suppl_3.589S. [DOI] [PubMed] [Google Scholar]
- 3.Kritchevsky D, Tepper SA, Czarnecki SK, Klurfeld DM. Atherogenicity of animal and vegetable protein : Influence of the lysine to arginine ratio. Atherosclerosis. 1982;41:429–431. doi: 10.1016/0021-9150(82)90208-8. [DOI] [PubMed] [Google Scholar]
- 4.Kritchevsky D. Vegetable protein and atherosclerosis. J Am Oil Chem Soc. 1979;56:135–140. doi: 10.1007/BF02671435. [DOI] [PubMed] [Google Scholar]
- 5.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104:947–956. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira RdCMdA, Molina MdCB, Zandonade E, Mill JG. Risco cardiovascular em vegetarianos e onívoros: um estudo comparativo. Arquivos Brasileiros de Cardiologia. 2007;89:237–244. doi: 10.1590/s0066-782x2007001600005. [DOI] [PubMed] [Google Scholar]
- 7.Chen CW, Lin YL, Lin TK, Lin CT, Chen BC, Lin CL. Total cardiovascular risk profile of Taiwanese vegetarians. Eur J Clin Nutr. 2007;62:138–144. doi: 10.1038/sj.ejcn.1602689. [DOI] [PubMed] [Google Scholar]
- 8.Szeto YT, Kwok TCY, Benzie IFF. Effects of a long-term vegetarian diet on biomarkers of antioxidant status and cardiovascular disease risk. Nutrition. 2004;20:863–866. doi: 10.1016/j.nut.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Alexander H, Lockwood LP, Harris MA, Melby CL. Risk Factors for Cardiovascular Disease and Diabetes in Two Groups of Hispanic Americans with Differing Dietary Habits. J Am Coll Nutr. 1999;18:127–136. doi: 10.1080/07315724.1999.10718840. [DOI] [PubMed] [Google Scholar]
- 10.Vega-López S, Lichtenstein AH. Dietary protein type and cardiovascular disease risk factors. Preventive Cardiology. 2005;8:31–40. doi: 10.1111/j.1520-037x.2005.3923.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacques H, Noreau L, Moorjani S. Effects on plasma lipoproteins and endogenous sex hormones of substituting lean white fish for other animal-protein sources in diets of postmenopausal women. Am J Clin Nutr. 1992;55:896–901. doi: 10.1093/ajcn/55.4.896. [DOI] [PubMed] [Google Scholar]
- 12.Gascon A, Jacques H, Moorjani S, Deshaies Y, Brun L-D, Julien P. Plasma lipoprotein profile and lipolytic activities in response to the substitution of lean white fish for other animal protein sources in premenopausal women. Am J Clin Nutr. 1996;63:315–321. doi: 10.1093/ajcn/63.3.315. [DOI] [PubMed] [Google Scholar]
- 13.Vega-López S, Yeum K-J, Lecker JL, Ausman LM, Johnson EJ, Devaraj S, Jialal I, Lichtenstein AH. Plasma antioxidant capacity in response to diets high in soy or animal protein with and without isoflavones. Am J Clin Nutr. 2005;81:43–49. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:960–966. doi: 10.1093/ajcn/85.4.960. [DOI] [PubMed] [Google Scholar]
- 15.Xiao CW. Health Effects of Soy Protein and Isoflavones in Humans. J. Nutr. 2008;138:1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 16.Teede HJ, Dalais FS, Kotsopoulos D, Liang Y-L, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira SR, Potter SM, Weigel R, Hannum S, Erdman JW, Jr, Hasler CM. Effects of feeding 4 levels of soy protein for 3 and 6 wk on blood lipids and apolipoproteins in moderately hypercholesterolemic men. Am J Clin Nutr. 2000;71:1077–1084. doi: 10.1093/ajcn/71.5.1077. [DOI] [PubMed] [Google Scholar]
- 18.Baum JA, Teng H, Erdman JW, Weigel RM, Klein BP, Persky VW, Freels S, Surya P, Bakhit RM, Ramos E, Shay NF, Potter S. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr. 1998;68:545–551. doi: 10.1093/ajcn/68.3.545. [DOI] [PubMed] [Google Scholar]
- 19.Dalais FS, Ebeling PR, Kotsopoulos D, McGrath BP, Teede HJ. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin Endocrinol. 2003;58:704–709. doi: 10.1046/j.1365-2265.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- 20.Tonstad S, Smerud K, Hoie L. A comparison of the effects of 2 doses of soy protein or casein on serum lipids, serum lipoproteins, and plasma total homocysteine in hypercholesterolemic subjects. Am J Clin Nutr. 2002;76:78–84. doi: 10.1093/ajcn/76.1.78. [DOI] [PubMed] [Google Scholar]
- 21.Puska P, Korpelainen V, Høie LH, Skovlund E, Lahti T, Smerud K. Soy in hypercholesterolaemia: a double-blind, placebo-controlled trial. Eur J Clin Nutr. 2002;56:352–357. doi: 10.1038/sj.ejcn.1601340. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein AH, Jalbert SM, Adlercreutz H, Goldin BR, Rasmussen H, Schaefer EJ, Ausman LM. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2002;22:1852–1858. doi: 10.1161/01.atv.0000033513.18431.a1. [DOI] [PubMed] [Google Scholar]
- 23.Blum A, Lang N, Peleg A, Vigder F, Israeli P, Gumanovsky M, Lupovitz S, Elgazi A, Ben-Ami M. Effects of oral soy protein on markers of inflammation in postmenopausal women with mild hypercholesterolemia. American Heart Journal. 2003;145:E7. doi: 10.1067/mhj.2003.115. [DOI] [PubMed] [Google Scholar]
- 24.Cuevas AM, Irribarra VL, Castillo OA, Yañez MD, Germain AM. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr. 2003;57:889–894. doi: 10.1038/sj.ejcn.1601622. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78:123–130. doi: 10.1093/ajcn/78.1.123. [DOI] [PubMed] [Google Scholar]
- 26.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EHF, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women. A randomized clinical trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Campbell C, Brown B, Dufner D, Thorland W. Effects of soy or milk protein during a high-fat feeding challenge on oxidative stress, inflammation, and lipids in healthy men. Lipids. 2006;41:257–265. doi: 10.1007/s11745-006-5095-5. [DOI] [PubMed] [Google Scholar]
- 28.Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of Two Types of Soy Milk and Dairy Milk on Plasma Lipids in Hypercholesterolemic Adults: A Randomized Trial. J Am Coll Nutr. 2007;26:669–677. doi: 10.1080/07315724.2007.10719646. [DOI] [PubMed] [Google Scholar]
- 29.West SG, Hilpert KF, Juturu V, Bordi PL, Lampe JW, Mousa SA, Kris-Etherton PM. Effects of Including Soy Protein in a Blood Cholesterol-Lowering Diet on Markers of Cardiac Risk in Men and in Postmenopausal Women with and without Hormone Replacement Therapy. J Womens Health. 2005;14:253–262. doi: 10.1089/jwh.2005.14.253. [DOI] [PubMed] [Google Scholar]
- 30.Thorp AA, Howe PR, Mori TA, Coates AM, Buckley JD, Hodgson J, Mansour J, Meyer BJ. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am J Clin Nutr. 2008;88:298–304. doi: 10.1093/ajcn/88.2.298. [DOI] [PubMed] [Google Scholar]
- 31.Park M-SC, Liepa GU. Effects of dietary protein and amino acids on the metabolism of cholesterol-carrying lipoproteins in rats. J. Nutr. 1982;112:1892–1898. doi: 10.1093/jn/112.10.1892. [DOI] [PubMed] [Google Scholar]
- 32.Rajamohan T, Kurup PA. Lysine:arginine atio of a protein influences cholesterol metabolism. Part 1 - Studies on sesame protein having low lysine:arginine ratio. Indian Journal of Experimental Biology. 1997;35:1218–1223. [PubMed] [Google Scholar]
- 33.Sanchez A, Rubano DA, Shavlik GW, Hubbard R, Horning MC. Cholesterolemic effects of the lysine/arginine ratio in rabbits after initial early growth. Archivos Latinoamericanos de Nutrición. 1988;38:229–238. [PubMed] [Google Scholar]
- 34.Kurowska EM, Carroll KK. Effect of high levels of selected dietary essential amino acids on hypercholesterolemia and down-regulation of hepatic LDL receptors in rabbits. Biochim Biophys Acta. 1992;1126:185–191. doi: 10.1016/0005-2760(92)90289-8. [DOI] [PubMed] [Google Scholar]
- 35.Kurowska EM, Carroll KK. Hypercholesterolemic responses in rabbits to selected groups of dietary essential amino acids. J. Nutr. 1994;124:364–370. doi: 10.1093/jn/124.3.364. [DOI] [PubMed] [Google Scholar]
- 36.Giroux I, Kurowska EM, Freeman DJ, Carroll KK. Addition of arginine but not glycine to lysine plus methionine–enriched diets modulates serum cholesterol and liver phospholipids in rabbits. J. Nutr. 1999;129:1807–1813. doi: 10.1093/jn/129.10.1807. [DOI] [PubMed] [Google Scholar]
- 37.Czarnecki SK, Kritchevsky D. The effect of dietary proteins on lipoprotein metabolism and atherosclerosis in rabbits. J Am Oil Chem Soc. 1979;56:388A. [Google Scholar]
- 38.Kritchevsky D, Tepper SA, Story JA. Influence of soy protein and casein on atherosclerosis in rabbits. Fed Proc. 1978;37:747. [Google Scholar]
- 39.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? Journal of the American College of Cardiology. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Yin W-H, Chen J-W, Tsai C, Chiang M-C, Young MS, Lin S-J. l-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clinical Nutrition. 2005;24:988–997. doi: 10.1016/j.clnu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, Celermajer DS. Oral -arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis. 1997;129:261–269. doi: 10.1016/s0021-9150(96)06044-3. [DOI] [PubMed] [Google Scholar]
- 42.Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J. Clin. Invest. 1996;97:1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxwell AJ, Anderson BE, Cooke JP. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vascular Medicine. 2000;5:11–19. doi: 10.1177/1358836X0000500103. [DOI] [PubMed] [Google Scholar]
- 44.Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, Dagre AG, Stamatelopoulos K, Protogerou A, Stamatelopoulos SF. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. International Journal of Cardiology. 2002;86:317–323. doi: 10.1016/s0167-5273(02)00413-8. [DOI] [PubMed] [Google Scholar]
- 45.Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral l-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. Journal of the American College of Cardiology. 1995;26:1054–1061. doi: 10.1016/0735-1097(95)00257-9. [DOI] [PubMed] [Google Scholar]
- 46.Mullen MJ, Wright D, Donald AE, Thorne S, Thomson H, Deanfield JE. Atorvastatin but not L-arginine improves endothelial function in type I diabetes mellitus: a double-blind study. Journal of the American College of Cardiology. 2000;36:410–416. doi: 10.1016/s0735-1097(00)00743-9. [DOI] [PubMed] [Google Scholar]
- 47.Bennett-Richards KJ, Kattenhorn M, Donald AE, Oakley GR, Varghese Z, Bruckdorfer KR, Deanfield JE, Rees L. Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int. 2002;62:1372–1378. doi: 10.1111/j.1523-1755.2002.kid555.x. [DOI] [PubMed] [Google Scholar]
- 48.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington: Carnegie Institute; 1919. [Google Scholar]
- 49.Schaefer EJ, Lichtenstein AH, Lamon-Fava S, Contois JH, Li Z, Goldin BR, Rasmussen H, McNamara JR, Ordovas JM. Effects of National Cholesterol Education Program Step 2 diets relatively high or relatively low in fish-derived fatty acids on plasma lipoproteins in middle-aged and elderly subjects. Am J Clin Nutr. 1996;63:234–241. doi: 10.1093/ajcn/63.2.234. [DOI] [PubMed] [Google Scholar]
- 50.Schakel S. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. J Food Comp and Anal. 2001;14:315–322. [Google Scholar]
- 51.Miyauchi K, Kayahara N, Ishigami M, Kuwata H, Mori H, Sugiuchi H, Irie T, Tanaka A, Yamashita S, Yamamura T. Development of a homogeneous assay to measure remnant lipoprotein cholesterol. Clin Chem. 2007;53:2128–2135. doi: 10.1373/clinchem.2007.092296. [DOI] [PubMed] [Google Scholar]
- 52.Hirano T, Ito Y, Yoshino G. Measurement of Small Dense Low-density Lipoprotein Particles. Journal of Atherosclerosis and Thrombosis. 2005;12:67–72. doi: 10.5551/jat.12.67. [DOI] [PubMed] [Google Scholar]
- 53.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clinica Chimica Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clinica Chimica Acta. 2006;371:163–168. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Kimura H. Immunoassay with stable polystyrene latex particles. J Immunol Methods. 1980;38:353–360. doi: 10.1016/0022-1759(80)90283-5. [DOI] [PubMed] [Google Scholar]
- 56.Vega-López S, Vidal-Quintanar RL, Fernandez ML. Sex and hormonal status influence plasma lipid responses to psyllium. Am J Clin Nutr. 2001;74:435–441. doi: 10.1093/ajcn/74.4.435. [DOI] [PubMed] [Google Scholar]
- 57.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 58.Harding SV, Zhao HL, Marinangeli CP, Day AG, Dillon HF, Jain D, Jones PJ. Red algal cellular biomass lowers circulating cholesterol concentrations in Syrian golden hamsters consuming hypercholesterolaemic diets. Br J Nutr. 2009 Jun;16:1–16. doi: 10.1017/S0007114509380046. [DOI] [PubMed] [Google Scholar]
- 59.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 60.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 61.Feskens EJM, Oomen CM, Hogendoorn E, Menotti A, Kromhout D. Arginine intake and 25-year CHD mortality: the Seven Countries Study. Eur Heart J. 2001;22:611–612. doi: 10.1053/euhj.2000.2330. [DOI] [PubMed] [Google Scholar]
- 62.Oomen CM, van Erk MJ, Feskens EJM, Kok FJ, Kromhout D. Arginine Intake and Risk of Coronary Heart Disease Mortality in Elderly Men. Arterioscler Thromb Vasc Biol. 2000;20:2134–2139. doi: 10.1161/01.atv.20.9.2134. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez ML. Guinea pigs as models for cholesterol and lipoprotein metabolism. J. Nutr. 2001;131:10–20. doi: 10.1093/jn/131.1.10. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez ML, Wilson TA, Conde AK, Vergara-Jimenez M, Nicolosi RJ. Hamsters and guinea pigs differ in their plasma lipoprotein cholesterol distribution when fed diets varying in animal protein, soluble fiber, or cholesterol content. J. Nutr. 1999;129:1323–1332. doi: 10.1093/jn/129.7.1323. [DOI] [PubMed] [Google Scholar]
- 65.Sugiyama K, Muramatsu K. Significance of the amino acid composition of dietary protein in the regulation of plasma cholesterol. J Nutr Sci Vitaminol. 1990;36:S105–S110. doi: 10.3177/jnsv.36.supplementii_s105. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka K, Sugano M. Effects of addition of sulfur-containing amino acids and glycine to soybean protein and casein on serum cholesterol levels of rats. J Nutr Sci Vitaminol. 1989;35:323–332. doi: 10.3177/jnsv.35.323. [DOI] [PubMed] [Google Scholar]
- 67.Morita T, Oh-hashi A, Takei K, Ikai M, Kasaoka S, Kiriyama S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997;127:470–477. doi: 10.1093/jn/127.3.470. [DOI] [PubMed] [Google Scholar]
- 68.Morrison A, Hokanson JE. The independent relationship between triglycerides and coronary heart disease. Vasc Health Risk Manag. 2009;5:89–95. [PMC free article] [PubMed] [Google Scholar]
- 69.Havel PJ. Update on Adipocyte Hormones: Regulation of Energy Balance and Carbohydrate/Lipid Metabolism. Diabetes. 2004;53:S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 70.Clarkson TB, Anthony MS, Potvin Klein K. Effects of estrogen treatment on arterial wall structure and function. Drugs. 1994;47:42–51. doi: 10.2165/00003495-199400472-00008. [DOI] [PubMed] [Google Scholar]
- 71.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-Arginine Supplementation in Peripheral Arterial Disease: No Benefit and Possible Harm. Circulation. 2007;116:188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 72.Abdelhamed AI, Reis SE, Sane DC, Brosnihan KB, Preli RB, Herrington DM. No effect of an L-arginine-enriched medical food (HeartBars) on endothelial function and platelet aggregation in subjects with hypercholesterolemia. American Heart Journal. 2003;145:E15. doi: 10.1067/mhj.2003.160. [DOI] [PubMed] [Google Scholar]
- 73.Maxwell AJ, Anderson B, Zapien MP, Cooke JP. Endothelial dysfunction in hypercholesterolemia is reversed by a nutritional product designed to enhance nitric oxide activity. Cardiovascular Drugs & Therapy. 2000;14:309–316. doi: 10.1023/a:1007886725480. [DOI] [PubMed] [Google Scholar]
- 74.Mariotti F, Huneau JF, Szezepanski I, Petzke KJ, Aggoun Y, Tome D, Bonnet D. Meal Amino Acids with Varied Levels of Arginine do Not Affect Postprandial Vascular Endothelial Function in Healthy Young Men. J. Nutr. 2007;137:1383–1389. doi: 10.1093/jn/137.6.1383. [DOI] [PubMed] [Google Scholar]
- 75.Wells BJ, Mainous AG, Iii, Everett CJ. Association between dietary arginine and C-reactive protein. Nutrition. 2005;21:125–130. doi: 10.1016/j.nut.2004.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.