Abstract
An open issue of retinal organization and function is the comprehension of the different tasks specifically performed by bipolar cells, the neurons that collect information from photoreceptors in the outer retina and convey the signal to the inner plexiform layer. Particularly interesting is to understand the unique contribution to the visual signal brought by cone bipolar cells, neurons typical of the mammalian retina and especially dedicated to receive synaptic input from cones. In all the species studied so far, it has been shown that cone bipolar cells occur in about ten different types, which form distinct clusters identified with a panel of both classical and modern genetic methods.
Reviewed here is current literature illustrating the occurrence of morphological, molecular and architectural features that confer to each bipolar cell type exclusive fingerprints, ultimately predicting the emergence of similarly unique, albeit still partially unraveled, functional properties. Thus, differences among cone bipolar cells lay the ground for the genesis in the outer retina of parallel channels, which convey to the inner retina separate information, among others, about contrast, chromatic features and temporal properties of the visual signal.
Keywords: bipolar cells, parallel pathways, glutamate receptors, ON and OFF channels
1. Introduction
That the retina is one of the most studied and best understood areas of the Central Nervous System can be deduced by simple bibliographic data. A recent PuMed search with the keyword “retina” retrieved about 110,000 scientific articles, while searches having as topics the names of other CNS organs, also representing areas of intense investigation in the field of neuroscience, and namely “hippocampus” and “cerebellum”, returned only 90,000 and 63,000 articles, respectively. This notwithstanding the fact that for all the three centers, the first papers listed in PubMed date back to the same period, around the year 1870.
Correspondingly, our notions about retinal development, organization and function are considerably broad compared to what is known for other CNS structures. For instance, albeit the concept of cytoarchitecture, defined as the different arrangement of cells in a tissue, was first used extensively as an attribute of the cerebral cortex, it is only for the vertebrate retina that the catalogue of constituting neuronal types has been virtually completed and their precise layering within the retinal tissue (and therefore a detailed description of cytoarchitecture) clarified. Similarly, numerous functional properties of retinal neurons have been elucidated, while the physiology of various types of cerebral cortex interneurons is still poorly understood.
And yet the retinal “mystery” is far from being completely solved and retinal research more complex than a trivial matter of adding in the right place the details of an otherwise self-explanatory puzzle. Many fundamental questions remain still unsettled.
One of the most intriguing open issues of retinal organization and function is the comprehension of the different tasks specifically performed by bipolar cells, the neurons that collect information from photoreceptors in the outer plexiform layer (OPL) and convey the signal to the next processing tier of the retina, the inner plexiform layer (IPL). Particularly intriguing is the yet partially undefined processing operated by cone bipolar cells, the neurons present in the retina of all mammals and especially dedicated to receive synaptic input from cone photoreceptors.
In the past years, increasing evidence has been provided that these cells come in roughly ten different types in virtually all mammalians studied, which include cats, rabbits, rats, mice, ground squirrels and primates. The different kinds of bipolar cells seem to constitute parallel channels operating multiple types of computation on the signal prior and after subsequent elaboration from amacrine cells and before final delivery to ganglion cells. However, the individual role and unique contribution to retinal processing provided by each type of cone bipolar cell are still elusive.
Here, we will review current literature illustrating the occurrence of morphological, molecular and architectural features that confer to each bipolar cell type exclusive fingerprints, ultimately predicting the emergence of similarly unique, albeit still partially unraveled, functional properties.
2. Cone bipolar cells: how many neurons?
Since the times of Santiago Ramon y Cajal’s pioneering studies, it has been known that the retina of mammals contains a single type of bipolar cell collecting information from rods and multiple types of bipolar cells connected to cones. Although cells with mixed rod-cone connections have been identified recently, it still holds true that, usually, rod bipolar cells do not contact cone photoreceptors, while bipolar cells exist that receive their input predominantly from cones.
Since the 80ties, the application of antibodies against the alpha isoform of the ubiquitary enzyme protein kinase C (PKC) to fish retinal tissue was observed to label cells mostly connected to rods (Negishi et al., 1988). Later observations extended the specificity of such antibody staining to the retina of mammals (Greferath et al., 1990), for which PKC has been traditionally used to visualize rod bipolar cells ever since.
PKC alpha antibodies allowed detailed description of morphology, topographical distribution and frequency of rod bipolar cells in rabbits and monkeys and later in other mammalians. Convergence of rods upon rod bipolars was calculated for different species. However, analogous antibodies or histochemical markers capable of distinguishing among the assortment of cone bipolars become available at a much slower rate and only quite recently most of the cell types have been associated to specific antigenic markers. It is likely that the different types of cone bipolar cells share strict similarities from a molecular point of view and therefore distinction based on antigen specificity is particularly difficult. A peculiarity one encounters also when dealing with ganglion cells, but not necessarily true, for instance, for amacrine cells, somewhat easier to separate in different types also based on their expression of specific antigens. The latter include, among others, antibodies to enzymes for the synthesis of classical neurotransmitters or peptides.
Difficulties in differential staining and small size of cone bipolar cells have made it correspondingly complex to estimate their precise number. For a long time, it has been believed that, given the fact that rods largely outnumber cones in most mammalian retinas, rod bipolar cells had to be more numerous than cone bipolar cells. This statement was quite common in specialized literature and even in textbooks up to the early 90ties (Wässle and Boycott, 1991), notwithstanding the knowledge that rod bipolar cells have wide and bushy dendritic arbors so that very large numbers of rods converge synaptically on each of them. One could have imagined a bottleneck in the rod-rod bipolar connectivity causing the number of rod bipolars to drop sensibly from that of rod photoreceptors. However, nobody really imagined that cone bipolars indeed outnumber rod bipolar cells, not only in specialized, cone-rich areas, like the visual streak of the rabbit, but also elsewhere, where cone density is lower.
A strategy of cell counting based on bipolar cell identification independent from staining and making use of high resolution images of retinal semithin sections allowed the evaluation of the absolute fraction of inner nuclear layer cells occupied by bipolar cells alone in the retina of the rabbit (Strettoi and Masland, 1995). Bipolar cells represent 40% of all the cell somata constituting the inner nuclear layer. Separate counts on retinal whole mounts stained with fluorescent PKC antibodies and nuclear dyes retrieved the fraction of inner nuclear layer cells occupied by rod bipolars only. Incidentally, this method was one of the first applications of confocal microscopy to the study of retinal morphology. The combination of the two counting strategies led to the unexpected discovery that in the rabbit retina, at all eccentricities, cone bipolars outnumber rod bipolars by 3–4 times (Strettoi and Masland,1995).
Recent studies allowed calculation of the densities of separate types of cone bipolar cells. None of them occupies a relevant fraction or represents a “dominant” category; rather, each of the different types covers the retina uniformly with a roughly similar frequency (Wässle et al., 2009). The total density of cone bipolar cells for the mouse retina has been estimated in about 40,000 cells/mm2 (Wässle et al., 2009). Since rod bipolar cells alone have an average density of 15,000 cells/mm2 (Strettoi and Volpini, 2002), the estimated cone bipolar:rod bipolar ratio for the retina of the mouse is 2.6:1. The total number of cone bipolar cells in the retina of the mouse can been estimated in about 540,000 cells (Strettoi and Volpini, 2002).
Evidently, the relatively high incidence of cone bipolar cells is not due to abundance of a single type. The numerosity of cone bipolar cells as a whole is partially due to the many types in which they occur. As a consequence, each cone has to diverge repeatedly, by means of parallel synaptic contacts, to access each one of the channels embodied by individual cone bipolar cell types. Such a divergence is particularly evident at the primate fovea, where 3–4 cone bipolar cells exist for each cone (Ahmad et al., 2003). In the mouse retina, a single cone makes connections with at least one cone bipolar cell of each category (Wässle et al., 2009). A primate cone pedicle can make up to 500 contacts and it is regarded to as one of the most complex synaptic apparatuses of the CNS (Wässle, 2004).
Because of the occurrence of multiple connections, the synaptic ending of a cone, the pedicle, can be compared to a high-transit subway station, where traffic is divided into multiple channels, each deputed to transfer a specific cargo. Channels are distinguishable on the basis of postsynaptic receptors on the membrane of cone bipolars, on the basis of additional, voltage sensitive channels modulating signal transfer, and by virtue of the type of connections established by each bipolar cell at the next synaptic station, in the IPL. Exactly like subway lines, which can be distinguished on a map as results of their different names, destinations and number of intersections
On the contrary, each rod, by means of its synaptic ending, the spherule, makes one single connection with the dendrite on one individual rod bipolar cell. A wealth of rods converges upon a single rod bipolar, therefore contributing to the extraordinary sensitivity of this neuron to light. In synthesis, the cone system privileges variety of function while the rod system favors sensitivity. This is reflected in functional properties of individual bipolar cells highlighted by single unit electrophysiological studies (Pang et al., 2004). It has been calculated that the sensitivity of a rod bipolar cells to 500 nm light is at least 20 times higher than that of a rod, as an effect of synaptic convergence. On the other hand, a depolarizing cone bipolar which receives a pure cone input has a threshold that is 2 Logarithmic unites higher than that of a rod bipolar of the same retina (Pang et al., 2004).
3. Cone bipolar cells: how many types?
One of the first studies documenting the variety of bipolar cell types present in a rodent retina was published by Euler et al., in 1996. The Authors recorded light responses from bipolar cells in a slice preparation of rat retina. Subsequently, recorded cells were injected with a fluorescent dye, revealing their detailed morphology. It appeared that even the retina of a rat, a typically nocturnal animal, for which vision is clearly rod-dominated, displayed an abundance of cone bipolar cell types, mainly distinguishable by virtue of the morphology and level of stratification of their axonal terminals in the IPL. Studies that followed demonstrated that the occurrence of multiple types of cone bipolar cells is a general rule for the retina of any mammal, from mice to humans. Variety is now documented by a panel of techniques, which comprise the use of available antibodies to selected cell types, intracellular injections with fluorescent molecules, gun-labeling with lipophilic dyes and, more recently, transgenic expression of fluorescent markers based on cell-type specific promoters (Martin and Grunert 1992; Haverkamp et al., 2003; Pignatelli and Strettoi, 2004; Ghosh et al., 2004; Dhingra et al., 2008). Studies based on different methods have produced remarkably similar results.
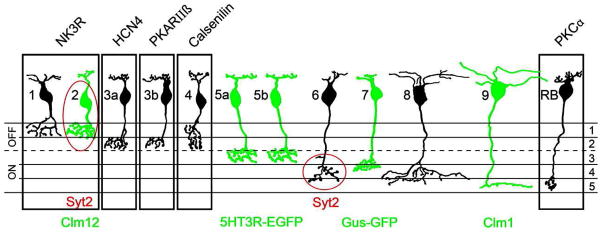
Morphologically, 9 different varieties of cone bipolar cells can be identified in the mammalian retina. Taking the mouse as a paradigm, two additional clusters can be further recognized that share morphologies with other types but exhibit different antibody staining properties (Figure 1). Independent density calculations lead to the conclusion that the catalogue of 11 types of cone bipolar cells and one type of rod bipolar cell is now complete (Wässle et al., 2009). Cone bipolar cells differ in the morphology of dendrites, which can be flat or spiny, sparse or highly ramified, in the OPL. Also, the number of cones each bipolar contacts, as well as their spectral sensitivity, might differ, particularly in the primate retina. Even more pronounced are the differences among axonal arbors in the IPL. A strict topological rule of retinal architecture establishes that cells which are depolarized at the light onset (ON-types) ramify in the innermost half of the IPL, also called sublamina ON or b, while cells which become hyperpolarized when the light is turned on (OFF-types) stratify in the outermost portion of the IPL, named sublamina OFF or a. Sublamina a can be further divided into two strata, 1 and 2, while sublamina b is separated in three strata, 3, 4 and 5. In the retina of monkeys, rats and mice, 5 varieties of cone bipolars have axonal endings localized to sublamina a, while 4 types have axons that ramify in sublamina b of the IPL. According to the most commonly used nomenclature, they are named CB1–5 and CB6–9 respectively (Ghosh et al., 2004). Hence, the simplest level of analysis of cone bipolar cells, based on the depth of stratification of their axonal arbors in the IPL, leads to a broad classification of these neurons into two large categories, and namely those with axon terminals in sublamina a or b of the IPL, respectively corresponding to the OFF or ON types identified by electrophysiology experiments.
Figure 1.
Summary diagram of the bipolar cell types of the mouse retina. A total of 11 cone bipolar cell and one rod bipolar cell types can be distinguished by immunocytochemical staining (outlines) or by GFP/Clomeleon expression in transgenic mouse lines (green cells).
(From Wässle et al., 2009)
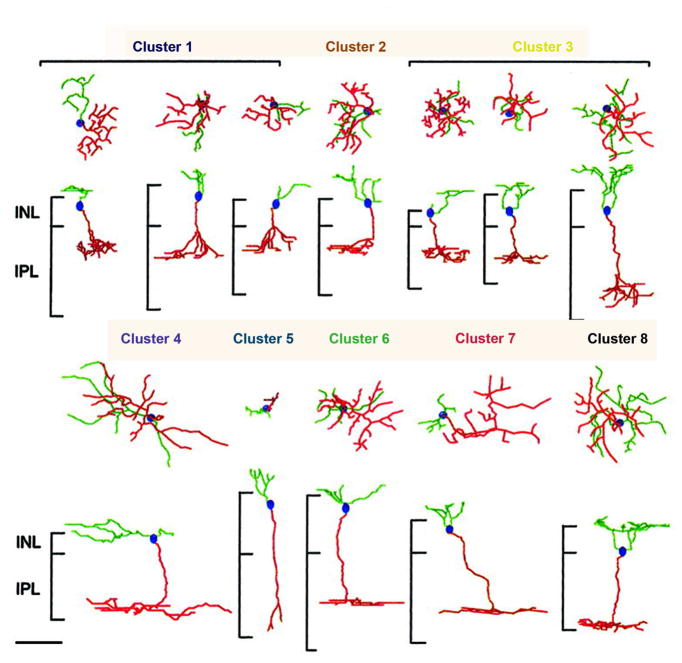
Besides depth of stratification in the IPL, cells differ in the axonal arbor diameter, in the thickness of the arborization itself, which can be wide or narrow, or in its shape, which can be highly ramified or sparse in cell more or less complex, respectively. Depth and width of axonal arbors obviously dictate types and number of candidate synaptic partners in the IPL. Cluster analysis of a wide cohort of cells from the retina of a mouse in which bipolar cells could be visualized thanks to a genetically directed reporter allowed identification of separate groups of bipolars based on shape, size, depth of stratification of axonal arbors and neurite branching pattern (Badea and Nathans, 2004) (Figure 2). It is important to note that this (genetically directed) method is independent from any immunological or histochemical staining but produced results very similar to other studies.
Figure 2.
Three-dimensional reconstructions of representative bipolar cells from eight clusters identified in the mouse retina by genetic analysis. Green, OPL arbors; red, IPL arbors; blue dots, cell body locations. The vertical bars to the left of each transverse view represent the vertical extents of the INL and the IPL. There is an approximately twofold variation in the thickness of the retinal layers at different eccentricities. Scale bar: 20 μm.
(From Badea and Nathans, 2004)
4. Differences for cell type identification: antibody staining and expression of fluorescent markers
Classification of cone bipolar cells has been made more objective by the identification of antibodies revealing specific molecular markers characteristic of different cell types. These include antibodies against calcium binding proteins, ionic channels, cell adhesion molecules, synaptic markers and neurotransmitter receptors. A recent paper by Wässle and collaborators (2009) describes five different types of cone bipolar cells of the mouse retina that can be discriminated with specific antibodies. Using genetic expression of fluorescent markers under cell-type specific promoters to identify additional cone bipolars, this study gives an account of all the cone bipolar cell types likely present in the mouse retina (Figures 1 and 3).
Figure 3.
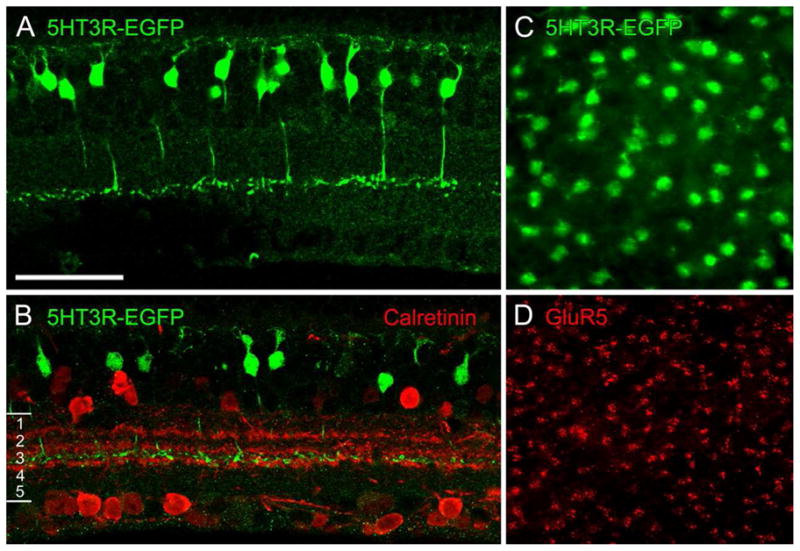
The mouse line shown here has type 5 cone bipolar cells expressing enhanced GFP (EGFP) under the control of the serotonin 3 receptor (5-HT3R). A, Vertical section through the retina of the 5-HT3R–EGFP mouse. B, Vertical section double labeled for GFP (green) and calretinin (red). C, D, Same field of a whole mount of a 5-HT3R–EGFP retina. C, Focus on the cell bodies of GFP-labeled bipolar cells. D, Focus on the cone pedicles marked by GluR5 immunostaining (red). Scale bar: A, B, 25 μm; C, D, 32 μm.
(From Wässle et al., 2009)
As mentioned before, rod bipolar cells are specifically recognized by antibodies against the enzyme PKC alpha. Type 1 (OFF) cone bipolar cells express neurokinin receptor 3 (NK3-R) and can be stained with anti-NK3 antibodies. Two (very similar) types of OFF cone bipolars can be labeled with antibodies against the ionic channel HCN4 and against the regulatory subunit IIβ of protein kinase A (PKARIIβ). These are type 3a and 3b cone bipolar cells, respectively. Antibodies against the calcium binding protein calsenilin reveal type 4 (OFF) cone bipolar cells, while antibodies against the vesicular protein synaptotagmin label type 2 (OFF) and type 6 (ON) cone bipolar cells. Mouse lines which express GFP, EGFP or other fluorescent markers (i.e. Chlomeleon, a calcium indicator) under specific promoters are available so that the remaining type of OFF cone bipolar cell (type 5) and 2 additional types of ON cone bipolar cells (types 7 and 9) are also labeled. Thus, with the exception of type 8 (ON) cone bipolar cell, we have now in our hands specific markers allowing visualization of individual categories of cone bipolar cells as whole populations.
5. Molecular basis of cone bipolar cell diversity: differences established in the OPL. ON versus OFF CB
Pioneering electrophysiology studies on cold blooded vertebrates (Kaneko, 1973; Schwartz, 1974) demonstrated that bipolar cells respond to light in a graded fashion, producing depolarizing or hyperpolarizing responses which reverse in polarity upon illumination of the peripheral portion of their dendritic field, so that ON-center bipolar cells hyperpolarize to peripheral stimulation and vice versa.
Such a dichotomy in light responses is established at the first synaptic station of the retina and it is due to the presence on the dendritic tips of different types of cone bipolar cells of different types of receptors for the aminoacid glutamate, the transmitter released by both rods and cones. All photoreceptors hyperpolarize in response to light. ON bipolar cells express a retinal specific, high affinity type of metabotropic receptor, named mGluR6, which is negatively coupled to a cationic-selective channel. Glutamate closes this channel that would otherwise keep ON bipolar cells depolarized. Therefore, the photoreceptor-ON bipolar cell synapse inverts light activated hyperpolarization of photoreceptors into a depolarizing response in ON bipolar cells.
OFF bipolar cells, on the contrary, express lower affinity ionotropic receptors of the AMPA/kainate type at their dendritic tips and hyperpolarizing light responses of photoreceptors are maintained at this sign-conserving synapse.
6. Transduction pathway of mGluR6
Slaughter and Miller (1981) were the first to demonstrate by pharmacological experiments the different components separating the ON and OFF signaling pathways within the retina (Masu et al., 1995; Slaughter and Miller, 1981; Vardi et al., 2000). They showed that 2-amino-4-phosphonobutyric acid (2-APB) blocked the light responses of ON but not OFF bipolar cells. They also demonstrated that 2-APB produced this effect by acting as an agonist to hyperpolarize ON bipolar cells closing an ionic channel, similar to the endogenous effects of glutamate released by photoreceptors in the dark. The depolarizing response to light of both rod and ON cone bipolar cells is due to the interaction of photoreceptor-released glutamate with a particular type of metabotropic receptor named mGluR6 (Masu et al., 1995; Ueda et al., 1997), exquisitely restricted to the retina. It appears that all ON bipolar cells express a single isoform of mGluR6 (Vardi et al., 2000), whose genetic ablation is confirmed to cause severe reduction of the amplitude of the b wave of the photopic ERG (Koyasu et al., 2008). Retinal ON bipolar cells make up over 70% of all bipolar cells. These comprise all rod bipolars as well as some cone bipolar cells. A transgenic mouse line was recently used to determine the total density of ON bipolar cells, which in central retina is 29,600 cells/mm2 (Wässle et al., 2009).
Considerable progress has been made in the last years to elucidate the molecular cascade activated by the glutamate-mGluR6 association. Glutamate, released from photoreceptors in the dark, binds to mGluR6 receptors localized to the dendritic tips of rod bipolars and ON cone bipolar cells and activates a specific splice variant of a G protein, named G0alpha, which ultimately closes the non-specific cationic channel (Dhingra et al., 2002; Henry et al., 2003). The identity of the postsynaptic channel that mediates synaptic transmission from photoreceptor to ON bipolar cells has been elusive for a long time. Very recently, evidence has been provided that the channel is likely to be a member of the family of TRP channels (melastatin-related transient receptor potential like channel), which functions similarly to, but it is distinct from, the cyclic nucleotide gated channel found in photoreceptors (see Henry et al., 2003). Opening of this channel generates the depolarizing current characteristic on the ON bipolar response to light (Morgans et al., 2009). It appears that, at least in the dendrites of rod bipolar cells, the transduction channel is composed of TRPM1, either as a homomer or in association with other TRP channels. ERG measurements from mice lacking TRPM1 receptors reveal the virtual absence of a b-wave (Shen et al., 2009).
Light responses require rapid inactivation of G0alpha, activated in the dark by mGluR6. Inactivation of G0alpha takes place by the latter hydrolyzing GTP and is accelerated by particular proteins known as RGS (regulators of G-protein signaling), which are localized on the dendritic tips of ON bipolar cells (Mojumder et al., 2009, Morgans et al., 2007). G0alpha constitutes the substrate of at least two RGS proteins, named RGS7 and RGS11, which have an obligate binding partner, Gbeta5. Genetic disruption of either RGS7 or RGS11 causes delays in the b-wave of the ERG, known to arise from ON-bipolar cell activity.
One difference among the population of retinal cells globally expressing mGluR6 receptors arises from the fact that RGS7 expression is present in dendritic tips of all rod-ON bipolar cells, but is missing in those of subsets of ON cone bipolars, whereas the converse is true for RGS11 staining. It appears that RGS7 and RGS11 regulate the kinetics of ON bipolar cells responses, with differential outcomes on the rod and cone pathways. In the cone pathway, RGS11 plays an accelerating role in the deactivation of G0alpha, which precedes activation of the depolarizing current in ON bipolar cells. Hence, the presence or absence, together with specificity of RGS proteins, act as variable regulators of kinetics of ON bipolar cell responses (Mojumder et al., 2009). This is in turn reflected in the temporal features of the ERG b-wave.
The activation and inactivation of G0alpha represents a major site for the modulation of the glutamate-sensitive cationic channel. Besides interacting with RGS proteins, it appears that G0alpha interacts with a retinal specific splice variant of PCP2, a presumptive guanine nucleotide dissociation inhibitor. PCP2, also known as L7, is uniquely expressed in cerebellar Purkinje cells and in retinal bipolar neurons, and it may function as a cell-type specific modulator for G protein-mediated cell signaling, particularly in regions of synaptic activity. PCP2 facilitates closure of the mGuR6 cation channel in the dark and shortens the rise time of the light response in ON bipolar cells (Xu et al., 2008).
7. OFF-Cone bipolar cells
ON-cone bipolar cells respond to light with a graded depolarization caused by an inward cationic current. On the contrary, OFF cone bipolars respond to light with a graded hyperpolarization caused by an outward cationic current named delta Ic. This is mediated by interaction of glutamate with AMPA/kainate receptors on the dendritic tips of OFF cone bipolar cells.
Studies of dorsal root ganglion neurons in culture show that exogenous application of glutamate elicits sustained trains of impulses in cells expressing AMPA receptors, while the response of neurons expressing kainate receptors is more transient. Moreover, AMPA receptors recover much more quickly from desensitization (Lee et al., 2004). So, AMPA receptors have more potent activation properties, and faster recovery than kainate receptors. Thus endogenous glutamate acting on neurons expressing different combinations of these two types of non-NMDA receptors has different functional consequences. Similarly, OFF bipolar cells with intrinsically different composition of AMPA and kainate receptors have different physiological properties.
For instance, in the primate retina it has been shown that midget bipolar cells of the OFF type express the AMPA receptor GluR1 subunits on their dendritic tips. These cells contact pedicles of Medium wavelength (M) and Long wavelength (L) sensitive cones, but not of Short wavelength (S) cones. Rather, kainate receptor GluR5 subunits are expressed in apposition to the synaptic surface of S-cone pedicles, indicating that OFF bipolar cells which receive an S cone input express kainate-type glutamate receptors (Puller et al., 2007). Hence, within OFF cone bipolar cells, at least two types can be distinguished on the basis of both their different type of glutamate receptors and chromatic input received form cones.
The photoreceptor-OFF cone bipolar synapse responds poorly to light increments as the AMPA/kainate receptors enter quickly in a desensitized, poorly conducting state. This means that during continuous release of glutamate from photoreceptors, in the dark, postsynaptic receptors on OFF bipolar cells are maintained in a desensitized condition. During light increments, glutamate concentration in the synaptic space decreases and ionotropic glutamate receptors can desensitize. Upon subsequent light decrements, their response will be robust again. Because of this feature, the OFF channel is well designed to signal light decrements in illumination levels but does no respond well to increases of light intensity (DeVries, 2000). Rather, this is the specific function of the ON pathway.
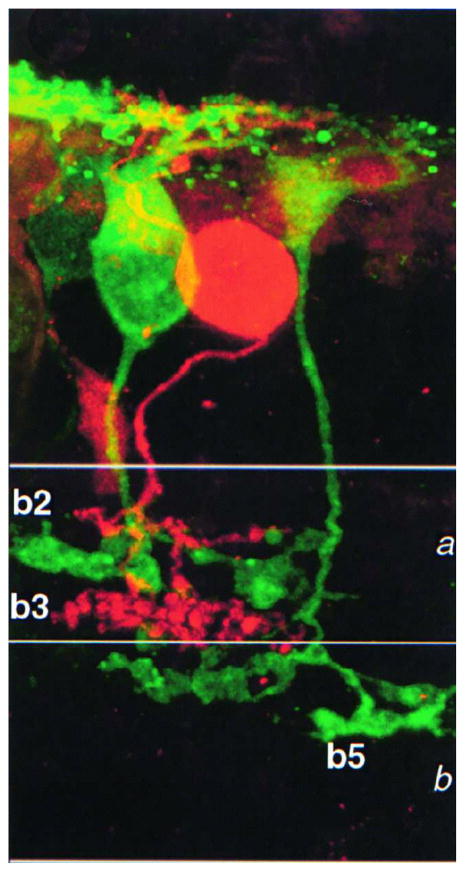
Electrophysiological and pharmacological studies show that different types of OFF cone bipolar cells express unique combinations of AMPA/kainate receptors, each conferring to the cell characteristic rates of recovery from desensitization (DeVries, 2000). Following a desensitizing application of glutamate, AMPA receptors have a recovery time constant of about 50 msec, while for kainate receptors this constant is much slower (about 1–2 seconds). Pharmacological and electrophysiological studies on the ground squirrel retina have shown that three different types of OFF cone bipolar cells, ending at three different depths in sublamina a of the IPL, use unique combinations of postsynaptic ionotropic receptors for glutamate, ultimately receiving three different types of input from an identical pool of cones (Figure 4). The different desensitization rates of the three cell types lead to exclusive functional properties for each of them: the cell type expressing AMPA receptors and recovering rapidly is well suited to signal transient components of the cone input, while another cone bipolar type, expressing kainate receptors with a slower desensitization rate, appears to enhance the steady component of the cone response (Figure 5). These intrinsic differences among OFF cone bipolar cells have profound effects on retinal physiology as they contribute to the genesis of different temporal channels at the first retinal synaptic station.
Figure 4.
Ground squirrel retinal preparation. The axon terminals of a b2 cone bipolar cell, selectively stained with an antibody to calbindin (in green), are sandwiched between the terminals of a b3 cell injected with Alexa 568 (in red).
(From DeVries, 2000)
Figure 5.
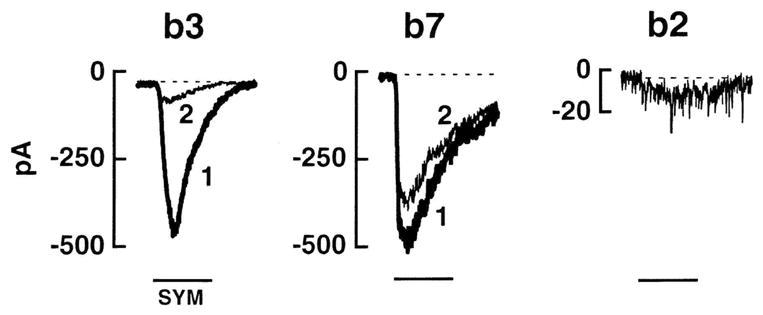
Types b3, b7 and b2 cone bipolar cells in a ground squirrel retinal preparation are distinguished by their responses to paired puffs of a kainate agonist, SYM 2081. b3 cell receptors remain desensitized for an extended period of time following exposure to SYM 2081. Hence, a first puff of SYM 2081 (1) elicits a large b3 cell response, while the second puff (2) produces a much smaller response. On the contrary, the receptors on b7 cells recover very quickly following exposure to SYM 2081 and, thus, give large responses to both puffs. Finally, the AMPA receptors on b2 cells fails to respond to SYM 2081.
(From DeVries, 2000)
It is well known that the retina represents information about object positions in the outside world in a bidimensional space. However, it appears that it also uses a third dimension and namely the depth in the IPL, to represent and/or to segregate the attribute of the visual stimuli (Figure 6). Hence, the different synaptic strata in the IPL correspond to different kinetics and thus constitute distinct temporal channels interacting with each others through connections with amacrine cells (Baccus, 2007).
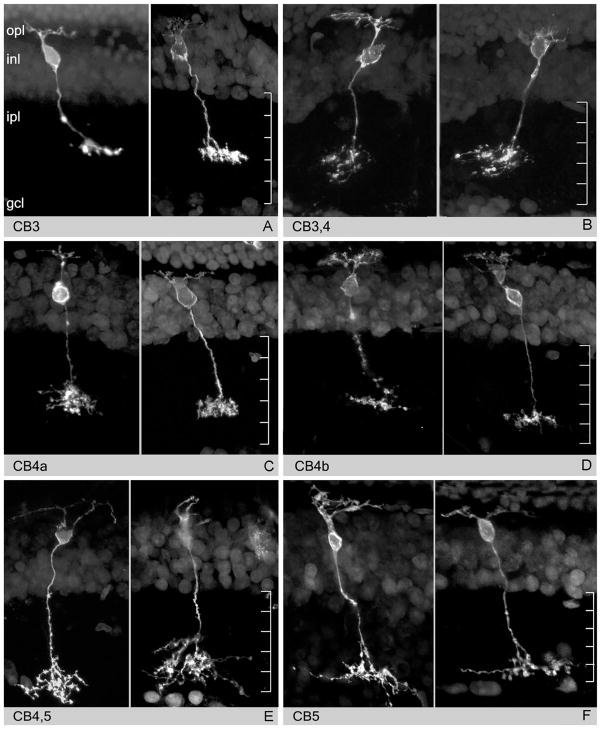
Figure 6.
Mouse retina. Examples of cone bipolar cells with axonal arbors in sublamina b of the IPL. Cells in E and F are two rarely encountered types. These cells have been visualized upon gun-delivery of DiI on vertical retinal sections. Differences in depth of stratification of axonal arbors in the IPL visible among OFF cone bipolar cells keep separate the temporal channels generated in the outer retina.
(Modified from Pignatelli and Strettoi, 2004)
Intrinsic differences among types of OFF cone bipolar are enhanced by an optimized synaptic microenvironment established within the terminal of each cone, the pedicle. Recent data show that the architecture of a pedicle is such that dendritic tips of bipolar cells carrying AMPA receptors sense quick fluctuations in the concentration of glutamate. This is achieved by dendrites penetrating within the depth of the pedicle. Dendrites of OFF bipolar cells expressing kainate receptor, instead, establish connections located on the external surface of the cone pedicle. This surface is reached by a steady flux of glutamate transmitter eliciting a sustained response in the bipolar cell. Thus, intrinsic capabilities of AMPA and kainate receptors to respond to transient and sustained stimuli, respectively, are enhanced by fine synaptic architecture at the cone ending (DeVries et al., 2006). The intricacy of circuitry and fine architecture of a cone pedicle is illustrated in Figure 7.
Figure 7.
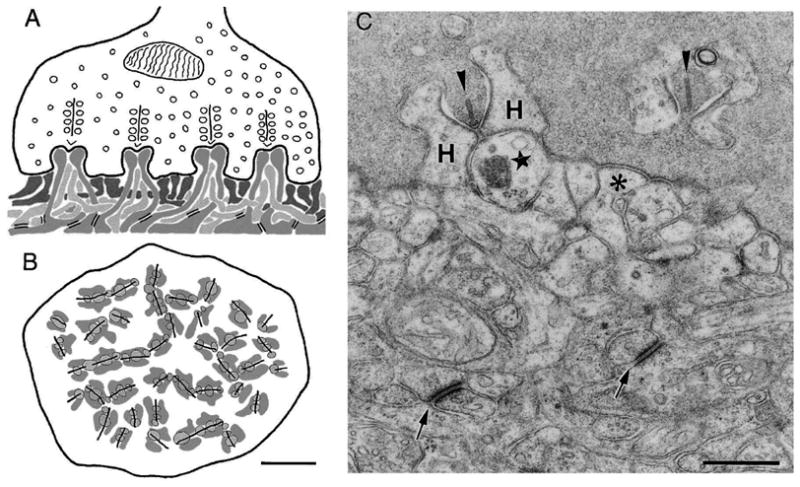
Organization of a primate cone pedicle A and B: vertical and horizontal diagrams of the pedicle. Four presynaptic ribbons, flanked by synaptic vesicles and four triads are shown. Invaginating dendrites of horizontal cells (medium gray) form the lateral elements, and invaginating dendrites of ON cone bipolar cells (light gray) form the central elements of the triads. OFF cone bipolar cell dendrites (dark gray) make flat contacts at the cone pedicle base. C. EM picture, showing shows the cone pedicle filled with vesicles and presynaptic ribbons (arrowheads). Postsynaptic processes are from an invaginting bipolar cell (star), from an horizontal cell (“H”)and from a flat bipolar cell (asterisk). Arrows point to desmosome-like junctions. Scale bars, 2 μm (B) and 0.5 μm (C).
(From Haverkamp et al., 2000)
It is likely that the different kinetics of the OFF cone bipolar cells are preserved in the inner retinal layers, so that sustained cone bipolar cells contribute mostly to the physiology of sustained, X-like ganglion cells, while transient cone bipolar cells feed transient, Y-like type ganglion cells. Inner retinal connections obviously participate in the emergence of the full panel of ganglion cell properties with the contribution of bipolar and amacrine cell synapses.
8. Contribution of other ionic channels to cone bipolar cell diversity
The rat retina contains cone bipolar cells which are respectively rich in T-type and in L-type Ca2+ channels. These, also known as low-voltage activated, open near the resting membrane potential and conduct a transient current in cells. Single cell electrophysiological studies, have shown that T-type channels are capable of generating low-threshold spikes (Hu et al., 2009). Within rat retinal cone bipolar cells particularly abundant in T-type Ca2+ currents, 3 different subgroups have been identified, each expressing a single dominant T channel subunit. Excitatory synaptic currents, especially transient components, can be increased by activation of T-type channels, which exhibit strong inactivation properties. Hence, the presence and different composition in Ca2+ channels can contribute in shaping different cone bipolar cell types, influencing their excitability and responses to glutamate (Hu et al., 2009).
Some bipolar cells express hyperpolarization-activated and cyclic-nucleotide-gated (HCN1) channels, thought to contribute in shaping the bipolar response to light, particularly in the temporal domain. The distribution varies greatly but consistently with bipolar cell types (Fyk-Kolodziej and Pourcho, 2007). Recordings from the rat retina show that each bipolar cell possesses a peculiar inventory of ionic channels of the HCN type that represent a true fingerprint (Ivanova and Muller, 2006). Particularly one OFF type shows high expression of HCN1 on its dendrites. On the contrary, rod bipolar cells selectively express HCN2 channels, which contribute to their typical response to light (Cangiano et al., 2007). The unique combination of HCN currents and other voltage-gated currents participate in determining the specific identity of cone bipolar cell types and can actually be used for classification of these neurons.
9. Additional molecular differences established in the outer retina
Single cell RT-PCR studies show that, within otherwise homogeneous bipolar cell types, variations in genetic expression exist at single unit level. Thus, each cell type expresses a primary pattern of genes plus some other variable genes. This could serve to further adjust the stimulus-response profile of each neuron (Hanna and Calkins, 2007).
An inwardly rectifying potassium channel, Kir2.4, was recently localized in ON bipolar cells (Dhingra et al., 2008) and likely contributes to shape their light response. Because Kir2.4 is a potassium channel and interacts with G01, it probably participates in the regulation of the resting potential, possibly regulated by G0.
Studies in the salamander retina have shown that a major difference between ON and OFF bipolar cells, besides the polarity of light response, is the phase delay as compared to that of the cone response. This is definitely higher in ON bipolar cells and could be due to retard caused by the second messenger G-protein cascade (Burkhardt et al., 2007). Thus, the second messenger G-protein pathway might introduce itself a delay in the response of ON as respect to that of OFF bipolar cells.
A key regulatory element in synaptic transmission is calcium clearance from the cytosol by high affinity calcium ATPases (PMCAs). It appears that the high sensitivity of the rod pathway is partially modulated by a particular plasma membrane Ca2+ATPase isoform (PMCA2) with high affinity for calcium calmodulin (Duncan et al., 2006). Amplitude and sensitivity of the rod-rod bipolar synaptic transmission are very much reduced in mice lacking the PMCA2 protein and this is reflected in a reduction and slowing down of the b wave of the scotopic ERG. Although it has been demonstrated that PMCAs are active in both rod and cone bipolar cells, biophysical measurements demonstrate that this particular form of calcium extrusion contributes to the sensitivity of the rod pathway, while it is much less involved in cone-initiated signaling (Duncan et al., 2006).
Pharmacological and morphological studies on the mouse retina (Mojumder et al., 2007) have risen the possibility of the presence of voltage-gated sodium channels in dendrites of some bipolar cells in the OPL. Recordings from human and rat cone bipolar cells show that indeed these neurons have voltage gated sodium channels sensitive to TTX and can generate prominent spikes upon depolarization. These channels might have the role of amplifying the release of neurotransmitter (Ohkuma et al., 2007) but also of enhancing the response kinetics and amplitude of those cone bipolar cells which express them (Ma et al., 2005).
10. Further differences among cone bipolar cells: transcription factors and expression of connexins
During development, retinal cell differentiation is tightly regulated by a cascade of transcription factors. At least three of them, and namely Vsx1, Bhlhb5 and Irx5 are tightly associated to the generation of a specific type of OFF cone bipolar cells (Cheng et al., 2005). Targeted deletion of Bhlhb5 results into a major reduction in the number of these neurons (Feng et al., 2006). In addition, during neurogenesis, cone bipolar cells are generated before rod bipolars, reflecting the order of presynaptic photoreceptor genesis (Morrow et al., 2008). Additional differences are reflected in the existence of molecular markers strictly related to genetic features of bipolar cells and revealed by microarray analysis. Gene expression patterns typical of cone bipolar cells can be identified, with at least five markers restricted to these cells and relatively unaffected by degeneration of rod bipolar cells (Kim et al., 2008). Thus, selective types of cone bipolar cells can be separated based on expression of specific transcription factors, indicating the existence of distinct genetic pathways. Additional criteria for separation are time of genesis during development and expression of unique markers linked to their genetic features.
In addition to regulate cell-specific development, transcription factors seem to control specific aspects of visual functions in retinal circuitry. Recently, it has been shown that mice lacking both Vsx1 and Irx5 have morphologically normal bipolar cells. However, response threshold, gain and range of light responses recorded from cohorts of ganglion cells appear lowered in OFF neurons selectively. Similarly, temporal contrast adaptation is reduced in OFF ganglion cells, while ON ganglion cells remain normal (Kerschensteiner et al., 2008). Thus, expression of bipolar specific transcription factors contributes to the differences between ON and OFF retinal pathways.
Additional distinctive elements among bipolar cell types are represented by expression of connexins participating in the composition of gap junctions in the IPL. Gap junctions ply a role as relevant functionally as that of chemical synapses, because they can be regulated by a number of extrinsic factors such as dopamine, calcium and pH and also because they regulate the function of whole neuronal networks.
Recent data show that most types of ON cone bipolar cells express connexin 36 (cx36) while at least one type of ON cone bipolar cell does express preferentially cx36 at the gap junctions with AII amacrine cell processes; connexin 45 is systematically missing from these cells (Han and Massey, 2005). These findings implicate that visual signals conveyed to the inner retina by different ON cone bipolar cells can be variably processed in virtue of different composition of electrical synapses.
Finally, there are differences among bipolar cells arising from their likely employment of different combinations of neurotransmitters. It has long been known that bipolar neurons release the excitatory transmitter glutamate. They correspondingly express dedicated glutamate transporters such as the vesicular transporter VGLUT1. However, in the retina of the cat a selective population of cone bipolar cells has been identified that express glutamate and VGLUT1, plus GABA and its synthetic enzyme GAD65. They also express the vesicular GABA transporter VGAT (Kao et al., 2004). These cells are identified as OFF cone bipolars in virtue of the level of stratification of their axonal arbors in the IPL. The presence of such subsets of bipolar cells within the global population of OFF cone bipolar cells predicts dedicated functional properties unique to these neurons.
11. Input and output differences among cone bipolar cells established in the IPL
Differences in the connectivity of cone bipolar cells in the inner retina are simply a reflection of the necessity of keeping separate the functional channels created in the outer retina. As examples of the existence of various paradigms of connections for the different types of cone bipolars one can take their variable contribution to the rod pathway as well as the presence of segregated co-fasciculation of processes in the IPL itself.
Classical ultrastructural studies on the cat retina based on 3D reconstructions of bipolar cells from continuous series of ultrathin sections examined by electron microscopy established the presence of 7 types of cone bipolar cells with quite different pattern of synaptic connections in the IPL (McGuire et al., 1984). Not only among the 7 types could one separate cells with axons ending in the two different sublaminae of the IPL, and thus presumptive OFF and ON cone bipolars. Among cells terminating in the same sublamina, pattern of specific and stereotypes connections could be recognized, splitting each of them in separate clusters. For example, cone bipolar cells could be distinguished based on the presence and number of connections with cells of the rod pathway: in the OFF sublamina of the IPL, only some cone bipolar cells establish reciprocal synapses with AII amacrine cells, the fundamental interneurons of the rod pathway. Similarly, in the ON sublamina of the IPL many, but not all, cone bipolar cells axonal endings make gap junctions with dendrites of AII amacrine cells. Hence, one can infer that the entity of rod-originated signals carried by each of the parallel channels represented by the different types of cone bipolars is not homogeneous.
Within the width of a and b sublaminae of the IPL, one can recognize further pattern of stratification that dictate synaptic circuitry: only processes of cells ultimately running in the same stratum have the opportunity to establish direct synaptic connections. One clear example of this “co-stratification” rule is represented by one type of ON cone bipolar cells, revealed in the retina of the rabbit by antibodies against the carbonate epitope CD-15 (Brown and Masland, 1999). Such cone bipolar cells have axonal endings expanding in large terminal varicosities which stratify within the processes of ON-starburst amacrine cells (Figure 8). These are well known amacrines involved in the process of direction selectivity, which makes a ganglion cell capable of responding to stimuli moving in a preferred direction. High resolution studies carried on by confocal microscopy on retinal preparations labeled with antibodies against CD-15 and the enzyme choline acetyl transferase (ChAT), labeling starburst amacrine cells, showed how strictly the cone bipolar terminals follow the pattern of ON starburst cells. Such an intimate association strongly suggests that the CD-15 cone bipolar cells provides an input to ON starburst amacrines and therefore participates in the physiology of ON and ON-OFF directional selective ganglion cells.
Figure 8.
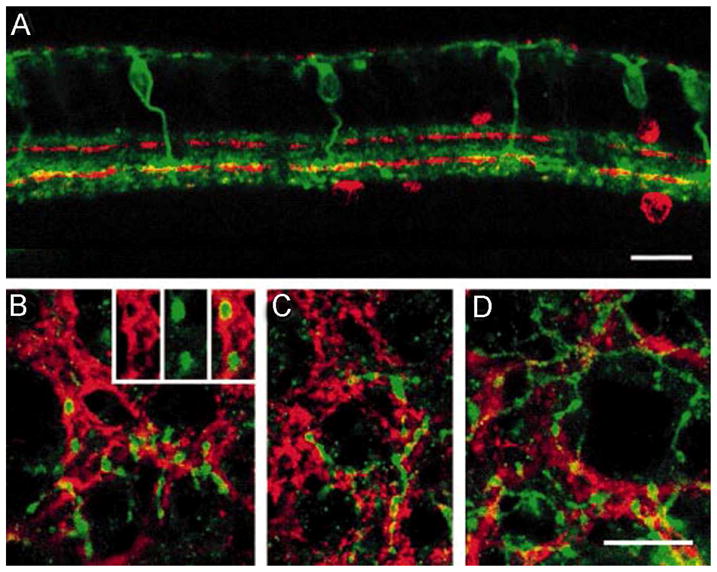
Vertical sections and wholemounts double labeled with antibodies against CD15 and against choline acetyltransferase (ChAT).
A: In this confocal image of a vertical section, the axons of the CD15-positive bipolar cells (green) can be observed to ramify above and within the ChAT-positive band created by the processes of the ON-starburst amacrine cells (in red). Places where the CD15-positive axons overlap with the starburst processes appear yellow. B–D: These confocal images of retinal wholemounts show how the axonal varicosities of the CD15-positive bipolar cells (green) follow the same pattern as the ChAT-positive processes. The inset in C shows how the varicosities penetrate the bundles of ChAT-positive processes.
(Modified from Brown and Masland, 1999)
While the occurrence of separate connections in the inner retina for different types of cone bipolar cells can be seen as a consequence of keeping separate already formed channels operating in parallel, additional features which reinforce distinction of these channels are generated in the inner retina itself. Specificity of amacrine input is among these features. Immunocytochemistry with antibodies against the widely diffused GABA and glycine receptors demonstrates a sharp distinction in their distribution with respect to bipolar cells in the retina of mammals. Bipolar cells of the ON channel (both rod and cone bipolars) are dominated by GABAergic input, while OFF cone bipolar cells are dominated by glycinergic input (Grunert, 2000). In particular, OFF cone bipolar cells receive a strong glycinergic input from AII amacrine cells. This input appears mediated from fast alpha1beta-containing channels (Ivanova and Muller, 2006). In general, it is known that glycine is used as a neurotransmitter by small-field amacrine cells, while GABA is used by wide-field amacrines. Although the precise functional significance of this differential distribution of inhibitory input is not known, it obviously arises from totally different types of amacrines, ultimately affecting bipolar cell physiology, particularly in the temporal domain.
12. Breaking the rules: mixed rod-cone bipolar cells in mammalian retinas
Electrophysiological recordings from ganglion cells in a “coneless” mouse suggested for the first time the existence of a direct pathway from rods to OFF cone bipolar cells (Soucy et al., 1998). Later, confocal and electron microscopy demonstrated the occurrence of symmetrical contacts involving rod spherules and some dendrites of OFF cone bipolar cells (Hack et al., 1999, Tsukamoto et al., 2001). Dendrites of such cone bipolars express ionotropic glutamate receptors at the site of apposition to rod spherules, but only some 20% of all the rods are involved in this particular type of connections.
Subsequent morphological evidence has been provided for direct connections between rods and OFF cone bipolar cells in the rabbit retina as well (Li and DeVries, 2004). This suggests that the alternative rod pathway may be a common feature of the mammalian retina, but its relative importance and significance differ between species.
Apparently, all three rod pathways are functional in the mouse retina, but operate under stimulus intensity ranges that are widely different, with the primary rod pathway being the most sensitive (Volgyi et al., 2004). It is anyway important to notice that not all the existing types of OFF cone bipolar cells establish connections with rods. In the mouse retina, type CB4, expressing the marker calselinin, establishes basal contacts with cones as well as with some rods. Also types 3a and 3b OFF cone bipolars receive a mixed cone-rod input. Thus, within OFF cone bipolar cells a distinction can be introduced between the two types making connections with both rods and cones and those that can be defined as “truly” cone selective (Haverkamp et al., 2008).
Among ON bipolar cells, those with a predominant or sole cone input can be distinguished from those with a mixed rod-cone input on the basis of their threshold. Studies on mice have shown that depolarizing cone bipolar cells with a mixed rod-cone input, whose axons end in strata 8 and 9 of the IPL, have a threshold similar to that of rod bipolar cells but a wider dynamic range; instead, depolarizing cone bipolar cells whose axonal endings terminate in strata 6–8 of the IPL, which receive a pure cone input, have a threshold 2 log units higher. So, similar cone bipolar, signaling light increments to ganglion cells, have different thresholds and thus different sensitivities (Wu, 2009).
Recently, bipolar cells which break the ON OFF stratification rule in the IPL have been described in the retina of rabbits (Hoshi et al., 2009). One type of ON cone bipolar cell (specifically labeled by calbindin antibodies) was found which establish ribbon synapses with dopaminergic amacrines and ganglion cells as their axons pass through the sublamina OFF of the IPL. Postsynaptic processes exhibited GluR4 glutamate receptors indicative of functional synapses. The establishment of these connections by a selective type of ON bipolar cell endows this neuron of unique output properties which are not present in other ON cone bipolar cells of different types.
13. Different reactions to inherited photoreceptor degeneration
Besides exhibiting morphological and molecular differences that subserve diverse functions in the normal retina, cone bipolar cells also seem to respond differently to pathological conditions. Studies on rodent models of Retinitis Pigmentosa, a genetic disorder in which a mutation in a retinal specific gene leads to the degeneration of rods followed by secondary death of cones, demonstrate that second order neurons undergo progressive changes collectively known as remodeling (Marc et al., 2003). Remodeling is usually regressive, as bipolar cells, and particularly rod bipolars, exhibit progressive retraction of dendrites, up to the complete atrophy of their dendritic arborizations, with concomitant loss of glutamate postsynaptic receptors (Strettoi and Pignatelli, 2000; Strettoi et al., 2002; Gargini et al., 2007). Electrophysiological studies show that regressive remodeling of bipolar cells is accompanied by progressive loss of glutamate sensitivity and capability to respond to exogenous applications of this neurotransmitter, normally released from rods and cones (Varela et al., 2003; Barhoum et al., 2008). Interestingly, rod bipolars loose dendrites and mGluR6 receptors before cone bipolars of the ON functional types (Strettoi et al., 2002). This reflects the later time of degeneration of cones. However, it is intriguing the recent finding that integrity of dendrites and glutamate responses are preserved for much longer times in cone bipolar cells of the OFF channel, normally expressing AMPA/kainate receptors (Puthussery et al., 2009). Given the fact that both ON and OFF cone bipolar cells are connected to the same pools of degenerating cones, and nourished through the same plexus of retinal blood vessels, it is hard to attribute these differences to extrinsic properties of the outer environment. Rather, it is more conceivable that intrinsic properties of the bipolar cells themselves, possibly related to the different glutamate receptors expressed on their dendritic tips, confer to these cells various abilities to resist deafferentation. Whatever its source might be, the ability of OFF cells to better survive photoreceptor degeneration is a very important property in view of the possibility to restore vision in individuals with photoreceptor degeneration by means of cellular transplantation or via direct stimulation of surviving retinal cells with electronic prostheses: evidently, OFF cone bipolar cells represent a better and more suitable platform for repair because of their intrinsic resistance to long term photoreceptor death.
14. Conclusions
We have summarized the existence of a collection of morphological, molecular, staining properties and functional features among bipolar cells of the mammalian retina, altogether supporting the concept of different functional channels, each of them devoted to convey key aspects of the visual scenery, such as contrast, color, time, etc. Many of the properties are generated at the retinal first synaptic station, in the outer plexiform layer. Some of the difference among channels are shaped and reinforced by selective and differential inhibitory input from amacrine cells in the inner retina. Finally, separate connectivity is established in the inner plexiform layer so that each parallel pathway is routed to partially distinct ganglion cells.
A challenge for future studies in retinal research is to clarify better the function of each channel and to unravel the molecular cascades leading to the genesis of these parallel pathways during retinal development.
Acknowledgments
Supported by the Italian National Research Council (CNR), NIH grant RO1 EY 12654, PRIN 2008 of the Italian Ministry of Education, The British Retinitis Pigmentosa Society, London.
We are grateful to R.H. Masland, L. Cervetto and L. Galli-Resta for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad KM, Klug K, Herr S, Sterling P, Schein S. Cell density ratios in a foveal patch in macaque retina. Vis Neurosci. 2003;20:189–209. doi: 10.1017/s0952523803202091. [DOI] [PubMed] [Google Scholar]
- Baccus SA. Timing and computation in inner retinal circuitry. Annu Rev Physiol. 2007;69:271–290. doi: 10.1146/annurev.physiol.69.120205.124451. [DOI] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Barhoum R, Martinez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, et al. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008;155:698–713. doi: 10.1016/j.neuroscience.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Costratification of a population of bipolar cells with the direction-selective circuitry of the rabbit retina. J Comp Neurol. 1999;408:97–106. [PubMed] [Google Scholar]
- Burkhardt DA, Fahey PK, Sikora MA. Retinal bipolar cells: temporal filtering of signals from cone photoreceptors. Vis Neurosci. 2007;24:765–774. doi: 10.1017/S0952523807070630. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Gargini C, Della Santina L, Demontis GC, Cervetto L. High-pass filtering of input signals by the Ih current in a non-spiking neuron, the retinal rod bipolar cell. PLoS One. 2007;2:e1327. doi: 10.1371/journal.pone.0001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Chow RL, Lebel M, Sakuma R, Cheung HO, et al. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev Biol. 2005;287:48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, et al. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, et al. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008;510:484–496. doi: 10.1002/cne.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, Yang H, Doan T, Silverstein RS, Murphy GJ, et al. Scotopic visual signaling in the mouse retina is modulated by high-affinity plasma membrane calcium extrusion. J Neurosci. 2006;26:7201–7211. doi: 10.1523/JNEUROSCI.5230-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Schneider H, Wässle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci. 1996;16:2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, et al. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyk-Kolodziej B, Pourcho RG. Differential distribution of hyperpolarization-activated and cyclic nucleotide-gated channels in cone bipolar cells of the rat retina. J Comp Neurol. 2007;501:891–903. doi: 10.1002/cne.21287. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghösh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Grünert U. Distribution of GABA and glycine receptors on bipolar and ganglion cells in the mammalian retina. Microsc Res Tech. 2000;50:130–140. doi: 10.1002/1097-0029(20000715)50:2<130::AID-JEMT5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Massey SC. Electrical synapses in retinal ON cone bipolar cells: subtype-specific expression of connexins. Proc Natl Acad Sci U S A. 2005;102:13313–13318. doi: 10.1073/pnas.0505067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna MC, Calkins DJ. Expression of genes encoding glutamate receptors and transporters in rod and cone bipolar cells of the primate retina determined by single-cell polymerase chain reaction. Mol Vis. 2007;13:2194–2208. [PubMed] [Google Scholar]
- Haverkamp S, Ghösh KK, Hirano AA, Wässle H. Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol. 2003;455:463–476. doi: 10.1002/cne.10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Specht D, Majumdar S, Zaidi NF, Brandstatter JH, et al. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101. doi: 10.1002/cne.21612. [DOI] [PubMed] [Google Scholar]
- Henry D, Burke S, Shishido E, Matthews G. Retinal bipolar neurons express the cyclic nucleotide-gated channel of cone photoreceptors. J Neurophysiol. 2003;89:754–761. doi: 10.1152/jn.00771.2002. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Bi A, Pan ZH. Differential expression of three T-type calcium channels in retinal bipolar cells in rats. Vis Neurosci. 2009;26:177–187. doi: 10.1017/S0952523809090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller F. Retinal bipolar cell types differ in their inventory of ion channels. Vis Neurosci. 2006;23:143–154. doi: 10.1017/S0952523806232048. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973;235:133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YH, Lassova L, Bar-Yehuda T, Edwards RH, Sterling P, et al. Evidence that certain retinal bipolar cells use both glutamate and GABA. J Comp Neurol. 2004;478:207–218. doi: 10.1002/cne.20221. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Liu H, Cheng CW, Demas J, Cheng SH, et al. Genetic control of circuit function: Vsx1 and Irx5 transcription factors regulate contrast adaptation in the mouse retina. J Neurosci. 2008;28:2342–2352. doi: 10.1523/JNEUROSCI.4784-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, et al. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, et al. Photopic electroretinograms of mGluR6-deficient mice. Curr Eye Res. 2008;33:91–99. doi: 10.1080/02713680701823232. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Labrakakis C, Joseph DJ, Macdermott AB. Functional similarities and differences of AMPA and kainate receptors expressed by cultured rat sensory neurons. Neuroscience. 2004;129:35–48. doi: 10.1016/j.neuroscience.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Ma YP, Cui J, Pan ZH. Heterogeneous expression of voltage-dependent Na+ and K+ channels in mammalian retinal bipolar cells. Vis Neurosci. 2005;22:119–133. doi: 10.1017/S0952523805222010. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Martin PR, Grunert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis. 2007;13:2163–2182. [PubMed] [Google Scholar]
- Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–7765. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, et al. Gbeta5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. Eur J Neurosci. 2007;26:2899–2905. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Chen CM, Cepko CL. Temporal order of bipolar cell genesis in the neural retina. Neural Dev. 2008;3:2. doi: 10.1186/1749-8104-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Ohkuma M, Kawai F, Horiguchi M, Miyachi E. Patch-clamp recording of human retinal photoreceptors and bipolar cells. Photochem Photobiol. 2007;83:317–322. doi: 10.1562/2006-06-15-RA-923. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol. 2004;476:254–266. doi: 10.1002/cne.20207. [DOI] [PubMed] [Google Scholar]
- Puller C, Haverkamp S, Grunert U. OFF midget bipolar cells in the retina of the marmoset, Callithrix jacchus, express AMPA receptors. J Comp Neurol. 2007;502:442–454. doi: 10.1002/cne.21315. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur J Neurosci. 2009;29:1533–1542. doi: 10.1111/j.1460-9568.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974;236:211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Masland RH. The organization of the inner nuclear layer of the rabbit retina. J Neurosci. 1995;15:875–888. doi: 10.1523/JNEUROSCI.15-01-00875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Volpini M. Retinal organization in the bcl-2-overexpressing transgenic mouse. J Comp Neurol. 2002;446:1–10. doi: 10.1002/cne.10177. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Iwakabe H, Masu M, Suzuki M, Nakanishi S. The mGluR6 5′ upstream transgene sequence directs a cell-specific and developmentally regulated expression in retinal rod and ON-type cone bipolar cells. J Neurosci. 1997;17:3014–3023. doi: 10.1523/JNEUROSCI.17-09-03014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Varela C, Igartua I, De la Rosa EJ, De la Villa P. Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision Res. 2003;43:879–885. doi: 10.1016/s0042-6989(02)00493-5. [DOI] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM. From retinal circuitry to eye diseases--in memory of Henk Spekreijse. Vision Res. 2009;49:992–995. doi: 10.1016/j.visres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sulaiman P, Feddersen RM, Liu J, Smith RG, et al. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J Neurosci. 2008;28:8873–8884. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]