Abstract
Objectives
In expert hands, the intra-thoracic esophago-gastric anastamosis usually provides a low rate of strictures and leaks. However, anastomoses can be technically challenging and time consuming when minimally invasive techniques are used. We present our preliminary results of a standardized 25mm/4.8mm circular stapled anastomosis using a trans-orally placed anvil.
Materials and Methods
We evaluated a prospective cohort of 37 consecutive patients offered minimally invasive Ivor Lewis Esophagectomy at a tertiary referral center. The esophagogastric anastomosis was created using a 25mm anvil (Orvil, Autosuture, Norwalk, CT) passed trans-orally, in a tilted position, and connected to a 90cm long PVC delivery tube through an opening in the esophageal stump. The anastomosis was completed by joining the anvil to a circular stapler (EEA XL 25mm with 4.8mm Staples, Autosuture, Norwalk, CT) inserted into the gastric conduit. Primary outcomes were leak and stricture rates.
Results
Thirty-seven patients (mean age 65 yrs) with distal esophageal adenocarcinoma (n=29), squamous cell cancer (n=5), or high-grade dysplasia in Barrett's Esophagus (n=3) underwent an Ivor Lewis Esophagectomy between October 2007 and August 2009. The abdominal portion of the operation was completed laparoscopically in 30 patients (81.1%). The thoracic portion was done using a muscle sparing mini-thoracotomy in 23 patients (62.2%) and thoracoscopic techniques in 14 patients (37.8%). There were no intra-operative technical failures of the anastomosis or deaths. Five patients had strictures (13.5%) and all were successfully treated with endoscopic dilations. One patient had an anastomotic leak (2.7%) that was successfully treated by re-operation and endoscopic stenting of the anastomosis.
Discussion
The circular stapled anastomosis with the transoral anvil allows for an efficient, safe and reproducible anastomosis. This straightforward technique is particularly suited to the completely minimally invasive Ivor Lewis Esophagectomy.
Keywords: Esophagectomy, Esophageal Cancer, Minimally Invasive, Anastomose, Stapler, Complications
Introduction
Distal esophageal adenocarcinoma is the most common type of esophageal cancer in Europe and in the U.S. 1. Surgical approaches to treat these lesions include the transhiatal esophagectomy and the transthoracic esophagectomy with the anastomosis in the chest or in the neck. Controversy exists regarding the best surgical approach, the extent of lymph nodal dissection, and the type of anastomosis that provides the lowest rate of leaks and strictures 2-3. Worldwide the feasibility and safety of minimally invasive surgical (MIS) techniques for esophagectomy has been demonstrated in multiple centers. Furthermore, the oncologic outcomes of minimally invasive esophagectomy may be comparable to those of open esophagectomy4.
Performing an intrathoracic esophagogastric anastamosis using MIS techniques can be technically challenging and time consuming. Despite increasing experience with several different techniques and advances in technology, anastomotic complications remain a source of morbidity and mortality. Anastomotic options include variations of traditional hand sewn anastomosis, side-to-side stapled anastomosis, or circular stapled anastomosis. Randomized trials have compared hand-sewn anastomoses with stapled anastomoses 5-12 and not shown a difference in the anastomotic leak rate and only one of these studies reported a higher stricture rate with the stapled anastomosis 7. Using the circular stapling technique may be less time-consuming than the hand-sewn technique and may possibly be performed with a shorter gastric conduit compared to the linear stapled technique 13. However, most surgeons use a traditional circular stapled anastomosis approach, which involves inserting the anvil through a large opening in esophageal stump and securing it with a purse-string suture 14. This step can be inefficient and time-consuming, particularly when using minimally invasive techniques.
Our objective is to present the outcomes of a consecutive series of patients who underwent Ivor Lewis Esophagectomy using a standardized 25mm/4.8mm circular stapled anastomosis with a transorally placed anvil. The primary outcomes of interest include anastomotic leak and stricture.
Materials and Methods
We evaluated a cohort of consecutive patients with distal esophageal lesions offered minimally invasive Ivor Lewis Esophagectomy at the University of California-San Francisco Medical Center from October 2007 to August 2009 using a clinical database prospectively maintained by a trained research coordinator. The database includes demographic information, pre-operative clinical measurements, and peri-operative and long-term outcomes of all patients who underwent esophageal surgery at UCSF. In addition, we examined all patients' electronic charts, operative reports and anesthesia records, discharge summaries, and follow-up clinic notes to search for possible missing outcomes. This study was approved by the UCSF institutional review board and informed consent was obtained from all patients.
Surgical and Anastomotic Technique
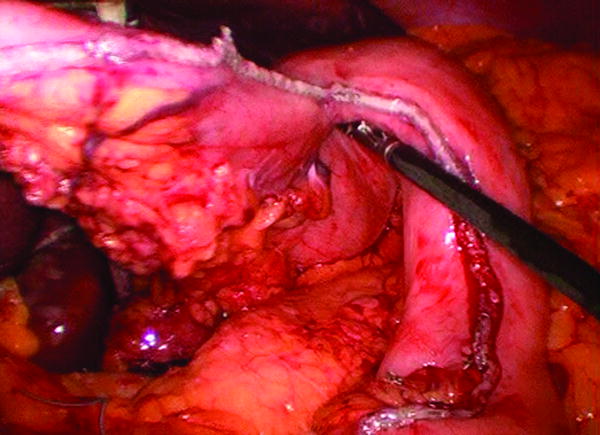
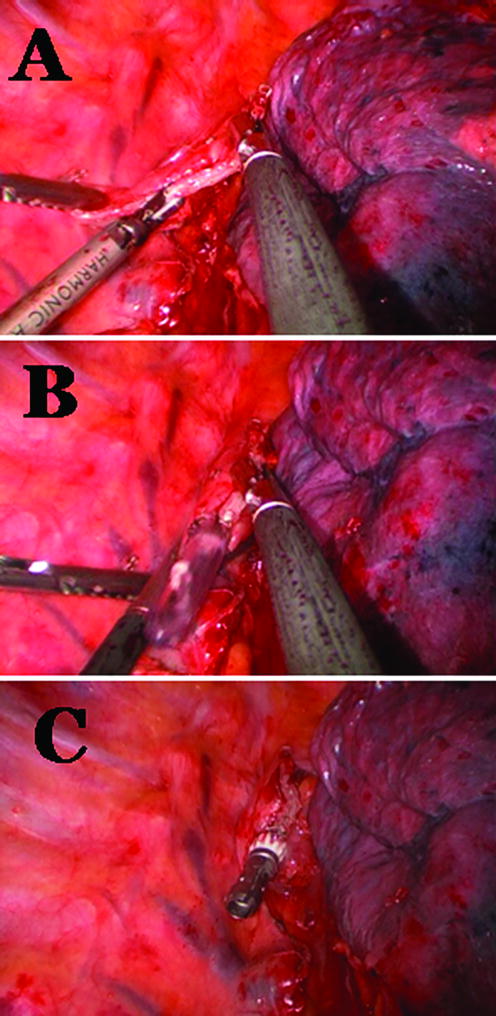
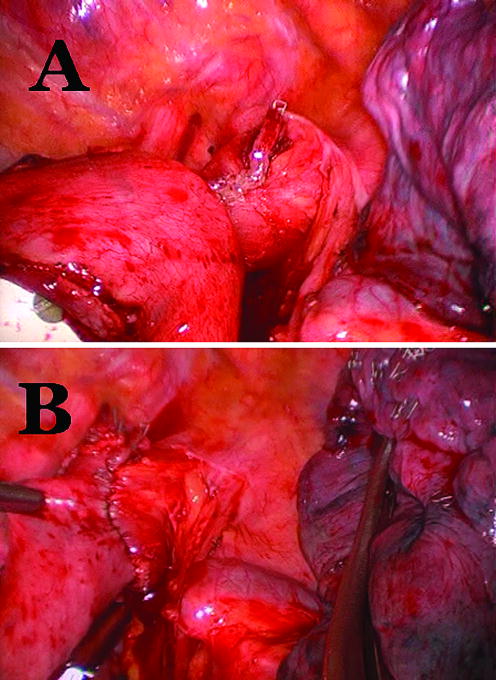
All patients were offered minimally invasive esophagectomy. First, a diagnostic laparoscopy was performed to determine resection using a laparoscopic approach would be possible. If there were no contra-indications six to seven ports were placed and the formal dissection was started. The operation included laparoscopic hiatal, distal esophageal, and gastroesophageal junction dissection. Lymph node dissections of the porta-hepatis, left gastric artery, and supra-pancreatic nodal groups were performed. Gastric conduit preparation was performed using multiple firings of a 4.8 mm linear stapler (Figure 1) (United States Surgical Corporation, Norwalk, CT). A pyloroplasty was performed and a feeding jejunostomy placed. The thoracic portion was completed using either a muscle sparing thoractomy in the 6th intercostal space or standard thoracoscopic ports and techniques. The thoracic portion of the operation included mobilization of the esophagus from the esophageal bed, subcarinal lymph node dissection and transection of the most superior aspect of the thoracic esophagus at the level of the thoracic inlet with a 4.8 mm linear stapler (United States Surgical Corporation, Norwalk, CT) above the divided azygous vein. The esophagogastric anastomosis was performed using a 25 mm anvil (OrVil, Autosuture, Norwalk, CT) passed trans-orally through a small opening in the stapled esophageal stump (Figure 2). The OrVil™ 25 mm Device is a pre-packaged commercially available device (OrVil, Autosuture, Norwalk, CT). It combines the anvil head, secured in the tilted position, mounted on a 90cm long PVC delivery tube and secured to the tube with a suture. The PVC delivery tube in inserted through the patient's mouth, delivered through a small opening in the stapled esophageal stump, and pulled from one of the thoracic port sites or incision to assist bringing the anvil shaft into the esophageal stump. A critical step of the procedure is then passing the tilted anvil head attached to the delivery tube through the posterior pharynx into the esophagus. As the patient's head is turned to the side and with a double-lumen endotracheal tube in place, we recommend that the anesthesiologist and an assistant are present for this portion of the procedure. The anvil head should be generously lubricated and its convex side directed and maintained toward the hard and soft palate. After the anvil enters the posterior pharynx, elevating the mandible, similar to a Jaw thrust maneuver, and briefly deflating the balloon of the double-lumen endotracheal tube, facilitates the anvil passage into the esophagus. After the anvil shaft has been exteriorized through the esophageal stump, the suture that holds it to the delivery tube is cut and the tube disconnected from the anvil while holding the anvil in place. The anastomosis was completed by joining the anvil to a circular stapler (EEA XL 25 mm with 4.8 mm Staples, Autosuture, Norwalk, CT) inserted into the gastric conduit (Figure 3A). Then, the EEA stapler and anvil were removed, the anastomosis inspected, and the gastric conduit opening was closed using an additional firing of a 4.8 mm linear stapler (United States Surgical Corporation, Norwalk, CT) (Figure 3B).
Figure 1.
Laparoscopic Gastric Conduit preparation
Figure 2.
Tran-oral introduction of the 25mm anvil in the esophageal stump (Orvil, Autosuture, Norwalk, CT). Small opening of the esophageal stump (A), initial delivery of the 90cm long PVC tube through the small opening in the stapled esophageal stump (B), and 254m anvil in the esophageal stump (C).
Figure 3.
Joining the anvil to a circular stapler (EEA XL 25mm with 4.8mm Staples, Autosuture, Norwalk, CT) (A) and final aspect of the anastomosis (B).
Definition of leak
Anastomotic leakage was defined as extravasation of water-soluble contrast medium by a radiographic contrast esophagogram or CT scan and/or clinical symptoms of leakage.
Definition of stricture
Stricture was suspected in patients with dysphagia, postprandial vomiting, or regurgitation. The diagnosis was confirmed by the inability to pass a standard video optic endoscope (10 mm diameter) through the esophagogastrostomy. The endoscopic procedures were performed by the author (GMC) in an outpatient endoscopy suite using a combination of narcotic analgesic and sedative hypnotics for conscious sedation. The patient was placed in the left lateral decubitus position. Once a stricture was confirmed, sequential balloon dilation was performed up to a maximum of 20mm. Dilation was routinely performed using a controlled radial expansion (CRE) Wireguided Esophageal / Pyloric 180cm dilator (Boston Scientific, Natick, MA). Three dilations of increasing diameter were performed with each balloon passage. Inflation pressures were monitored using the Alliance II integrated inflation system (Boston Scientific, Natick, MA). Patients were followed for clinical response and underwent repeat endoscopy if their symptoms did not improve.
Peri-operative (≤ 30 days) and long-term (> 30 days) complications were documented and included, but were not limited to use of unexpected drug therapy or imaging, use of total parenteral nutrition, blood transfusion, superficial wound infection, cardiac arrhythmias, pleural space or lung infections, hospital stay greater than twice the median stay, use of diagnostic or therapeutic endoscopy, reoperation, or death.
Results
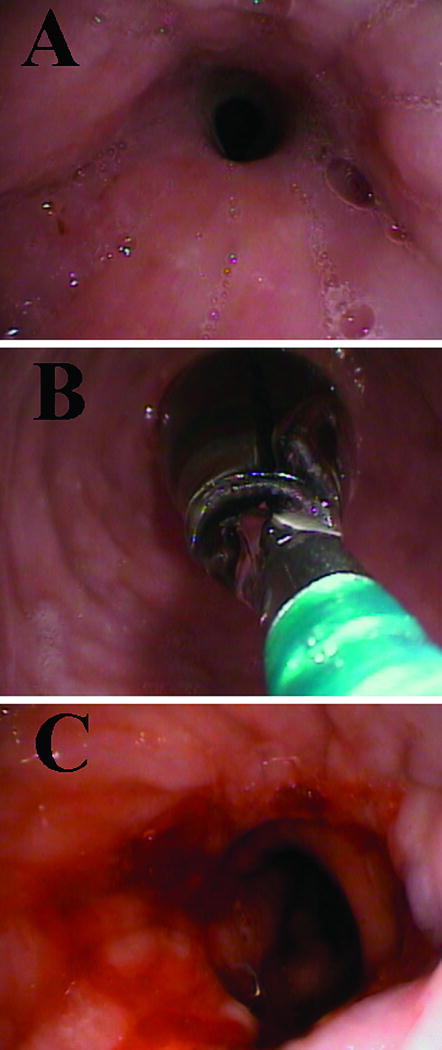
Thirty-seven consecutive patients (mean age 67 years; range 45 to 85 yrs) were offered minimally invasive Ivor Lewis Esophagectomy between October 2007 and August 2009. Demographic data, clinical characteristics, and pathologic staging are shown in Table 1. The abdominal portion of the operation was completed laparoscopically in 30 patients (81.1%). The thoracic portion was completed using a mini-thoracotomy in 23 patients (62.2%) and a thoracoscopic technique in 14 (37.8%). Median operative time was 275 minutes (range 210 to 420 minutes). Proximal and distal margins were negative in all patients. A median of 15 lymph nodes (range 8 to 33) were dissected from each specimen, with a median of 3 (range 0 to 18) histologically positive nodes. No intra-operative technical failures of the anastomosis or deaths occurred. The median hospital stay was 10 days (range 7 to 30) and the median follow-up was 11 months (range 1.5 to 23 months). Twenty-three complications occurred in 16 patients (43%) (Table 2). Five patients were diagnosed with anastomotic stricture (13.5%) and symptoms occurred an average of 20 days after surgery (range 12 to 28). All patients were successfully treated with two (n=2) or three sessions (n=3) of endoscopic balloon dilations (Figure 4). A leak was detected in one patient (2.7%,). The patient was a 65 year-old woman with squamous cell carcinoma of the distal esophagus, chronic esophageal wall disease manifested by Plummer-Vinson Syndrome with a history of multiple dilations for esophageal webs, and rheumatoid arthritis for which she was on steroid therapy. A leak from the esophagogastrostomy became apparent on post-operative day number 14 after a normal esophagogram was obtained on post-operative day number 11. The patient was successfully treated with re-operation, drainage, pleural flap, and endoscopic stenting of the anastomosis. She recovered without any further problems.
TABLE 1.
Demographic and Clinical Characteristics and Pathologic Staging.
| N = 37 | |
|---|---|
| Age (years), mean ± SD (range) | 65 ± 8.7 (42 to 85) |
| Sex, M:F | 32:5 |
| Tumor histology, n (%) | |
| Adenocarcinoma | 29 (78%) |
| Squamous Cell Carcinoma | 5 (14%) |
| High Grade Dysplasia in Barrett Esophagus | 3 (8%) |
| Tumor Location, n (%) | |
| Distal Esophagus, GE Junction | 34 (92%) |
| Mid Thoracic Esophagus | 3 (8%) |
| Neo-adjuvant treatment, n (%) | |
| Radiation only | 3 (8%) |
| Chemotherapy only | 2 (5%) |
| Chemotherapy and Radiation | 15 (41%) |
| Pathological Stage AJCC Classification | |
| High Grade Dysplasia in Barrett Esophagus | 3 (8.1%) |
| 0 | 4 (10.8%) |
| I | 3 (8.1%) |
| II | 14 (37.8%) |
| III | 12 (32.4%) |
| IV | 1 (2.7%) |
TABLE 2.
Complications after Surgery.
| N = 16 (43.2%) | |
|---|---|
| Atrial fibrillation | 8 |
| Anastomotic stricture | 5 |
| Pneumonia | 3 |
| Superficial Surgical Site Infection | 2 |
| Anastomotic leak | 1 |
| Deep vein thrombosis | 1 |
| Post-operative Bleeding | 1 |
| Clostridium difficile diarrhea | 1 |
| Pneumothorax | 1 |
Figure 4.
Endoscopic view of an esophago-gastric anastomosis with a stricture (A), with the balloon dilation in place (B) and after successful dilation (C).
Discussion
Although mortality and morbidity from esophageal cancer surgery is decreasing, complications of the esophagogastric anastomosis are a source of significant concern 3, 15-17. We have found that constructing a circular stapled anastomosis with the transoral anvil allows for a standardized esophagogastric anastomosis. It is a straightforward and reproducible technique that is particularly suited to the minimally invasive thoracoscopic approach, and has a low leak and stricture rate.
Anastomotic leaks are a concern with all types of esophagogastric anastomoses. Prior studies have suggested that intrathoracic anastomotic leaks may be associated with greater morbidity and mortality than cervical anastomotic leaks after transhiatial esophagectomy 18. However, recent reports have shown similar related morbidity rate due to a leak of a neck or intrathoracic anastomosis3 and also similar stricture, leak, mortality, or five year survival rates when comparing a hand sewn cervical versus a stapled intrathoracic anastomosis14. Unlike the leak rates reported with a hand-sewn technique during a cervical anastomosis, the intrathoracic anastomotic leak rate seem not to differ by type of anastomosis (hand sewn versus stapled) 8, 19. Factors such as body habitus, peripheral vascular disease, and neoadjuvant therapy may influence the esophagogastric anastomotic leak rate and have not been controlled for in many prior studies.
There are several treatment options for intrathoracic esophageal anastomotic leaks including surgical re-exploration and repair or consevative therapy including external drainage, total parenteral nutrition, and nasogastric decompression 20. In addition, temporary esophageal stents have been used more frequently to treat anastomotic leaks. Several studies have reported successful treatment of intrathoracic anastomotic leaks using temporary esophageal stents placed endoscopically 20-21. We had one patient with an anastomotic leak (2.7%) using the transoral circular stapled intrathoracic anastomosis that was successfully treated with re-operation, drainage, pleural flap, and endoscopic stenting.
Anastomotic strictures are another important technical complication of esophagogastric anastomoses. The stricture rate using different intrathoracic anastomotic techniques can be difficult to determine because there is no standardized reporting system for strictures. Therefore, the results of studies comparing the stricture rate between hand sewn and stapled intrathoracic esophagogastric anastomosis vary; there is no consistent trend favoring one technique over the other. In general, authors have reported a spectrum ranging from postoperative dysphagia (22% to 73%) to radiologically or endoscopically noted narrowings not needing intervention, to strictures necessitating multiple dilations (13% to 40%) when using a traditional circular stapling technique 22. We report a 13.8% stricture rate with two patients requiring three endoscopic dilatations with the last endoscopy showing a patent anastomosis. At the time of final endoscopy all patients were eating and drinking without difficulty.
There is concern that there may be an association between anvil size and the risk of stricture 23. However, two recent studies compared different anvil sizes (25, 29, and 33mm) and found no correlation between anvil size and dysphagia or stricture 14, 19. We use a 25 mm transoral anvil and have a low stricture rate. We suspect that stricture formation is multifactorial including patient characteristics and operative factors such as blood supply to the conduit and tension at the anastomosis.
Complications specific to the trans-oral passage of the OrVil™ 25 mm device have been reported and are rare13. They consist of premature dislodging of the anvil from the delivery tube necessitating manual or endoscopic removal of the anvil or hypopharyngeal or esophageal mucosal injuries. These complications can usually be prevented by gentle and appropriate handling during the trans-oral passage of the anvil.
To decrease morbidity, minimally invasive techniques have been applied to esophagectomies. Recently, several series have described the feasibility and safety of minimally invasive Ivor-Lewis Esophagectomy24. The extent of minimally invasive techniques has ranged from a laparoscopic abdominal component with a thoracotomy or mini-thoracotomy, to a thoracoscopic thoracic component and an open abdominal procedure. We used a minimally invasive abdominal component in the majority of our patients and a thoracoscopic technique in one-third of the patients. Long-term oncologic outcomes using minimally invasive techniques are still being investigated, but in our series, lymph node retrieval seems similar to retrieval when using standard open techniques. In addition, using a transoral anvil technique seems more efficient and may decrease operative time.
In summary, we report our experience with intrathoracic esophagogastric anastomoses using a transoral anvil during minimally invasive Ivor-Lewis Esophagectomy. We have found that it is a safe technique with preliminary results showing an anastomotic leak rate and stricture formation on the low end of reported ranges. Furthermore, the transoral anvil improves the technical feasibility of the intrathoracic esophagogastric anastomosis during completely minimally invasive esophagectomy.
Acknowledgments
This publication was supported by Grant Number KL2 RR024130 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research (GMC)
Footnotes
This paper was presented at the 23rd European Association for Cardio-Thoracic Surgery Annual Meeting, Vienna, Austria. October, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 2.Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, Mazumdar M. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248(2):221–226. doi: 10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]
- 3.Martin LW, Swisher SG, Hofstetter W, Correa AM, Mehran RJ, Rice DC, Vaporciyan AA, Walsh GL, Roth JA. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg. 2005;242(3):392–402. doi: 10.1097/01.sla.0000179645.17384.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santillan AA, Farma JM, Meredith KL, Shah NR, Kelley ST. Minimally invasive surgery for esophageal cancer. J Natl Compr Canc Netw. 2008;6(9):879–884. doi: 10.6004/jnccn.2008.0066. [DOI] [PubMed] [Google Scholar]
- 5.Craig SR, Walker WS, Cameron EW, Wightman AJ. A prospective randomized study comparing stapled with handsewn oesophagogastric anastomoses. J R Coll Surg Edinb. 1996;41(1):17–19. [PubMed] [Google Scholar]
- 6.Laterza E, de' Manzoni G, Veraldi GF, Guglielmi A, Tedesco P, Cordiano C. Manual compared with mechanical cervical oesophagogastric anastomosis: a randomised trial. Eur J Surg. 1999;165(11):1051–1054. doi: 10.1080/110241599750007883. [DOI] [PubMed] [Google Scholar]
- 7.Law S, Fok M, Chu KM, Wong J. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg. 1997;226(2):169–173. doi: 10.1097/00000658-199708000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luechakiettisak P, Kasetsunthorn S. Comparison of hand-sewn and stapled in esophagogastric anastomosis after esophageal cancer resection: a prospective randomized study. J Med Assoc Thai. 2008;91(5):681–685. [PubMed] [Google Scholar]
- 9.Okuyama M, Motoyama S, Suzuki H, Saito R, Maruyama K, Ogawa J. Handsewn cervical anastomosis versus stapled intrathoracic anastomosis after esophagectomy for middle or lower thoracic esophageal cancer: a prospective randomized controlled study. Surg Today. 2007;37(11):947–952. doi: 10.1007/s00595-007-3541-5. [DOI] [PubMed] [Google Scholar]
- 10.Valverde A, Hay JM, Fingerhut A, Elhadad A. Manual versus mechanical esophagogastric anastomosis after resection for carcinoma: a controlled trial. French Associations for Surgical Research Surgery. 1996;120(3):476–483. doi: 10.1016/s0039-6060(96)80066-3. [DOI] [PubMed] [Google Scholar]
- 11.Suturing or stapling in gastrointestinal surgery: a prospective randomized study. West of Scotland and Highland Anastomosis Study Group. Br J Surg. 1991;78(3):337–341. doi: 10.1002/bjs.1800780322. [DOI] [PubMed] [Google Scholar]
- 12.Urschel JD, Blewett CJ, Bennett WF, Miller JD, Young JE. Handsewn or stapled esophagogastric anastomoses after esophagectomy for cancer: meta-analysis of randomized controlled trials. Dis Esophagus. 2001;14(3-4):212–217. doi: 10.1046/j.1442-2050.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NT, Hinojosa MW, Smith BR, Chang KJ, Gray J, Hoyt D. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg. 2008;248(6):1081–1091. doi: 10.1097/SLA.0b013e31818b72b5. [DOI] [PubMed] [Google Scholar]
- 14.Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg. 2003;238(6):803–812. doi: 10.1097/01.sla.0000098624.04100.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarela AI, Tolan DJ, Harris K, Dexter SP, Sue-Ling HM. Anastomotic leakage after esophagectomy for cancer: a mortality-free experience. J Am Coll Surg. 2008;206(3):516–523. doi: 10.1016/j.jamcollsurg.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Sauvanet A, Mariette C, Thomas P, Lozac'h P, Segol P, Tiret E, Delpero JR, Collet D, Leborgne J, Pradere B, Bourgeon A, Triboulet JP. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201(2):253–262. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Viklund P, Lindblad M, Lu M, Ye W, Johansson J, Lagergren J. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg. 2006;243(2):204–211. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil PK, Patel SG, Mistry RC, Deshpande RK, Desai PB. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol. 1992;49(3):163–167. doi: 10.1002/jso.2930490307. [DOI] [PubMed] [Google Scholar]
- 19.Blackmon SH, Correa AM, Wynn B, Hofstetter WL, Martin LW, Mehran RJ, Rice DC, Swisher SG, Walsh GL, Roth JA, Vaporciyan AA. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg. 2007;83(5):1805–1813. doi: 10.1016/j.athoracsur.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hunerbein M. Treatment of oesophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Br J Surg. 2009;96(8):887–891. doi: 10.1002/bjs.6648. [DOI] [PubMed] [Google Scholar]
- 21.Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg. 2008;12(7):1168–1176. doi: 10.1007/s11605-008-0500-4. [DOI] [PubMed] [Google Scholar]
- 22.Williams VA, Watson TJ, Zhovtis S, Gellersen O, Raymond D, Jones C, Peters JH. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc. 2008;22(6):1470–1476. doi: 10.1007/s00464-007-9653-6. [DOI] [PubMed] [Google Scholar]
- 23.Wong J, Cheung H, Lui R, Fan YW, Smith A, Siu KF. Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery. 1987;101(4):408–415. [PubMed] [Google Scholar]
- 24.Maloney JD, Weigel TL. Minimally invasive esophagectomy for malignant and premalignant diseases of the esophagus. Surg Clin North Am. 2008;88(5):979–990. doi: 10.1016/j.suc.2008.05.016. [DOI] [PubMed] [Google Scholar]