Abstract
HLA class II allele DRB1*0401 is associated with predisposition to Rheumatoid Arthritis in humans as well as collagen-induced arthritis in mice. Predominantly females develop arthritis in humans and DR4 transgenic mice; however the mechanism of sex-bias is still unknown. We have investigated the molecular basis by which DR4 is associated with sex-bias of arthritis. Here we show that differential antigen-specific immune mechanisms in DR4 male and female mice lead to increased susceptibility in female mice. B cells are hyperactive and present DR-restricted peptides robustly in females compared to males. Antigen-specific response showed that females produced B cell modulating cytokines like IL-13 while males produced IFNγ. Male transgenic mice have higher number of T and B regulatory cells. An exogenous supply of 17β estradiol in male mice led to enhanced expression of DR4 and antigen-specific response to DR4-restricted peptides. On the other hand, castration increased the incidence of arthritis. We propose that sex-bias in arthritis involves B cells and presentation of antigen by HLA-DR4 leading to activation of autoreactive cells and autoantibodies production in females, while regulatory B cells in males protect them from pathogenesis. The transgenic mice expressing RA susceptible haplotype simulate human RA and may be valuable to study gender differences observed in patients.
Keywords: Rheumatoid Arthritis, Transgenic mice, HLA-DR4, Sex-bias, Antigen presentation
Introduction
Rheumatoid arthritis (RA) is a chronic disease mainly characterized by inflammation of the synovial joints leading to joint damage and disability. It occurs two to three times more often in women than in men with about 70% of patients being women. Sex-bias of disease and its remission during pregnancy suggest a role of hormones in the pathogenesis of arthritis, although data in humans and animal models of RA has been controversial (1). Some in vitro studies have suggested that gender differences in cytokine production may lead to differential immune responses in men and women (2, 3). Predisposition to develop rheumatoid arthritis is associated with inheritance of certain DR alleles that share sequences at 3rd hypervariable region with DRB1*0401, called ‘shared epitope’ (4). On the other hand, the motif I/D/E/A (DRB1*0402) at 67,70,71,74 is associated with non-susceptibility to RA. DR4 occurs in linkage with DQ7 (DQB1*0301) and DQ8 (DQB1*0302). The DRB1*0401/DQ8 haplotype has been associated with rheumatoid arthritis in some ethnic population while *0401/DQ7 is associated with severity in others (5, 6). Although the mechanisms by which HLA alleles affect degrees of RA susceptibility is not understood, the T cell selection by susceptible and resistant alleles in thymus has been suggested as a possible mechanism (7). Penetrance of the HLA shared-epitope genotype and onset of RA is influenced by sex, with RA being most penetrant in women with *0401 genotype (8) and the onset of arthritis being earlier in females than males (9).
Patients with arthritis produce autoantibodies like rheumatoid factor (RF) and anti cyclic citrullinated peptide antibodies (ACPAs) of synovial proteins including type II collagen (10-12). Immunization of certain strains of mice with type II collagen induces inflammatory polyarthritis, collagen-induced arthritis (CIA), with histopathological similarities to rheumatoid arthritis. A major difference between human RA and CIA in mice has been the lack of sex-bias and production of autoantibodies like RF and ACPAs in the latter. Previous studies with mice lacking endogenous class II molecules (Aβo) and expressing HLA-DQ8 (HLA-DQA1*0301/DQB1*0302) showed that they were highly susceptible to CIA (13). The DQ8-restricted development of arthritis could be modulated by the DR polymorphism similar to that observed in humans (14). Our recent studies with CIA in AEo mice (complete class II deletion) showed that *0401 and *0401/DQ8 mice developed arthritis and produced RFs and ACPAs (15, 16). On the other hand, *0402 and *0402/DQ8 mice were resistant to developing CIA (16). Further, a sex-bias was observed in CIA in *0401 mice with predominantly female mice developing arthritis leading us to hypothesize that DR4 renders sex-bias in arthritis. In this study, we analyzed the immunological basis for DR4-mediated sex-bias in susceptibility to develop arthritis. We show that DR-restricted antigen presentation via B cells regulates sex-bias of arthritis suggesting B cell depletion therapy in patients may work differently in both sexes.
Materials and Methods
Transgenic mice
All transgenic mice, DRB1*0401, DRB1*0402, DRB1*0401/DQ8, DRB1*0402/DQ8 and DQ8 were generated as described previously (15, 16). All mice are on B6 background and lacked all endogenous MHC class II chains, AE-/-. Mice of both sexes (8-12 weeks of age) used in this study were bred and maintained in the pathogen-free Immunogenetics Mouse Colony at the Mayo Clinic, Rochester, MN in accordance with the Animal Use and Care Committee. All the experiments were carried out with the approval of Institute’s Animal use and care committee. For convenience, DRB1*0402 and DRB1*0401 transgenic mice will be referred to as *0402 and *0401 respectively and double transgenic mice expressing both DR4 and DQ8 molecules as *0401/DQ8 and *0402/DQ8 in the manuscript.
Frozen PBMCs of RA patients and healthy individuals were used in accordance with the IRB protocol.
Flow cytometry
The expression of DR, DQ and H2E on PBLs of transgenic mice were analyzed by flow cytometry using mAbs: L227 (anti-DR), IVD12 (anti-DQ), SPLV3 (anti-DQ), 14-4-4s (anti-Eα), Y17 (anti-Eβ). Conjugated antibodies for CD1, CD3, CD4, CD5, CD8, CD19, CD25 and B220 (BD Pharmingen, CA) were also used. All cell surface markers were done with cells pooled from 2 mice/strain and experiments were repeated 2-3 times. Intracellular staining for FoxP3 was performed using antibodies obtained from eBioscience (San Diego, CA) as described elsewhere (16).
DR4 expression on cells from RA patients and healthy samples was analyzed by FACS after staining with biotinylated anti-DR4 antibody (One Lambda, CA) as primary and streptavidin conjugated secondary antibody. For sorting CD4 cells, cells were stained with OKT4 and FAC sorted (Beckton Dickinson).
Induction and evaluation of CIA
CIA was induced in transgenic mice and monitored for the onset and progression of arthritis as described previously. The arthritic severity of mice was evaluated as described previously with a grading system for each paw of 0-3 (16). The mean arthritic score was determined using arthritic animals only.
B cell and DC isolation
Splenic cells were harvested for isolation of B and dendritic cells from mice primed with CII or its derived peptides. CD4+ cells, Dendritic and B cells were isolated by labeling cells with micro beads coupled to CD4, CD11c and CD45R respectively. Cells were sorted by automated cells sorter (AutoMACS magnetic cell separator). Cells with 99% or more purity were utilized for experiments.
T cell proliferation Assay
Mice were immunized with 200 μg of CII emulsified 1:1 in CFA (Difco) intradermally at the base of the tail and one hind footpad. Ten days postimmunization, draining lymph nodes/ spleen were removed and cultured in vitro as described previously (15). Results are calculated as Δ cpm (mean cpm of triplicate cultures containing Ag- mean cpm of medium) or stimulation index (S.I.). Stimulation index of 2 or more was taken as positive response.
For presentation of antigens by APCs, isolated B cells and DCs were cultured in the presence or absence of antigen and sorted CD4+ cells from primed mice. 5×105 CD4+ cells were cultured in 1:1 ratio with B cells or DCs and CII-derived peptides, 254-273 and 284-303. B cells were irradiated for antigen presentation assays. Experiments were done 3 times with cells pooled from 2 mice /experiment.
For presentation of CII-derived peptide 254-273 by CD4 T cells of RA patients and healthy individuals, 5×105 sorted CD4 cells were cultured with irradiated APCs from DRB1*0401 mice in the presence or absence of CII-peptide 254-273. PBMCs were also cultured with the peptide. Proliferation was determined by counting tritium incorporation.
Peptides
All peptides were synthesized and purified at Mayo peptide core facility. Peptides used in this study are: CII-derived peptides 254-273, TGGKPGIAGFKGEQGPKGEP, 284-303-PAGEEGKRGARGEPGGVGPI, 304-323-GPPGERGAPGNRGFPGQDGL, and citrullinated CII-derived peptide, 304-323 GPPGEcit GAPGNRGFPGQDGL
BrdU Labeling
Mice were given 5-bromo-2-deoxyuridine (BrdU; Sigma, St. Louis, MO) in their drinking water at a concentration of 0.8 mg/ml until the day of experiment, 10-15 days. BrdU was dissolved in sterile water and was changed daily. BrdU incorporated into DNA was detected using BrdU flow kit using an APC anti-BrdU antibody (Becton Dickinson, San Jose, CA) as described in the package insert.
Cytokines
Cytokines were measured using the Bio-Plex protein array system with the mouse cytokine 23-plex panel as per manufacturer’s instructions and analyzed with Bio-Plex manager 2.0 software (Bio-Rad laboratories, Hercules, CA). Some cytokines were also tested by Capture ELISA using commercial kits (BD biosciences).
Real time PCR
Levels of TGF-β and IL-23 mRNA from in vitro cultures were analyzed using Real time PCR as described previously (16).
Castration
Males were anesthetized and incision was made on the scrotal sac. The testis was separated and cauterized distally at two points, once at the cauda epididymis and second from the spermatic blood vessel. The scrotal skin was closed with two stainless steel clips. Males were placed back into their cage on a warming table until ambulant. The animals were monitored daily for two weeks then the clips were removed. Castrated mice were rested for one more week before use for in vitro and in vivo experiments to stabilize hormones and any responses due to stress of surgery.
Exogenous supply of Estradiol 17
17β estradiol pellets (Innovative research of America, Fl) which release hormone slowly for up to 90 days (2.5mg/pellet) were implanted under the skin between shoulder blades by using Trochar gauge after anesthetizing mice with 2.5% avertin. For in vitro experiments, mice were used 21 days post implantation of 17β estradiol pellets to stabilize hormones and any response to implantation.
Statistical analysis
The difference in the incidence of arthritis between groups was analyzed using the Fisher’s exact test. One-sided P value of less than 0.05 was considered significant. Comparison of cytokine levels, T cell proliferation, expression of transgene and onset of arthritis between male and female were done using 2 tailed Student’s t test.
Results
Presence of DR4 skews arthritis towards sex-bias
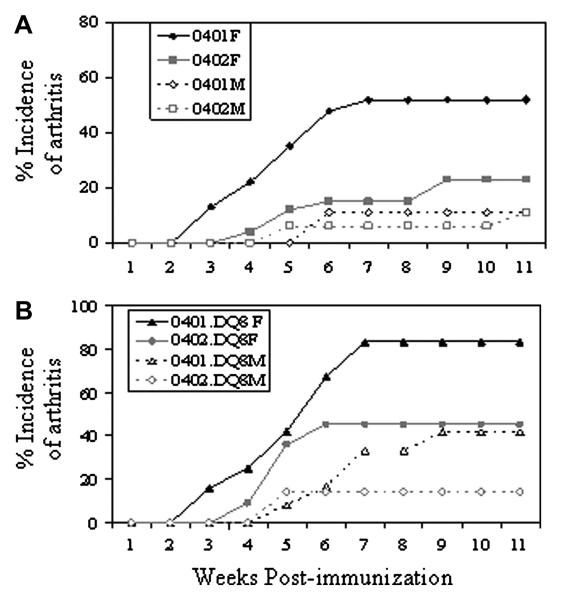
Based on our previous studies in *0401 and DQ8 mice (15), we hypothesized that DR4 has a role in sex-bias in arthritis and tested it in double transgenic mice expressing both DRB1*0401 and DQ8 molecules. *0401/DQ8 female mice developed CIA with significantly higher incidence compared to males (83% and 41% respectively, P=0.04), with a female to male a ratio of 2:1 (Figure 1, Table 1), similar to that observed in human RA patients.
Figure 1.
Collagen-induced arthritis in HLA-DRB1*0401 and DRB1*0402 and double transgenic *0401/DQ8 and *0402/DQ8 mice shows that predominantly female mice develop arthritis. A) Incidence of arthritis in *0401 and *0402 male and female mice following immunization with type II collagen. B) Incidence of arthritis in *0401/DQ8 and *0402/DQ8 male and female mice shows that *0402 protects mice from developing arthritis. Number of mice used in each group and significance of difference in disease incidence is given in Table 1.
Table1.
Collagen-induced arthritis in male and female transgenic mice.
| Mice | Incidence (%) |
Onset | Severity |
|---|---|---|---|
| 0401/DQ8 | |||
| M | 5/12 (41) | 7±1 | 6.3±1.5 |
| F | 10/12 (83) | 5.2±1.5 | 6.4±1.3 |
| Total | 15/24(63) | 5.5±1.6 | 6.4±1.2 |
| 0402/DQ8 | |||
| M | 2/14 (14) | 5±0 | 7±1.4 |
| F | 5/11(45) | 5±0.7 | 6.8±1.8 |
| Total | 7/25 (28) | 5±0.5 | 6.8±1.6 |
| 0402 | |||
| M | 2/17 (12) | 8±4.2 | 3.5±3.5 |
| F | 6/26 (23) | 6.3±2.1 | 4.8±2.9 |
| Total | 8/43 (19) | 6.7±2.5 | 4.5±2.9 |
| DQ8 | |||
| M | 6/9(67) | 6.2±2.1 | 6.2±3.5 |
| F | 8/11 (73), | 5.6±2.6 | 7.2±1.8 |
| Total | 14/20 (70) | 5.8±2.3 | 6.7±2.5 |
*0401/DQ8 Female vs Male, p=0.04
*0402.DQ8 vs DQ8, Male, p=.02
*0401/DQ8 vs *0402/DQ8, p=0.02
*0402/DQ8 vs DQ8, p=0.005
We have shown that DRB1*0402 is associated with protection from arthritis in transgenic mice similar to humans (16, Figure 1). Here we determined if protection provided by *0402 is biased in both sexes. There was a significant decrease in incidence of CIA in *0402/DQ8 mice compared to DQ8 mice (p=0.005) confirming a protective role of *0402. Interestingly, even though incidence of arthritis was very low in *0402/DQ8 mice (28%), they still showed a gender-bias with predominantly female mice developing arthritis, at F: M ratio of 3:1.
Female mice show higher antigen specific response than males
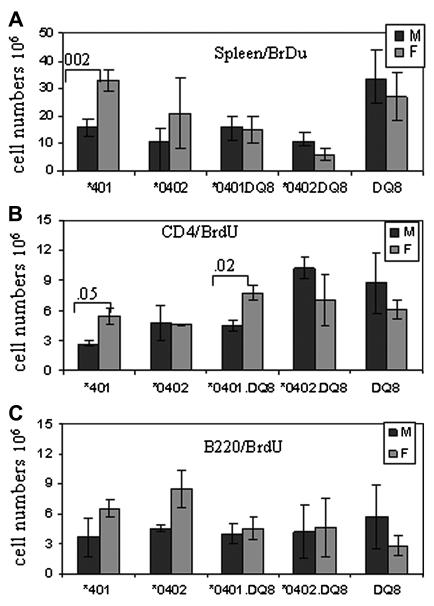
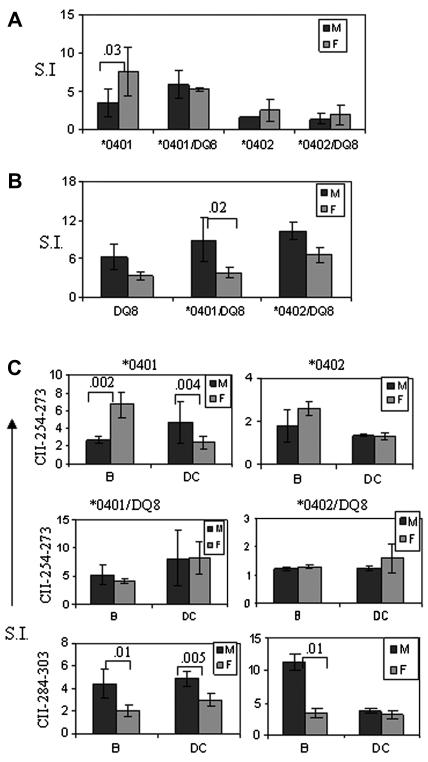
As females developed CIA more often, we determined in vivo antigen-specific activation in both sexes by BrdU staining. In vivo response reflected a higher CII-reactive T and B cell response in *0401 female mice compared to males as observed by BrdU staining in CII primed mice (Figure 2A-C). T cell repertoire in both sexes showed a higher proliferation of CD4+ Vβ8+ cells in females as analyzed by FACS 26 vs 29, p<0.05 (data not shown). We further determined if decreased number of activated cells in males is due to cell death by staining BrdU positive cells with apoptosis markers. Males did show a non-significant increase in cell death (10% and 7% CD4 cells in males and females respectively) in proliferating cells (data not shown).
Figure 2.
BrdU staining in vivo in CII-primed mice showed a higher cell proliferation in female *0401 mice compared to male mice in A) spleen cells, B) CD4 T cells and C) B cells. S.I - stimulation index. N=3-5 in each experiment.
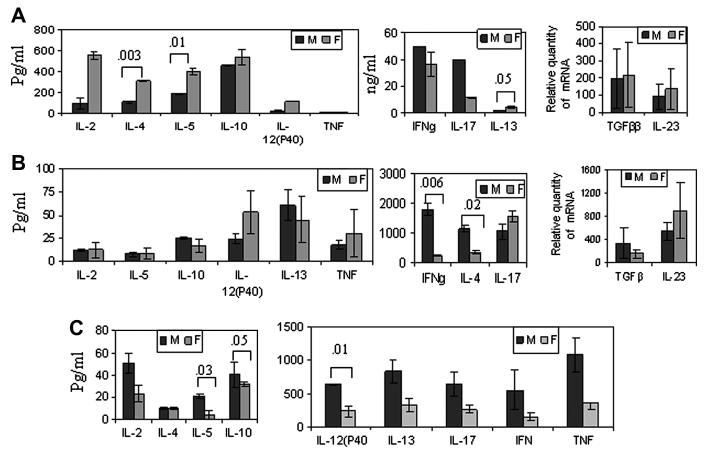
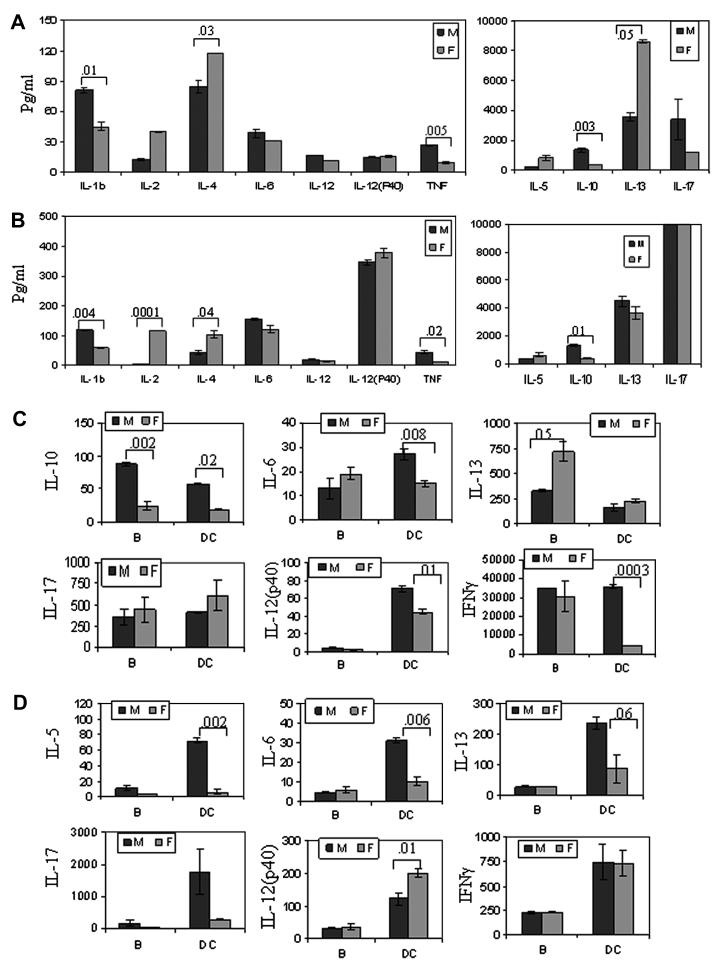
Both sexes of *0401 mice produced a differential CII-specific cytokine profile (Figure 4). *0401 male mice produced higher amounts of IFNγ and IL-17 cytokines while female mice produced higher amounts of IL-4, IL-13 and IL-12 (P40) and IL-23 (Figure 3A). In contrast, DQ8 males produced increased levels of all cytokines compared to female mice with significant differences for IL-10 and IL-12 (p40) (Figure 3C). Most of the cytokines produced in *0401/DQ8 mice were similar in both sexes except IFNγ and IL-4, which were produced at significantly higher levels by males (Figure 3B). DQ8 mice produced higher levels of IL-12(p40) and TNF compared to *0401 and *0401/DQ8 mice suggesting DR4 allele can modulate DQ-restricted response and it is sex-specific. This data clearly showed that DQ-restricted presentation in males and DR-restricted presentation in females lead to activation of different pathways resulting in a different cytokine milieu. Males generally produced a Th0 profile while females had higher levels of immunomodulating cytokines.
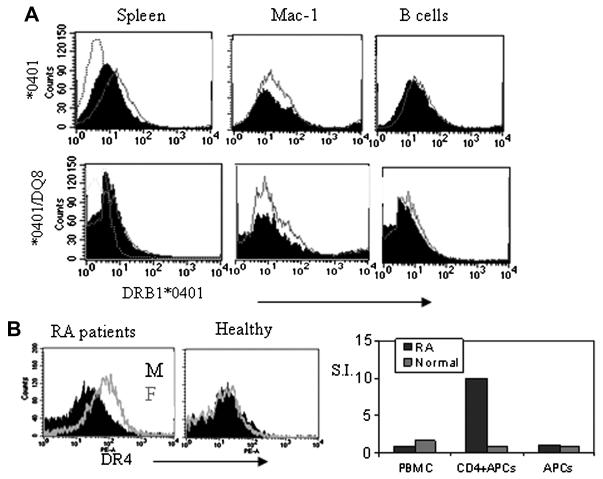
Figure 4.
Expression of HLA-DR4 is higher in females than males in humans and transgenic mice. A) Expression of DR4 in male (black) and female (Grey line) *0401 and *0401/DQ8 transgenic mice. B) A representative FACs analysis of expression of DR4 in female and male RA patients (N=4) and healthy individuals (N=2). Sorted CD4 cells from DR4+ RA patient (female) responded to CII-derived DR4 restricted peptide 254-273 while those from normal healthy DR4 positive (female) individual did not respond.
Figure 3.
In vitro cytokine production in male and female A) *0401, B) *0401/DQ8 and C) DQ8 mice. DRB1*0401 female mice produced significantly higher amounts of B cell modulating cytokines while DQ8 males produced elevated levels of all cytokines. All cytokines were measured from culture supernatants from an in vitro recall challenge with CII of the splenocytes isolated from CII-primed mice. Expression of TGFβ and IL-23 were quantified by real-time PCR, expression of GADPH was used as an internal control. The expression of cytokines in LNCs stimulated with CII relative to that in medium control was calculated by the ΔΔCt method. Data are presented as means ± SD of at least three different mice. The detection limit for all cytokines was 1-2 pg./ml.
Sex-specific presentation of DR and DQ-restricted collagen-derived peptides
As DR4 female mice have increased incidence of arthritis and generate a robust response to CII compared to males, we analyzed the expression of DR and DQ transgenes to determine if it is varied between the sexes. We have previously shown that DR4 expression is observed with a higher mean flow intensity (MFI) in female mice than male *0401 mice (15). *0401 and *0401/DQ8 mice showed similar findings in splenic cells and APCs although the difference in MFI was not significant between the sexes (Figure 4A). However, this phenomenon was unique to DR4 as MFI of DR3 in DR3.AEo mice did not show any difference between sexes (data not shown). Further, we compared DR expression in humans using frozen PBMCs from DR4 positive RA patients and healthy individuals (Figure 4B). Among patients, women expressed higher levels of DR4 compared to men (N=4), although healthy PBMCs (N=2) did not show any difference in expression levels. We tested if presentation of DR4-restricted CII-254-273 peptide is critical in RA patients by comparing response to the peptide in a patient and healthy control (both females) (Figure 4B). CD4+ T cells of only RA patient generated a response to this peptide suggesting DR4-restricted response is critical for pathogenesis. Our in vivo observations along with the DR4 expression results led us to hypothesize that immune response to DR-restricted peptides may be sex-specific. We tested this hypothesis by measuring T cell response to DR4 and DQ8 restricted CII peptides in transgenic mice. In *0401 mice, females mounted a higher in vitro T cell response to CII-peptide 254-273 than male mice did (Figure 5A). Interestingly, male mice mounted a significantly higher response to DQ8-restricted CII-peptide 284-303 compared to females in all strains, DQ8, *0401/DQ8 and *0402/DQ8 mice (Figure 5B). However, T cell response to CII-peptide 254-273 was similar in both sexes in *0401/DQ8 mice, that may be due to epistatic interaction between DR and DQ molecules. Even though *0401/DQ8 mice showed similar T cell response in both sexes, female mice developed arthritis with increased incidence than males suggesting a differential cytokine profile by activated cells and other factors like regulatory cells may lead to a difference in CIA incidence.
Figure 5.
Female mice present DR4-restricted CII peptide robustly while male mice present DQ8-restricted peptides efficiently. In vitro T cell response to A) DR4-restricted CII-derived peptide 254-273 in transgenic mice and B) DQ8-restricted CII peptide 284-303 in male and female transgenic mice. C) DR4 and DQ8-restricted antigen presentation by B cells and dendritic cells differs in both sexes. B cells and DCs were isolated from spleens of primed mice. B cells were irradiated for antigen presentation experiments. In vitro T cell response to DR4-restricted CII-peptide 254-273 was significantly higher in females compared to males in *0401 mice when presented by B cells while presentation by DCs showed a robust response by males. In *0401/DQ8 mice, antigen presentation by B cells and DCs was similar in both sexes. DRB1*0402/DQ8 mice did not respond to DR4-restricted peptide. DQ8-restricted CII-derived peptide 284-303 was presented robustly in male mice by both B cells and DCs in *0401/DQ8 mice. CIA resistant *0402/DQ8 mice showed a statistical significant difference between male and female mice with B cells as APCs. Numbers in the histograms show significant p value for the comparison between male and female mice. P value <0.05 was considered significant. S.I.-stimulation index, N=4-6 for each experiment.
B cells and DCs present peptides differentially in both sexes
Since we observed a differential presentation of DR and DQ-restricted CII peptides in both sexes, we further investigated if antigen presentation by various APCs also differed between sexes. In *0401 mice, B cells in females and DCs in males present CII-derived peptide 254-273 efficiently (p<0.002 and p<0.004 respectively) (Figure 5 C). Antigen presentation via B cells and DCs results in activation of different pathways as reflected in cytokine profile produced by *0401 mice (Figure 6A, 6B). Females produced B cell modulating cytokines like IL-4 and IL-13 in significantly higher amounts while males showed higher production of the Th1/Th2 cytokines IL-1b, TNF-α and IL-10 (Figure 6A, data not shown). Both sexes produced high IFNγ levels (not shown). Though DCs presented peptide 254-273 less efficiently than B cells in females, high IL-2 levels suggest they do activate T cells (Figure 6B).
Figure 6.
Cytokines produced (pg/ml) in response to CII-derived peptides by B cells and DCs show a different profile in male and female transgenic mice. Cytokine produced in response to DR4-restricted CII-derived peptide 254-273 in vitro by A) B cells and B) DCs in *0401 mice. Both sexes produced IFNγ above detection limits when presented by B cells and DCs (not shown). Cytokines produced in *0401/DQ8 mice in response to presentation of C) DR4-restricted CII peptide 254-273 and D) DQ8-restricted CII peptide 284-303 by B cells and DCs.
In *0401/DQ8 mice, DR4-restricted peptide was presented similarly in both sexes, though cytokine profile showed high IL-10 production by males and IL-13 by females when presented via B cells. In contrast, presentation by DCs lead to significantly higher amounts of IL-10 and Th1 cytokines in males compared to females (Figure 6C, data not shown). However, DQ8-restricted CII-peptide 284-303 was presented more efficiently by both APCs in males compared to female mice (Figure 5C). When presented by DCs, it lead to higher production of IL-17 and immuno-modulating cytokine IL-13 in males while females produced significantly higher amounts of IL-12(p40) and IL-6 (Figure 6D). These data suggest a sex-specific presentation of and immune response to DR and DQ-restricted peptides by B cells and DCs.
Females have lower number of regulatory cells
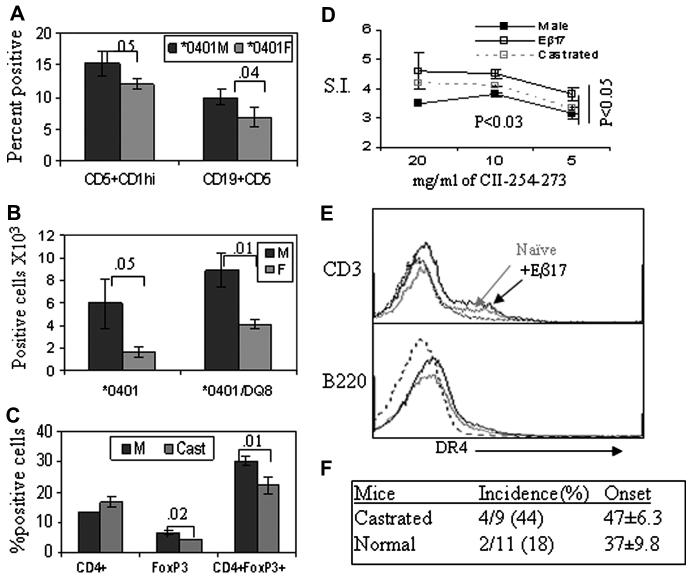
Since *0401 male mice produced much higher levels of IL-10 in response to antigen presentation by B cells, we determined presence of B regulatory cells in mice. B cells expressing CD5+CD1hi have been suggested to behave as regulatory cells and produce IL-10. *0401 male mice had higher percent of B regulatory cells compared to females (Figure 7A). Recently, a contribution of CD4+CD25+ T regulatory cells has been suggested in gender-differences in autoimmunity (17). We determined if similar phenomenon was responsible for protection from arthritis in male mice. *0401 and *0401/DQ8 male mice have higher numbers of T regulatory cells compared to females suggesting hormones may be involved in peripheral regulation of these cells (Figure 7B). To determine if regulatory cell numbers can be modulated by hormones in periphery, we analyzed FoxP3+ and CD4+FoxP3+ cells in castrated males. The total number of FoxP3+ and CD4+FoXP3+ cells decreased after castration compared to normal males (Figure 7C) suggesting hormones influence T regulatory cells.
Figure 7.
Estrogen modulates antigen presentation, regulatory cells and collagen-induced arthritis. A) B regulatory cells, CD19+, CD5+, CD1hi, in male and female *0401 transgenic mice. CD5+CDhi cells were enumerated after gating on CD19+ cells from spleen. B) A comparison of CD4+CD25+FoxP3 T regulatory cells in naïve male and female transgenic mice, n=5 each group. C) CD4 and regulatory T cells, CD4+FoxP3+, in *0401 castrated and control mice. Numbers denote p value for comparison between male and female mice. D) In vitro T cell response in male *0401 mice with or without exogenous 17Eβ estradiol tablets and castrated mice primed with CII-derived peptide 254-273 shows that mice with exogenous supply of estradiol presented the peptide much more efficiently than controls. Castrated mice also showed a much higher proliferation compared to control mice. N=3 each group, p<0.03 at 10mg/ml and p<0.05 at 5 mg/ml CII peptide 254-273 for comparison between control and treated or castrated mice. E) Expression of DR molecule in *0401 male mice with or without estradiol showed higher expression in the latter (black line) compared to control mice without estradiol (gray line). Dotted line is isotype control. The graph represents 2 of the 5 mice tested. F) Collagen-induced arthritis in castrated and normal *0401 male mice showed an increase in incidence of arthritis in castrated mice compared to controls.
Role of hormones in DR-restricted response
To understand the role of female hormone estrogen, especially in context of presentation of DR-restricted peptides, we implanted slow release estradiol tablets in male *0401 mice and tested for presentation of CII 254-273 peptide (Figure 7D). There was a significant higher antigen-specific response in Estradiol implanted versus untreated mice and castrated mice. Estradiol pellets led to an increase in the expression of DR compared to non-treated mice, in particular in B cells and CD3+ cells (Figure 7E), although it was variable from mouse to mouse. To test the role of male hormones, arthritis was induced in *0401 castrated mice( Figure 7F). . The incidence of arthritis doubled in castrated mice compared to those that were not castrated, 44% and 18% respectively, suggesting androgen might be protective
Discussion
Autoimmune diseases including rheumatoid arthritis occur more often in women than men, however the antecedent/ cause remains unknown. While many models replicate histopathology, there was no good animal model to study the mechanism of sex-bias in arthritis. Based on the present data and our previous observations (15), we hypothesized a role of DR4 in sex bias of arthritis. We have studied the immunological basis for DR4-mediated sex-specific pathogenesis. Our data show that *0401 females mount a more robust response to CII and DR4-restricted CII peptide than males. On the other hand, *0401/DQ8 mice generate similar responses in both sexes suggesting that DR modulates DQ-restricted response. However, a sex-specific CII-specific cytokine profile in *0401/DQ8 mice suggests that different subsets of T cells are being activated. Interestingly, males generate a more robust response to DQ8-restricted peptide than female mice. However, not all DQ8-restricted peptides are presented in sex-biased manner, 2 of the 3 DQ8-restricted CII-derived peptides tested were presented much more efficiently in males (not shown). We chose 1 of the 2 CII-peptides, 284-303, to further study presentation by B cells and DCs in both sexes. In females, B cells present DR-restricted peptides much more efficiently than DQ-restricted peptides while in males DCs present both DR and DQ-restricted peptides efficiently. Our data suggest that immune response to CII results in a sex-specific cytokine milieu. The role of various cytokines in pathogenesis of arthritis has been documented (18). In humans, cytokines produced in response to non-specific activation is sex-specific, with males producing higher levels of Th1/Th2 cytokines compared to females (3). Present observations confirm this finding in male *0401 mice. In male mice, presentation of peptides via DCs lead to production of Th1, IL-17 and IL-10 while females produced IL-2 and Th17 cytokines. The role of IL-17 in arthritis is controversial with some studies in favor while others have shown that IL-17 in concert with Th1 may be involved in protection (19, 20). Indeed, human data regarding the role of IL-17 in arthritis is not clear (18). The present data support the findings in RA patients and other disease model where significant proportion of IL-17 producing cells also produces IFNγ (21, 22).
The data in this study showed that the APC and the restricting MHC molecule contributed to the type of immune response in both sexes. In response to DR-restricted presentation by B cells, *0401 females produced IL-4 and IL-13 while males produced IL-10. Both IL-4 and IL-13 increase the expression of MHC antigens and stimulate proliferation of and antibody production by B cells, while IL-10 decreases expression of these antigens on activated monocytes (23-25). A gender variation in IL-13 production has been shown in experimental model for multiple sclerosis (26). Although not directly shown, IL-13 may modulate immune response by upregulation of class II expression and higher antibody production in female mice as observed here. Our findings are supported by high levels of IL-13 in RA patients than healthy individuals and its correlation with CCP positivity (27). In *0401 males, regulatory B cells, CD5+CD1hi, may be involved in IL-10 production and suppressing inflammation by inhibiting presentation. This data is supported by previous observations with regulatory B cells (28).
The present data together with previous observations (15, 29) suggest that B cells are critical as APCs for arthritis development in addition to antibody production. Depletion of B cells in humans has shown varying degree of efficacy in treating autoimmune diseases including RA (30). There is no available statistical data to determine if the effectiveness of B cell depletion differs between sexes. Our observations suggest that outcome of B cell depletion for RA patients may differ between sexes. Anti-CD20 treatment may be more effective in males compared to females due to the presence of higher number of B regulatory cell. However, it may need to be initiated early in the course of autoimmune disease before the long-lived plasma pool is established. Recently, a study with small numbers of RA patients on anti-CD20 treatment showed that 65% of female patients responded to the treatment compared to 100% of male patients (31).
Estrogen has been shown to be involved in modulating B cell responses by increasing their survival (32). We observed an increase in B220+ cells in male mice implanted with 17Eβ which might be due to increased survival of B cells. Male RA patients have higher levels of estradiol compared with healthy controls (33). Estrogen has also been shown to modulate antigen presentation and production of proinflammatory cytokines (34, 35). Data in this study support a role of estrogen in enhancing immune response as observed in *0401 male mice implanted with estradiol pellets. An increase in disease incidence in castrated male suggests an imbalance in estrogen/ testosterone levels or a protective role of male hormones.
Recent investigations have shown that ACPAs are associated with DRB1 shared epitope and precede the onset of RA, making citrullinated proteins strong candidates for driving the autoimmune response in this disease (36,37). Thus, posttranslational conversion of peptidyl arginine to peptidyl citrulline by peptidylarginine deiminase enzymes (PAD) is an essential feature of many autoantigens in RA. Estrogen modulates expression of and can increase production of PAD enzymes (38). Since the immunodominant DR4-restricted CII peptide does not have any arginine, we tested native and citrullinated CII-derived peptides that are non-responders in native form. Native peptide primed mice generated a recall response in vitro with citrullinated peptide (data not shown) delineating how DR4 can contribute to sex-bias in this model.
From the present data and available literature, one can speculate that the increased expression of PAD enzyme could cause an increase in citrullination of peptides, not only in the uterus, but other tissues including synovial tissue resulting in the citrullination of multiple synovial specific autoantigens. Presentation of modified antigens by DRB1*0401 molecule can lead to activation of CD4 T and B cells and increased expression of class II molecules on activated T cells. Estrogen can increase B cell survival leading to production of autoantibodies and differential antigen presentation by DR molecule resulting in a pro-inflammatory cytokine milieu. We hypothesize that differences in posttranslational modification of arginine in both sexes may dictate the final antigen-specific immune response. It can be speculated that in males there is less citrullination of the arginine residue due to lower levels of PAD enzymes making them available for methylation. Arginine methylation of Stat1 by protein arginine methyltransferases (PRMTs) is required for transcriptional activity (39). We did observe higher IFNγ production in males compared to females in this model. Studies to prove the above hypothesis using these transgenic mice are under way and may explain increased autoimmunity in females.
Acknowledgement
We thank Julie Hanson and her staff in the Mayo Immunogenetic mouse colony for breeding and care of the mice. We thank Michele Smart for tissue typing of transgenic mice.
We are indebted to Drs C. Benoist and D. Mathis for the class II-deficient (MHCIIΔ/Δ) mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cuotolo M. Sex and rheumatoid arthritis: mouse model versus human disease. Arthritis Rheum. 2007;56:1–3. doi: 10.1002/art.22322. [DOI] [PubMed] [Google Scholar]
- 2.Bouman A, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 2004;52:19–26. doi: 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 3.Giron-Gonzalez JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 4.Winchester R, Dawyer E, Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheum. Dis. Clin. North. Am. 1992;18:761–83. [PubMed] [Google Scholar]
- 5.Taneja V, Mehra NK, Chandershekaran AN, Ahuja RK, Singh YN, Malaviya AN. HLA-DR4-DQw8 but not DR4-DQw7 haplotypes occur in Indian patients with rheumatoid arthritis. Rheumatol. Int. 1992;11:251–55. doi: 10.1007/BF00301502. [DOI] [PubMed] [Google Scholar]
- 6.Stephens HA, Sakkas LI, Vaughan RW, Teitsson I, Welsh KI, Panayi GS. HLA-DQw7 is a disease severity marker in patients with rheumatoid arthritis. Immunogenetics. 1989;30:119–22. doi: 10.1007/BF02421540. [DOI] [PubMed] [Google Scholar]
- 7.Moller E, Bohme J, Valugerdi MA, Ridderstad A, Olerup O. Speculations on the mechanism of HLA association with autoimmune diseases and the specificity of autoreactive T lymphocytes. Immuno. Rev. 1990;118:5–19. doi: 10.1111/j.1600-065x.1990.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JM, Han J, Singh R, Moxley G. Sex influences on the penetrance of HLA shared-epitope genotypes for rheumatoid arthritis. Am. J. Hum. Genet. 1996;58:371–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Jawaheer D, Lum RF, Gregersen PK, Criswell LA. Influence of male sex on disease phenotype in familial rheumatoid arthritis. Arthritis Rheum. 2006;54:3087–94. doi: 10.1002/art.22120. [DOI] [PubMed] [Google Scholar]
- 10.He X, Kang AH, Stuart JM. Accumulation of T cells reactive to type II collagen in synovial fluid of patients with rheumatoid arthritis. J. Rheumatol. 2000;27:589–593. [PubMed] [Google Scholar]
- 11.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, Saigo SK, Morinobu A, Koshiba M, Kuntz KM, Kamae I, Kumagai S. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. ANN. Intern. Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 12.Huseyin U, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engström A, Serre G, Burkhardt H, Thunnissen MM, Holmdahl R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra HS, David CS. CD4 and CD8 T Cells in Susceptibility/Protection to Collagen-Induced Arthritis in HLA-DQ8 Transgenic Mice: Implications for Rheumatoid Arthritis. J. Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 14.Taneja V, Griffiths MM, Luthra HS, David CS. Modulation of HLA-DQ-restricted collagen-induced arthritis by HLA-DRB1 polymorphism. Int. Immunol. 1998;10:1449–1457. doi: 10.1093/intimm/10.10.1449. [DOI] [PubMed] [Google Scholar]
- 15.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum. 2007;56:69–78. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 16.Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra HS, David CS. Delineating the role of HLA-DR4 shared epitope in susceptibility versus resistance to develop arthritis. J. Immunol. 2008;181:2869–77. doi: 10.4049/jimmunol.181.4.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 2005;175:5591–95. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 18.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 20.Irmler IM, Gajda M, Bräuer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J. Immunol. 2007;179:6228–36. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 21.Shen H, Goodall JC, Gaston JS Hill. Frequency and phenotype of the peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid factor. Arthritis Rheum. 2009;60:1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 22.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J. Neuroimmunol. 2007;183:96–110. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 23.de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, deVries JE. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 24.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 25.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, Ferrara P. Interleukin 13 is a B cell stimulating factor. J. Exp. Med. 1994;179:135–43. doi: 10.1084/jem.179.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha S, Kaler LJ, Proctor TM, Teuscher C, Vandenbark AA, Offner H. IL-13-mediated gender difference in susceptibility to autoimmune encephalomyelitis. J. Immunol. 2008;180:2679–85. doi: 10.4049/jimmunol.180.4.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hitchon CA, Alex P, Erdile LB, Frank MB, Dozmorov I, Tang Y, Wong K, Centola M, El-Gabalawy HS. A distinct multicytokine profile is associated with anti-cyclical citrullinated peptide antibodies in patients with early untreated inflammatory arthritis. J. Rheumatol. 2004;31:2336–46. [PubMed] [Google Scholar]
- 28.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Taneja V, Krco CJ, Behrens M, Luthra HS, Griffiths MM, David CS. B cells are important as antigen presenting cells for induction of MHC-restricted arthritis in transgenic mice. Mol. Immunol. 2007;44:2988–96. doi: 10.1016/j.molimm.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 31.Roll P, Dorner T, Tony HP. Anti-CD20 therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:1566–1575. doi: 10.1002/art.23473. [DOI] [PubMed] [Google Scholar]
- 32.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell. J. Clin. Invest. 2002;109:1625–33. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tengstrand B, Carlström K, Felländer-Tsai L, Hafström I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J. Rheumatol. 2003;30:2338–43. [PubMed] [Google Scholar]
- 34.Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538–47. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 35.Whitacre CC. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 36.Kinloch A, Lundberg K, Venables P. The pathogenic role of antibodies to citrullinated proteins in rheumatoid arthritis. Exp. Rev. Clin. Immunol. 2006;2:365–75. doi: 10.1586/1744666X.2.3.365. [DOI] [PubMed] [Google Scholar]
- 37.Van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–21. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 38.Senshu T, Akiyama K, Nagata S, Watanabe K, Hikichi K. Peptidylarginine deiminase in rat pituitary: sex difference, estrous cycle-related changes, and estrogen dependence. Endocrinology. 1989;124:2666–70. doi: 10.1210/endo-124-6-2666. [DOI] [PubMed] [Google Scholar]
- 39.Mowen MA, Schurter BT, Fathman JW, David M, Glincher LH. Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes 2004. Mol. Cell. 15:559–71. doi: 10.1016/j.molcel.2004.06.042. [DOI] [PubMed] [Google Scholar]