Abstract
Cytosolic phospholipase A2α (cPLA2α, Group IVA phospholipase A2) is a central mediator of arachidonate release from cellular phospholipids for the biosynthesis of eicosanoids. cPLA2α translocates to intracellular membranes including the Golgi in response to a rise in intracellular calcium level. The enzyme’s calcium-dependent phospholipid-binding C2 domain provides the targeting specificity for cPLA2α translocation to the Golgi. However, other features of cPLA2α regulation are incompletely understood such as the role of phosphorylation of serine residues in the catalytic domain and the function of basic residues in the cPLA2α C2 and catalytic domains that are proposed to interact with anionic phospholipids in the membrane to which cPLA2α is targeted. Increasing evidence strongly suggests that cPLA2α plays a role in regulating Golgi structure, tubule formation and intra-Golgi transport. For example, recent data suggests that cPLA2α regulates the transport of tight junction and adherens junction proteins through the Golgi to cell-cell contacts in confluent endothelial cells. However, there are now examples where data based on knockdown using siRNA or pharmacological inhibition of enzymatic activity of cPLA2α affects fundamental cellular processes yet these phenotypes are not observed in cells from cPLA2α deficient mice. These results suggest that in some cases there may be compensation for the lack of cPLA2α. Thus, there is continued need for studies employing highly specific cPLA2α antagonists in addition to genetic deletion of cPLA2α in mice.
Keywords: cytosolic phospholipase A2, Golgi, arachidonic acid, calcium, trafficking
1. Introduction
Since the discovery of a high molecular weight, arachidonic acid preferring cytosolic phospholipase A2 (cPLA2), now classified as Group IVA cPLA2 (cPLA2α), there has been a great deal of progress in elucidating its biochemical properties, regulation and function [1–8]. The interest in cPLA2α stems from its role as the first regulatory enzyme in the pathway for the production of oxygenated metabolites of arachidonic acid [9].
Among the 20 enzymes classified as PLA2s in mammals, cPLA2α is the only member that exhibits preference for the sn-2 arachidonyl group [6, 10–13]. cPLA2α is expressed in many cell types and is highly regulated in order to control the cellular levels of free arachidonic acid. The function of cPLA2α in mediating the regulated release of arachidonic acid for the production of eicosanoids is well established [14–18]. These potent lipid mediators have diverse functions in regulating normal processes and contributing to disease pathogenesis [9]. In cells involved in promoting acute inflammation in response to tissue injury such as neutrophils, macrophages and mast cells, cPLA2α activation results in the production of numerous lipid mediators including leukotrienes, prostanoids and platelet-activating factor [14–18]. These mediators have diverse functions in promoting inflammation and contributing to its resolution. The coordinated action of secreted lipid mediators is regulated in a cell type specific manner by the differential expression of specific G-protein coupled receptors that initiate diverse signal transduction pathways and biological responses [19–21].
In addition to its role in initiating eicosanoid production, there is data indicating that cPLA2α plays a role in regulating the structure and trafficking through the Golgi apparatus. Evidence is emerging that phospholipases and acyltransferases regulate trafficking and membrane tubulation by modification of the local lipid composition that affects membrane curvature [22–27]. This review will highlight new information about the localization and functional role of cPLA2α at the Golgi.
2. Calcium-dependent translocation of cPLA2α to the Golgi
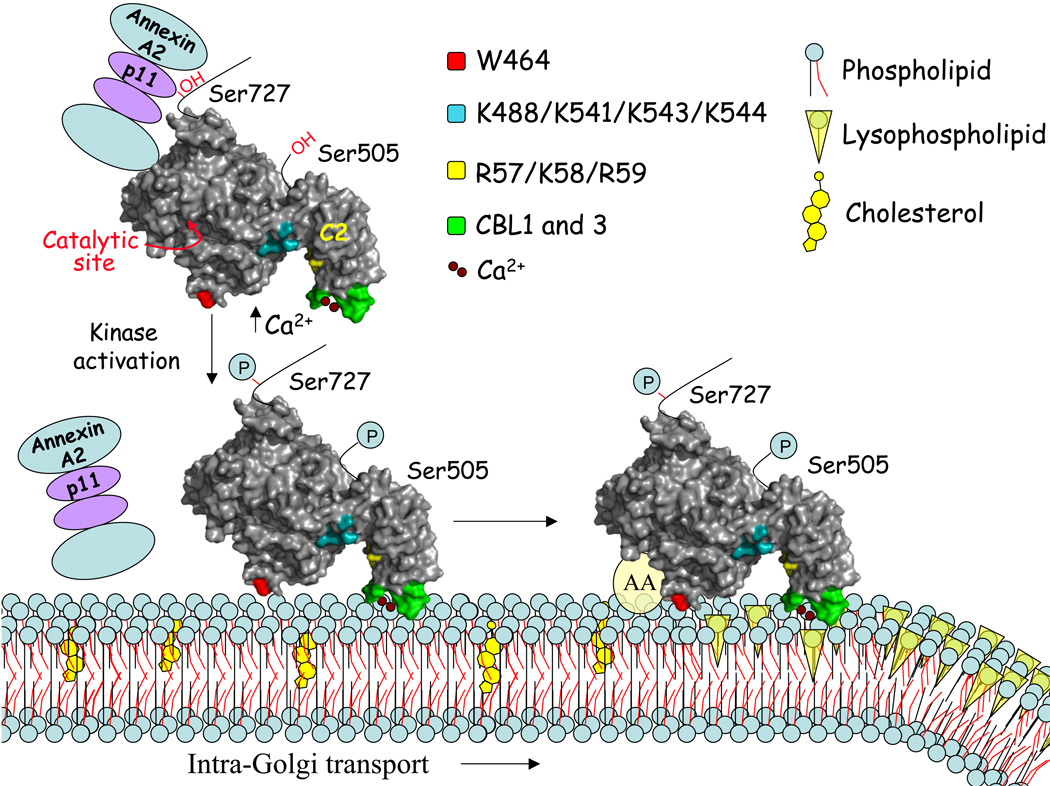
cPLA2α is regulated by post-translational mechanisms such as increases in levels of intracellular calcium [Ca2+]i, phosphorylation and interaction of basic residues with anionic membrane components (Fig. 1) [4, 5]. Agonists that increase [Ca2+]i stimulate cPLA2α-dependent release of arachidonic acid by promoting translocation of cPLA2α from the cytosol to membrane [28–32]. This is a critical regulatory step necessary for cPLA2α to access phospholipid substrate. The cPLA2α C2 domain has a high affinity for calcium, which binds to anionic residues in the calcium binding loops (CBLs) [33–36]. Calcium binding reduces the negative electrostatic potential of the surface exposed CBLs allowing the surrounding hydrophobic residues in CBL1 and CBL3 to penetrate membrane (Fig. 1) [37–40]. In contrast, calcium binding to other C2 domains, such as the protein kinase C alpha (PKCα) C2 domain, induces an "electrostatic switch" from electronegative to electropositive promoting the interaction of basic residues with anionic phospholipids [41–45]. Differences in the properties of C2 domains determines the lipid binding specificity in vitro and the membrane targeting specificity in cells [46–50]. The cPLA2α C2 domain preferentially binds to phosphatidylcholine (PC) and mediates the calcium-dependent translocation of cPLA2α to the Golgi, endoplasmic reticulum and nuclear envelope (Fig. 1) [31, 34, 38, 51, 52]. The PKCα C2 domain exhibits calcium-dependent binding to anionic phospholipids and translocates to the inner leaflet of the plasma membrane, which is enriched in the negatively charged phospholipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) [44, 46–50].
Fig. 1. Proposed mechanism of cPLA2α localization and function on the Golgi.
The N-terminal C2 domain of cPLA2α is attached to a large catalytic domain that contains the catalytic site Ser/Asp dyad, and the sites phosphorylated by MAPKs (Ser505) and MAPK-interacting kinases (Ser727). The hydroxyl group of Ser727 interacts with p11/Annexin A2 complexes maintaining cPLA2α in an inactive state. Phosphorylation of Ser727 causes disassociation of the bulky p11/Annexin A2 complex allowing the calcium-dependent interaction of cPLA2α with the Golgi membrane. Calcium binding to the cPLA2α C2 domain reduces the negative electrostatic potential of the surface exposed CBLs allowing the surrounding hydrophobic residues (green) in CBL1 and CBL3 to penetrate the membrane. The basic residues (R57/K58/R59) (yellow) in the C2 domain of cPLA2α form the proposed site for interaction with C-1-P. Calcium-dependent binding of cPLA2α to the Golgi positions the catalytic domain on the membrane, which is stabilized by interaction of Trp464 (red) in the catalytic domain with the membrane. There is evidence that association of cPLA2α with the Golgi is influenced by changes in cholesterol content. Phosphorylation at Ser505 increases the hydrolytic activity of cPLA2α on the membrane perhaps by promoting a conformational change due to its proximity to the flexible linker that connects the catalytic and C2 domains. A patch of basic residues (K488/K541/K543/K544) (teal) in the catalytic domain also regulates the ability of cPLA2α to release arachidonic acid from the Golgi. These residues are necessary for activation of cPLA2α in vitro by polyphosphoinositides, however, the endogenous anionic components in the Golgi that interact with this basic site have not been identified. Therefore the ability of cPLA2α to release arachidonic acid (AA) and form lysophospholipids in the Golgi involves increases in calcium, phosphorylation and interaction of basic residues with anionic components in the membrane. Lysophospholipids generated at the rims of the Golgi cisternae by cPLA2α are thought to induce positive membrane curvature for formation of tubules that connect the Golgi stacks and promote intra-Golgi transport. Surface representation of the x-ray crystal structure of cPLA2α (PDB: 1CJY) was made using PYMOL.
In early studies it was observed that cPLA2α translocates to the perinuclear region including the nuclear envelope in a variety of cells in response to increases in [Ca2+]i [29, 30, 53]. This was particularly interesting in light of work showing localization of 5-lipoxygenase, 5-lipoxygenase activating protein and leukotriene C4 synthase to the nuclear envelope suggesting that this may be a site for production of leukotrienes [54–56]. Subsequent work using organelle markers and Golgi disrupting agents clearly demonstrated that cPLA2α also translocates to the Golgi apparatus, which in most cells is located adjacent to the nucleus [31]. Translocation of cPLA2α is regulated by both the amplitude and duration of [Ca2+]i. The concentration of calcium required for binding to Golgi is lower than for association with the endoplasmic reticulum (ER)/nuclear envelope [31, 32]. This is consistent with data showing that cPLA2α preferentially translocates to the Golgi in response to physiological increases in [Ca2+]i that occurs with agonists such as ATP and serum, and to Golgi and the ER/nuclear envelope in response to calcium ionophores, which cause a supra-physiological rise in [Ca2+]i [31]. Serum induces a typical capacitative increase in [Ca2+]i and potently stimulates arachidonic acid release. The initial transient increase in calcium from intracellular stores promotes the rapid translocation of cPLA2α to Golgi [57]. However the subsequent influx of extracellular calcium due to store depletion is essential for stable binding of cPLA2α to Golgi and for arachidonic acid release [57]. This is evident from results showing that chelating extracellular calcium, which blocks calcium influx but not the initial calcium transient from intracellular stores, prevents serum-stimulated arachidonic acid release. Therefore a rapid transient increase from intracellular stores in this model in itself is not sufficient for cPLA2α to release arachidonic acid [57]. However a low amplitude sustained calcium increase due to influx of extracellular calcium that occurs in the absence of a rapid calcium transient from intracellular stores, results in a slow rate of cPLA2α translocation to Golgi and low level arachidonic acid release [57]. Collectively, studies have established the importance of extracellular calcium influx and the duration of calcium elevation for stable binding of cPLA2α to Golgi and arachidonic acid release [32, 57–59].
3. Role of the phospholipid binding specificity in targeting cPLA2α to Golgi
Most C2 domains are calcium-dependent phospholipid binding domains that function to promote interaction of proteins to membrane. In several cell models, cPLA2α C2 domain has been shown to localize to the same intracellular membranes as full-length cPLA2α in response to calcium increases indicating that it is responsible for the membrane targeting specificity [22, 31, 48]. Hydrophobic residues in the CBLs in the cPLA2α C2 domain are required for calcium-dependent binding to PC rich vesicles in vitro and localization to intracellular membranes in cells (Fig. 1) [38, 39, 51, 60, 61]. The sites of cPLA2α localization, Golgi, ER and nuclear envelope, are the sites for synthesis of PC [62, 63]. It has been suggested that a high local concentration of PC on internal membranes functions to lower the [Ca2+]i necessary for cPLA2α C2 domain to bind to these membrane [47, 48].
The enrichment of PC in intracellular membranes may be sufficient to provide the targeting specificity of cPLA2α to internal membranes. However, the role for an additional lipid or protein in regulating cPLA2α targeting to internal membranes cannot be ruled out. There are studies suggesting that ceramide-1-phosphate (C-1-P), produced from ceramide kinase (CERK), is an endogenous regulator of cPLA2α that acts by enhancing calcium-dependent membrane binding and activity [64–66]. In vitro studies demonstrated that C-1-P acts through the cPLA2α C2 domain to increase the calcium-dependent binding of cPLA2α to PC-containing mixed micelles and acts as an allosteric activator by decreasing the dissociation constant [67]. C-1-P is a much better activator of cPLA2α in vitro when a mixed-micelle assay consisting of PC in Triton X-100 detergent micelles is used versus PC liposomes. For example the presence of 5 mole% C-1-P in Triton X-100/PC mixed micelles leads to a ~15-fold activation of cPLA2α [67], whereas with PC liposomes the activation is ~2-fold (M. Gelb and C. Leslie, unpublished) [65]. This is reminiscent of the activation reported by PI(4,5)P2, where the activation is much more pronounced with mixed micelles than with liposomes [57, 68]. This tends to suggest that when the PC is at low surface packing density (mixed-micelles), cPLA2α is more dependent on anionic lipid additives for binding to membranes and activation. This creates controversy for the role of these anionic lipids in regulating the action of cPLA2α in cells. Although a PC liposome is only a model of a cell membrane, it is arguably a better model than a PC-detergent mixed particle containing mostly Triton X-100.
The interaction of cPLA2α with C-1-P in vitro is mediated in part by basic residues (R57/K58/R59) in the C2 domain, and mutations in these residues impair agonist-induced translocation of cPLA2α (Fig. 1) [69, 70]. The major route of C-1-P production is the phosphorylation of ceramide by CERK. Knockdown of CERK by siRNA results in a partial reduction in C-1-P levels and reduces agonist-induced arachidonic acid release and translocation of cPLA2α in A549 cells [64, 66]. Collectively these studies have led to the hypothesis that C-1-P produced from CERK is a proximal activator of cPLA2α, necessary for its C2 domain-dependent membrane binding and ability to release arachidonic acid. This is an attractive hypothesis since CERK localizes to the Golgi for production of C-1-P [66, 71]. However, results of recent studies do not corroborate these conclusions. Generation of CERK−/− mice confirmed that CERK plays a major role in production of N-palmitoyl-C-1-P in mast cells, macrophages and cerebellum although in some cells (neutrophils) CERK is not the major pathway for C-1-P production [70, 72]. However, cPLA2α-dependent release of arachidonic acid and eicosanoid production is identical in wild type and CERK−/− macrophages and kidney fibroblasts despite a greater reduction in C-1-P compared to cells treated with siRNA [70, 72]. In addition, no differences are observed in wild type and CERK−/− mice in a cPLA2α-dependent model of rheumatoid arthritis [72]. Additional results using a specific CERK inhibitor suggest that the lack of effect on cPLA2α regulation in CERK−/− cells and mice is not due to compensatory mechanisms. The specific CERK inhibitor NVP-231 significantly reduces cellular levels of C-1-P but has no effect on arachidonic acid release or PGE2 production in several cell types stimulated with a variety of agonists [73]. These results do not support a role for the CERK-dependent production of C-1-P as a generalized mechanism for regulating cPLA2α and eicosanoid production. The results raise the question of why siRNA knockdown of CERK inhibits arachidonic acid release but this is not observed in CERK−/− cells or with a specific CERK inhibitor. It also raises questions about the basis for the translocation defect observed with cPLA2α mutated at the putative C-1-P binding site in the C2 domain [70]. It is possible that there are other components on the Golgi that bind to this site. Alternatively mutating this site may result in subtle conformational alteration of the CBLs and affect Ca2+-dependent translocation in cells. Clearly more studies are required to resolve the discrepancies regarding the role of CERK and C-1-P in regulating cPLA2α.
4. Regulation of cPLA2α activity at the Golgi
Calcium-dependent translocation of cPLA2α positions the catalytic domain on the membrane for interaction with phospholipid substrate (Fig. 1). A comparison of the calcium-induced translocation of full-length cPLA2α and the C2 domain to Golgi revealed that the C2 domain acts as a calcium sensor and translocates in direct proportion to [Ca2+]i [74]. Translocation of full-length cPLA2α is slightly slower but it remains associated with the Golgi in a calcium-independent fashion unlike the C2 domain, which rapidly dissociates as [Ca2+]i decreases. There is little experimental data that well defines the basis of catalytic domain binding to the interface of the Golgi membrane in cells. The only clear result is that a tryptophan residue on the membrane-exposed face of the catalytic domain contributes to the prolonged calcium-independent residence of cPLA2α at the Golgi [74].
Translocation to membrane is necessary but not sufficient for cPLA2α to release arachidonic acid. The hydrolytic activity of cPLA2α is independently regulated by phosphorylation and by interaction of an anionic membrane component with basic residues in the catalytic domain (Fig.1). Phosphorylation on Ser-505 has been studied most extensively since it is the site for MAPKs, a common signaling pathway that regulates the release of arachidonic acid [4, 75]. cPLA2α that is completely dephosphorylated is active when assayed in vitro and phosphorylation on Ser-505 modestly enhances activity by about 2-fold [57, 75, 76, 77]. Ser-505 phosphorylation is not sufficient for cPLA2α to release arachidonic acid but acts synergistically with increases in calcium [75, 78–80]. Phosphorylation of cPLA2α on Ser-505 has been reported to increase the membrane binding affinity at low calcium [81]. However, wild type cPLA2α and cPLA2α Ser-505A mutant exhibit similar rates of translocation to Golgi in response to physiological agonists, but the mutant releases less arachidonic acid [57, 82]. These results suggest that phosphorylation at Ser-505 enhances release of arachidonic acid but not calcium-dependent translocation, which is consistent with in vitro data showing that Ser-505 phosphorylation increases catalytic activity but not calcium-dependent interfacial binding [57].
The phosphorylation sites of cPLA2α, Ser-505 and Ser-727, are located in a flexible region that is not near either the membrane binding face or the active site (Fig. 1). This raises questions as to how phosphorylation regulates cPLA2α function. It has been suggested that phosphorylation of Ser-505, which is located near the interdomain flexible linker region, may promote optimal orientation of the C2 and catalytic domains for efficient catalysis [83]. Phosphorylation at Ser-505 by MAPKs is generally accompanied by phosphorylation at Ser-727, which occurs by kinases downstream of MAPK such as MAPK-interacting kinase [80, 84, 85]. Ser-727 phosphorylation plays a novel role in regulating the interaction of cPLA2α with p11 (S100A10)/annexin A2 complexes (Fig. 1) [86]. This bulky complex binds to the hydroxy group of Ser-727, which maintains cPLA2α in an inactive state by interfering with membrane binding in vitro and blocking translocation of cPLA2α to peri-nuclear membranes in cells (Fig. 1)[86–88]. The interaction of p11/annexin A2 complex with cPLA2α is prevented by phosphorylation of Ser-727 thus allowing calcium-dependent interaction of cPLA2α with membrane and release arachidonic acid [86].
The activity cPLA2α is enhanced by several types of polyphosphoinositides through interaction with a patch of basic residues (K488/K541/K543/K544) in the catalytic domain [68, 89–92]. It was shown that PI(4,5)P2 facilitates binding of cPLA2α to PC/Triton X-100 mixed micelles and promotes activation of cPLA2α in a calcium-independent manner [68, 91]. However, other studies have shown that PI(4,5)P2 in PC liposomes enhances cPLA2α activity in the presence but not in the absence of calcium [57, 89]. This is reminiscent of studies with C-1-P described above showing that cPLA2α activation by the anionic additive is much more pronounced with mixed-micelles versus liposomes. In vitro data showing that PI(4,5)P2 primarily acts to enhance catalytic activity and not interfacial binding is consistent with cellular data showing that wild type cPLA2α and the K488N/K543N/K544N mutant exhibit similar rates of translocation to Golgi in response to serum but the mutant releases less arachidonic acid (Fig. 1) [57]. In addition, it has been shown that translocation of cPLA2α to internal membranes induced by loading cells with PI(4,5)P2 occurs in the presence but not in the absence of intracellular calcium [92]. Although polyphosphoinositides activate cPLA2α in vitro and when loaded into cells, further studies are needed to definitively identify the endogenous anionic component on the Golgi that activates cPLA2α through the basic residues in the catalytic domain.
5. Function of cPLA2α at the Golgi
Several studies have provided evidence that cPLA2α regulates the structure and function of the Golgi. The expression of cPLA2α in LLC-PK1 kidney epithelial cells, which are deficient in cPLA2α, causes disruption of the Golgi cisternae and blocks the constitutive transport of aquaporin-2 to the plasma membrane [93]. The effect on Golgi structure is suggested to be due to the ability of cPLA2α to generate free fatty acids and lysophospholipids that modify the properties of the Golgi membrane.
cPLA2α also plays a role in regulating the structure and function of the Golgi that occurs by modifying cellular cholesterol content (Fig. 1). Increasing cellular cholesterol induces vesiculation of the Golgi apparatus that occurs by a cPLA2α- and dynamin-dependent mechanism [94]. Loading cells with cholesterol induces the translocation of cPLA2α to the Golgi and an increase in arachidonic acid release. Vesiculation of the Golgi by cholesterol is blocked by a cPLA2α inhibitor suggesting that enzymatic production of free fatty acids and lysophospholipids are involved. Conversely it has been shown that lowering Golgi cholesterol inhibits the association of cPLA2α with the Golgi resulting in a decrease in the release of arachidonic acid and accumulation of caveolin-1 in the Golgi [95]. The ability of a cPLA2α inhibitor to block transport of caveolin-1 from the Golgi supports a role for cPLA2α activity in regulating trafficking. These studies highlight the important role of membrane lipid composition in regulating the association of cPLA2α with the Golgi and a role for cPLA2α in regulating Golgi structure and transport.
There is considerable evidence that modification of Golgi lipid composition by phopholipases and acyltransferases influences membrane curvature and regulates tubulation and vesicle formation (Fig. 1) [22, 23, 96, 97]. cPLA2α has recently been shown to be required for formation of tubules that connect the Golgi stacks and for regulation of intra-Golgi transport [22]. When transport through the Golgi is activated, cPLA2α is recruited to the rims of the Golgi cisternae and to rim-associated tubules. Similar translocation to Golgi of the cPLA2α C2 domain during transport suggests a role for local calcium increases perhaps from the high intraluminal levels in the Golgi. cPLA2α is also associated with the Golgi during steady state trafficking. The function of cPLA2α is specific for regulating tubule formation and intra-Golgi trafficking but not formation of Golgi vesicles or other trafficking pathways [22]. cPLA2α is proposed to act by generating lysophospholipids at the rims of the Golgi cisternae to promote positive membrane curvature for formation of tubules (Fig. 1).
Several studies have identified a role for cPLA2α and its association with Golgi in regulating the function of endothelial cells. Endothelial cells line the luminal surfaces of blood vessels and serve a barrier function due to tight junctions and endothelial adherens [98]. Endothelial cells proliferate during wound healing for formation of new blood vessels and during tumor formation. The proliferation of sub-confluent human umbilical vein endothelial cells, and cell cycle entry, is blocked by the cPLA2α inhibitor pyrrolidine-1 and siRNA knockdown of cPLA2α [99]. It is known that sub-confluent endothelial cells release greater amounts of arachidonic acid than confluent cells [100]. The decrease in proliferation and arachidonic acid release in confluent endothelial cells correlates with a redistribution of cPLA2α from the cytosol and nucleus to the Golgi [99]. Localization to Golgi does not require an increase in [Ca2+]i and correlates with inhibition of cPLA2α due to interaction with annexin A1 [101]. It is suggested that annexin-1 at the Golgi sequesters cPLA2α and prevents direct interaction with Golgi membrane and association with substrate.
However, recent findings have demonstrated that localization of cPLA2α at the Golgi in confluent endothelial plays a functional role in regulating cell-cell junction formation [102]. The translocation of cPLA2α to the Golgi correlates with the initial maturation of adherens junctions, and disruption of junctions using a VE-cadherin blocking antibody causes cPLA2α to dissociate from the Golgi. Results suggest that VE-cadherin clustering may provide signals for promoting translocation of cPLA2α to the Golgi. Once on the Golgi cPLA2α regulates the transport of junction proteins through and/or from the Golgi to cell-cell contacts, which is blocked by siRNA knockdown of cPLA2α. Treating confluent endothelial cell with cPLA2α inhibitors causes accumulation of proteins that form adherens junction (VE-cadherin) and tight junctions (occludin, claudin-5) in the Golgi suggesting that cPLA2α enzymatic activity is required for transport of junction proteins [102]. Therefore although cPLA2α localization to Golgi in confluent endothelial cells may dampen the agonist-induced release of arachidonic acid, cPLA2α maintains a level of activity that is required for regulating Golgi trafficking. The regulation of junction formation in endothelial cells by cPLA2α is not due to the production of prostaglandins [102]. These studies implicate a role for formation of the immediate products of cPLA2α action, free fatty acids and lysophospholipids, or a non-prostanoid lipid mediator, in regulating Golgi function although the actual mechanism remains to be defined.
6. The need for specific PLA2 inhibitors
Studies presented above provide strong evidence that cPLA2α plays a role in regulating fundamental cellular processes such as Golgi trafficking. This raises the question of why the cPLA2α knockout mouse does not exhibit more profound phenotypic alterations. It is certainly possible that there is adaptation in response to the genetic ablation of cPLA2α in mice and compensatory pathways come into play. It is unlikely that cells would rely on a single pathway for regulating important physiological cell functions. This is illustrated in studies that clearly show a role for cPLA2α in regulating tubulation and intra-Golgi transport, yet these processes function normally in fibroblasts from the cPLA2α knockout mouse [22]. An siRNA screen to determine the role of other PLA2s of the Group IV, VI, VII and VIII families, found that silencing of only the GVIIIA PLA2 inhibited Golgi transport in cPLA2α-deficient fibroblasts [22]. The cPLA2α knockout mouse has been very useful in elucidating the role of cPLA2α in disease, but compensatory pathways may obscure our understanding of the full functional role of cPLA2α. This argues for the importance of developing specific inhibitors for mammalian PLA2s to more accurately evaluate the role of these important enzymes in regulating normal cellular processes and in disease pathogenesis.
Probably the most reliable cPLA2α inhibitors for use in cellular studies are the pyrrolidines developed at Shionogi and the indoles developed at Wyeth [103, 104]. These compounds inhibit cPLA2α in the sub-micromolar range using a number of different in vitro lipolysis assays and they block cPLA2α-dependent arachidonate release in mammalian cells in the 0.01–1 µM range [103–107]. The pyrrolidine and indole inhibitors have recently been shown to be efficacious in mouse models showing a role for cPLA2α in collagen-induced arthritis and in experimental autoimmune encephalomyelitis, respectively [108, 109]. They do not inhibit the human cPLA2β and cPLA2γ isoforms but data on the mouse isoforms are not yet available [105, 110]. However, recent data show that the pyrrolidines and indoles work about as well on mouse cPLA2ζ as on cPLA2α in vitro [111]. In fact, these compounds block arachidonate release in mouse lung fibroblasts prepared from cPLA2α deficient mice, suggesting that cPLA2ζ can contribute at least some of the arachidonate in these cells. There is no data on the effect of these inhibitors on purified human cPLA2ζ or on mouse and human cPLA2δ and cPLA2ε. The groups of Lehr and Kokotos have reported on a series of ketone-containing compounds that are also potent inhibitors of cPLA2α [112, 113]. Studies in the author's laboratories are underway to fully explore the specificity of cPLA2α inhibitors on the complete set of human and mouse Group IV PLA2 isoforms.
In summary, we have available sub-micromolar cPLA2α inhibitors that work in cell models, but the full reliability of these compounds must await further analysis in terms of cross-specificity with other cPLA2 isoforms. Such inhibitors, when combined with siRNA and gene deletion, will lead to a comprehensive understanding of the function of individual members of the Group IV cPLA2 family.
Acknowledgement
The authors acknowledge funding from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alonso F, Henson PM, Leslie CC. A cytosolic phospholipase in human neutrophils that hydrolyzes arachidonoyl-containing phosphatidylcholine. Biochim. Biophys. Acta. 1986;878:273–280. doi: 10.1016/0005-2760(86)90156-6. [DOI] [PubMed] [Google Scholar]
- 2.Leslie CC, Voelker DR, Channon JY, Wall MM, Zelarney PT. Properties and purification of an arachidonoyl-hydrolyzing phospholipase A2 from a macrophage cell line, RAW 264.7. Biochim. Biophys. Acta. 1988;963:476–492. doi: 10.1016/0005-2760(88)90316-5. [DOI] [PubMed] [Google Scholar]
- 3.Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc. Natl. Acad. Sci. USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark JD, Schievella AR, Nalefski EA, Lin L-L. Cytosolic phospholipase A2. J. Lipid Mediat. Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Clark JD, Lin L-L, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 7.Sharp JD, White DL, Chiou XG, Goodson T, Gamboa GC, McClure D, Burgett S, Hoskins J, Skatrud PL, Sportsman JR, Becker GW, Kang LH, Roberts EF, Kramer RM. Molecular cloning and expression of human Ca2+-sensitive cytosolic phospholipase A2. J. Biol. Chem. 1991;266:14850–14853. [PubMed] [Google Scholar]
- 8.Kramer RM, Roberts EF, Manetta J, Putnam JE. The Ca2+-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J. Biol. Chem. 1991;266:5268–5272. [PubMed] [Google Scholar]
- 9.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–18755. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 10.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem. Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 11.Leslie CC. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2. Prostagl. Leukotr. Essen. Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Hanel AM, Schüttel S, Gelb MH. Processive interfacial catalysis by mammalian 85-kilodalton phospholipase A2 enzymes on product-containing vesicles: application to the determination of substrate preferences. Biochemistry. 1993;32:5949–5958. doi: 10.1021/bi00074a005. [DOI] [PubMed] [Google Scholar]
- 13.Sundler R, Winstedt D, Wijkander J. Acyl-chain selectivity of the 85 kDa phospholipase A2 and of the release process in intact macrophages. Biochem. J. 1994;301:455–458. doi: 10.1042/bj3010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 15.Diaz BL, Fujishima H, Sapirstein A, Bonventre JV, Arm JP. Participation of cytosolic phospholipase A2 in eicosanoid generation by mouse bone marrow-derived mast cells. Adv. Exp. Med. Biol. 2002;507:41–46. doi: 10.1007/978-1-4615-0193-0_7. [DOI] [PubMed] [Google Scholar]
- 16.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 17.Gijón MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that do and do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J. Biol. Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 18.Rubin BB, Downey GP, Koh A, Degousee N, Ghomashchi F, Nallan L, Stefanski E, Harkin DW, Sun C, Smart BP, Lindsay TF, Cherepanov V, Vachon E, Kelvin D, Sadilek M, Brown GE, Yaffe MB, Plumb J, Frinstein S, Glogauer M, Gelb MH. Cytosolic phospholipase A2-α is necessaary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune reponse to pulmonary infection. cPLA2-α does not regulate neutrophil NADPH oxidase activity. J. Biol. Chem. 2005;280:7519–7529. doi: 10.1074/jbc.M407438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans JF. The cysteinyl leukotriene receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:117–122. doi: 10.1016/s0952-3278(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 21.Okuno T, Yokomizo T, Hori T, Miyano M, Shimizu T. Leukotriene B4 receptor and the function of its helix 8. J. Biol. Chem. 2005;280:32049–32052. doi: 10.1074/jbc.R500007200. [DOI] [PubMed] [Google Scholar]
- 22.San Pietro E, Capestrano MG, Polishchuk EV, DiPentima A, Trucco A, Zizza P, mariggio S, Pulvirenti T, Sallese M, Tete S, Mironov AA, Leslie CC, Corda D, Luini A, Polishchuk RS. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLos Biol. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 25.Voeltz GK, Prinz WA. Sheets, ribbons and tubules - how organelles get their shape. Nat. Mol. Cell Biol. 2007;8:258–264. doi: 10.1038/nrm2119. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerberg J, Chernomordik LV. Synaptic membranes bend to the will of a neurotoxin. Science. 2005;310:1626–1627. doi: 10.1126/science.1122439. [DOI] [PubMed] [Google Scholar]
- 27.Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C. Equivalent effect of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 28.Channon J, Leslie CC. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line, RAW 264.7. J. Biol. Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- 29.Schievella AR, Regier MK, Smith WL, Lin L-L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J. Biol. Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 30.Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J. Biol. Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 31.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J. Biol. Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi T, Kume K, Hirose K, Yokomizo T, Iino M, Itoh H, Shimizu T. Critical duration of intracellular Ca2+ response required for continuous translocation and activation of cytosolic phospholipase A2. J. Biol. Chem. 1999;274:5163–5169. doi: 10.1074/jbc.274.8.5163. [DOI] [PubMed] [Google Scholar]
- 33.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J. Biol. Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 34.Nalefski EA, Slazas MM, Falke JJ. Ca2+-signaling cycle of a membrane-docking C2 domain. Biochemistry. 1997;36:12011–12018. doi: 10.1021/bi9717340. [DOI] [PubMed] [Google Scholar]
- 35.Ball A, Nielsen R, Gelb MH, Robinson BH. Interfacial membrane docking of cytosolic phospholipase A2 C2 domain using electrostatic potential-modulated spin relaxation magnetic resonance. Proc. Natl. Acad. Sci. U S A. 1999;96:6637–6642. doi: 10.1073/pnas.96.12.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu G-Y, McDonagh T, Hsiang-Ai Y, Nalefski EA, Clark JD, Cumming DA. Solution structure and membrane interactions of the C2 domain of cytosolic phospholipase A2. J. Mol. Biol. 1998;280:485–500. doi: 10.1006/jmbi.1998.1874. [DOI] [PubMed] [Google Scholar]
- 37.Davletov B, Perisic O, Williams RL. Calcium-dependent membrane penetration is a hallmark of the C2 domain of cytosolic phospholipase A2 whereas the C2A domain of synaptotagmin binds membranes electrostatically. J. Biol. Chem. 1998;273:19093–19096. doi: 10.1074/jbc.273.30.19093. [DOI] [PubMed] [Google Scholar]
- 38.Perisic O, Paterson HF, Mosedale G, Lara-González S, Williams RL. Mapping the phospholipid-binding surface and translocation determinants of the C2 domain from cytosolic phospholipase A2. J. Biol. Chem. 1999;274:14979–14987. doi: 10.1074/jbc.274.21.14979. [DOI] [PubMed] [Google Scholar]
- 39.Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2. J. Biol. Chem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- 40.Nalefski EA, Wisner MA, Chen JZ, Sprang SR, Fukuda M, Mikoshiba K, Falke JJ. C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity. Biochemistry. 2001;40:3089–3100. doi: 10.1021/bi001968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray D, Honig B. Electrostatic control of the membrane targeting of C2 domains. Molecular Cell. 2002;9:145–154. doi: 10.1016/s1097-2765(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 42.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nalefski EA, Newton AC. Membrane binding kinetics of protein kinase C betaII mediated by the C2 domain. Biochemistry. 2001;40:13216–13229. doi: 10.1021/bi010761u. [DOI] [PubMed] [Google Scholar]
- 44.Corbalán-García S, Rodríguez-Alfaro JA, Gómez-Fernández JC. Determination of the calcium-binding sites of the C2 domain of protein kinase Cα that are critical for its translocation to the plasma membrane. Biochem. J. 1999;337:513–521. [PMC free article] [PubMed] [Google Scholar]
- 45.Kohout SC, Corbalan-Garcia S, Gomez-Fernandez JC, Falke JJ. C2 domain of protein kinase calpha: elucidation of the membrane docking surface by site-directed fluorescence and spin labeling. Biochemistry. 2003;42:1254–1265. doi: 10.1021/bi026596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans JH, Gerber SH, Murray D, Leslie CC. The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells. Mol. Biol. Cell. 2004;15:371–383. doi: 10.1091/mbc.E03-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbin JA, Evans JH, Landgraf KE, Falke JJ. Mechanism of specific membrane targeting by C2 domains: Localized pools of target lipid enhance Ca2+ affinity. Biochemistry. 2007;46:4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahelin RV, Rafter JD, Das S, Cho W. The molecular basis of differential subcellular localization of C2 domains of protein kinase C-a and group IVA cytosolic phospholipase A2. J. Biol. Chem. 2003;278:12452–12460. doi: 10.1074/jbc.M212864200. [DOI] [PubMed] [Google Scholar]
- 49.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim. Biiophys. Acta. 2006;1761:838–849. doi: 10.1016/j.bbalip.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Evans JH, Murray D, Leslie CC, Falke JJ. Specific translocation of protein kinase Cα to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol. Biol. Cell. 2006;17:56–66. doi: 10.1091/mbc.E05-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalefski EA, McDonagh T, Somers W, Seehra J, Falke JJ, Clark JD. Independent folding and ligand specificity of the C2 calcium-dependent lipid binding domain of cytosolic phospholipase A2. J. Biol. Chem. 1998;273:1365–1372. doi: 10.1074/jbc.273.3.1365. [DOI] [PubMed] [Google Scholar]
- 52.Hixon MS, Ball A, Gelb MH. Calcium-dependent and -independent interfacial binding and catalysis of cytosolic group IV phospholipase A2. Biochemistry. 1998;37:8516–8526. doi: 10.1021/bi980416d. [DOI] [PubMed] [Google Scholar]
- 53.Peters-Golden M, Song K, Marshall T, Brock T. Translocation of cytosolic phospholipase A2 to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem. J. 1996;318:797–803. doi: 10.1042/bj3180797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brock TG, McNish RW, Peters-Golden M. Translocation and leukotriene synthetic capacity of nuclear 5-lipoxygenase in rat basophilic leukemia cells and alveolar macrophages. J. Biol. Chem. 1995;270:21652–21658. doi: 10.1074/jbc.270.37.21652. [DOI] [PubMed] [Google Scholar]
- 55.Christmas P, Weber BM, McKee M, Brown D, Soberman RJ. Membrane localization and topology of leukotriene C4 synthase. J. Biol. Chem. 2002;277:28902–28908. doi: 10.1074/jbc.M203074200. [DOI] [PubMed] [Google Scholar]
- 56.Woods JW, Evans JF, Ethier D, Scott S, Vickers PJ, Hearn L, Heibein JA, Charleson S, Singer II. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J. Exp. Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucker DE, Ghosh M, Ghomashchi F, Loper R, Suram S, St. John B, Girotti M, Bollinger JG, Gelb MH, Leslie CC. Role of phosphorylation and basic residues in the catalytic domain of cytosolic phospholipase A2α in regulating interacial kinetics and binding and cellular function. J. Biol. Chem. 2009;284:9596–9611. doi: 10.1074/jbc.M807299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang W-C, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. J. Biol. Chem. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 59.Rzigalinski BA, Blackmore PF, Rosenthal MD. Arachidonate mobilization is coupled to depletion of intracellular calcium stores and influx of extracellular calcium in differentiated U937 cells. Biochim. Biophys. Acta. 1996;1299:342–352. doi: 10.1016/0005-2760(95)00224-3. [DOI] [PubMed] [Google Scholar]
- 60.Nalefski EA, Falke JJ. Location of the membrane-docking face on the Ca2+-activated C2 domain of cytosolic phospholipase A2. Biochemistry. 1998;37:17642–17650. doi: 10.1021/bi982372e. [DOI] [PubMed] [Google Scholar]
- 61.Gerber SH, Rizo J, Sudhof TC. The top loops of the C2 domains from synaptotagmin and phospholipase A2 control functional specificity. J. Biol. Chem. 2001;276:32288–32292. doi: 10.1074/jbc.C100108200. [DOI] [PubMed] [Google Scholar]
- 62.Heneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol. Biol. Cell. 2002;13:3148–3161. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J. Biol. Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 65.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Freiberg EJHJ, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 66.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Merrill AH, Cho W, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J. Lipid Res. 2007;48:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Chi W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2a and enhances the interaction of the enzme with phosphatidylcholine. J. Biol. Chem. 2005;280:17601–17607. doi: 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- 68.Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J. Biol. Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- 69.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J. Biol. Chem. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 70.Lamour NF, Subramanian P, Wijesinghe DS, Stahelin RV, Bonventre JV, Chalfant CE. Ceramide 1-phosphate is required for the translocation of Group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J. Biol. Chem. 2009;284:26897–26907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carre A, Graf C, Stora S, Mechtcheriakova D, Csonga R, Urtz N, Billich A, Baumruker T, Bornancin F. Ceramide kinase targeting and activity determined by its N-terminal pleckstrin homology domain. Biochem. Biophys. Res. Commun. 2004;324:1215–1219. doi: 10.1016/j.bbrc.2004.09.181. [DOI] [PubMed] [Google Scholar]
- 72.Graf C, Zemann B, Rovina P, Urtz N, Schanzer A, Reuschel R, Mechtcheriakova D, Muller M, Fischer EH, Reichel C, Huber S, Dawson J, Meingassner JG, Billich A, Niwa S, Badegruber R, Van Veldhoven PP, Kinzel B, Baumruker T, Bornancin F. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J. Immunol. 2008;180:3457–3466. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- 73.Graf C, Klumpp M, Habig M, Rovin a P, Billich A, Baumruker T, Oberhauser B, Bornancin F. Targeting ceramide metabolism with a potent and specific ceramide kinase inhibitor. Mol. Pharmacol. 2008;74:925–932. doi: 10.1124/mol.108.048652. [DOI] [PubMed] [Google Scholar]
- 74.Evans JH, Leslie CC. The cytosolic phospholipase A2 catalytic domain modulates association and residence time at Golgi membranes. J. Biol. Chem. 2004;279:6005–6016. doi: 10.1074/jbc.M311246200. [DOI] [PubMed] [Google Scholar]
- 75.Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 76.Bayburt T, Gelb MH. Interfacial catalysis by human 85 kDa cytosolic phospholipase A2 on anionic vesicles in the scooting mode. Biochemistry. 1997;36:3216–3231. doi: 10.1021/bi961659d. [DOI] [PubMed] [Google Scholar]
- 77.Nemenoff RA, Winitz S, Qian N-X, Van Putten V, Johnson GL, Heasley LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J. Biol. Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 78.Qiu Z-H, Gijón MA, de Carvalho MS, Spencer DM, Leslie CC. The role of calcium and phosphorylation of cytosolic phospholipase A2 in regulating arachidonic acid release in macrophages. J. Biol. Chem. 1998;273:8203–8211. doi: 10.1074/jbc.273.14.8203. [DOI] [PubMed] [Google Scholar]
- 79.Lin L-L, Lin AY, Knopf JL. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc. Natl. Acad. Sci. USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hefner Y, Borsch-Haubold AG, Murakami M, Wilde JI, Pasquet S, Schieltz D, Ghomashchi F, Yates JR, 3rd, Armstrong CG, Paterson A, Cohen P, Fukunaga R, Hunter T, Kudo I, Watson SP, Gelb MH. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J. Biol. Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 81.Das S, Rafter JD, Kim KP, Gygi SP, Cho W. Mechanism of group IVA cytosolic phospholipase A2 activation by phosphorylation. J. Biol. Chem. 2003;278:41431–41442. doi: 10.1074/jbc.M304897200. [DOI] [PubMed] [Google Scholar]
- 82.Evans JH, Fergus DJ, Leslie CC. Inhibition of the MEK1/ERK pathway reduces arachidonic acid release independently of cPLA2 phosphorylation and translocation. BMC Biochemistry. 2002;3:30. doi: 10.1186/1471-2091-3-30. [Computer File] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, Somers WS. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 84.Börsch-Haubold AG, Bartoli F, Asselin J, Dudler T, Kramer RM, Apitz-Castro R, Watson SP, Gelb MH. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J. Biol. Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- 85.de Carvalho MGS, McCormack AL, Olson E, Ghomashchi F, Gelb MH, Yates JR, III, Leslie CC. Identification of phosphorylation sites of human 85-kDa cytosolic phospholipase A2 expressed in insect cells and present in human monocytes. J. Biol. Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- 86.Tian W, Wijewickrama GT, Kim JH, Das S, Tun MP, Gokhale N, Jung JW, Kim KP, Cho W. Mechanism of regulation of Group IVA phospholipase A2 activity by Ser727 phosphorylation. J. Biol. Chem. 2008;283:3960–3971. doi: 10.1074/jbc.M707345200. [DOI] [PubMed] [Google Scholar]
- 87.Yao X-L, Cowan MJ, Gladwin MT, Lawrence MM, Angus CW, Shelhamer JH. Dexamethasone alters arachidonate release from human epithelial cells by induction of p11 protein synthesis and inhibition of phospholipase A2 activity. J. Biol. Chem. 1999;274:17202–17208. doi: 10.1074/jbc.274.24.17202. [DOI] [PubMed] [Google Scholar]
- 88.Wu T, Angus CW, Yao X-L, Logun C, Shelhamer JH. p11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85-kDa cytosolic phospholipase A2. J. Biol. Chem. 1997;272:17145–17153. doi: 10.1074/jbc.272.27.17145. [DOI] [PubMed] [Google Scholar]
- 89.Das S, Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. J. Biol. Chem. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- 90.Leslie CC, Channon JY. Anionic phospholipids stimulate an arachidonoyl-hydrolyzing phospholipase A2 from macrophages and reduce the calcium requirement for activity. Biochim. Biophys. Acta. 1990;1045:261–270. doi: 10.1016/0005-2760(90)90129-l. [DOI] [PubMed] [Google Scholar]
- 91.Six DA, Dennis EA. Essential Ca2+-independent role of the group IVA cytosolic phospholipase A2 C2 domain for interfacial activity. J. Biol. Chem. 2003;278:23842–23850. doi: 10.1074/jbc.M301386200. [DOI] [PubMed] [Google Scholar]
- 92.Casas J, Gijon MA, Vigo AG, Sanchez Crespo M, Balsince J, Balboa MA. Phosphatidylinositol 4,5-bisphosphate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Mol. Biol. Cell. 2006;17:155–162. doi: 10.1091/mbc.E05-06-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choukroun GJ, Marshansky V, Gustafson CE, McKee M, Hajjar RJ, Rosenzweig A, Brown D, Bonventre JV. Cytosolic phospholipase A2 regulates Golgi structure and modulates intracellular trafficking of membrane proteins. J. Clin. Invest. 2000;106:983–993. doi: 10.1172/JCI8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grimmer S, Ying M, Walchli S, van Deurs B, Sandvig K. Golgi vesiculation induced by cholesterol occurs by a dynamin- and cPLA2-dependent mechanism. Traffic. 2005;6:144–156. doi: 10.1111/j.1600-0854.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 95.Cubells L, Vila de Muga S, Tebar F, Bonventre JC, Balsinde J, Pol A, Grewal T, Enrich C. Annexin A6-induced inhibition of cytoplasmic phospholipse A2 is linked to caveolin-1 export from the Golgi. J. Biol. Chem. 2008;283:10174–10183. doi: 10.1074/jbc.M706618200. [DOI] [PubMed] [Google Scholar]
- 96.Yang J-S, Gad H, Lee SY, Mironov AA, Zhang L, Beznoussenko GV, Valente C, Tracchio G, Bonsra AN, Du G, Baldanzi G, Graziani A, Bouroin S, Frohman MA, Luini A, Hsu VW. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nature Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morikawa RK, Aoki J, Kano F, Murata M, Yamamoto A, Tsujimoto M, Arai H. Intracellular phospholipase A1γ (iPLA1γ) is a novel factor involved in coat protein complex I- and Rab6-independent retrograde transport between the endoplasmic reticulum and the Golgi complex. J. Biol. Chem. 2009;284:26620–26630. doi: 10.1074/jbc.M109.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Herbert SP, Ponnambalam S, Walker JH. Cytosolic phospholipase A2-α mediates endothelial cell proliferation and is inactivated by association with the Golgi apparatus. Mol. Biol. Cell. 2005;16:3800–3809. doi: 10.1091/mbc.E05-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whately RE, Satoh K, Zimmerman GA, McIntyre TM, Prescott SM. Proliferation-dependent changes in release of arachidonic acid from endothelial cells. J. Clin. Invest. 1994;94:1889–1900. doi: 10.1172/JCI117539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herbert SP, Odell AF, Ponnambalam S, Walker JH. The confluence-dependent interaction of cytosolic phospholipase A2-α with annexin A1 regulates endothelial cell prostaglandin E2 generation. J. Biol. Chem. 2007;282:34468–34478. doi: 10.1074/jbc.M701541200. [DOI] [PubMed] [Google Scholar]
- 102.Regan-Klapisz E, Krouwer V, Langelaar-Makkinje M, Nallan L, Gelb M, Gerritsen H, Verkleij AJ, Post JA. Golgi-assocaited cPLA2α regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol. Biol. Cell. 2009;20:4225–4234. doi: 10.1091/mbc.E08-02-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seno K, Okuno T, Nishi K, Murakami Y, Watanabe F, Matsuura T, Wada M, Fujii Y, Yamada M, Ogawa T, Okada T, Hashizume H, Kii M, Hara S, Hagishita S, Nakamoto S, Yamada K, Chikazawa Y, Ueno M, Teshirogi I, Ono T, Ohtani M. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J. Med. Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- 104.Lee KL, Foley MA, Chen L, Behnke ML, Lovering FE, Kirincich SJ, Wang W, Shim J, Tam S, Shen MWH, Khor SP, Xu X, Goodwin DG, Ramarao MK, Nickerson-Nutter C, Donahue F, Ku MS, Clark JD, McKew JC. Discovery of ecopladib, an indole inhibitor of cytosolic phospholipase A2α. J. Med. Chem. 2007;50:1380–1400. doi: 10.1021/jm061131z. [DOI] [PubMed] [Google Scholar]
- 105.Ghomashchi F, Stewart A, Hefner Y, Ramanadham S, Turk J, Leslie CC, Gelb MH. A pyrrolidine-based specific inhibitor of cytosolic phospholipase A2α blocks arachidonic acid release in a variety of mammalian cells. Biochim. Biophys. Acta. 2001;1513:160–166. doi: 10.1016/s0005-2736(01)00349-2. [DOI] [PubMed] [Google Scholar]
- 106.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2-α. J. Biol. Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 107.Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, Seno K. Characterization of a novel inhibitor of cytosolic phospholipase A2α, pyrrophenone. Biochem. J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tai N, Kuwabara K, Kobayashi M, yamada K, Ono T, Seno K, Gahara Y, Ishizaki J, Hori Y. cytosolic phospholipase A2 alpha inhibitor, pyrroxyphene, displays anti-arthritic and anti-bone destructive action in a murine arthritis model. Inflamm. Res. 2009;59:53–62. doi: 10.1007/s00011-009-0069-8. [DOI] [PubMed] [Google Scholar]
- 109.Marusic S, Thakker P, Pelker JW, Stedman NL, Lee KL, McKew JC, Han L, Xu X, Wolf SF, Borey AJ, Cui J, Shen MW, Donahue F, hassan-Zahraee M, Leach MW, Shimizu T, Clark JD. Blockade of cytosolic phospholipase A2α prevents experimental autoimmune encephalomyelitis and diminishes development of Th1 and Th17 responses. J. Neuroimmunol. 2008;204:29–37. doi: 10.1016/j.jneuroim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 110.McKew JC, Lee KL, Shen MW, Thakker P, Foley MA, Behnke ML, Hu B, Sum F-W, Tam S, Hu Y, Chen L, Kirincich SJ, Michalak R, Thomason J, Ipek M, Wu K, Wooder L, Ramarao MK, Murphy EA, Goodwin DG, Albert L, Xu X, Donahue F, Ku MS, Keith J, Nickerson-Nutter C, Abraham WM, Williams C, Hegen M, Clark JD. Indole cytosolic phospholipase A2α inhibitors: discovery and in vitro and in vivo characterization of 4-{3-[5Chloro-2-(2-{[3,4-dichlorobenzyl)sulfonyl]amino}ethyl)-1- (diphenylmethyl)-1H-indol-3-yl]propyl}benzoic Acid, Efipladib. J. Med. Chem. 2008;51:3388–3413. doi: 10.1021/jm701467e. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh M, Loper R, Ghomashchi F, Tucker DE, Bonventre JV, Gelb MH, Leslie CC. Function, activity and membrane targeting of cytosolic phospholipase A2ζ in mouse lung fibroblasts. J. Biol. Chem. 2007;282:11676–11686. doi: 10.1074/jbc.M608458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludwig J, Bovens S, Brauch C, Elfringhoff AS, Lehr M. Design and synthesis of 1-indol-1-yl-propan-2-ones as inhibitors of human cytosolic phospholipase A2α. J. Med. Chem. 2006;49:2611–2620. doi: 10.1021/jm051243a. [DOI] [PubMed] [Google Scholar]
- 113.Kokotos G, Kotsovolou S, Six DA, Constantinou-Kokotou V, Beltzner CC, Dennis EA. Novel 2-oxoamide inhibitors of human group IVA phospholipase A2. J. Med. Chem. 2002;45:2891–2893. doi: 10.1021/jm025538p. [DOI] [PubMed] [Google Scholar]