1. Introduction
Paraplegia from spinal cord ischemia is a devastating complication of thoracoabdominal aortic aneurysm repair (Schepens et al., 2009; Svensson et al., 1993). Compared with conventional open surgery repair, endovascular stent-graft surgery is a relatively safe procedure that decreases postoperative mortality. However, it does not eliminate the occurrence of ischemic spinal cord injury (Stone et al., 2006). Protective techniques including epidural cooling, distal aortic perfusion, and cerebrospinal fluid drainage have been applied in patients to decrease the incidence and severity of injury (Cambria et al., 2000; Coselli et al., 2002; Safi et al., 1997; Safi et al., 2003; Tiesenhausen et al., 2000; Tschop et al., 2008), but better understanding of both mechanisms of injury and preclinical evaluation of interventions should serve to further reduce morbidity associated with this disorder.
Genomic expression is important in the response of spinal cord to ischemic injury. Mouse spinal cord injury models allow study of overexpression or targeted deletion of specific proteins and their role in the evolution of spinal cord injury. At the same time, post-ischemic responses persist for weeks to months after ischemia and use of a model allowing long-term recovery to allow definition of the full course of disease offers potentially greater clinical relevance.
Currently, two mouse models of spinal cord ischemia have been reported. Lang-Lazdunski et al. (Lang-Lazdunski et al., 2000) developed a model of combined aortic, left subclavian and internal mammary artery cross-clamping for 9 or 11 min. Motor functional deficits of the hind limbs and histological damage of spinal cord were assessed within 1–2 days after injury. While also employed by others (Casey et al., 2005; Stone et al., 2009a; Stone et al., 2009b), there are no reports of survival studies beyond 48 hours. Gaviria et al. (Gaviria et al., 2002) developed a photochemical occlusion model in C57Bl/6Jmice and irradiated the dorsal surface of the T9 vertebral laminae mice with a 560-nm wavelength-light after intravenous injection of Rose Bengal. Post-ischemic MRI changes were observed at 24 hours (Gaviria et al., 2006). Again, long-term recovery has not been reported.
The current study investigated a modification of aortic cross-clamping in mice to develop a clinically relevant and simplified model of spinal cord ischemia for use in long-term survival studies.
2. Materials and methods
The Duke Institutional Animal Care and Use Committee approved this study. Male C57Bl/6J mice (8–10 weeks of age, 20–25 grams, Jackson Laboratories, Bar Harbor, ME) were used in all investigations.
2.1. Ischemic model
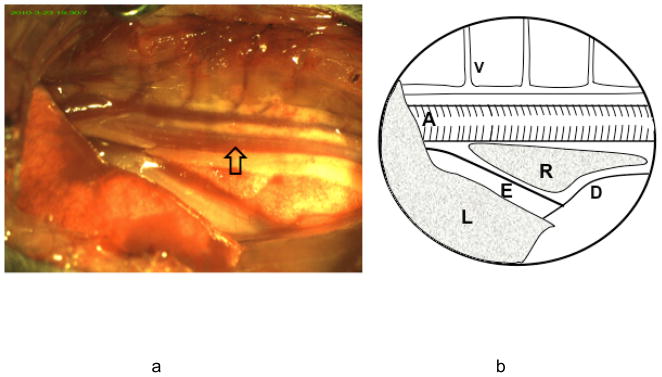
Mice were fasted over night with free access to water. On the day of surgery, mice were anesthetized with 5% isoflurane and the trachea was orally intubated with a 20 gauge intravenous catheter (Insyte-W, Becton Dickinson, Sandy, UT). Both lungs were mechanically ventilated with a tidal volume of 0.7 ml and a rate of 105 breaths per minute in 40% O2 balanced with N2. Inspired isoflurane was decreased to 1.8% during surgical preparation and ischemia. Rectal temperature was maintained at 37.0 ± 0.5°C by automatic surface heating and cooling. Mice were placed in the right lateral decubitus position. The surface of the left chest was prepared and covered with the surgical drapes. A 1.0 cm skin incision was made along the 8th rib and the serratus muscle was cut to expose the 8th and 9th ribs. The lower lobe of left lung was clearly visible through thin chest wall and inflating/deflating with the ventilator rhythm. The inferior edge of the left lung was considered as a landmark to access the thoracic cavity to prevent potential lung injury. The superficial layer of the intercostal muscle was cauterized (High Temperature Loop Cautery, Bovie Medical Co., St. Petersburg, FL) allowing gentle insertion of the tip of a closed surgical scissor into the thoracic cavity and slowly widening the intercostal space by opening the scissor. Two small wire retractors were applied to the edges of the opened chest wall and pulled toward both rostral and caudal directions. The thoracic aorta was immediately visible in the surgical field. Neighboring structures including the azygos vein, esophagus, diaphragm, and both lungs, could be seen when the retractors were positioned (Figure 1).
Figure 1.
a) Surgical view of the thoracic aorta and neighboring structures. The chest cavity is accessed through the intercostal space between the 8th and 9th ribs. The aorta is readily visualized in the middle of the posterior wall of the chest and covered by the parietal pleura. b): Thoracic structures. The azygos vein (V) and its branches are located above the aorta (A). It receives blood return from the chest wall. The esophagus (E) is located below the aorta and enters the abdominal cavity through the diaphragm (D). The lower lobe of the left lung (L) is located lateral to the mediastinal structures. A small part of the right lung (R) is visible between aorta and esophagus.
Mice were randomly assigned to sham (0 min, n=5), 8 min (n=5), 10 min (n=5) or 12 min (n=7) ischemia groups. A small human use aneurysm clip was placed on the aorta at the level of T8. The toe skin color of the hind legs immediately turned pale while the front legs remained well perfused. The clip was removed at the end of the defined ischemia interval. The wound was first closed by suturing the serratus muscle. The tidal volume was transiently increased to expel air from the thorax before completely closing the serratus. The skin was closed with interrupted suture and antibiotic ointment was applied to the wound. Isoflurane was discontinued. Mice were continuously ventilated until spontaneous respiration resumed. The trachea was extubated upon recovery of the righting reflex (typically within 5 min after isoflurane discontinuation). Animals randomized to the sham group underwent all procedures with the exception of aortic occlusion.
2.2. Spinal cord blood flow measurement
Spinal cord surface blood flow change was monitored in 5 additional mice using a laser Doppler probe. Mice were anesthetized and intubated. Both lungs were mechanically ventilated. A laminectomy was performed at the level of L1 to expose the spinal cord. A sheath, used to fix the laser Doppler probe in position, was placed on the vertebra and glued to the surrounding muscles. Then mice were placed in the lateral position and prepared for aortic cross-clamping as described above. The laser Doppler probe was inserted into the sheath before aortic cross-clamping with the probe surface opposing the dura. An aneurysm clip was placed on the thoracic aorta for 10 min. Laser Doppler flow velocity was recorded every min for 5 min prior to ischemia, during ischemia and for 5 min post-ischemia. The severity of blood flow reduction was calculated as (mean LDF reading during ischemia or post-ischemia/mean LDF reading prior to ischemia) × 100%.
2.3. Neurological evaluation
Open field BBB score
At 1 hour, and 1, 3, 5, and 7 days post-injury, the mice were placed on a 50×50 cm surface and the movement of both hind limbs was observed for 5 min. A BBB locomotor score (range 1–21, normal = 21) (Basso et al., 1996) was assigned for each hind limb by an observer blinded to group assignment. The score of each of the two hind limbs was averaged and used for data analysis.
Rotarod performance
At 1 hour, and 1, 3, 5, and 7 days post-injury, the mice were placed on an accelerated rotating rod (4 to 40 rpm, ENV-577M, Med Associates INC, Georgia, Vermont). The latency to fall from the rod was automatically recorded by the action of the mouse dropping from the rotating rod. Three trials were performed at each session and the best performance (i.e., longest latency to fall from the rod) from the three trials was selected for analysis. The mouse was allowed to rest for 15 min between trials.
2.4. Histological evaluation
At 7 days post-injury, the mice were anesthetized with isoflurane. The trachea was intubated and the lungs were mechanically ventilated. In situ transcardiac intra-aortic perfusion was performed with 30 ml of saline followed by 30 ml of 10% formalin (pH 7.4). The mice were stored in the refrigerator overnight after which the lumbar segment of spinal cord was harvested and paraffin embedded. Serial coronal sections (5 μm thick) were collected and stained with hematoxylin and eosin. An observer blinded to group assignment, using light microscopy, counted neurons with normal histologic features in the ventral horn. The number of surviving neurons from both horns was averaged for further analysis.
2.5. Evaluation of post-ischemia long-term survival
After left lateral thoracotomy, 10 mice were randomly assigned to 10 min of aortic cross-clamping or sham surgery (n = 5 per group). Open field BBB score and rotarod performance were evaluated at 1 h, and 1, 3, 5, 7, 14, and 28 days after surgery as described above.
2.5. Statistical Analysis
The number of surviving neurons, BBB scores and rotarod latencies are presented as mean ± SEM. Rotarod latencies and BBB scores were compared with repeated measures ANOVA with days post-injury as the repeated measure. One-way ANOVA was used to compare the number of surviving neurons. Post hoc testing, when indicated by a significant F ratio, was performed using Fisher’s protected least squares difference test. A p value <0.05 was considered to be statistically significant.
3. Results
A total of 37 mice were entered into this study (27 with ischemia and 10 shams). In the experiment defining the ischemic duration, all mice in the ischemic groups had some degree of hind limb weakness at 1 hour post-ischemia. Four of 7 mice subjected to 12 min of ischemia died on 2–7 days post-injury. The remaining survived for the full 7-day observation interval. All sham mice survived. In the experiment evaluating long-term survival, one mouse in the ischemia group died at 10 days while the remaining 4 mice survived the full 28 day observation interval
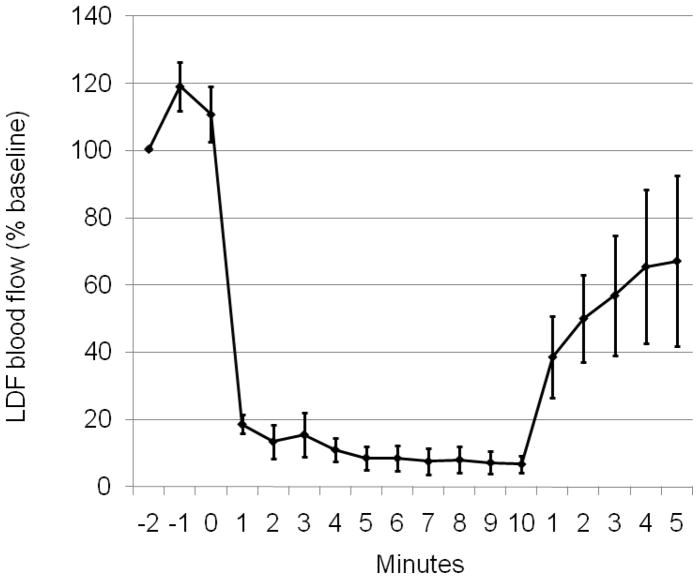
Spinal cord blood flow velocity was measured in 5 mice by laser Doppler. Thoracic aortic cross-clamping caused blood flow velocity on the dorsal surface of the lumbar spinal cord to decrease to 9.3 ± 0.4 % of pre-ischemia values. Spinal cord blood flow velocity recovered to 58 ± 10% of pre-ischemia values at 5 min post-ischemia (Figure 2).
Figure 2.
Ischemia induced lumbar spinal cord blood flow changes. Blood flow velocity was measured using a laser Doppler probe and expressed as % baseline (mean ± S.E.M, n=5).
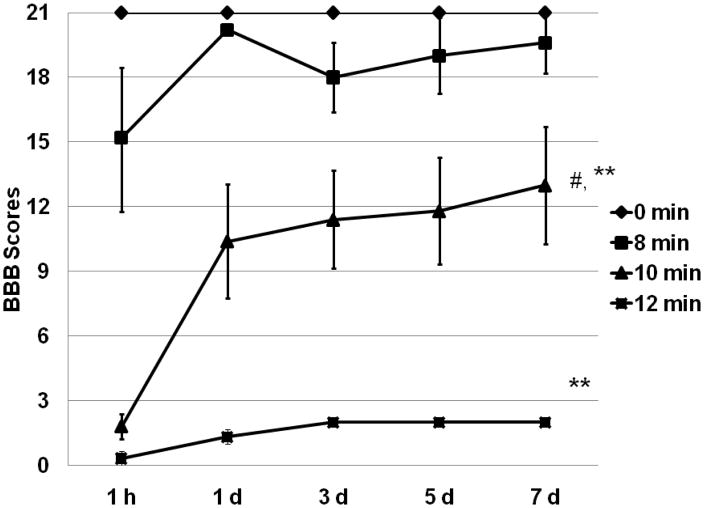
A main effect was present for locomotor BBB score (p <0.01). Scores in sham mice were normal and not affected by the thoracotomy (Figure 3). In the 8 min ischemia group, the BBB score declined to 15 at 1 hour post-injury and then recovered to almost normal by 24 hours. The mean score decreased to 18 at 3 days post-injury, and then recovered slowly, but this decrease was not statistically significant. There was no difference from the sham group at 7 days post-ischemia (p = 0.6). In the mice exposed to 10 or 12 min of ischemia, a more severe decline in BBB scores and a slower recovery were observed. BBB scores in both groups were worse when compared to either 8 min ischemia or sham surgery at 7 days post-ischemia (P <0.01). Twelve min ischemia in surviving mice resulted in a persistent locomotor function deficit that was significantly different from the 10 min ischemia group (p = 0.045).
Figure 3.
Ischemia induced locomotor function deficit. For clarity, BBB scores are presented as mean ± S.E.M. Post-ischemia locomotor function deficit was associated with ischemia duration. # P <0.05, vs. 12 min, ** P < 0.01, vs. 0 min or 8 min ischemia.
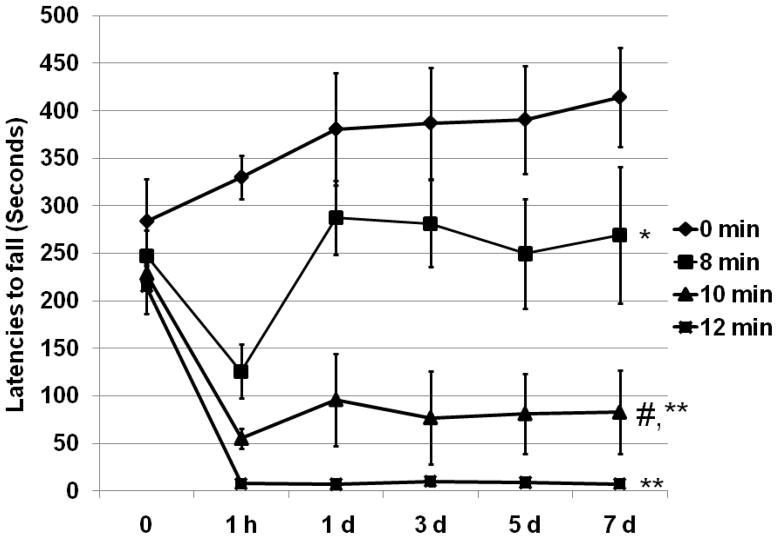
Rotarod performance was assessed by comparing latencies to fall from the rotating rod (Figure 4). All mice subjected to aortic cross-clamping had shorter latencies to fall, compared to the sham group (p = 0.025, 8 min vs. 0 min; p < 0.01, 10 min or 12 min vs. 0 min). Mice in the 8 min ischemia group had a similar pattern of functional deficit, as defined by BBB score, and recovered faster than those subjected to 10 or 12 min of ischemia (p < 0.01). There was no significant performance difference between the 10 min group and those mice surviving 12 min ischemia.
Figure 4.
Post-thoracic aortic clamping rotarod performance. Latency to fall from the rotating rod (seconds) is presented as mean ± S.E.M. All ischemia durations produced a deficit (p <0.05) compared to sham. * P <0.05, vs. 0 min, ** P <0.01, vs. 8 min, # P < 0.05, vs. 12 min ischemia.
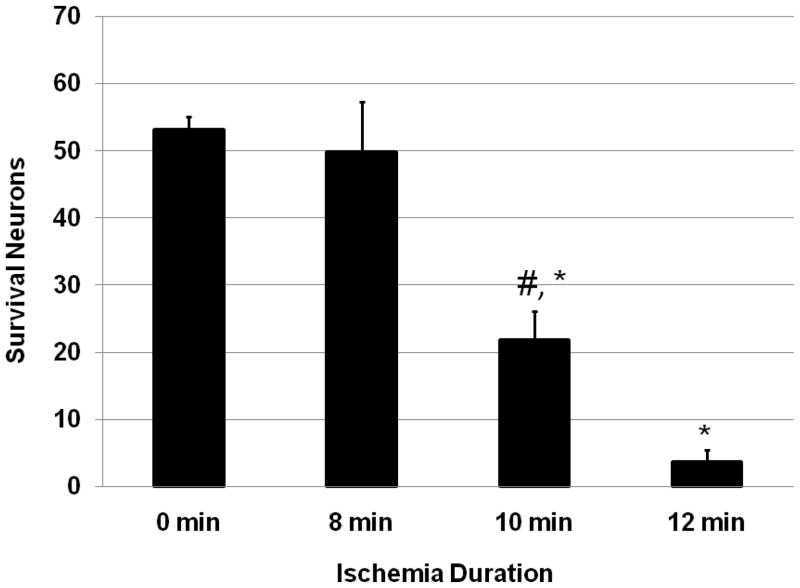
The severity of histological damage was associated with aortic cross-clamping duration (Figure 5). Sham mice had no necrotic or eosinophic neurons in the gray matter of the lumbar spinal cord. In mice subjected to aortic cross-clamping there were numerous neutrophils and mononuclear phagocytes in the ischemic gray matter. Some areas in the ventral horn were occupied by these inflammatory cells, especially in mice subjected to 10 or 12 min of ischemia (Figure 6). Eight min of ischemia produced less histological damage and the number of surviving neurons in the ventral horn was not significantly different from sham mice (p = 0.6). However, 10 and 12 min of ischemia resulted in severe neuronal loss in the ventral horns compared to either sham or 8 min ischemia (p <0.01). Mice subjected to 12 min ischemia had more severe histological damage than those exposed to 10 min ischemia (p = 0.045).
Figure 5.
Number of ventral horn neurons surviving ischemia. Normal neurons in the ventral horn were counted at 7 days post-ischemia and expressed as mean ± S.E.M. * P < 0.01, vs. 0 min, # P < 0.05, vs. 12 min ischemia.
Figure 6.
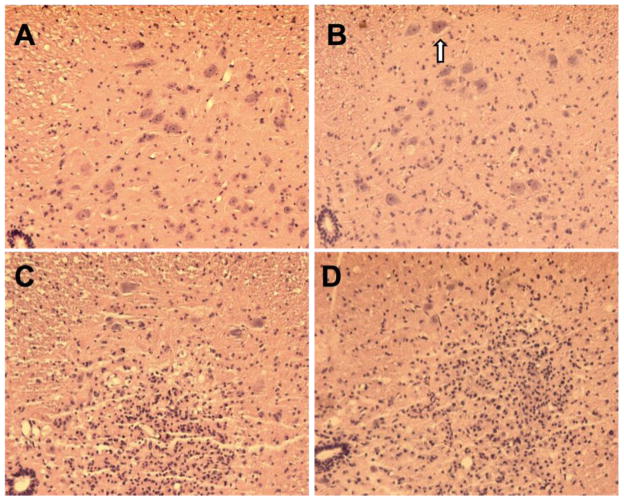
Histological change of lumbar spinal cord at 7 days after thoracic aortic clamping. All sections were stained with hematoxylin and eosin. A. 0 min of ischemia, normal histology was seen in the ventral horn; B. 8 min of ischemia, few infiltrated eosinphilic neurons (arrow) were found in the ventral horn; C. 10 min of ischemia, infiltrated inflammatory cells were evident in the ventral horn and the number of surviving neurons was decreased. D. 12 min of ischemia, a large area of the ventral horn was infarcted and occupied by infiltrated inflammatory cells. Few neurons remained.
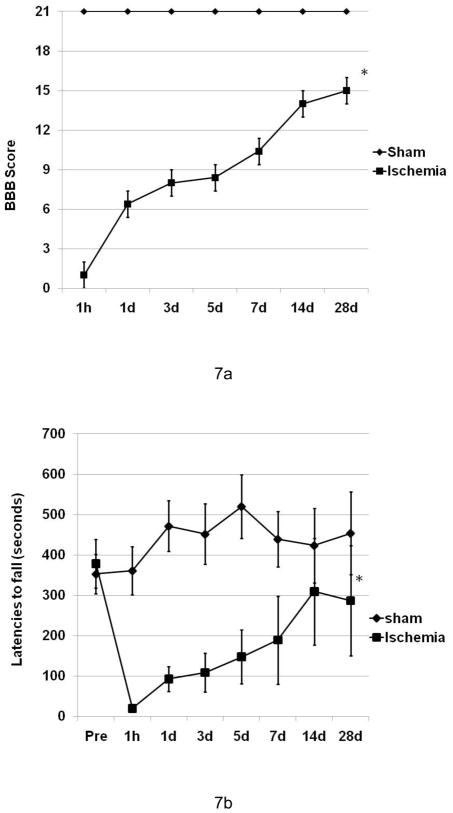
In the long-term survival experiment, 80% of the injured mice survived for the full 28-day observation interval. All mice in sham group survived and had no functional deficits. A significant difference was found in both open filed BBB score and rotarod performance (p <0.01) (Figure 7a and 7b) at 28 days. Ten min thoracic aorta clamping resulted in severe functional deficit at 1 h and the injured mice had a 50–60% functional recovery at 28 days post-ischemia.
Figure 7.
Post-ischemia neurological function deficit in a recovery period of 28 days. BBB scores (a) and rotarod latencies (b) are expressed as mean ± S.E.M. (n=4–5). Ischemia resulted in a significant motor function deficit even at 28 days post-ischemia (P < 0.01).
4. Discussion
We developed a simplified mouse model of spinal cord ischemia using the aortic cross-clamping technique used in larger species. By lowering the level of aortic cross-clamping to the middle segment of the thoracic aorta, mice were able to survive long-term. In addition, by using a left thoracotomy, immediate access to the thoracic aorta was provided and did not require disruption of bony structures or interruption of flow in the inferior vena cava. Thus, it is less invasive and facilitates recovery from surgery necessary to create the ischemic insult. Mice in the sham group did not have any motor functional deficit even at 1 h after surgery when compared with baseline values, indicating that the thoracotomy procedure, itself, was well tolerated. The severity of neurological deficit was closely associated with ischemia duration and loss of ventral horn motor neurons. During aortic occlusion, blood flow velocity in the dorsal lumbar spinal cord fell to <10 % of baseline flow, confirming severe lumbar spinal cord ischemia. The results also demonstrated that 10 min of aorta cross-clamping is the optimal ischemia duration to study the pathological mechanism and therapy development in the C57Bl/6J mouse should long-term outcome measurements be desired. This is based on a mortality rate of 20%, but persistent functional deficits over a 28-day recovery interval. Strain differences have been reported for sensitivity to cerebral ischemia (Wellons et al. 2000). It is plausible that other mouse strains will respond differently to this form of spinal cord ischemia.
With 12 min ischemia, 4 of 7 mice died at post-injury 2–7 days for undefined reasons, but plausibly due to organ (kidney, gut) failure. Surviving mice showed a persistent neurological deficit. Lang-Lazdunski et al. (Lang-Lazdunski et al., 2000) gave a subcutaneous injection of heparin (400 IU/kg) in their model. We did not use heparin prior to ischemia. The use of heparin might provide a different post-ischemic recovery. In order to determine if the lack of heparin in this model caused thrombosis and further to spinal cord ischemia, we examined the spinal cord histology at 24 h following 12 min of aortic clamping. Dead neurons were found in the ventral horn. However, no thrombosis was in adjacent vessels (data not shown). We examined the distal aorta during aortic clamping and no abnormal signs were found. Finally, toe color rapidly returned to normal after removal of the aneurysm clip. Thus, we could not find evidence that is that 10 min of aortic clamping led to aortic thrombosis, complicating ischemia caused by aortic cross-clamping.
The vertebral artery arises from the subclavian artery and gives off branches to supply the spinal cord. To obtain more reliable ischemia, Lang Lazdunski et al. (Lang-Lazdunski et al., 2000) clamped the subclavian artery to further decrease spinal cord blood flow. In our model, spinal cord blood flow was decreased by an average of >90% from of baseline after thoracic aortic clamping only. This was similar to the post-ischemic blood flow change of Tiara and Marsala (Taira and Marsala, 1996) who subjected rats to mid-thoracic endo-aortic balloon occlusion. To our knowledge a method of intra-aortic balloon occlusion has not been developed in the mouse. Thoracic aortic occlusion was sufficiently severe to induce spinal cord ischemia in our model, and it appears that it is not necessary to add subclavian artery occlusion, plausibly decreasing post-operative surgical morbidity. There were no deaths in mice subjected to sham surgery or ischemia intervals of 10 min or less during one week post-ischemia. This indicates that the surgical procedure allows full recovery by avoiding surgical manipulation of vital structures in the mediastinum. The total anesthesia/surgery time required to perform the procedure was less than 30 min. Ten min of ischemia induced a reliable injury and injured mice had 100% motor deficit in the first 24 hours and recovered about 30–50% of normal motor function by 7 days post-ischemia.
Several groups have reported that spontaneous recovery of sensory motor function occurs after spinal cord injury in rodents (Farooque et al., 2006; Gulino et al., 2007; Lapointe et al., 2006; Lee et al., 2009; Weidner et al., 2001; Wolpaw and Tennissen, 2001). This recovery is strain-dependent (Lapointe et al., 2006), gender-related (Farooque et al., 2006) and use-dependent (Wolpaw and Tennissen, 2001). A small proportion of nerve fibers spared by the original injury were found to grow new connections to other cells. Called sprouting, this re-growth occurs spontaneously without therapeutic intervention. When sprouting is prevented, functional recovery does not occur (Weidner et al., 2001). It was also found that synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection (Gulino et al., 2007). Enhanced H-reflex response promotes functional recovery (Lee et al., 2009). Our study also showed that motor deficit severity is associated with ischemia duration and that this also affects rate of spontaneous functional recovery. Ung et al. (Ung et al., 2007) reported that post-injury BBB scores reached a spontaneous recovery plateau by 28 days. Guertin et al. (Guertin, 2005) also chose post-injury 28 days as a period for quantitatively assessing hind limb movement recovery without intervention in adult paraplegic mice. Thereby, we decided to use a 28 day recovery interval in the long-term survival experiment.
Body temperature and blood glucose are two important factors affecting outcome in ischemic injury. Therefore, body temperature and glucose control are likely to be crucial to obtain a stable injury in this model besides fully cross-clamping the aorta.
There were few necrotic neurons remaining in the ventral horn at 7 days after either 10 or 12 min ischemia. We assume that most degenerated or dead neurons had undergone phagocytosis by this time. We stained some slides from one mouse subjected to 12 min ischemia and euthanasia at 24 hours post-injury. Numerous neurons in the ventral horn exhibited cytoplasmic eosinophilia and pyknotic homogenous nuclei. Infarct was mainly localized in the central part of the intermediate zone and the dorsomedial aspect of the dorsal horn and was characterized by destruction of normal tissue, infiltrated neutrophils and mononuclear phagocytes, and gliosis. This pattern is consistent with previous experimental studies of aortic occlusion-induced spinal cord ischemia in other animal models (Kanellopoulos et al., 1997; Marsala and Yaksh, 1994; Taira and Marsala, 1996; Yamamoto et al., 1994).
Because less surgical invasiveness is required in this model, the injured mice were able to quickly recover and did not have surgical complications confounding spinal cord ischemia. In the experiment evaluating post-ischemia long-term survival, 80% (4 out of 5) mice survived for the full 28 day observation interval, all of them having had severe motor functional deficits immediately after injury.
5. Conclusion
Aortic cross-clamping at a middle thoracic level (T8) via left lateral thoracotomy is a simple procedure to produce spinal cord ischemia in mice. This model generates reliable injury similar to the results shown in larger animal models. More importantly, mice readily recover from the surgical insult allowing study of long-term recovery from spinal cord ischemia to be extended to the mouse.
Acknowledgments
This study was supported, in part, by United States Public Health Service Grant R21NS063108.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–56. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Cambria RP, Davison JK, Carter C, Brewster DC, Chang Y, Clark KA, Atamian S. Epidural cooling for spinal cord protection during thoracoabdominal aneurysm repair: A five-year experience. J Vasc Surg. 2000;31:1093–102. doi: 10.1067/mva.2000.106492. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Black JH, Szabo C, Frosch M, Albadawi H, Chen M, Cambria RP, Watkins MT. Poly(adenosine diphosphate ribose) polymerase inhibition modulates spinal cord dysfunction after thoracoabdominal aortic ischemia-reperfusion. J Vasc Surg. 2005;41:99–107. doi: 10.1016/j.jvs.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Coselli JS, Lemaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–9. doi: 10.1067/mva.2002.122024. [DOI] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–7. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Gaviria M, Bonny JM, Haton H, Jean B, Teigell M, Renou JP, Privat A. Time course of acute phase in mouse spinal cord injury monitored by ex vivo quantitative MRI. Neurobiol Dis. 2006;22:694–701. doi: 10.1016/j.nbd.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Gaviria M, Haton H, Sandillon F, Privat A. A mouse model of acute ischemic spinal cord injury. J Neurotrauma. 2002;19:205–21. doi: 10.1089/08977150252806965. [DOI] [PubMed] [Google Scholar]
- Guertin PA. Semiquantitative assessment of hindlimb movement recovery without intervention in adult paraplegic mice. Spinal Cord. 2005;43:162–6. doi: 10.1038/sj.sc.3101701. [DOI] [PubMed] [Google Scholar]
- Gulino R, Dimartino M, Casabona A, Lombardo SA, Perciavalle V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci Res. 2007;57:148–56. doi: 10.1016/j.neures.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos GK, Kato H, Wu Y, Dougenis D, Mackey M, Hsu CY, Kouchoukos NT. Neuronal cell death in the ischemic spinal cord: the effect of methylprednisolone. Ann Thorac Surg. 1997;64:1279–85. doi: 10.1016/S0003-4975(97)00903-X. discussion 86. [DOI] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Matsushita K, Hirt L, Waeber C, Vonsattel JP, Moskowitz MA, Dietrich WD. Spinal cord ischemia. Development of a model in the mouse. Stroke. 2000;31:208–13. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- Lapointe NP, Ung RV, Bergeron M, Cote M, Guertin PA. Strain-dependent recovery of spontaneous hindlimb movement in spinal cord transected mice (CD1, C57BL/6, BALB/c) Behav Neurosci. 2006;120:826–34. doi: 10.1037/0735-7044.120.4.826. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jakovcevski I, Radonjic N, Hoelters L, Schachner M, Irintchev A. Better functional outcome of compression spinal cord injury in mice is associated with enhanced H-reflex responses. Exp Neurol. 2009;216:365–74. doi: 10.1016/j.expneurol.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Marsala M, Yaksh TL. Transient spinal ischemia in the rat: characterization of behavioral and histopathological consequences as a function of the duration of aortic occlusion. J Cereb Blood Flow Metab. 1994;14:526–35. doi: 10.1038/jcbfm.1994.65. [DOI] [PubMed] [Google Scholar]
- Safi HJ, Miller CC, 3rd, Azizzadeh A, Iliopoulos DC. Observations on delayed neurologic deficit after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1997;26:616–22. doi: 10.1016/s0741-5214(97)70060-0. [DOI] [PubMed] [Google Scholar]
- Safi HJ, Miller CC, 3rd, Huynh TT, Estrera AL, Porat EE, Winnerkvist AN, Allen BS, Hassoun HT, Moore FA. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg. 2003;238:372–80. doi: 10.1097/01.sla.0000086664.90571.7a. discussion 80-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens MA, Heijmen RH, Ranschaert W, Sonker U, Morshuis WJ. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg. 2009;37:640–5. doi: 10.1016/j.ejvs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Stone DH, Albadawi H, Conrad MF, Entabi F, Stoner MC, Casey PJ, Cambria RP, Watkins MT. PJ34, a poly-ADP-ribose polymerase inhibitor, modulates visceral mitochondrial activity and CD14 expression following thoracic aortic ischemia-reperfusion. Am J Surg. 2009a;198:250–5. doi: 10.1016/j.amjsurg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Stone DH, Brewster DC, Kwolek CJ, Lamuraglia GM, Conrad MF, Chung TK, Cambria RP. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44:1188–97. doi: 10.1016/j.jvs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Stone DH, Conrad MF, Albadawi H, Entabi F, Stoner MC, Cambria RP, Watkins MT. Effect of PJ34 on Spinal Cord Tissue Viability and Gene Expression in a Murine Model of Thoracic Aortic Reperfusion Injury. Vasc Endovascular Surg. 2009b;43:444–51. doi: 10.1177/1538574409333582. [DOI] [PubMed] [Google Scholar]
- Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–68. discussion 68–70. [PubMed] [Google Scholar]
- Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27:1850–8. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- Tiesenhausen K, Amann W, Koch G, Hausegger KA, Oberwalder P, Rigler B. Cerebrospinal fluid drainage to reverse paraplegia after endovascular thoracic aortic aneurysm repair. J Endovasc Ther. 2000;7:132–5. doi: 10.1177/152660280000700207. [DOI] [PubMed] [Google Scholar]
- Tschop J, Czerner S, Nuscheler M, Thiel M. Epidural cooling. Neuroprotective treatment of thoracoabdominal aortic aneurysms. Anaesthesist. 2008;57:988–97. doi: 10.1007/s00101-008-1414-y. [DOI] [PubMed] [Google Scholar]
- Ung RV, Lapointe NP, Tremblay C, Larouche A, Guertin PA. Spontaneous recovery of hindlimb movement in completely spinal cord transected mice: a comparison of assessment methods and conditions. Spinal Cord. 2007;45:367–79. doi: 10.1038/sj.sc.3101970. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–8. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–43. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Takano H, Kitagawa H, Kawaguchi Y, Tsuji H. Changes of evoked action potentials and histology of the spinal cord, and hind limb dysfunction in spinal cord ischemia of cats. J Spinal Disord. 1994;7:285–95. [PubMed] [Google Scholar]