Abstract
Chronic inflammation is strongly associated with approximately 1/5th of all human cancers. Arising from combinations of factors such as environmental exposures, diet, inherited gene polymorphisms, infections, or from dysfunctions of the immune response, chronic inflammation begins as an attempt of the body to remove injurious stimuli; however, over time, this results in continuous tissue destruction and promotion and maintenance of carcinogenesis. Here we focus on intestinal inflammation and its associated cancers, a group of diseases on the rise and affecting millions of people worldwide. Intestinal inflammation can be widely grouped into inflammatory bowel diseases (ulcerative colitis and Crohn's disease) and celiac disease. Long-standing intestinal inflammation is associated with colorectal cancer and small-bowel adenocarcinoma, as well as extraintestinal manifestations, including lymphomas and autoimmune diseases. This article highlights potential mechanisms of pathogenesis in inflammatory bowel diseases and celiac disease, as well as those involved in the progression to associated cancers, most of which have been identified from studies utilizing mouse models of intestinal inflammation. Mouse models of intestinal inflammation can be widely grouped into chemically induced models; genetic models, which make up the bulk of the studied models; adoptive transfer models; and spontaneous models. Studies in these models have lead to the understanding that persistent antigen exposure in the intestinal lumen, in combination with loss of epithelial barrier function, and dysfunction and dysregulation of the innate and adaptive immune responses lead to chronic intestinal inflammation. Transcriptional changes in this environment leading to cell survival, hyperplasia, promotion of angiogenesis, persistent DNA damage, or insufficient repair of DNA damage due to an excess of proinflammatory mediators are then thought to lead to sustained malignant transformation. With regards to extraintestinal manifestations such as lymphoma, however, more suitable models are required to further investigate the complex and heterogeneous mechanisms that may be at play.
1. Evidence of Inflammation and Cancer
Chronic inflammation is characterized by persistently activated immune cells in which there is a vicious cycle of tissue destruction and repair due to either irremovable injurious stimuli or a dysfunction in any component of the normal inflammatory response. Sources of chronic inflammation include infectious agents, physical and chemical agents such as environmental exposures and dietary carcinogens, sustained wounds or trauma, gastric fluids, bile acids, or urine reflux, and dysfunctions of the immune system. The consequential tissue atrophy and increased cellular proliferation in an environment of damaging free radicals can lead to many pathological conditions, including atherosclerosis, autoimmune diseases, inflammatory diseases, and cancer [1]. Chronic inflammation is strongly associated with and is proposed to be at the root of approximately 1/5th of all human cancers [2].
After more than 100 years of correlating inflammation and cancer, it is now widely acknowledged that chronic inflammatory conditions can both pave the way for and sustain conditions favorable for carcinogenesis and tumor progression [3,4]. Although the molecular mechanisms of this causal relationship remain to be elucidated further, there is strong evidence in support of this correlation stemming from abundant epidemiological and molecular histopathological studies.
1.1 Epidemiological Correlations
1.11 Infectious agents
The principal cause of 18% of all cancer cases worldwide affecting 1.2 million people per year are infectious agents [5,6]. Liver cancers are strongly associated with hepatitis B/C virus infection; cholangiocarcinoma with liver flukes; stomach cancer and mucosa-associated lymphoid tissue (MALT) lymphoma with Helicobacter pylori infection; urinary tract and colorectal cancers with schistosomiasis; lymphoid tissue cancers with Epstein-Barr virus; non-Hodgkin's lymphoma and Kaposi's sarcoma with human immunodeficiency virus and/or human herpes virus type 8; cervix and uterine cancers with certain strains of human papilloma virus; and ovarian cancers with gonorrhea, chlamydia, or human papilloma virus [3,4,7]. These chronic infections serve as irremovable, injurious stimuli to the immune system, and contribute to the persistent activation of immune cells, or chronic inflammation. It is important to note that viral agents such as human papilloma virus may also act through other mechanisms aside from chronic inflammation; such as through direct production of oncogenic proteins [6].
1.12 Chemical/Physical agents
Many inflammation-derived pathological conditions with known etiology have been well documented, including lung airway inflammation due to smoking and air pollution. Lung cancer has become the leading cause of cancer death among women, affecting more than 71,000 in 2007, attributed to increasing smoking rates among women [8]. In addition to smoking, exposure to air pollution, specifically fine particulate matter from vehicle exhaust, has been associated with lung cancer and adverse cardiovascular/respiratory events, including development of chronic obstructive pulmonary disease (COPD), respiratory infections, and heart failure through occupational and population based studies [9,10]. Smoking and exhaust particles destroy cilia and lung epithelial tissue, increase mucus production, and thus increase susceptibility to respiratory infections, which also contribute to inflammation [11]. Studies have confirmed consistently higher levels of C-reactive protein (CRP) in circulating blood of lung cancer patients even after a five-year latency period [12], demonstrating constant activation of the immune system and suggesting the presence of inflammatory mediators in the lung cancer microenvironment [13]. In addition, peroxides and nitrosamines found in exhaust and in smoking are suggested to produce reactive oxygen and nitrogen species (RONS) through metabolic activation of reactive intermediates or activation of CYP enzymes, which in turn is thought to be mutagenic and immunotoxic [14,15]. Other similar forms of recurring tissue atrophy and persistent activation of the immune response, as well as mutagenicity resulting from long-term exposures include asbestos fiber and silica particle-derived lung cancers, bladder inflammation and consequential cancer due to urinary catheters or chronic indwelling, gastric acid reflux and esophageal cancer, talcum powder use and endometriosis/ovarian cancer [16], and exposure to UV light and skin inflammation/melanoma. Although these associations to neoplasms are evident among many populations, the nature of the local, as well as systemic immune response to the stimuli and hence tumor microenvironment varies from person to person due to polymorphisms in antioxidant, DNA repair, and immune response/cytokine genes. Duration and dose of exposures are also important determinants of individual response variation.
1.13 Autoimmunity and other unknown etiologies
In addition to chronic infection or environmental exposures, chronic inflammatory states can arise from many other pathological conditions and etiologies, including immune imbalances such as in autoimmunity, and other unknown mechanisms. Patients with autoimmune disease such as Sjőgren's syndrome, rheumatoid arthritis, multiple sclerosis, systemic lupus erythromatosus, and inflammatory bowel diseases that display characteristics similar to those of autoimmune diseases demonstrate increased susceptibility to hematopoetic cancers such as non-Hodgkin's T-cell lymphomas and mucosa-associated lymphoid tissue lymphomas compared to the general population [17-23]. Autoimmune diseases are generally more prevalent in females compared to males, possibly due to differences in hormonal factors, though the exact reasons for this are unknown. Mechanisms responsible for the development of lymphomas arising from autoimmunity also remain to be elucidated; however, chronic antigenic stimulation, deficiencies in immune surveillance, and in the case of intestinal autoimmune diseases, increased intestinal permeability to environmental carcinogens, have been proposed [24,25]. Given the relatively long latency between disease and lymphomagenesis, a multistep process of genetic instability and consequential alterations is probable for disease progression. For example, in rheumatoid arthritis patients, inflammation-induced DNA damage in the form of microsatellite instability, as well as strand breaks in peripheral lymphocytes and the repression of DNA mismatch repair genes, have been observed [26,27].
In addition to autoimmune diseases, the etiology of inflammation-associated prostate cancer remains to be elucidated further. It has been recently proposed that chronic inflammation contributes to the dysplasia–adenocarcinoma sequence in the prostate. Sources of inflammation have not been clarified; although infection, immune dysfunction, dietary carcinogens such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), and urine reflux have all demonstrated the potential to chronically activate the innate immune system [2].
Although idiopathic inflammatory diseases of the colon, such as ulcerative colitis, Crohn's disease, and celiac disease, are associated with the development of small bowel or colonic lymphomas as described previously, inflammatory bowel diseases (IBDs) are associated most strongly with the development of colorectal cancer, increasing the relative risk 0.5-1% per year after 7 years of colitis [28-30]. Crohn's disease patients share a similar increased risk for small bowel adenocarcinoma, as do celiac disease patients, who are at increased risk for small bowel adenocarcinomas as well as cancers of the pharynx and esophagus [25,31]. Extraintestinal cancers, such as hepatobiliary cancers, also have been observed in IBD patients at variable rates [31]. Because the causes of these inflammatory diseases are complex and multi-faceted, many models have been created to mimic characteristic symptoms in rodents in hopes of clarifying how underlying inflammation in the colon can lead to inflammation-associated cancers.
1.2 Histopathological evidence and the inflammatory microenvironment
In addition to the strong correlations of cancer occurrence preceded by chronic inflammatory states derived from epidemiological evidence, there is a wealth of molecular histopathological evidence of inflammation playing a vital role throughout the process of carcinogenesis in many types of cancers. The presence of an inflammatory infiltrate in and around tumors has been recognized for over a century. Positive staining of CD45+ leukocytes in the tumor and in areas surrounding the tumor suggests attraction of immune cells towards and into the tumor [3]. The inflammatory component of tumors, as well as the surrounding stroma, has been found to contain many types of inflammatory cells, including mononuclear cells such as tumor-associated macrophages, dendritic cells, eosinophils, mast cells, and lymphocytes [32,33]. These cell types as well as the tumor cells themselves are able to produce cytokines and chemokines that will further attract inflammatory cells and result in production of a wide array of reactive oxygen and nitrogen species, various proteases, and pro-angiogenic growth factors that can favor tumor growth and sustainability.
In addition to the inflammatory cell infiltrate of developing tumors, there is also evidence of the presence and involvement of inflammatory cells in pre-neoplastic tissue such as polyps or dysplastic tissue. Pre-malignant tumors may be seen as “wounds to be healed” by the immune system, in which tumor growth is mediated by neighboring stromal cells via upregulation of genes involved in prostaglandin synthesis to mediate tissue repair such as cyclo-oxygenase 2 (COX-2), cell survival via upregulation of nuclear factor (NF)-κB, promotion of cell proliferation, and eventually angiogenesis [34,35]. Tumors arising without persistent infection or irritation, such as mammary adenocarcinoma, also contain an inflammatory infiltrate composed of activated macrophages; further supporting the role of the inflammatory microenvironment in tumorigenesis.
1.3 Use of anti-inflammatory agents in prevention of carcinogenesis
Finally, the involvement of inflammation in carcinogenesis has been solidified further by the use of non-steroidal anti-inflammatory drugs (NSAIDs) in the prevention of many types of cancers, including colon cancer and prostate cancer [36,37]. Risk has been shown to be reduced dramatically when NSAIDs such as COX-2 inhibitors are administered in low doses over long periods of time for multiple types of cancers. Most NSAIDs inhibit COX function and prevent platelet synthesis of prostaglandins or platelet aggregation, which is thought to be a mechanism in the inhibition of carcinogenesis. However, some NSAIDs that are known to have no effect on COX function also have chemopreventative effects, potentially through inducing apoptosis, enhancing immune surveillance, or by cell-cycle regulation [38]. It is worth noting that several high-dose COX-2-specific NSAID treatments have resulted in adverse cardiovascular events, including myocardial infarction and stroke. This has caused several large clinical studies to be halted [39,40,41]. Mechanisms are believed to center around a physiological imbalance between the accumulation of the COX-1-dependent thromboxane and decreased production of the COX-2-dependent prostacyclin, which opposes the thrombogenic effects of thromboxane [42]. Therefore, other treatments, such as mild COX-2 selective inhibitors or nonselective COX inhibitors, are preferred for prevention or treatment.
In addition to the protective effect of NSAIDs, there are many other anti-inflammatory therapeutic agents that have been used in studies of prevention of inflammation-associated carcinogenesis. Monoclonal anti-tumor necrosis factor α (TNF-α), currently used in therapy as infliximab, has been administered to dampen TNF-α mediated inflammation [43]; dexamethasone as a glucocorticoid receptor agonist [44]; statins acting in cholesterol checkpoint regulation of inflammation [45]; agonists of vitamin D3 as inducers of innate and adaptive regulatory function [46]; peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists as repressing inflammatory transcriptional programs [47]; and IL-17A as mediating protection in T cell-mediated colitis [48], in addition to the many other monoclonal antibodies targeting components of the innate immune response. The administration of prebiotics/probiotics have also gained interest in their abilities to restore balance to the gastrointestinal microbiota and reduce intestinal inflammation, although whether or not these agents act as anti-inflammatory agents is yet to be clarified [49]. However, there still remains ample room for improvement in prevention and therapy for inflammation-associated cancers.
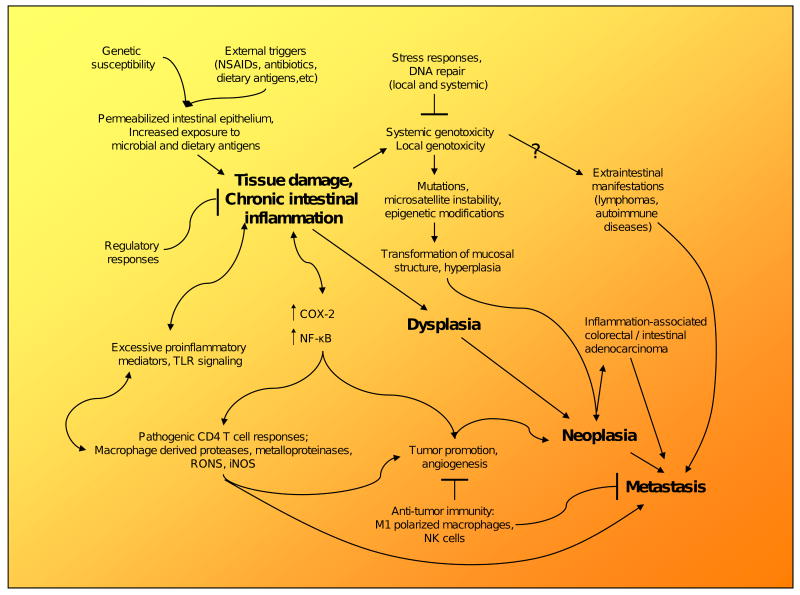
2. Overview of Mechanisms in Inflammation-Associated Carcinogenesis
Mechanisms involved in the progression of chronic inflammation to cancer, as well as the role of inflammation in cancer and cancer-related inflammation have been scrutinized extensively, leading to the acceptance of several paradigms. Not only can processes involved in chronic inflammation promote malignant transformation of tissue, termed the extrinsic pathway, but tissues “initiated” by a series of genomic mutations such as in oncogenes can also change the transcriptome of cells to express an inflammatory microenvironment, termed the intrinsic pathway [50,51]. Both of these pathways are thought to be involved in further promotion of chronic inflammation and tumor growth through induction of genetic instability, upregulation of key regulators such as NF-κB and pro-inflammatory cytokines, suppression of antitumor immunity, and proliferation and survival of malignant cells.
In the setting of chronic inflammation, monocytes are activated by T cell-derived cytokines and differentiate into immature dendritic cells and macrophages in tissues. Plasma cells become terminally differentiated B cells, and T and B cells both are in turn antigen-activated by macrophages and dendritic cells. In tissue that has already been “initiated” carrying key genetic mutations and in the “promotion” stage of carcinogenesis, continuous recruitment of monocytes and maintenance of proliferation of macrophages is sustained through cytokines, such as interferon-γ (IFN-γ), both through the intrinsic and extrinsic pathways involving the tissue itself, the surrounding stroma, and infiltrating inflammatory cells. Persistent activation and recruitment of leukocytes, especially innate immune cells, including macrophages and dendritic cells, can cause continuous tissue destruction and atrophy via release of a myriad of substances. The latter include neutral proteases, matrix metalloproteinases, elastase, collagenase, plasminogen activator, acid hydrolase, phosphatases, lipases, plasma proteins, complement components, reactive metabolites of oxygen, eicosanoids, cytokines/chemokines (IL-1, IL-8, TNF-α), growth factors (PDGF, EGF, FGF, VEGF, TGF-β), and nitric oxide among other reactive metabolites [4,52,53]. Cytokines released in response to this tissue destruction can induce upregulation of key genes thought to be necessary for malignant transformation of chronically inflamed tissue, such as COX-2 and NF-κB, which are thought to inhibit apoptosis, promote angiogenesis, modulate cellular adhesion, and induce proinflammatory mediators such as prostaglandin E2, in addition to further recruitment of other innate and adaptive cell types [52,54,55]. Inflammasomes present in cells of the innate immune system, particularly macrophages, also have the ability to activate pro-inflammatory caspases that can activate IL-1β, leading further to a potent inflammatory response [56].
The damaged epithelial cells consequential to the macrophage-derived reactive intermediates are usually replaced by cell division from resident progenitor cells. Cell division in the setting of these DNA damaging agents results in increased risk of mutation and genetic instability, leading to malignant transformation. In addition, resident stem cells can acquire genetic and epigenetic changes necessary for transformation into cancer stem cells in this environment [57]. Furthermore, chronic inflammation and associated tissue injury could also lead to depletion of the indigenous stem cell niche either through continuous proliferation or interference with niche signaling [58]. These empty stem cell niches could then be reoccupied by bone marrow-derived stem cells or tissue progenitor cells, which if incompletely reprogrammed by niche signals, may become cancer stem cells or contribute to the tumor mass [59]. Chronic inflammation also promotes bone marrow stem cells to enter circulation, increases their homing to sites of inflammation, and promotes heterotypic cell fusion of myelo-lymphoid cells and non-hematopoietic cells [60].
Once a tissue has transformed into a malignant tumor, recruited inflammatory cells can migrate readily through the extracellular matrix in response to proteolytic enzymes, and given their inherent mobile nature, may facilitate epithelial cell invasion into stromal compartments and tumor growth. Cytokines released by the tumor-associated macrophages (TAMs) can promote epithelial cell proliferation and can stimulate angiogenesis and lymphangiogenesis. Given the appropriate conditions, properly activated TAMs (M1 polarized) are able to correctly attack tumor cells, the signals from the microenvironment of the tumor, and the surrounding stroma involving NF-κB gear macrophage activation (M2 polarized) towards a growth advantage of the tumor [61,62]. By secreting extracellular proteases and cytokines such as IL-10, tumor cells and macrophages are able to inhibit cytotoxic T cells and, therefore, aid in suppression of the immune response against the tumor [4,52]. The inflammatory microenvironment of malignant tissue, including the tumor stroma, therefore aids in orchestrating further progression of the tumor. Further detailed mechanisms of inflammation-associated carcinogenesis have also been reviewed in detail elsewhere [35,50,63].
3. Intestinal Inflammation and Associated Cancers
3.1 Chronic intestinal inflammation
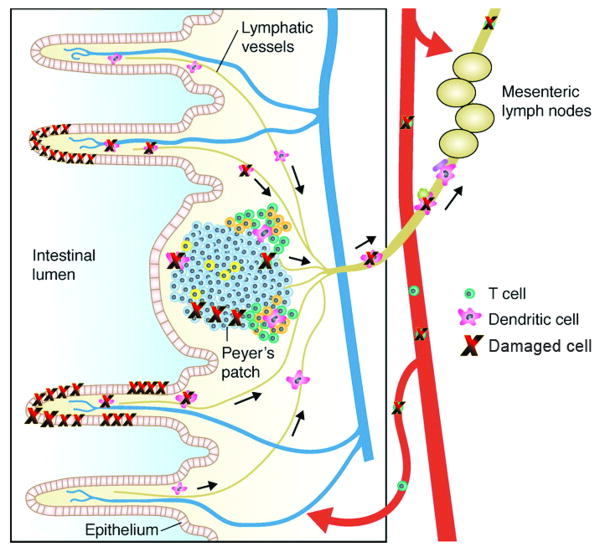
Chronic inflammatory diseases of the intestinal tract include IBDs and celiac disease, which account for the majority of known cases. The most common forms of IBDs include ulcerative colitis and Crohn's disease. Clinically, intestinal inflammation in ulcerative colitis is limited most commonly to the colon, whereas Crohn's disease and celiac disease encompass both the small and large intestinal tract. The GI tract is a very complicated system to study because billions of microbes reside in both the proximal and distal portions of the colon, co-existing in a symbiotic fashion. Microbes are generally considered to be pathogenic to the body, but in the colon, various programs involving basal, constitutive immune surveillance have been established by the immune system to allow for tolerance to these organisms without mounting an overt immune response. A dysregulated immune response, including loss of tolerance against commensal bacteria in the intestinal lumen caused by transient breaks in the mucosal barrier, has been heavily implicated in the pathogenesis of colitis and other forms of inflammatory bowel disease [64].
3.1.1 Ulcerative colitis
Ulcerative colitis is an intermittent disease characterized by cycles of exacerbation or “disease flares” followed by periods of remission. Patients mainly suffer from abdominal pain, vomiting, diarrhea, hematochezia, and weight loss. Pathology is characterized by continuous ulceration of mucosal tissue in the colon, also usually involving the rectum and extending proximally. Pathology does not involve the small intestines. In addition, presence of crypt abscesses, extensive lymphocyte infiltration into the lamina propria, and loss of crypt architecture are common features of the disease [65]. Pathogenesis is thought to involve loss of integrity of the mucosal barrier of the intestinal epithelium, in which the mucosal immune system elicits a dysregulated immune response against the normal commensal microflora in immunocompetent individuals. The cytokine profile of inflammatory cells demonstrates excess IL-13, IFN-γ, TNF-α, IL-8, and IL-4 production, with a generally polarized Th2-like immune response [66].
Treatment is adjusted depending on the individual, involving aminosalicylates, corticosteroids, immunomodulators, and TNF-α blockers such as infliximab. Surgical removal of the colon is also performed as a potentially curative procedure in extreme cases.
3.1.2 Crohn's disease
Crohn's disease is another form of IBD in which patients may develop severe inflammation of not only the colon but also of the entire span of the gastrointestinal tract, commonly involving the small intestine. Crohn's disease is also a chronic relapsing disease in which disease flare-ups are followed by periods of remission. Patients suffer from abdominal pain, vomiting, diarrhea, malnutrition, and weight loss. Pathology is characterized by patchy discontinuous ulcerations in which inflammation is transmural, spanning the entire length of the intestinal wall, in contrast to ulcerative colitis where only the mucosa is affected. Granulomas as well as crypt abscesses, leukocyte infiltration into lamina propria, blunting of the intestinal villi, formation of fistulae (obstructing abnormal connections of the intestines), and intestinal stenosis are common features of the disease [67]. Crohn's disease is associated with elevated IL-12/IL-23 and IFN-γ/IL-17 production, or a Th1/Th17 polarized immune response. The disease has a strong genetic link to mutations in the CARD15 (NOD2) gene, TNFSF, IL23R, and more recently XBP1, which serve as partial explanations for pathogenesis of Crohn's disease [68]. CARD15/NOD2 mutations result in decreased microbial detection and reduced production of Paneth-cell and mucosal defensins, which are endogenously occurring antibiotics with microbicidal activity. Lack of defensins results in decreased epithelial barrier function as a part of the innate immune response [69]. CARD15/NOD2 mutations are also thought to result in uncontrolled NF-κB activation and consequential release of pro-inflammatory cytokines [70]. TNFSF encodes for the tumor necrosis factor super family of genes involved in pro-inflammatory responses as well as in apoptosis, whereas IL23R encodes the receptor for the cytokine IL-23, which is highly expressed in Crohn's disease and representative of the adaptive immune response. XBP1, also found to be mutated in ulcerative colitis patients, is an endoplasmic reticulum (ER) stress-response protein, which when lacking in epithelial cells, promotes a pro-inflammatory response [71]. Therefore, the combination of increased permeability and decreased barrier function of the epithelial cells, as well as the dysregulated immune response against the commensal bacteria, are thought to be responsible for pathogenesis. Treatment regimens are similar to those for ulcerative colitis, including TNF-α blockers such as infliximab, corticosteroids, and immunomodulatory agents. Disease-specific monoclonal antibodies such as anti-IL-12/p40, which targets both IL-12 and IL-23, the key cytokines of the Th1-mediated immune response, have recently shown surprising efficacy and hold promise for therapy [65,72].
3.13 Celiac disease
Celiac disease is a more common disorder, occurring in 1% of the population [73], separately classified from IBDs and genetically determined. Most patients carry the heritable human leukocyte antigen (HLA)-DQ2 or –DQ8 haplotype, which codes for a complex on antigen-presenting cells for presentation of gluten-derived peptides to T cells. Inflammation is caused by dietary gluten, present in wheat, barley, and rye in which the gluten-derived peptides are presented to T cells, provoking a Th1 pro-inflammatory response and damaging the intestinal epithelial layer [74]. The expression of the HLA-DQ2 or -DQ8 molecules is, therefore, not sufficient for disease development. Patients may or may not demonstrate gastrointestinal symptoms, depending on the clinical manifestation of the disease. Latent celiac disease, for example, may develop into celiac disease in the future, or may be present in a person who had the disease who is presently able to eat gluten and has normal intestinal pathology. Similarly, silent or asymptomatic celiac disease occurs in patients without gastrointestinal symptoms, although they may manifest non-intestinal symptoms such as other autoimmune disorders, including dermatitis herpetiformis. Active or classical celiac disease is characterized by diarrhea, abdominal pain, weight loss, metabolic bone disease, and anemia [75]. The proximal small intestine, potentially involving the entire small intestine, is affected, demonstrating varying grades of villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis, depending on the patient and severity of disease [76]. Positive serological tests for autoantigens, including antibodies to tissue transglutaminase, also characterize celiac disease. Pathology is thought to be dependent on the development of a chronic anti-gluten T cell response involving secretion of IFN-γ and proliferation of an inflammatory response in the lamina propria and intestinal epithelium, assuming ingestion of gluten [77]. Exposure to gliadin peptides, derived from digested gluten, results in preferential presentation by HLA-DQ2 or -DQ8 molecules to pathogenic CD4 T cells in the lamina propria, resulting in a Th1 pro-inflammatory response and damage to the intestinal epithelium [78]. In addition, intraepithelial TCRγδ T cells in the small intestine can interact with NKG2D ligands on stressed or damaged enterocytes through the NKG2D-activating receptor, which induces further cytotoxicity, contributing to villous atrophy [79]. Treatment of celiac disease involves a strict gluten-free diet for life, which usually results in a rapid clinical response, commonly without the reversal of histopathological alterations of the gut.
3.2 Association of chronic intestinal inflammation with colorectal cancer and small bowel adenocarcinomas
Chronic inflammation, through complex mechanisms involving persistently activated immune cells and DNA damage in the setting of proliferation, is associated most commonly with development of cancer at the site of inflammation. In ulcerative colitis patients, in which inflammation is restricted most commonly to the colon, duration and severity of inflammation correlate with the cumulative probability of colorectal cancer development, ranging from 2% at 10 years of colitis to 18% after 30 years [80]. Crohn's disease patients with the most severe inflammation restricted to the small intestine have a 12- to 60-fold increased risk of developing small-bowel adenocarcinoma compared to the normal population [81]. Unfortunately, the prognosis of small-bowel adenocarcinoma is poor, with mortality at 1-2 years being as high as 30-60% [82,83]. Crohn's disease patients with colonic involvement, or Crohn's colitis, have a relative risk for colorectal cancer of 23.8 compared to 4.3 in the general Crohn's population [84]. Others determined relative risk for colorectal cancer to be 5.6 for Crohn's patients with exclusively colonic involvement, 3.2 for those with ileocolitis, and 1.0 for those with only ileal involvement [81,85,86]. These observations demonstrate the heterogeneity of the disease and consequential differences in the risk for either colorectal cancer or small-bowel adenocarcinoma. Crohn's disease patients with a similar extent and severity of inflammatory involvement in the colon as ulcerative colitis patients have similar risks for development of colorectal cancer [86]. Celiac disease, which is generally restricted to the small intestine, is strongly associated with development of small-bowel adenocarcinoma, with patients having an 80-fold greater risk than the general population [24,75,87].
3.3 Association of chronic intestinal inflammation with extraintestinal cancers including lymphoma
Many cases of pediatric and young adult IBD patients developing non-Hodgkin's T cell lymphomas have been described. In these patients, the inflammatory process itself, as well as, immunosuppressive or chemotherapeutic therapy, can increase the risk of lymphomas, including colonic lymphoma and gut-mucosa-associated lymphoid tissue (GALT) lymphoma [88]. Case reports have described ulcerative colitis patients developing malignant colonic or rectal lymphoma [89-91], as well as non-lymphoid cancers such as rectal carcinoma and hepatobiliary carcinoma [31]. In addition to ulcerative colitis, Crohn's disease patients have also demonstrated evidence of lymphomas, including hepatic T cell lymphoma [92] and non-Hodgkin's T cell lymphomas [93], especially after immunomodulatory treatments such as azathioprine or anti-TNF therapy [94,95]. Although many meta-analyses have been performed on cohort studies or case series, to date, no case-controlled trial in IBD patients has been carried out to specifically evaluate severity of inflammation to lymphoma risk. However, H. pylori infection and its associated chronic inflammation have been found to increase the risk of gastric lymphoma [96]. In addition, in a large matched case-control study of rheumatoid arthritis patients, chronic inflammation itself, independent of treatment, was found to increase significantly the risk of lymphoma [97]. Therefore, chronic inflammation of the small and large intestine may also increase risk for extra-intestinal cancers, including lymphomas.
In addition to increased risk of small-bowel adenocarcinoma development, 8-13% of celiac disease patients develop non-Hodgkin's enteropathy-associated T cell lymphoma [23], which is a much higher percentage than when compared to ulcerative colitis or Crohn's disease patients. When celiac disease is left untreated, patients have a 43-fold higher relative risk of developing small-bowel lymphoma, a 12-fold higher relative risk of developing esophageal cancer, and a 10-fold higher relative risk of developing oralpharyngeal squamus carcinoma [23-25]. Other extra-intestinal malignancies include cancer development in the liver, spleen, thyroid, skin, nasal sinus, brain, and B cell lymphomas or other extra-intestinal lymphomas [23,24]. Elderly celiac disease patients with other autoimmune diseases in addition to celiac disease, such as dermatitis herpetiformis and autoimmune thyroiditis, also have a much higher risk of developing lymphoma [23]. Aside from extra-intestinal cancer development, celiac disease patients also may develop other autoimmune disorders, such as insulin-dependent diabetes, Sjőgrens syndrome, thyroid disease, cardiomyopathy, and autoimmune neurological disorders [25].
4. Mouse Models of Intestinal Inflammation and Cancer
A plethora of mouse models are now available to study an aspect of chronic inflammation thought to play an important role in the pathogenesis of IBDs and in inflammation-associated carcinogenesis. Most mechanistic studies of celiac disease involve the use of human biopsies, and the use of mouse models to study pathogenesis is limited. However, new transgenic mouse models mimicking the HLA-DQ variants and celiac disease progression are emerging. Though no single model captures all of the clinical features and manifestations of the human diseases, each has contributed to the understanding of the multifaceted and heterogeneous mechanisms thought to be involved. For more detailed reviews on current mouse models of inflammatory bowel diseases see [98,99].
4.1 Chemically induced models
Chemically induced models of inflammation and inflammation-associated carcinogenesis in healthy wild-type mice and in mice genetically susceptible to colitis or colitis-associated cancers are used commonly by many investigators due to their ease of induction of colitis-like symptoms and associated pathology as well as their high reproducibility. The most widely used models of chemically induced intestinal inflammation (Table 1) have been divided into those that disrupt the mucosal/epithelial barrier and/or that involve hapten-induced hypersensitivity reactions in the induction of intestinal inflammation.
Table 1.
Chemically-induced models of intestinal inflammation.
| Model | Description | Reference |
|---|---|---|
| DSS | Direct epithelial toxicity leading to acute and chronic colonic inflammation | [100, 101] |
| TNBS/DNBS in ethanol | Delayed type hypersensitivity immune response to hapten bound to proteins, inducing both acute and chronic inflammation, similar to Crohn's disease | [123,127] |
| Oxazolone in ethanol | Haptenizing agent inducing a Th2 type experimental colitis similar to ulcerative colitis | [132] |
| DNCB in acetic acid | Intrarectal administration leading to epithelial injury, and acute and chronic inflammation | [102] |
| Carrageenan | Degraded carrageenan leads to mucosal inflammation of the cecum within a week | [103],[104] |
4.1.1 Disruption of the epithelial barrier
One such experimental model of colitis and colitis-associated neoplasia is cyclic administration through the drinking water of dextran sulfate sodium (DSS), a non-genotoxic sulfated polysaccharide [100,101]. Acute and chronic colonic inflammation can be induced depending on dose and duration. In healthy wild-type mice, DSS administration is characterized by gross bleeding in the stool, diarrhea, and weight loss, especially after cyclic administration, with one cycle usually consisting of 5-7 days of DSS followed by 7-14 days of normal water. Pathology of the colon is similar to that seen in ulcerative colitis, consisting of ulcerations, erosion of the epithelial barrier, and extensive lymphocyte and granulocyte infiltration [100,105]. Administration of four cycles of DSS, followed by an extended four-month period of normal water, leads to dysplasia followed by development of colorectal cancer, a sequence also observed in IBD patients [101]. Notably, acute DSS administration is not sufficient to induce tumor development, thus leading to many studies utilizing carcinogen-induced initiation followed by DSS-induced tumor promotion. DSS is thought to induce direct metabolic epithelial toxicity (further discussed in 4.2.3) in the basal crypts, which compromises the mucosal barrier and allows mucosal intrusion of enteric microbiota and their products. Resultant inflammation in response to this disruption is driven predominantly by innate rather than adaptive immune cell types [100,106-108], making the DSS model useful for studying the contribution of innate mechanisms, as well as the disruption of the integrity of the epithelial barrier in colitis. Notably, the lack of a major role of the adaptive immune response in DSS colitis was found by the development of severe colitis in C.B-17SCID (SCID) and Rag1/2-/- mice due to DSS, at least during the acute phase [108]. Although DSS itself is not genotoxic [109], it can activate macrophages and other inflammatory cells both directly and indirectly [100,110], producing immune profiles similar to those seen in genetic models of immune-mediated colitis [111-114].
In addition to DSS administration alone, DSS can be administered following an initial dose of a colon carcinogen such as azoxymethane or 1,2-dimethylhydrazine to initiate carcinogenesis. Colorectal cancer development is, therefore, much faster in this scheme of treatment compared to many cycles of DSS treatment alone, and it is useful to study the role of inflammation in the promotion of carcinogenesis after initiation [115-118]. The DSS-induced colitis model has also been used in many other transgenic mice in addition to immunodeficient mice, most of which are genetically susceptible to intestinal inflammation. For example, mice deficient in TLR4 or MyD88, which are involved in bacterial sensing and activating immune responses, when treated with DSS have more severe symptoms and pathology than DSS-treated wild-type mice. Similar results are seen in Tir8-/- mice, which are incapable of regulating TLR signaling in intestinal epithelial cells or dendritic cells [119], as well as in DNA repair-deficient mice, such as Ogg1-/- [120], Aag-/- [121], and recently, Atm-/- mice [122].
4.1.2 Hapten-induced colitis
Intrarectal administration of ethanol-dissolved 2,4,6-trinitrobenzene sulfonic acid (TNBS), dinitrobenzene sulfonic acid (DNBS), or oxazolone (4-ethoxymethylene-2-phenyl-2-oxazoline-5-one) in wild-type mice will result in a local mixed Th1/Th2 colitis depending on the hapten, susceptibility of the mouse strain, and conditions of administration. Ethanol is used to break the mucosal barrier, whereas TNBS/DNBS/oxazolone haptenizes proteins autologous or derived from the microflora, which then renders them as immunogenic, leading to priming of antigen-specific T-cells. The hapten-induced colitis models, similar to DSS-colitis, also have been used in many types of transgenic mice deficient in immunoregulation or susceptible to immune-mediated colitis and inflammation-associated adenocarcinoma such as SCID mice, CD4-/- and CD8-/- mice [123], CD1-/- mice [124], Epstein Barr virus-induced gene 3 (EBI3)-deficient mice [125], and IFN-γ-/- mice [126].
A single dose of TNBS may be administered at the start of the experiment that results in an acute inflammatory response characterized by upregulation of Th1 cytokines within 2-3 days, or a delayed-type hypersensitivity reaction may be developed by an initial sensitization step with TNBS given either intrarectally or via the skin, which then requires a second administration after about 6 days to induce the delayed-type hypersensitivity reaction to the haptenized proteins [127]. CD4+ T cells are heavily implicated in the IL-12-driven Th1 immune response, resulting in transmural colitis reminiscent of Crohn's disease [123,128]. Compared to the DSS-induced colitis model, the cytokine patterns are, therefore, distinctly different [129]. In TNBS-susceptible SJL/J mice, resultant acute mucosal inflammation is mediated by excessive cytokine production of Th1 cytokines, including IFN-γ, TNF-α, and IL-12, accompanied by infiltration of neutrophils and macrophages into the mucosa and submucosal layers [130]. Chronic colitis more reminiscent of IBDs induced by weekly injections of TNBS is characterized by increased production of IL-23 and IL-17 due to repeated induction of delayed-type hypersensitivity reactions and fibrotic lesions [131]. DNBS-induced colitis is also similar to TNBS-induced colitis, in which extensive tissue damage and an acute inflammatory infiltrate similar to Crohn's disease is CD4+ T cell dependent [123].
Unlike TNBS/DNBS colitis, oxazolone-induced colitis is a Th2-type experimental colitis with a strong histological resemblance to ulcerative colitis that is dependent on NK-T cell derived IL-13 production as well as T cell derived IL-4 and IL-5 production [124,132]. The rapidly developing colitis is usually confined to the distal colon and exhibits neutrophil and lymphocyte infiltration into the mucosa that is accompanied by strong TGF-β induction, which then limits the extent, duration, and severity of the inflammation [132].
4.2 Genetic models of intestinal inflammation
The development of a wide variety of transgenic and knock-out mouse models of genetic traits have given insight into the pathogenesis and the association of chronic intestinal inflammation with colonic adenocarcinoma, as well as into the polygenic and heterogeneous nature of IBDs. Studied most frequently are those involved in promotion of inflammation by impairment of epithelial barrier function and bacterial sensing, innate immune signaling, immune regulation, and stress-response signaling (Table 2).
Table 2.
Genetic models of intestinal inflammation.
| Epihelial Barrier Function | Ref. |
|---|---|
| DN N-cadherin | [137] |
| Keratin 8 | [138] |
| O-glycan | [107] |
| MDR1α | [141] |
| TLR4 or TLR2 | [145] |
| MyD88 | [145] [146] |
| Tir8 | [119] |
| Muc2 | [139] |
| Nod2 | [144] |
| IKK-γ (NEMO)/IKKαβ in IECs | [148] |
| IKK-β in IECs | [149] |
| Trefoil factor | [140] |
| Nhe3 (Slc9a3-deficient mice) | [142] |
| Octn2 | [143] |
| Immunoregulation | |
| T cell regulation | |
| Tgfβ1 | [150] |
| DN TGF-βR2 in T cells | [151] |
| TGF-βR2/Rag2 with H. hepaticus infection | [154] |
| Smad3 | [152] |
| IL-2 | [155] |
| IL-2Rα | [156] |
| IL-2/β2M | [157] |
| IL-10 | [158,159] |
| IL-10R2/TGF-βR2 | [161] |
| IL-10/Leptin | [162] |
| IL-10/iNOS | [163] |
| CRF2-4 | [160] |
| B cell regulation | |
| TCR | [164] |
| TCRα | [164] |
| TCRα/IFNγ | [165] |
| TCRα/STAT6 | [166] |
| TCRβ/p53 | [167,168] |
| MHCII | [164] |
| Signal Transducing | |
| Stat3 in myeloid cells | [169,170,171] |
| Stat6 | [172] |
| Stat4 Tg | [173] |
| Gαi2 | [112] |
| A20 | [177,178] |
| A20/MyD88 | [179] |
| A20/Rag1 | [178] |
| A20/TNF | [178] |
| A20/TNFR1 | [178] |
| NF-κB p55-/-/p65+/- | [154] |
| Tbx21/Rag2 | [188,189] |
| Socs-1 | [191,192] |
| Others | |
| TNFΔARE | [193] |
| CD40L Tg | [194] |
| IL-7 Tg | [195] |
| WASP | [196] |
| Cathepsin D | [197] |
| Stress Response | |
| Gpx1/Gpx2 | [198] |
| Xbp1 in IECs | [71] |
All models are KO unless indicated; DN: dominant negative, IECs: intestinal epithelial cells, Tg: transgenic
4.2.1 Epithelial barrier function
An anatomically and immunologically compromised intestinal epithelial barrier allows direct contact of the intestinal mucosa with the luminal bacteria and plays a crucial role in the development and maintenance of IBDs by initiating chronic inflammatory responses. Crohn's disease patients demonstrate increased epithelial permeability early on in disease reoccurrence, and first degree relatives of both Crohn's disease and ulcerative colitis patients also exhibit abnormal intestinal permeability [133]. Celiac disease patients also demonstrate increased intestinal permeability compared to healthy relatives [134]. In addition, it has been shown that Crohn's disease is associated with variants of the intracellular pattern-recognition receptor gene CARD15 (NOD2), as well as the organic cation transporters OCTN1 and OCTN2, which are involved in intestinal epithelial barrier function [135,136]. Mouse models recapitulating defects in epithelial barrier function have, therefore, played a large role in the understanding of pathogenesis of IBDs and celiac disease.
Dominant negative N-cadherin transgenic mice, expressing the mutant form in intestinal epithelial cells, develop areas of porous epithelium and altered intercellular adhesion leading to spontaneous chronic inflammatory bowel disease similar to Crohn's disease, eventually leading to intestinal neoplasia [137]. Mice deficient in Keratin-8, a structural intermediate filament protein, also spontaneously develop intestinal inflammation and colorectal hyperplasia [138]. Deficiency of vital structural components of the protective mucosal layer, such as core 3-derived O-glycans [107], Muc2 [139], and trefoil factor [140], have demonstrated either spontaneous colitis development due to increased intestinal permeability or increased sensitivity to agents such as DSS and impaired mucosal healing. In addition to structural components, genes encoding transporters and exchangers in intestinal epithelial cells, such as MDR1α (P-glycoprotein), SLC9A-3 (NHE3 or Na+/H exchanger 3), and OCTN2 (carnitine transporter), are also affected and regulated by innate immune responses to luminal bacterial flora, as knockout mouse models demonstrate spontaneous transmural colitis, colitis restricted to the distal colonic mucosa, or spontaneous villous atrophy mimicking Crohn's disease, respectively [141-143]. Interestingly, mice deficient for pattern recognition receptors or bacterial-sensing genes such as NOD2, TLR4, TLR2, and MyD88 do not develop spontaneous intestinal inflammation, demonstrating the necessity of additional triggers for colitis development; however, TLR4-, TLR2-, and MyD88-deficient mice have an increased susceptibility to DSS-induced colitis [144-147]. NOD2-deficient mice, however, have excessive IL-12 production due to TLR2 activation, and studies have suggested inhibitory cross-regulation between several bacterial sensors, giving insights into the loss-of-function mutations of NOD2 seen in Crohn's disease. Furthermore, the lack of NF-κB activation specifically in intestinal epithelial cells (IKKγ and IKKβ deficiency) leads to an exaggerated response to the luminal bacterial flora [148,149], demonstrating the protective role of the intestinal epithelial cells to the luminal bacterial flora independent of the immune system.
4.2.2 Immunoregulation
Many genetic defects in immune regulation have been studied in mice that lead to development of colitis that involve regulatory cytokines and their receptors, pro-inflammatory cytokines, regulators of NF-κB signaling and cytokine responses, and associated signal-transduction molecules, and other soluble mediators. These studies have led to evidence supporting excessive immune activation and insufficient downregulation, as also seen in intestinal immune cells from IBD patients.
4.2.2.1 T and B cell Regulation
Commonly studied mouse models of deficiencies in T cell regulatory cytokines include TGF-β1 and its signal transducer Smad3, IL-2, and IL-10. The lack of regulatory cells in TGF-β1-deficient and dominant-negative TGF- βR2 in T cells result in diffuse multiorgan inflammation and death as early as 5 weeks and development of severe colitis and mononuclear infiltration, respectively [150,151]. Smad3 deficiency, resulting in loss of TGF- β1 signal transduction, similarly results in chronic intestinal inflammation and causes death within 1 to 8 months [152]. The autoimmune phenotype is rescued in TGF- β1/Rag2 double-knockout mice, which live up to 8 months, but when infected with H. hepaticus develop neoplasia of the cecum and colon with granulocytic inflammation of the mucosa, similar to Rag2-deficient mice infected with H. hepaticus [153,154].
IL-2 is important for T cell clonal expansion and activation-induced cell death, a mechanism underlying peripheral tolerance, and plays a crucial role in expansion of the regulatory T cell subsets. IL-2 and IL-2 receptor α-deficient mice develop unremitting spontaneous colitis and systemic wasting disease due to impaired T cell activation-induced cell-death mechanisms, as well as decreased CD4+CD25+ T cells. Due to their relatively long lifespan, IL-10/β2M (β2-microglobulin) double-knockout mice also develop a milder spontaneous colitis followed by carcinoma of the colon and rectum [155,156].
IL-10 is also a crucial anti-inflammatory cytokine produced by many cells types involved in the generation of the Tr1 subset of regulatory T cells. The popularly utilized IL-10-/- mice are characterized by a mild disease progression involving spontaneous CD4+ Th1-driven enterocolitis, dependent on IFN-γ for onset and IL-12 for sustaining disease and progression to adenocarcinoma, occurring from 5-6 months of age [158,159]. Similar to the IL-10-/- mice, deficiency of CRF2-4, a subunit of the IL-10 receptor, results in development of splenomegaly and chronic colitis [160]. Whereas the IL-10R2/TGFβR2 double-knockout mouse develops rapid fulminant ulcerative colitis within weeks after birth, other double–knockouts, including IL-10/Leptin and IL-10/iNOS, develop similar mild colitis compared to IL-10-/- mice, and their genetic status does not confer protection or rescue the phenotype of the IL-10 deficiency [161-163].
In addition to T cell regulation, loss of B cell regulation by deficiencies in the T cell receptor (TCR)-associated genes such as TCR, TCR-α, TCR-β, major histocompatibility complex II (MHCII), and associated double knockouts (Table 2), induces spontaneous unremitting colitis and wasting of varying severity driven by effector subsets producing IL-4, independent of STAT6 or IFNγ [164-168]. Preferential differentiation of intestinal CD4 T cells into Th2 T cells and polyclonal expansion and activation of B cells with autoantibody production in the colon contributes to disease progression, including dysplasia and adenocarcinoma, in these models.
4.2.2.2 Signal-transducing molecules and transcription factors
Mouse models with alterations in signal-transduction molecules, including signal transducer and activator of transcription 3 (STAT3), STAT6, STAT4, Gαi2, A20, NF-κB subunits, the transcription factor T-bet, and suppressor of cytokine signaling-1 (SOCS1), develop forms of spontaneous enterocolitis. STAT3 is a critical signal transducer in the pathway of IL-10 among other cytokines. STAT3 deficiency specific to myeloid-derived cells or to the bone marrow results in segmental, granulomatous transmural colitis in both the small and large intestine resembling Crohn's disease, dependent on continuous inflammatory cell activation and a Th1-polarized response to bacterial antigens, which evolves into colorectal adenocarcinoma within several months [169,170]. Double knockouts involving STAT3 combined with IL-12p40 or RAG2 deficiency results in complete ablation of enterocolitis, establishing the dependency of STAT3-deficiency driven colitis on the presence of adaptive immune cells and IL-12p40 [171]. Deficiency of STAT6, involved in IL-4-mediated activities similarly results in colitis as in STAT3-deficient mice [172]. STAT4 is a regulatory transcription factor involved with IL-12/IL-23 receptor-mediated signaling. Transgenic mice overexpressing STAT4 develop chronic transmural colitis characterized by abnormal IL-12-driven Th1 responses against bacterial antigens [173], supporting the role of STAT3 deficiency in colitis development.
Gαi2 encodes for the α-subunit of the Gi2 protein, a heterotrimeric G-protein regulating downstream signal transduction through adenylyl cyclase found in lymphocytes and intestinal epithelial cells among other cell types. Gαi2-/- mice develop alterations in T cell maturation and function, including defective chemotactic migration of thymic and colonic T cells, which contributes to a Th1/Th17-driven colitis after 3-4 weeks of age and colorectal cancer within 3-4 months [112,174,175]. A defective epithelial barrier prior to clinically active inflammation and defective numbers and regulatory function of B cells in the mesenteric lymph nodes also have been observed, implicating both epithelial cells and immune cells in colitis development in this model [167]. Histopathological features are similar to ulcerative colitis in humans.
A20 is a widely expressed de-ubiquitinating cytoplasmic protein whose expression is induced rapidly in macrophages upon TNF-dependent TLR stimulation and acts as an inhibitor of NF-κB signaling and resultant gene expression of proinflammatory cytokines. Therefore, A20 functions as a negative feedback regulator of TLR-induced signals. A20-deficient mice develop spontaneous multiorgan inflammation and cachexia, leading to premature death due to inability to terminate proinflammatory responses through NF-κB signaling [177,178]. Intestinal inflammation in this model is characterized by increased activated T cells and granulocytes with crypt abscess formation. Double-knockout models with TNF or TNF receptor 1, and Rag1 demonstrate similar phenotypes to the single A20 knockout, indicating the role of A20 in regulating TNF and T cell-independent signals [178]. However, the A20/MyD88 double-knockout rescues the spontaneous multiorgan inflammation phenotype, demonstrating the requirement for MyD88 in the development of spontaneous Th1-mediated inflammation [179].
Though NF-κB is usually thought of as the inducer of innate proinflammatory gene expression, as demonstrated in the models described previously, NF-κB can also mediate inhibitory signals. Upregulation of the p50 subunit of NF-κB results in decreased TNF-α production [180]. H. hepaticus-infected NF-κB p50-/-p65+/- mice develop severe colitis with increased Th1 cytokine expression, indicating the protective role of p50 in the innate immune response [181].
T-bet (Tbx21) is another transcription factor acting as a master regulator of Th1 cytokine signaling involved in both the innate and adaptive immune systems. Mice deficient in T-bet are interestingly resistant to experimental encephalomyelitis, experimental colitis, and Helicobacter felis-induced gastric cancer [182-185]. T-bet-deficient mice overproduce Th2 cytokines accompanying spontaneous airway inflammation [186], and T-bet-deficient NOD mice are protected from diabetes [187].
However, in Tbx21/Rag2 double-knockout mice, a dysregulated cytokine response to luminal bacterial flora in the gut mucosa results in spontaneous colitis development from 4 weeks of age [188,189]. The lack of adaptive immune cells in this model suggests that T-bet deficiency in only the innate immune system is sufficient to create spontaneous and communicable ulcerative colitis due to the development of a niche for a colitogenic microbial community. Interestingly, this colitogenic microbial community, thought to consist mainly of anaerobic bacteria, is also transmissible to offspring as well as to co-housed Rag2-deficient or wild-type mice and is sufficient to induce colitis [189]. Notably, in celiac disease, upper-bowel lesions are associated with a marked infiltration of the mucosa with Th1 cells secreting IFN-γ and expressing T-bet [190].
Finally, SOCS1 is a member of the STAT-induced STAT inhibitor family (SSI) containing an SH2 domain that is expressed primarily in thymocytes and acts as a physiological regulator of proinflammatory cytokines. Deficiency of SOCS1 leads to spontaneous inflammation and lymphocyte-mediated perinatal lethality [191]. When SOCS1 expression is restored to only T and B cells in SOCS1-deficient mice rescuing the perinatal lethality, the mice spontaneously develop colitis at 3 months of age and colorectal carcinoma with p53 mutations by 6 months of age, dependent on IFN-γ and STAT1 hyperactivation [192]. SOCS1 has, therefore, been implicated as an important regulator of the IFN-γ and STAT1 signaling pathways in chronic inflammation and associated carcinogenesis.
4.2.2.3 Other components of T cell activation and effector function
Other mouse models with alterations in aspects of immunoregulation resulting in excessive effector T cell function also recapitulate features of IBDs. Excessive TNF biosynthesis caused by dysregulated mRNA processing through deletion of the AU-rich elements (ARE) leads to polyarthritis and chronic intestinal inflammation localized to the terminal ileum [193]. Chronic intestinal inflammation in this model is dependent on the presence of lymphocytes, TNFR1, and TNFRII, resulting in the TNFΔARE homozygous mice dying between 5-12 weeks of age. Similarly, transgenic mice overexpressing CD40 ligand (CD40L), a member of the TNF family, providing signals for naïve T cell activation, develop multiorgan inflammation, including colitis, due to continuous expression of proinflammatory cytokines and presence of activated T cells [194]. Transgenic mice overexpressing IL-7, which is synthesized in intestinal epithelial cells and regulates proliferation and differentiation of lymphocytes in the gut mucosa, develop ulcerative colitis-like chronic intestinal inflammation at 4-12 weeks of age [195]. Overexpression of IL-7, therefore, results in a strong pathogenic destructive T cell response against the luminal bacteria, similar to what is seen in the serum of ulcerative colitis patients. Just as important are proteins involved in cytoskeletal reorganization during T lymphocyte activation, such as the Wiskott-Aldrich syndrome protein (WASP). WASP-deficient mice develop intestinal inflammation characterized by lymphocytic and granulocytic infiltrates into the lamina propria by 4 months of age [196].
In addition to proteins mediating excessive T cell effector function, deficiency in cathepsin D, a lysosomal aspartic proteinase, results in atrophy of the ileal mucosa causing intestinal necroses, anorexia, fulminant loss of T and B cells in the thymus and spleen, and death within 26 days [197]. Normal function of cathepsin D is thus necessary for limited proteolysis of cell growth regulators and genes involved in tissue homeostasis.
4.2.3 Antioxidant/Stress response
Effective activation of stress responses in intestinal epithelial cells against metabolic byproducts of the luminal bacteria, even with intact epithelial barriers and immune systems, are essential in gut homeostasis and prevention of chronic inflammation. Glutathione peroxidases (GPX) are antioxidant enzymes, reducing hydrogen peroxide by glutathione oxidation. Double-knockouts of Gpx1 and Gpx2 result in ileocolitis between 2-7 weeks of age and dysplasia and adenocarcinoma commonly in the terminal ileum after 4 months of age [198]. In addition to Gpx deficiencies, deficiency of the transcription factor XBP1 in intestinal epithelial cells, involved in the ER stress-response pathway, as well as in development and maintenance of secretory cells, results in spontaneous enteritis and Paneth-cell apoptosis [71]. Intestinal inflammation can, therefore, emanate solely from aberrations in Xbp1 of intestinal epithelial cells due to exaggerated responses against luminal bacterial antigens and instigation of proinflammatory cytokines.
4.3 Adoptive transfer models of colitis
Selective transfer of immune cell types into immunodeficient mice (RAG-1 knockout, RAG-2 knockout, and SCID mice) specifically allows testing sufficiency of the transferred cell type to induce intestinal inflammation in an immunodeficient host. The use of these types of models has elucidated the predominant role of pathogenic T cells and T cell-mediated colitis in mucosal inflammation.
4.3.1 Pathogenic CD4+ T cell transfer
Adoptive transfer of naïve CD4+CD45RBhigh T helper cells into immunodeficient SCID or Rag deficient mice is the model used most commonly for induction of adoptive-transfer colitis, which takes about 5-10 weeks [199,200]. The clinical presentation of adoptive transfer-induced colitis is similar in most variants, including diarrhea, weight loss, and wasting, and involves the entire colon. Crypt abscesses, mononuclear cell infiltrates, hyperplasia of the epithelium and ulcerations are also common. Th1 effector cells mediate disease progression because T cells from recipient mice produce IFN-γ and administration of antibodies against TNF-α, IL-12 and IFN-γ, but not IL-4, prevents disease development [200,201]. The transfer of naïve T cells in inducing colitis development is dependent on these Th1 responses to antigens of the luminal bacteria because mice treated with antibiotics or harboring a reduced microbiota have ameliorated disease [202]. Therefore, after transfer, the naïve T cells react to antigens of the luminal bacteria and expand rapidly in the lymph nodes and colonic mucosa, becoming oligoclonal, pathogenic Th1-effector cells due to the absence of regulatory T cells.
The protective role of regulatory T cells has also been demonstrated with this model, with cotransfer of the CD4+CD45RBlow memory T-helper cells protecting against colitis induction. More specifically, the CD25+FoxP3+ regulatory T cells within the CD4+CD45RBlow population, which produces the anti-inflammatory cytokines IL-10 and TGF-β1, is responsible for the abrogation of colitis [203]. In addition to CD4+CD45Bhigh transfer, L-selectin has also been used as a marker for selection (CD4+CD62L+) for pathogenic T cells (which also demonstrate CD45RBhigh expression), giving rise to a chronic bowel inflammation similar to that of CD4+CD45RBhigh adoptive transfer, ameliorated by blocking the IL-6 receptor pathway [184,204].
In an infectious model of adoptive transfer, transfer of SheLAg-specific (soluble antigen found in H. hepaticus lysates) CD4+ T cell clones into H. hepaticus-infected RAG-/- mice induces colitis characterized by cecal and colonic inflammation with epithelial hyperplasia 7-8 weeks after transfer, whereas uninfected recipients do not develop colitis [205]. Therefore, pathogenic T cells responding to a single bacterial antigen can drive colitis [205,206].
4.3.2 CD3εTg26 model
Transgenic mice with overexpression of human CD3ε develop abnormalities in the thymus that leads to arrest of T and natural killer cell development and altered architecture of thymic stromal cells [207]. Transfer of wild-type bone marrow depleted of T cells into this transgenic model leads to Th1-mediated colonic inflammation and wasting similar to ulcerative colitis within 5-8 weeks that is characterized by TNF-α, IFN-γ, and IL-12 secretion by activated CD4+ T cells derived from the thymus [207]. T cells arising from aberrant thymic selection due to altered thymic microenvironments are, therefore, able to induce severe colitis.
4.3.3 Transfer of hsp60-specific CD8 T cells
Adoptive transfer of a heat-shock protein (HSP) 60-specific CD8 T cell clone into T cell receptor (TCR)-β-deficient mice, which are immunodeficient, induces severe small-intestinal inflammation. Intestinal pathology is driven by autoimmune hsp60 CD8 T cells that react to cellular hsp60 through presentation on MHC class I and leads to excessive TNF-α production [208].
4.4 Spontaneous mouse models of colitis
Several strains of mice without any induced genetic alterations or disruptions spontaneously develop various forms of intestinal inflammation, mimicking the complex, multifactorial aspects of human IBDs. The SAMP1/YitFc substrain develops spontaneous transmural intestinal inflammation localized to the distal small intestine similar to Crohn's disease [209,210]. Both Th1 and Th2 pathways mediate disease progression, and increased epithelial permeability precedes onset of inflammation. Alterations in frequency and location of epithelial cells, such as Paneth and goblet cells, are also characteristic of SAMP1/YitFc mice, which may play a role in initiation of intestinal inflammation. Similar susceptibility genes as to Crohn's disease patients such as PPAR-γ have been identified, making this model worthy of further investigation [210].
The C3H/HeJBir substrain is LPS unresponsive due to a lack of TLR-4 and spontaneously develops Th1-mediated transmural colitis of the cecum and proximal colon mucosa at 3-4 weeks of age, which resolves at 3 months of age [211]. Pathogenesis of colitis involves hyper-reactive CD4 T cells and B cells against antigens derived from luminal bacteria due to defects in the innate response to TLR ligands. Antibodies produced against bacterial proteins have been studied to determine immunodominant antigens such as flagellins, which are able to activate effector cells [212].
4.5 The role of luminal bacteria in mouse models of IBDs
The critical role of luminal bacteria in the pathogenesis of IBDs in mouse models has been demonstrated by amelioration or ablation of disease development either in germ-free mice, restricted flora mice, or in mice treated with antibiotics [167,213-215]. Although a few models remain to be tested in a germ-free/reduced bacterial flora state or with antibiotic treatment, almost every model has demonstrated direct dependence on the luminal bacteria for pathogenesis. Exposure to enteric bacterial antigens and the consequential activation of lymphocytes represents a common mechanism in mouse models of IBDs, whether the defect lies in epithelial barrier function, in immune dysregulation, or in stress response. Exceptions to dependence on the presence of luminal bacteria are demonstrated by the TGF-β1-deficient mice and the adoptive transfer model of hsp60-specific CD8 T cells into TCR-β-deficient mice, in which intestinal inflammation develops despite the lack of enteric bacteria. TGF-β1-deficient mice develop multiorgan inflammation, most severely in the heart and lungs, due to abnormal innate responses arising from antigens not only from the luminal bacteria [216]. The adoptive transfer of primed hsp60-reactive CD8 T cells results in autoimmune reactions specifically against cellular hsp60 proteins, causing pathology of the small intestine with mechanisms distinct from other models and independent of bacterial antigens.
4.6 Mouse models of celiac disease
As described previously, celiac disease is genetically dependent on the presence of susceptible HLA haplotypes and exposure to gluten peptides. Humanized HLA-DQ8 transgenic mice have been created mimicking the human β57 polymorphism to study the anti-gluten T cell response and ability to recruit crossreactive TCR repertoires [217,218] Sensitization with the native gluten peptides induced a TCR repertoire with a stronger response to the deamidated gluten peptides [218]. Others have found these mice have increased intestinal permeability and numbers of intestinal epithelial lymphocytes after gliadin sensitization and gliadin oral gavage [219]. However, the role in disease onset or progression is not possible to determine in this model because they do not develop enteropathy. Humanized HLA-DR3-DQ2 transgenic mice lacking murine MHC class II genes and also transgenic for human CD4 have also been created and used to study HLA-DQ2 restricted T cells after immunization with α-gliadin peptides [220]. Even after exposure to different forms of dietary gluten peptides, mice developed only low-penetrance enteropathy and intestinal inflammation, demonstrating the presence of additional factors to pathogenesis of celiac disease in this humanized mouse model.
4.7 Mouse models of sporadic and hereditary forms of colorectal cancer
Aside from inflammation-mediated colorectal cancer, as studied by IBD and celiac disease models, several forms of sporadic colorectal cancer are driven by heritable genetic alterations. It is important to note that inflammation still plays an important role, as demonstrated by dense lymphocytic infiltrates in the polyps and tumors. In addition, COX-2 specific NSAIDs have demonstrated efficacy in treatment of benign polyps in familial adenomatous polyposis (FAP) patients, who are prone to sporadic colorectal cancer [221]. The multiple intestinal neoplasia (ApcMin/+) mouse contains a mutation in the Apc gene, which is homologous to adenomatous polyposis coli (APC) in humans, used to model FAP and sporadic colorectal carcinogenesis [222]. Defects in APC, a tumor suppressor gene, are responsible for both FAP and promotion of sporadic colorectal cancer. The Min/+- mice can spontaneously develop many polyps throughout the small intestine and tumors in the colon. Several studies utilizing NSAIDs have demonstrated success in prevention of colorectal tumors in ApcMin mice [223], whereas others have not shown efficacy in treatment of established polyposis with NSAIDs [224].
5. Proposed Mechanisms of Intestinal Inflammation and Associated Cancers
5.1 Pathogenesis of inflammatory bowel diseases
Animal models of intestinal inflammation mimicking IBDs have allowed for examination of multiple aspects of acute and chronic inflammation and its progression to dysplasia and cancer, as well as the evaluation of novel therapeutic regimens. From these studies, it is generally accepted that strong immune responses driven by pathogenic effector CD4+ T cells against the commensal luminal bacteria are due to multiple defects resulting in immune-mediated tissue damage. These defects encompass innate immunity, regulation of cytokines and their downstream signal transducers, the epithelial barrier and pattern recognition receptors, and stress responses to byproducts of the microbiota. The importance of T cells and their interaction with dendritic cells in mediating intestinal inflammation have also been revealed. However, most models are exaggerated by the fact that they represent either complete genetic deficiencies or overexpression in one or two of these aspects, which may not involve an intact immune system. Thus, humans, most of whom have intact immune systems, may require a combination of genetic alterations involved in multiple pathways along with exposure to environmental factors for pathogenesis of chronic intestinal inflammation.
In both ulcerative colitis and in Crohn's disease patients, an increase in Foxp3+CD4+CD25+ regulatory T cells is seen in the lamina propria compared to healthy controls, indicating that the lack of this regulatory cell type itself is not responsible for chronic intestinal inflammation [225]. The regulatory T cells that are present, however, may have intrinsically impaired immunosuppressive function, or the tissue environment may be resistant to immunosuppression. IL-10 has been implicated as a critical cytokine mediating the immunosuppressive effects of both innate immune cells and T cells in many mouse models of colitis. IL-10 is produced locally by the regulatory T cells themselves and also by other cell types such as innate immune cells, epithelial cells, and other T cell subsets that may differentiate to become IL-10-producing cells. IL-10 is thought to mediate these immunosuppressive effects via activation of negative feedback loops, such as the SOCS proteins, which inhibit IL-6 and other proinflammatory cytokines [226]. In addition, alterations in T cell responsiveness to TGF-β, which maintains expression of Foxp3 in CD4+CD25+ cells, may also contribute to human IBDs because patients have demonstrated high levels of SMAD7, a negative regulator of TGF-β signaling [227]. Impaired regulatory function in IBD patients is also demonstrated by reduced numbers of a lamina propria CD8+ T cell subset responding to epithelial-derived antigens, such as gp180, and exerting regulatory function [228]. Interestingly, IBD patients have an abnormally increased appearance of Vδ1-positive TCRγδ T cells in the peripheral blood, which predominantly should only reside within the intestinal intraepithelial lymphocyte population in healthy humans. These cells contribute to oral tolerance or prevent exaggerated immune responses to food and bacterial antigens present in the microflora, exerting a protective role in colitis [229].
In addition to negative regulation of effector T cells and regulatory cell populations in the lamina propria and intestinal intraepithelial lymphocytes, negative regulation of TLR signaling through NOD2 in intestinal dendritic cells is required to prevent production of proinflammatory cytokines and effector T cell responses against the luminal bacteria. Dendritic cells are conditioned by factors released by intestinal epithelial cells, and they constitutively migrate to the mesenteric lymph nodes, which may drive the development of both local and systemic regulatory T cell responses suppressing intestinal inflammation [226]. Defects in both adaptive and innate immune reactions involving signals for differentiation of T cell subsets by dendritic cells and macrophages and their reaction to the luminal bacteria, therefore, play an important role in pathogenesis of IBDs.
In addition, the role of commensal enteric bacteria in IBDs as constant antigenic stimulants for pathogenic T-cell responses with subsequent chronic tissue damage is also affirmed by several lines of clinical evidence. IBDs are localized to intestinal segments with the highest microbial density [230,231]; fecal stream diversion can treat Crohn's disease, whereas restoration of fecal flow results in reoccurrence [232]; and antibiotics have been used to treat Crohn's disease/ileocolitis, whereas probiotics can prevent relapse of ulcerative colitis [233,234]. It has also been observed that microbial composition and metabolic activity are altered in Crohn's disease and ulcerative colitis [235-238]. Mucosal association of bacteria is increased in IBD patients, demonstrating enhanced mucosal invasion in active Crohn's disease [239,240]. Polymorphisms in bacterial pattern recognition receptors and intracellular microbial processing are also associated with increased susceptibility to Crohn's disease [241]. Enteric and fungal antigens elicit T cell and serologic responses in both Crohn's disease and ulcerative colitis [242], further underlying the role of enteric bacteria in IBD pathogenesis.
5.1.1 Specific mechanisms of pathogenesis in ulcerative colitis
Ulcerative colitis is characterized generally as a Th2 cytokine-mediated disease involving IL-5 and IL-13 producing T or NKT cells, although other Th1 cytokines, such as TNF-α. also play important roles. Pathological manifestations are similarly modeled through experimental colitis either by being induced chemically or by spontaneous immune colitis in several genetic models. Importantly, IL-10 secreting Lactococcus lactis can ameliorate DSS-induced intestinal pathology [228], demonstrating once again the importance of IL-10 in controlling intestinal inflammation. Although histopathological features are indeed similar to ulcerative colitis involving focal ulcerations only involving the mucosa of the colon, neutrophil infiltration, and crypt abscess formation, this model exaggerates the transient epithelial breaches that may actually occur in patients. In parallel to Th2-cell activation, epithelial cells can present bacterial antigens directly to natural killer cells that then produce IL-13, contributing to epithelial cell apoptosis and increased epithelial permeability [244]. Sustainment of inflammation through release of proinflammatory cytokines such as TNF-α contribute to the actions of IL-13, further enhancing permeability and leakage of bacterial antigens. Alterations in the number and activity of sulfate-reducing bacteria, as well as an increase in hydrogen sulfide levels in the feces of ulcerative colitis patients, may contribute to impaired short-chain fatty acid metabolism and epithelial cell starvation [245], further enhancing epithelial permeability.
5.1.2 Specific mechanisms of pathogenesis in Crohn's disease
Multiple studies have demonstrated an exaggerated CD4 Th1/17-type response in the gut of Crohn's disease patients characterized by polarized expression of IL-12, IFN-γ, IL-2, IL-17, IL-21 and TNF-α [246,247]. Most Th1-mediated colitis mouse models exemplify similar Th1 cytokine patterns and also display similar histological features such as transmural inflammation spanning the small intestine in humans. Macrophages and dendritic cells activated by luminal bacteria that have penetrated the epithelial barrier secrete proinflammatory cytokines, recruiting CD4 T cells that differentiate into pathogenic Th1/17 effector cells, producing IFN-γ and TNF-α and further Th17 differentiation. Variants of IL23R, encoding the receptor that further drives proliferation and clonal expansion of Th17 cells when activated, are found in Crohn's disease patients. Differentiated Th1 effector cells from patients are also resistant to apoptosis and have increased cell cycling. These inappropriate and excessive immune responses lead to increased concentrations of cytokines, damaging free radicals, lipid mediators, and overexpression of matrix metalloproteinases in fibroblasts, which ultimately results in tissue damage characterized by ulceration and fistulae.
NOD2 mutations in Crohn's disease patients cause exacerbated proinflammatory responses due to uncontrolled signaling through TLR and impaired IL-10 production and associated regulatory responses in intestinal dendritic cells. Other genes associated with Crohn's disease include OCTN, which is expressed in epithelial cells, macrophages, and T cells, impairing the ability of cells to pump xenobiotics and amino acids across the cell membrane; and guanylate kinase DLG5, which impairs epithelial polarity. These genes may be important in epithelial-barrier function, potentially leading to bacterial-product exposure [136,248].
5.2 Mechanisms underlying celiac disease
As described previously, celiac disease arises in genetically susceptible individuals due to an excessive cell-mediated immune response to gluten and gluten-derived peptides that are presented preferentially by HLA-DQ2 or -8 due to their affinity for negatively charged residues to activate T cells. The resultant inflammation is restricted to the mucosa of the upper small intestine involving an increase in the density of intraepithelial TCRγδ lymphocytes and transformation of the mucosal structure to a flat mucosa with short or absent villi. This uncontrolled activation and expansion of these lymphocytes is due to epithelial cell secretion of IL-15, which upregulates natural killer receptors on intraepithelial lymphocytes and lowers the TCR activation threshold, leading to their activation and acquisition of natural-killer-like activity [71]. In addition to the epithelial cells, macrophages also secrete IL-15, suggesting T cell independent innate immune activation. The activated intraepithelial lymphocytes damage the epithelium by cytolysis through recognition and engagement with epithelial NKG2D ligands such as major histocompatibility complex class I chain-related gene (MICA), which is also upregulated in response to IL-15 [78].
5.3 Similarities of celiac disease with IBDs
The production of proinflammatory cytokines is increased by increased epithelial permeability and resultant activation of innate immune cells and T cells due to the influx of luminal bacterial antigens in the case of IBDs or exposure to gluten peptides in the case of celiac disease. The result is resistance to apoptosis and a deficient response to regulatory cells and immunosuppressive cytokines, which in turn leads to a vicious cycle of tissue destruction and chronic inflammation. Both celiac disease and IBDs have overlapping variants of genes such as those involved in intestinal permeability, including DLG5, and enhanced immunological sensitivity to antigens, such as proinflammatory genes including IL18RAP [249]. In addition, a recent study has found that the risk of developing IBD is tenfold higher in celiac disease patients compared to the general population, supporting shared genetic factors [249,250].
5.4 The inflammation to dysplasia sequence followed by cancer
Chronic intestinal inflammation-associated colorectal carcinogenesis is thought to occur via a stepwise progression beginning with epithelial hyperplasia, leading to various grades of dysplasia, adenoma, and then to adenocarcinoma (Figure 1). Once a tumor is initiated, immunosuppression of anti-tumor immunity, promotion of angiogenesis, and chronically stimulated cell proliferation promotes tumorigenesis during chronic inflammation. Anergy of effector T cells and their conversion to regulatory cells by dendritic cells through contact with indoleamine 2,3 dioxygenase (IDO) released by tumor cells, and COX-2-dependent upregulation of IDO in tolerogenic antigen presenting cells, contributes to suppression of potential anti-tumor responses [74]. Prior to tumorigenesis, an accumulation of genetic alterations consisting of aneuploidy and microsatellite instability drives transformation early on from regenerating epithelia to adenocarcinoma, which are frequently detected in dysplastic and cancerous colon tissue from ulcerative colitis patients [230]. Genetic instability is, therefore, an early event. We have shown recently that systemic genotoxicity in the form of DNA strand breaks is also an early event in chemical and genetic models of chronic intestinal inflammation (Figure 2 [251,252]). Genotoxicity as well as genetic instability in general is also associated with accelerated telomere shortening in colonocytes of ulcerative colitis patients, and this worsens with progression to adenocarcinoma [253].
Figure 1.
Mechanisms of intestinal inflammation-mediated carcinogenesis. Persistent antigenic stimulation in the intestinal tract through a permeabilized epithelial barrier results in chronic inflammation. Dysfunctions of the epithelial barrier itself, the innate and adaptive immune responses, DNA repair and stress responses, as well as related downstream signaling pathways can result in transcriptional changes promoting malignant transformation of the tissue, as well as extraintestinal manifestations.
Figure 2.
Systemic genotoxicity of intestinal inflammation (Adapted from [251]). Tissue atrophy from persistent inflammation results in genotoxicity to surface epithelial cells as well as to infiltrating leukocytes. Damaged resident leukocytes may then migrate into the peripheral circulation through the lymph nodes, or circulating activated effector cells may cause genotoxicity to proximal circulating leukocytes through oxidative burst.
The accumulation of DNA damage and the resulting genetic alterations over time may arise from multiple sources during chronic intestinal inflammation. Inflammation characterized by large amounts of TNF-α and other proinflammatory mediators are capable of inducing chronic mucosal damage themselves or via upregulation of matrix metalloproteinases. Activated innate immune cells release reactive oxygen and nitrogen species during oxidative burst, mediating oxidative and nitrative base damage as well as DNA single- and double-strand breaks. Cytokines released also upregulate inducible nitric oxide synthase (iNOS) in macrophages and epithelial cells, resulting in increased local production of nitric oxide. IBD patients have increased expression of NOS-2 and COX-2 in inflamed mucosa, contributing to further perpetuation of inflammation-associated oxidative stress, which remains elevated throughout carcinogenesis [30]. Other reactive metabolites, such as lipid peroxidation intermediates, are also capable of causing DNA base transitions such as G to A mutations and C to T transitions through deamination reactions, accounting for about 80% of mutations seen in the P53 gene of ulcerative colitis-associated neoplasms [245]. DNA damage in the setting of quickly proliferating and regenerating epithelial cells can then cause fixation of mutations as described previously. Patients with intestinal inflammation harboring polymorphisms resulting from germline mutations or somatic gene mutations in various immune response genes, antioxidant and stress response genes, and DNA repair genes may further accumulate DNA damage and mutations, accelerating progression to dysplasia, adenoma, and to cancer.
In addition to direct DNA damage-mediated mutations and DNA transitions, hypermethylation of promoter regions of genes can cause gene silencing, as seen in the cyclin-dependent kinase inhibitor P16 in inflamed intestinal tissue, as well as, in adjacent normal epithelia and in cancerous tissue [245]. Microsatellite instability (MSI), commonly observed in IBDs, is also thought to be induced by hypermethylation of the DNA mismatch repair genes, MutL homologue 1 (hMLH1), MutS homologue 2 (hMSH2), and MutS homologue 6 (hMSH6). Epigentic alterations such as CpG methylation appear to increase in density from inflamed tissue to dysplasia and carcinoma, which has been proposed to be partially caused by premature aging and rapid turnover of cells during chronic inflammation [254]. Celiac disease-associated small-bowel adenocarcinoma, usually affecting the jejunum, is also associated with hypermethylation-induced high-frequency MSI and loss of hMLH1 and hMSH2, indicating defective mismatch repair [255]. Interestingly, high frequency MSI associates with better survival in sporadic and hereditary colorectal cancer, gastric, pancreatic, and small-bowel adenocarcinomas. High frequency MSI tumors have fewer mutations of the APC and P53 genes, and more frequent mutations of the β-catenin (CTNNB1) and transforming growth factor β receptor type II genes. Intense lymphocytic infiltrates are also characteristic to these tumors, perhaps contributing to yielding a survival advantage [256].
In ulcerative colitis, tumor suppressor gene mutation and loss of heterozygosity of P53 precedes dysplasia and occurs before aneuploidy along with hypermethylation of P16, whereas activating mutations in KRAS and loss of function of APC occurs late in the carcinoma sequence. In Crohn's-associated small-intestinal adenocarcinoma largely affecting the ileum, mutation frequencies in APC are much lower, and nuclear localization of β-catenin is less frequent compared to colorectal adenocarcinoma, which is morphologically similar [241]. However, decreased E-cadherin expression, hypermethylation-induced MSI, P53 overexpression, and decreased expression of P16 and P21 early in the development of adenocarcinoma are characteristics observed also in colorectal adenocarcinoma specimens [257-260]. Because cancerous tissues are examined more commonly than normal tissue, further mechanistic insight is needed to elucidate the sequence of genetic alterations in the development of small-bowel adenocarcinoma, although existing data suggest an adenoma-carcinoma sequence similar to colorectal cancer, given an estimated one third of solitary small-bowel adenomas transform into invasive adenocarcinoma [261].
5.5 The role of intestinal inflammation in extraintestinal cancers
Chronic intestinal inflammation also associates with extraintestinal cancers, such as various types of intestinal and non-intestinal lymphomas, and other solid cancers such as rectal and hepatobiliary carcinoma [262]. For example, in celiac disease-associated T cell lymphoma, the large involvement of intraepithelial lymphocytes in the pathogenesis of celiac disease leads to their aberrant clonal expansion and activation, favoring the development of enteropathy-associated T cell lymphoma. Intraepithelial lymphocytes that have acquired a natural killer phenotype and lowered their TCR activation threshold renders them to become autoreactive and susceptible to malignant transformation [24].
Systemic circulation of cytokines and inflammatory mediators as well as distribution of bacterial components through the mesenteric lymph nodes and peripheral circulation may contribute to extraintestinal manifestations of IBDs and celiac disease. Systemic genotoxicity resulting from chronic intestinal inflammation to circulating lymphocytes and erythroblasts, which correlates to disease activity and an activated immune response, may mediate genetic alterations and further promote the immune response, leading to malignant transformation of not only lymphoid tissues but also potentially distant tissues [252]. Evidence for inflammatory activation and hyper-responsiveness in chronic intestinal inflammation in distant immune cells such as in the lymph nodes or in the peripheral circulation may also contribute factors necessary for tumorigenesis. Additional extraintestinal manifestations such as autoimmune diseases, including arthritis, skin diseases, uveitis, and hepatic inflammation, are thought to arise from excessive proliferation of anaerobic bacteria in the small intestine, indicating the role of circulating endogenous bacterial components in the pathogenesis [215]. A persistently activated immune response to stimuli such as bacterial components, food components, or other antigens is, therefore, capable of eliciting a systemic response, including persistent DNA damage and immune dysregulation, potentially contributing to extraintestinal manifestations such as lymphomas (summarized in Figure 1).
6. Conclusion
Although the correlation between chronic inflammation and cancer has been recognized for over a century, mechanisms involved in the pathogenesis of chronic inflammation itself, as well as the sequence of events thought to be involved in the progression to cancer, are still actively being investigated. Numerous studies have been and will continue to be carried out targeting components of the inflammatory response and its associated effects in an effort to abrogate inflammation-associated carcinogenesis.
Inflammatory bowel diseases such as ulcerative colitis and Crohn's disease associate with colorectal cancer as well as small-intestinal adenocarcinoma development, whereas celiac disease associates with small-bowel adenocarcinoma development. In addition to cancer arising from the site of inflammation, these diseases also associate with extraintestinal manifestations, including lymphomas, hepatobiliary and rectal cancer, as well as other autoimmune diseases. Chronic intestinal inflammation is characterized by dysfunction of multiple pathways, including the innate and regulatory functions of the immune response, epithelial-barrier function, signal transduction, and stress response/DNA repair in response to persistent antigens deriving from the luminal bacteria or food peptides, such as gluten. The heterogeneous involvement of multiple genes and the diverse types of polymorphisms observed in patients has made this group of diseases complex to model and study. Nevertheless, genetic aspects thought to be involved in pathogenesis as well as through genetic linkage analyses of patients have been translated into overexpression or knockouts in mice. This had led to improved understanding of the intricate involvement of multiple pathways in the pathogenesis of chronic intestinal inflammation. Importantly, the role of luminal bacteria in pathogenesis of disease has been highlighted by the amelioration or complete inhibition of disease development in mice treated with antibiotics or reared under germ-free conditions. These pivotal findings have led to an increased awareness of the role of the luminal bacteria as a potentially modifiable risk factor for individualized interventions. New initiatives, such as the NIH-funded human microbiome project, can be expected to increase our knowledge beyond the complex ecosystem of the gut and towards a deeper understanding of the intricate relationship between the microflora and host. In doing so, novel prevention and treatment options may be introduced.
Although this review has focused on mouse models of intestinal inflammation-mediated cancers, useful insights have been acquired from chemical as well as some transgenic models in the rat. Chemically induced IBDs and associated colorectal cancer development via AOM or DMH injections followed by DSS and DNBS/TNBS/ethanol administration have been recapitulated in multiple rat strains. In addition, PhIP administered in the food of rats is able to induce preneoplastic lesions in the colon; however, these have not been found to have an inflammatory component [263]. Other rat models, including Pirc rats, which carry a knockout allele of Apc modeling FAP patients, are phenotypically more similar to humans than the ApcMin mouse model of sporadic or heritable colorectal cancer [264]. Further details and various other models in the rat are reviewed elsewhere [265, 266].
Mechanisms involved in the progression to dysplasia and cancer of the intestinal tract also have been studied extensively in several of the reviewed mouse models and through patient biopsies, illuminating the role of accumulation of genetic and epigenetic alterations in several tumor-suppressor genes, cell-cycle regulator genes, and DNA repair genes thought to be caused by an overload of DNA damage beginning early on in the sequence of inflammation to dysplasia. Continuous involvement of the inflammatory response after malignant transformation, including promotion of angiogenesis and sustainment and growth of the tumor, also has been confirmed. However, mechanisms involved in cancers developing outside the direct site of inflammation still remain to be explored, although systemic genotoxicity and a dysregulated immune response may play important roles in the development of such genetic alterations and microenvironments necessary for carcinogenesis. Additional studies in suitable models will be necessary for elucidation of these mechanisms, as well as for the validation of therapeutic agents targeting specific aspects of the sequence of inflammation to cancer.
Table 3.
Adoptive transfer models of intestinal inflammation
| Model | Description | Reference |
|---|---|---|
| CD4+CD45RBhi | Colonic inflammation mediated by pathogenic Th1 effector cells reacting to commensal microflora | [199] |
| CD4+CD62Lhi | Similar pathogenesis to CD4+CD45RBhi model using L-selectin as marker for pathogenic T cells | [204] |
| SheLAg specific CD4+ T cells | Transfer of SheLAg specific CD4+ T cells into H. hepaticus infected RAG-/- mice causes colitis due to a single bacterial antigen | [205] |
| CD3εTg26 | Transfer of wildtype bone marrow into transgenic mice with a high copy number of human CD3ε results in Th1 mediated colonic inflammation | [207] |
| Hsp60-specific CD8+ T cells | Autoimmune intestinal pathology caused by reaction to cellular Hsp60 | [208] |
Acknowledgments
This work was supported in part by NIH grant ES09519 (RS), the Jonsson Comprehensive Cancer Foundation (RS), a TRP grant (L618-B11) from the FWF (AS and RS) and a UCLA-NIEHS training grant in Molecular Toxicology (AW).
Funding sources for authors: NIH: Grant # ES09519 (RS), UCLA NIEHS Training Grant in Molecular Toxicology (AW)
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotran RS, Kumar V, Collins T. Robbins Pathological Basis of Disease. sixth. WB Saunders; Philadelphia: 1999. [Google Scholar]
- 2.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart BW, Kleihues P. World Cancer Report, IARC. 2003. [Google Scholar]
- 6.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 7.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society Cancer Facts and Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 9.Dominici F, Peng R, Bell M, Pham L, McDermott A, Zeger S, Samet J. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laden F, Hart J, Smith T, Davis M, Garshick E. Cause-specific mortality in the unionized US trucking industry. Environ Health Perspect. 2007;115:1192. doi: 10.1289/ehp.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spurzem J, Thompson A, Daughton D, Mueller M, Linder J, Rennard S. Chronic inflammation is associated with an increased proportion of goblet cells recovered by bronchial lavage. Chest. 1991;100:389–393. doi: 10.1378/chest.100.2.389. [DOI] [PubMed] [Google Scholar]
- 12.Siemes C, Visser LE, Coebergh JWW, Splinter TAW, Witteman JCM, Uitterlinden AG, Hofman A, Pols HAP, Stricker BHC. C-reactive protein levels, variation in the c-reactive protein gene, and cancer risk: The Rotterdam study. J Clin Oncol. 2006;24:5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 13.Peebles K, Lee J, Mao J, Hazra S, Reckamp K, Krysan K, Dohadwala M, Heinrich E, Walser T, Cui X. Inflammation and lung carcinogenesis: applying findings in prevention and treatment. Expert Rev Anticancer Ther. 2007;7:1405–1421. doi: 10.1586/14737140.7.10.1405. [DOI] [PubMed] [Google Scholar]
- 14.Hecht S. Tobacco and cancer: approaches using carcinogen biomarkers and chemoprevention. Ann N Y Acad Sci. 1997;833:91–111. doi: 10.1111/j.1749-6632.1997.tb48596.x. [DOI] [PubMed] [Google Scholar]
- 15.Parke D, Sapota A. Chemical toxicity and reactive oxygen species. Int J Occup Med Environ Health. 1996;9:331–340. [PubMed] [Google Scholar]
- 16.Merritt Melissa A, Green Adèle C, Nagle Christina M, Penelope M Webb, Australian Ovarian Cancer Study Group Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus MN, Robinson D, Mak V, Moller H, Isenberg DA. Incidence of cancer in a cohort of patients with primary Sjogren's syndrome. Rheumatology. 2006;45:1012–1015. doi: 10.1093/rheumatology/kei281. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Shakra M, Gladman D, Urowitz M. Malignancy in systemic lupus erythematosus. Arthritis Rheum. 1996;39:1050–1054. doi: 10.1002/art.1780390625. [DOI] [PubMed] [Google Scholar]
- 19.Matteson E, Hickey A, Maguire L, Tilson H, Urowitz M. Occurrence of neoplasia in patients with rheumatoid arthritis enrolled in a DMARD Registry. Rheumatoid Arthritis Azathioprine Registry Steering Committee. J Rheumatol. 1991;18:809–814. [PubMed] [Google Scholar]
- 20.Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001;60:433–441. doi: 10.1136/ard.60.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoenfeld Y, Gershwin M. Cancer and autoimmunity. Elsevier; Amsterdam: 2000. [Google Scholar]
- 22.Abu-Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis Rheum. 1993;36:460–464. doi: 10.1002/art.1780360405. [DOI] [PubMed] [Google Scholar]
- 23.Tlaskalova H, Tuckova L, Pospisil M, Stepankova R. Malignancy in coeliac disease and dermatitis herpetiformis. In: Shoenfeld Y, Gershwin M, editors. Cancer and Autoimmunity. Elsevier; Amsterdam: 2000. pp. 105–110. [Google Scholar]
- 24.Green PHR, Jabri B. Celiac disease and other precursors to small-bowel malignancy. Hematol Oncol Clin North Am. 2003;17:611–624. doi: 10.1016/s0889-8553(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 25.Catassi C, Bearzi I, Holmes GKT. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79–S86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Chang DK, Goel A, Boland CR, Bugbee W, Boyle DL, Firestein GS. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J Immunol. 2003;170:2214–2220. doi: 10.4049/jimmunol.170.4.2214. [DOI] [PubMed] [Google Scholar]
- 27.Altindag O, Karakoc M, Kocyigit A, Celik H, Soran N. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem. 2007;40:167–171. doi: 10.1016/j.clinbiochem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TIA. Increased risk of intestinal cancer in Crohn's disease: A meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 29.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 30.Itzkowitz SH, Yio X. Inflammation, Cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 31.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Giorgi V, Salvini C, Chiarugi A, Paglierani M, Maio V, Nicoletti P, Santucci M, Carli P, Massi D. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod-treated basal cell carcinoma. Int J Dermatol. 2009;48:312–321. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 33.Chaux P, Moutet M, Faivre J, Martin F, Martin M. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 costimulatory molecules of the T-cell activation. Lab Invest. 1996;74:975–984. [PubMed] [Google Scholar]
- 34.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S. Cancer Issue: Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 36.Slattery ML, Samowitz W, Hoffman M, Ma KN, Levin TR, Neuhausen S. Aspirin, NSAIDs, and colorectal cancer: possible involvement in an insulin-related pathway. Cancer Epidemiol Biomarkers Prev. 2004;13:538–545. [PubMed] [Google Scholar]
- 37.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elder D, Paraskeva C. Induced apoptosis in the prevention of colorectal cancer by non-steroidal anti-inflammatory drugs. Apoptosis. 1999;4:365–372. doi: 10.1023/a:1009696505108. [DOI] [PubMed] [Google Scholar]
- 39.Hampton T. Officials halt NSAID prevention trials. JAMA. 2005;293:664–665. doi: 10.1001/jama.293.6.664. [DOI] [PubMed] [Google Scholar]
- 40.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. The Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 41.Scott DGI, Watts RA. Increased risk of cardiovascular events with coxibs and NSAIDs. The Lancet. 2005;365:1537–1537. doi: 10.1016/S0140-6736(05)66445-8. [DOI] [PubMed] [Google Scholar]
- 42.McAdam B, Catella-Lawson F, Mardini I, Kapoor S, Lawson J, FitzGerald G. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanagarajan N, Nam JH, Al Noah Z, Murthy S. Disease modifying effect of statins in dextran sulfate sodium model of mouse colitis. Inflamm Res. 2008;57:34–38. doi: 10.1007/s00011-007-6177-4. [DOI] [PubMed] [Google Scholar]
- 44.van Meeteren ME, Meijssen MAC, Zijlstra FJ. The effect of dexamethasone treatment on murine colitis. Scand J Gastroenterol. 2000;35:517–521. doi: 10.1080/003655200750023787. [DOI] [PubMed] [Google Scholar]
- 45.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauch UG, Obermeier F, Grunwald N, Dunger N, Rath HC, Scholmerich J, Steinmeyer A, Zugel U, Herfarth HH. Calcitriol analog ZK191784 ameliorates acute and chronic dextran sodium sulfate-induced colitis by modulation of intestinal dendritic cell numbers and phenotype. World J Gastroenterol. 2007;13:6529–6537. doi: 10.3748/wjg.v13.i48.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takaki K, Mitsuyama K, Tsuruta O, Toyonaga A, Sata M. Attenuation of experimental colonic injury by thiazolidinedione agents. Inflamm Res. 2006;55:10–15. doi: 10.1007/s00011-005-0002-8. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prisciandaro L, Geier M, Butler R, Cummins A, Howarth G. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1906–1914. doi: 10.1002/ibd.20938. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 51.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, DuBois RN. Inflammatory mediators and nuclear receptor signaling in colorectal cancer. Cell Cycle. 2007;6:682–685. doi: 10.4161/cc.6.6.4030. [DOI] [PubMed] [Google Scholar]
- 54.Buchanan FG, Holla V, Katkuri S, Matta P, DuBois RN. Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 2007;67:9380–9388. doi: 10.1158/0008-5472.CAN-07-0710. [DOI] [PubMed] [Google Scholar]
- 55.Yu Y, Wan Y, Huang C. The biological functions of NF-kappaB1 (p50) and its potential as an anti-cancer target. Curr Cancer Drug Targets. 2009;9:566. doi: 10.2174/156800909788486759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Reya T, Morrison S, Clarke M, Weissman I. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 58.Morrison S, Spradling A. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonda T, Tu S, Wang T. Chronic Inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 60.Rizvi A, Swain J, Davies P, Bailey A, Decker A, Willenbring H, Grompe M, Fleming W, Wong M. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA. 2006;103:6321–6325. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 62.Hayden MS, West AP, Ghosh S. NF-[kappa]B and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 63.Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 65.Strober W. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsukada Y, Nakamura T, Iimura M, Iizuka B, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 67.Hanauer S, Sandborn W. Management of Crohn's disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 68.Heap GA, van Heel DA. The genetics of chronic inflammatory diseases. Hum Mol Genet. 2009;18:R101–106. doi: 10.1093/hmg/ddp001. [DOI] [PubMed] [Google Scholar]
- 69.Wehkamp J, Koslowski M, Wang G, Stange E. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol. 2008;1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 70.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 71.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EES, Higgins DE, Schreiber S, Glimcher LH. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Neurath MF, Salfeld J, Veldman GM, Schwertschlag U, Strober W, Anti-IL-12 Crohn's Disease Study Group Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 73.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 74.Festen EAM, Szperl AM, Weersma RK, Wijmenga C, Wapenaar MC. Inflammatory bowel disease and celiac disease: Overlaps in the pathology and genetics, and their potential drug targets. Curr Drug Targets Immune Endocr Metabol Disord. 2009;9:199–218. doi: 10.2174/187153009788452426. [DOI] [PubMed] [Google Scholar]
- 75.Green PHR, Jabri B. Coeliac disease. The Lancet. 2003;362:383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 76.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: The “No Man's Land” of gluten sensitivity. Am J Gastroenterol. 2009:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 78.Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64:505–519. doi: 10.1111/j.1398-9995.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 79.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 80.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 82.Feldstein Richard C, Sood Shivani, Katz Seymour. Small bowel adenocarcinoma in Crohn's disease. Inflamm Bowel Dis. 2008;14:1154–1157. doi: 10.1002/ibd.20393. [DOI] [PubMed] [Google Scholar]
- 83.Solem C, Harmsen W, Zinsmeister A, Loftus E., Jr Small intestinal adenocarcinoma in Crohn's disease: a case-control study. Inflamm Bowel Dis. 2004;10:32–35. doi: 10.1097/00054725-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Gyde SN, Prior P, Macartney JC, Thompson H, Waterhouse JA, Allan RN. Malignancy in Crohn's disease. Gut. 1980;21:1024–1029. doi: 10.1136/gut.21.12.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ekbom A, Adami HO, Helmick C, Zack M. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357–359. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 86.Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662–2669. doi: 10.3748/wjg.14.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Potter D, Murray J, Donohue J, Burgart L, Nagorney D, van Heerden J, Plevak M, Zinsmeister A, Thibodeau S. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073–7077. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 88.Cucchiara S, Escher JC, Hildebrand H, Amil-Dias J, Stronati L, Ruemmele FM. Pediatric inflammatory bowel diseases and the risk of lymphoma: should we revise our treatment strategies? J Pediatr Gastroenterol Nutr. 2009;48:257–267. doi: 10.1097/mpg.0b013e31818cf555. [DOI] [PubMed] [Google Scholar]
- 89.Abulafi A, Fiddian R. Malignant lymphoma in ulcerative colitis. Dis Colon Rectum. 1990;33:615–618. doi: 10.1007/BF02052219. [DOI] [PubMed] [Google Scholar]
- 90.Renton P, Blackshaw A. Colonic lymphoma complicating ulcerative colitis. Br J Surg. 1976;63:542–545. doi: 10.1002/bjs.1800630712. [DOI] [PubMed] [Google Scholar]
- 91.Lenzen R, Borchard F, Lubke H, Strohmeyer G. Colitis ulcerosa complicated by malignant lymphoma: case report and analysis of published works. Br Med J. 1995;36:306–310. doi: 10.1136/gut.36.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beigel F, Jurgens M, Tillack C, Subklewe M, Mayr D, Goke B, Brand S, Ochsenkuhn T. Hepatosplenic T-cell lymphoma in a patient with Crohn's disease. Nat Rev Gastroenterol Hepatol. 2009;6:433–436. doi: 10.1038/nrgastro.2009.87. [DOI] [PubMed] [Google Scholar]
- 93.Musso M, Porretto F, Crescimanno A, Bondi F, Polizzi V, Scalone R. Crohn's disease complicated by relapsed extranodal Hodgkin's lymphoma: prolonged complete remission after unmanipulated PBPC autotransplant. Bone Marrow Transplant. 2000;26:921–923. doi: 10.1038/sj.bmt.1702621. [DOI] [PubMed] [Google Scholar]
- 94.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn's disease: Benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–1024. doi: 10.1016/s0016-5085(00)70353-2. [DOI] [PubMed] [Google Scholar]
- 95.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: A meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1310–1311. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 97.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundstrom C. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 98.Elson C, Cong Y, McCracken V, Dimmitt R, Lorenz R, Weaver C. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 99.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 101.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 102.MacPherson B, Pfeiffer C. Experimental colitis. Digestion. 1976;14:424–452. doi: 10.1159/000197966. [DOI] [PubMed] [Google Scholar]
- 103.Edalat M, Mannervik B, Axelsson L. Selective expression of detoxifying glutathione transferases in mouse colon: effect of experimental colitis and the presence of bacteria. Histochem Cell Biol. 2004;122:151–159. doi: 10.1007/s00418-004-0688-7. [DOI] [PubMed] [Google Scholar]
- 104.Marcus R, Watt J. Colonic ulceration in young rats fed degraded carrageenan. Lancet. 1971;2:765–766. doi: 10.1016/s0140-6736(71)92130-1. [DOI] [PubMed] [Google Scholar]
- 105.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 106.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 109.Mori H, Ohbayashi F, Hirono I, Shimada T, Williams GM. Absence of genotoxicity of the carcinogenic sulfated polysaccharides carrageenan and dextran sulfate in mammalian DNA repair and bacterial mutagenicity assays. Nutr Cancer. 1984;6:92–97. doi: 10.1080/01635588509513812. [DOI] [PubMed] [Google Scholar]
- 110.Shintani N, Nakajima T, Sugiura M, Murakami K, Nakamura N, Kagitani Y, Mayumi T. Proliferative effect of dextran sulfate sodium (DSS)-pulsed macrophages on T cells from mice with DSS-induced colitis and inhibition of effect by IgG. Scand J Immunol. 1997;46:581–586. doi: 10.1046/j.1365-3083.1997.d01-169.x. [DOI] [PubMed] [Google Scholar]
- 111.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10-/-mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–739. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 112.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 113.McPherson M, Wei B, Turovskaya O, Fujiwara D, Brewer S, Braun J. Colitis immunoregulation by CD8+ T cell requires T cell cytotoxicity and B cell peptide antigen presentation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G485–G492. doi: 10.1152/ajpgi.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nemetz N, Abad C, Lawson G, Nobuta H, Chhith S, Duong L, Tse G, Braun J, Waschek JA. Induction of colitis and rapid development of colorectal tumors in mice deficient in the neuropeptide PACAP. Int J Cancer. 2008;122:1803–1809. doi: 10.1002/ijc.23308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki R, Miyamoto S, Yasui Y, Sugie S, Tanaka T. Global gene expression analysis of the mouse colonic mucosa treated with azoxymethane and dextran sodium sulfate. BMC Cancer. 2007;7:84. doi: 10.1186/1471-2407-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Colussi C, Fiumicino S, Giuliani A, Rosini S, Musiani P, Macri C, Potten CS, Crescenzi M, Bignami M. 1, 2-Dimethylhydrazine-induced colon carcinoma and lymphoma in msh2 (-/-) mice. J Natl Cancer Inst. 2001;93:1534–1540. doi: 10.1093/jnci/93.20.1534. [DOI] [PubMed] [Google Scholar]
- 118.Kohno H, Suzuki R, Sugie S, Tanaka T. Beta-Catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005;96:69–76. doi: 10.1111/j.1349-7006.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao J, Seril DN, Lu GG, Zhang M, Toyokuni S, Yang AL, Yang GY. Increased susceptibility of chronic ulcerative colitis-induced carcinoma development in DNA repair enzyme Ogg1 deficient mice. Mol Carcinog. 2008;47 doi: 10.1002/mc.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Westbrook AM, Schiestl RH. Atm deficient mice exhibit increased sensitivity to dextran sulfate sodium-induced colitis characterized by elevated DNA damage and persistent immune activation. Cancer Res. 2010;70:1875–1884. doi: 10.1158/0008-5472.CAN-09-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qiu B, Vallance B, Blennerhassett P, Collins S. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178–1182. doi: 10.1038/13503. [DOI] [PubMed] [Google Scholar]
- 124.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 125.Iijima H, Neurath M, Nagaishi T, Glickman J, Nieuwenhuis E, Nakajima A, Chen D, Fuss I, Utku N, Lewicki D. Specific regulation of T helper cell 1–mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Camoglio L, te Velde A, de Boer A, ten Kate F, Kopf M, van Deventer S. Hapten-induced colitis associated with maintained Th1 and inflammatory responses in IFN-receptor-deficient mice. Eur J Immunol. 2000;30:1486–1495. doi: 10.1002/(SICI)1521-4141(200005)30:5<1486::AID-IMMU1486>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 127.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neurath M, Fuss I, Kelsall B, Meyer zum Buschenfelde KH, Strober W. Effects of IL-12 and antibodies to IL-12 on established granulomatous colitis in mice. Ann NY Acad Sci. 1996;795:368–370. doi: 10.1111/j.1749-6632.1996.tb52695.x. [DOI] [PubMed] [Google Scholar]
- 129.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis, Inflamm. Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.te Velde A, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- 131.Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1-and Th2-mediated inflammatory bowel disease with NF- B decoy oligonucleotides. J Clin Invest. 2005;115:3057–3071. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz K, Binder V. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vogelsang H, Schwarzenhofer M, Oberhuber G. Changes in gastrointestinal permeability in celiac disease. Dig Dis. 1998;16:333–336. doi: 10.1159/000016886. [DOI] [PubMed] [Google Scholar]
- 135.Hugot J, Chamaillard M, Zouali H, Lesage S, Cézard J, Belaiche J, Almer S, Tysk C, O'Morain C, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 136.Peltekova V, Wintle R, Rubin L, Amos C, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- 137.Hermiston M, Gordon J. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1237. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 138.Baribault H, Penner J, Iozzo R, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 139.Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AWC. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 140.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 141.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- 142.Laubitz D, Larmonier C, Bai A, Midura-Kiela M, Lipko M, Thurston R, Kiela P, Ghishan F. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shekhawat PS, Srinivas SR, Matern D, Bennett MJ, Boriack R, George V, Xu H, Prasad PD, Roon P, Ganapathy V. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2 -/-) mice. Mol Genet Metab. 2007;92:315–324. doi: 10.1016/j.ymgme.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 145.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 147.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 148.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 149.Zaph C, Troy A, Taylor B, Berman-Booty L, Guild K, Du Y, Yost E, Gruber A, May M, Greten F. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 150.Shull M, Ormsby I, Kier A, Pawlowski S, Diebold R, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gorelik L, Flavell RA. Abrogation of TGF[beta] signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 152.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 154.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A, Horak I. Ulcerative colitislike disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 156.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 157.Sohn K, Shah S, Reid S, Choi M, Carrier J, Comiskey M, Terhorst C, Kim Y. Molecular genetics of ulcerative colitis-associated colon cancer in the interleukin 2-and 2-microglobulin-deficient mouse. Cancer Res. 2001;61:6912–6917. [PubMed] [Google Scholar]
- 158.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 159.Rennick DM, Fort MM. Lessons from genetically engineered animal models: XII. IL-10-deficient (IL-10-/-) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–833. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 160.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang S, Bloom S, Norian L, Geske M, Flavell R, Stappenbeck T, Allen P. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Medicine. 2008;5:e41. doi: 10.1371/journal.pmed.0050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siegmund B, Sennello JA, Lehr HA, Batra A, Fedke I, Zeitz M, Fantuzzi G. Development of intestinal inflammation in double IL-10-and leptin-deficient mice. J Leukoc Biol. 2004;76:782–786. doi: 10.1189/jlb.0404239. [DOI] [PubMed] [Google Scholar]
- 163.McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G90–99. doi: 10.1152/ajpgi.2000.279.1.G90. [DOI] [PubMed] [Google Scholar]
- 164.Mombaerts P, Mizoguchi E, Grusby M, Glimcher L, Bhan A, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 165.Mizoguchi A, Mizoguchi E, Bhan A. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor alpha mutant mice. Gastroenterology. 1999;116:320–326. doi: 10.1016/s0016-5085(99)70128-9. [DOI] [PubMed] [Google Scholar]
- 166.Okuda Y, Takahashi I, Kim J, Ohta N, Iwatani K, Iijima H, Kai Y, Tamagawa H, Hiroi T, Kweon M. Development of colitis in signal transducers and activators of transcription 6-deficient T-cell receptor -deficient mice: A potential role of signal transducers and activators of transcription 6-independent interleukin-4 signaling for the generation of Th2-biased pathological CD4+ T cells. Am J Pathol. 2003;162:263–271. doi: 10.1016/s0002-9440(10)63817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Morotomi M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–2398. [PubMed] [Google Scholar]
- 168.Funabashi H, Uchida K, Kado S, Matsuoka Y, Ohwaki M. Establishment of a Tcrb and Trp53 genes deficient mouse strain as an animal model for spontaneous colorectal cancer. Exp Anim. 2001;50:41–47. doi: 10.1538/expanim.50.41. [DOI] [PubMed] [Google Scholar]
- 169.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–50. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 170.Welte T, Zhang S, Wang T, Zhang Z, Hesslein D, Yin Z, Kano A, Iwamoto Y, Li E, Craft J. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kobayashi M, Kweon M, Kuwata H, Schreiber R, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 173.Wirtz S, Finotto S, Kanzler S, Lohse AW, Blessing M, Lehr HA, Galle PR, Neurath MF. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF-plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- 174.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Galphai2 (-/-) mice is an intrinsic naive CD4 (+) T cell disorder selective for the Galphai2 subunit. Int Immunol. 2003;15:1359–1367. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 175.Elgbratt K, Bjursten M, Willen R, Bland PW, Hörnquist EH. Aberrant T-cell ontogeny and defective thymocyte and colonic T-cell chemotactic migration in colitis-prone Gai2-deficient mice. Immunology. 2007;122:199–209. doi: 10.1111/j.1365-2567.2007.02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohman L, Franzén L, Rudolph U, Harriman GR, Hultgren HE. Immune activation in the intestinal mucosa before the onset of colitis in Galphai2-deficient mice. Scand J Immunol. 2000;52:80–90. doi: 10.1046/j.1365-3083.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 177.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappa B and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 179.Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kastenbauer S, Ziegler-Heitbrock H. NF-kappaB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect Immun. 1999;67:1553–1559. doi: 10.1128/iai.67.4.1553-1559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Erdman SE, Fox JG, Dangler CA, Feldman D, Horwitz BH. Cutting edge: Typhlocolitis in NF-{{kappa}}B-deficient mice. J Immunol. 2001;166:1443–1447. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- 182.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bettelli E, Sullivan B, Szabo S, Sobel R, Glimcher L, Kuchroo V. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stoicov C, Fan X, Liu J, Bowen G, Whary M, Kurt-Jones E, Houghton J. T-bet knockout prevents Helicobacter felis-induced gastric cancer. J Immunol. 2009;183:642–649. doi: 10.4049/jimmunol.0900511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park JW, Min HJ, Sohn JH, Kim JY, Hong JH, Sigrist KS, Glimcher LH, Hwang ES. Restoration of T-box-containing protein expressed in T cells protects against allergen-induced asthma. J Allergy Clin Immunol. 2009;123:479–485. doi: 10.1016/j.jaci.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 187.Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J Immunol. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fina D, Sarra M, Caruso R, Del Vecchio Blanco G, Pallone F, MacDonald TT, Monteleone G. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008;57:887–892. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 191.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 192.Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, Kato S, Yoshimura A. IFN{gamma}-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kontoyiannis D, Pasparakis M, Pizzaro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 194.Clegg C, Rulffes J, Haugen H, Hoggatt I, Aruffo A, Durham S, Farr A, Hollenbaugh D. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–1122. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 195.Watanabe M, Ueno Y, Yajima T, Okamoto S, Hayashi T, Yamazaki M, Iwao Y, Ishii H, Habu S, Uehira M, Nishimoto H, Ishikawa H, Hata J, Hibi T. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Snapper S, Rosen F, Mizoguchi E, Cohen P, Khan W, Liu C, Hagemann T, Kwan S, Ferrini R, Davidson L. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 197.Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Köster A, Hess B, Evers M, Von Figura K. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 199.Morrissey P, Charrier K, Braddy S, Liggitt D, Watson J. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Powrie F, Leach M, Mauze S, Caddie L, Coffman R, Hunig T. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 201.Brimnes Jens, Reimann Jörg, Nissen Mogens H, Claesson Mogens H. Enteric bacterial antigens activate CD4+ T cells from scid mice with inflammatory bowel disease. Eur J Immunol. 2001;31:23–31. doi: 10.1002/1521-4141(200101)31:1<23::aid-immu23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 202.Aranda R, Sydora B, McAllister P, Binder S, Yang H, Targan S, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 203.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 205.Kullberg M, Andersen J, Gorelick P, Caspar P, Suerbaum S, Fox J, Cheever A, Jankovic D, Sher A. Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope. Proc Natl Acad Sci USA. 2003;100:15830–15835. doi: 10.1073/pnas.2534546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Iqbal N, Oliver J, Wagner F, Lazenby A, Elson C, Weaver C. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hollander GA, Simpson SJ, Mizoguchi E, Nichogiannopoulou A, She J, Gutierrez-Ramos JC, Bhan AK, Burakoff SJ, Wang B, Terhorst C. Severe colitis in mice with aberrant thymic selection. Immunity. 1995;3:27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 208.Steinhoff U, Brinkmann V, Klemm U, Aichele P, Seiler P, Brandt U, Bland PW, Prinz I, Zügel U, Kaufmann SH. Autoimmune intestinal pathology induced by hsp60-specific CD8 T cells. Immunity. 1999;11:349–358. doi: 10.1016/s1074-7613(00)80110-7. [DOI] [PubMed] [Google Scholar]
- 209.Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, Imaoka A, Okada Y, Umesaki Y. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sugawara K, Olson TS, Moskaluk CA, Stevens BK, Hoang S, Kozaiwa K, Cominelli F, Ley KF, McDuffie M. Linkage to peroxisome proliferator-activated receptor- in SAMP1/YitFc mice and in human Crohn's disease. Gastroenterology. 2005;128:351–360. doi: 10.1053/j.gastro.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 211.Sundberg J, Elson C, Bedigian H, Birkenmeier E. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994;107:1726–1735. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 212.Brandwein S, McCabe R, Cong Y, Waites K, Ridwan B, Dean P, Ohkusa T, Birkenmeier E, Sundberg J, Elson C. Spontaneously colitic C3H/HeJBir mice demonstrate selective antibody reactivity to antigens of the enteric bacterial flora. J Immunol. 1997;159:44–52. [PubMed] [Google Scholar]
- 213.Schuppler M, Lotzsch K, Waidmann M, Autenrieth IB. An abundance of Escherichia coli is harbored by the mucosa- associated bacterial flora of interleukin-2-deficient mice. Infect Immun. 2004;72:1983–1990. doi: 10.1128/IAI.72.4.1983-1990.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McGhee JR, Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Farrell R, LaMont J. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:41–62. doi: 10.1016/s0889-8553(01)00004-8. [DOI] [PubMed] [Google Scholar]
- 216.Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, Gress RE, Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995;146:264–275. [PMC free article] [PubMed] [Google Scholar]
- 217.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: A model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169:5595–600. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 218.Hovhannisyan Z, Weiss A, Martin A, Wiesner M, Tollefsen S, Yoshida K, Ciszewski C, Curran SA, Murray JA, David CS, Sollid LM, Koning F, Teyton L, Jabri B. The role of HLA-DQ8 β57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature. 2008;456:534–538. doi: 10.1038/nature07524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G217–25. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 220.de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, Jackson DC, Ladhams J, Allison J, McCluskey J. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol. 2009;182:7440–7450. doi: 10.4049/jimmunol.0900233. [DOI] [PubMed] [Google Scholar]
- 221.Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z, Zala G, Flury R, Seelentag W, Roth J. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1090–1097. doi: 10.1007/BF02234627. [DOI] [PubMed] [Google Scholar]
- 222.Moser A, Pitot H, Dove W. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 223.Ritland S, Gendler S. Chemoprevention of intestinal adenomas in the ApcMin mouse by piroxicam: kinetics, strain effects and resistance to chemosuppression. Carcinogenesis. 1999;20:51–58. doi: 10.1093/carcin/20.1.51. [DOI] [PubMed] [Google Scholar]
- 224.Reuter B, Zhang XJ, Miller M. Therapeutic utility of aspirin in the ApcMin/+ murine model of colon carcinogenesis. BMC Cancer. 2002;2:19–27. doi: 10.1186/1471-2407-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T Cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Coombes J, Maloy K. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19:116–126. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 227.Monteleone G, Kumberova A, Croft N, McKenzie C, Steer H, MacDonald T. Blocking Smad7 restores TGF- 1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 229.Soderstrom K, Bucht A, Halapi E, Gronberg A, Magnusson I, Kiessling R. Increased frequency of abnormal gamma delta T cells in blood of patients with inflammatory bowel diseases. J Immunol. 1996;156:2331–2339. [PubMed] [Google Scholar]
- 230.Eckburg P, Relman DA. The role of microbes in Crohn's disease. Clin Infect Dis. 2007;44:256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 231.Sartor B. Bacteria in Crohn's disease: mechanisms of inflammation and therapeutic implications. J Clin Gastroenterol. 2007;41:S37–43. doi: 10.1097/MCG.0b013e31802db364. [DOI] [PubMed] [Google Scholar]
- 232.D'Haens G, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 233.Prantera C, Scribano ML. Antibiotics and probiotics in inflammatory bowel disease: why, when, and how. Curr Opin Gastroenterol. 2009;25:329–333. doi: 10.1097/MOG.0b013e32832b20bf. [DOI] [PubMed] [Google Scholar]
- 234.Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm M, Weismueller J, Beglinger C, Stolte M. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Frank D, St Amand A, Feldman R, Boedeker E, Harpaz N, Pace N. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bibiloni R, Mangold M, Madsen K, Fedorak R, Tannock G. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn's disease and ulcerative colitis patients. J Med Microbiol. 2006;55:1141–1149. doi: 10.1099/jmm.0.46498-0. [DOI] [PubMed] [Google Scholar]
- 237.Scanlan P, Shanahan F, O'Mahony C, Marchesi J. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. J Clin Microbiol. 2006;44:3980–3988. doi: 10.1128/JCM.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Marchesi J, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, Wilson I, Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 239.Mylonaki M, Rayment N, Rampton D, Hudspith B, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:481–487. doi: 10.1097/01.mib.0000159663.62651.4f. [DOI] [PubMed] [Google Scholar]
- 240.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 241.Barrett J, Hansoul S, Nicolae D, Cho J, Duerr R, Rioux J, Brant S, Silverberg M, Taylor K, Barmada M. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dotan I. Serologic markers in inflammatory bowel disease: tools for better diagnosis and disease stratification. Expert Rev Gastroenterol Hepatol. 2007;1:265–274. doi: 10.1586/17474124.1.2.265. [DOI] [PubMed] [Google Scholar]
- 243.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 244.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 245.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 246.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333. doi: 10.1136/gut.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kakazu T, Hara J, Matsumoto T, Nakamura S, Oshitani N, Arakawa T, Kitano A, Nakatani K, Kinjo F, Kuroki T. Type 1 T-helper cell predominance in granulomas of Crohn's disease. Am J Gastroenterol. 1999;94:2149–2155. doi: 10.1111/j.1572-0241.1999.01220.x. [DOI] [PubMed] [Google Scholar]
- 248.Stoll M, Corneliussen B, Costello CM, Waetzig GH, Mellgard B, Koch WA, Rosenstiel P, Albrecht M, Croucher PJP, Seegert D. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nat Genet. 2004;36:476–480. doi: 10.1038/ng1345. [DOI] [PubMed] [Google Scholar]
- 249.Festen EA, Szperl AM, Weersma RK, Wijmenga C, Wapenaar MC. Inflammatory bowel disease and celiac disease: Overlaps in the pathology and genetics, and their potential drug targets. Endocr Metab Immune Disord Drug Targets. 2009;9:199–218. doi: 10.2174/187153009788452426. [DOI] [PubMed] [Google Scholar]
- 250.Leeds JS, Horoldt BS, Sidhu R, Hopper AD, Robinson K, Toulson B, Dixon L, Lobo AJ. Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand J Gastroenterol. 2007;42:1214–1220. doi: 10.1080/00365520701365112. [DOI] [PubMed] [Google Scholar]
- 251.Westbrook AM, Wei B, Braun J, Schiestl R. More damaging than we think: Systemic effects of intestinal inflammation. Cell cycle. 2009;8:2482–2483. doi: 10.4161/cc.8.16.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Westbrook AM, Wei B, Braun J, Schiestl RH. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–4834. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.O'Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH, Crispin DA. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 254.Issa J, Ahuja N, Toyota M, Bronner M, Brentnall T. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 255.Potter D, Murray J, Donohue J, Burgart L, Nagorney D, van Heerden J, Plevak M, Zinsmeister A, Thibodeau S. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073–7077. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 256.Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 257.Zhang MQ, Chen ZME, Wang HL. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma: a comparison with colorectal adenocarcinoma. Mod Pathol. 2006;19:573–580. doi: 10.1038/modpathol.3800566. [DOI] [PubMed] [Google Scholar]
- 258.Chan RCF, Katelaris PH, Stewart P, Lin BPC. Small bowel adenocarcinoma with high levels of microsatellite instability in Crohn's disease. Hum Pathol. 2006;37:631–634. doi: 10.1016/j.humpath.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 259.Wheeler JMD, Warren BF, Mortensen N, Kim HC, Biddolph SC, Elia G, Beck NE, Williams GT, Shepherd NA, Bateman AC. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut. 2002;50:218–223. doi: 10.1136/gut.50.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rashid A, Hamilton S. Genetic alterations in sporadic and Crohn's-associated adenocarcinomas of the small intestine. Gastroenterology. 1997;113:127–135. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 261.Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C. Pathogenesis and risk factors of small bowel adenocarcinoma: A colorectal cancer sibling? Am J Gastroenterol. 2005;100:703–710. doi: 10.1111/j.1572-0241.2005.40605.x. [DOI] [PubMed] [Google Scholar]
- 262.Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, Aufses AH., Jr Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56:2914–2921. doi: 10.1002/1097-0142(19851215)56:12<2914::aid-cncr2820561232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 263.Kühnel D, Taugner F, Scholtka B, Steinberg P. Inflammation does not precede or accompany the induction of preneoplastic lesions in the colon of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine-fed rats. Arch Toxicol. 2009;83:763–768. doi: 10.1007/s00204-009-0406-2. [DOI] [PubMed] [Google Scholar]
- 264.Amos-Landgraf J, Kwong L, Kendziorski C, Reichelderfer M, Torrealba J, Weichert J, Haag J, Chen K, Waller J, Gould M. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci USA. 2007;104:4036–4041. doi: 10.1073/pnas.0611690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Uronis J, Threadgill D. Murine models of colorectal cancer. Mamm Genome. 2009;20:261–268. doi: 10.1007/s00335-009-9186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hoffmann J, Pawlowski N, Kühl A, Höhne W, Zeitz M. Animal models of inflammatory bowel disease: an overview. Pathobiology. 2000;70:121–130. doi: 10.1159/000068143. [DOI] [PubMed] [Google Scholar]