Abstract
Purpose
Reporting long-term toxicities in trials of chemo-irradiation (CRT) of head and neck cancer (HNC) has mostly been limited to observer-rated maximal grades ≥3. We evaluated this reporting approach for dysphagia by assessing 1) patient-reported dysphagia (PRD), and 2) objective swallowing dysfunction through videofluoroscopy (VF), in patients with various grades of maximal observer-reported dysphagia (ORD).
Methods
62 HNC patients completed quality-of-life questionnaires periodically through 12m post-CRT. Five PRD items were selected: three dysphagia-specific questions, an Eating-Domain, and “Overall Bother”. They underwent VF at 3m and 12m, and ORD (Common Terminology Criteria for Adverse Events) scoring every 2 months. We classified patients into four groups (0-3) according to maximal ORD scores documented 3-12 months post-CRT, and assessed PRD and VF summary scores in each group.
Results
Differences in ORD scores among the groups were considerable throughout the observation period. In contrast, PRD scores were similar between Groups 2 and 3, and variable in Group 1. VF scores were worse in Group 3 compared to 2 at 3m but similar at 12m. In Group 1, PRD and VF scores from 3 through 12 months were close to Groups 2 and 3 if ORD score 1 persisted, but were similar to Group 0 in patients whose ORD scores improved by 12m.
Conclusions
Patients with lower maximal ORD grades, especially if persistent, had similar rates of PRD and objective dysphagia as patients with highest grades. Lower ORD grades should therefore be reported. These findings may have implications for reporting additional toxicities besides dysphagia.
Keywords: dysphagia, swallowing, head and neck cancer, chemoirradiation, quality of life, CTCAE v3
Introduction
Decision making in oncology relies on weighing the potential benefits vs. the toxicities of specific therapies. However, such decisions are often limited due to inadequacies commonly found in the reporting of adverse events (AEs) in clinical trials1-3. Problematic aspects of toxicity reporting in clinical trials in oncology include underreporting of recurrent AEs and inconsistent and incomplete characterization and reporting of high grade AEs 2,3.
Examination of the reporting of late AEs in key phase II 4-17 or III 18-29 studies of chemoradiation (CRT) of head and neck cancer (HNC) published since 2000, revealed that almost all studies reported only the rates of high grade (≥3) observer-rated AEs. In order to assess the adequacy of reporting observer-rated high grade late AEs, we evaluated a specific AE, dysphagia, which has emerged in recent years as a major sequel of CRT for HNC and an important determinant of long-term quality of life (QOL) 30,31,32,33. We have evaluated dysphagia prospectively through three modalities: observer-rated scores (ORD), patient-rated scores (PRD), and objective evaluations of swallowing dysfunction. These evaluations, made synchronously at several time points after CRT, allowed us to assess the adequacy of the reporting of high grade ORD. In this study we have evaluated the severity of late dysphagia, measured by PRD and objective swallowing dysfunction, in patient groups stratified according to the highest ORD grades. Our belief is that the findings of this study may be relevant to the reporting of other AEs as well.
Patients and Methods
This is an analysis of data obtained in a prospective, longitudinal study of CRT for HNC. Full details of the study were previously published 34. The study was approved by the Institutional Review Board of the University of Michigan and all patients signed a study-specific consent form. Eligible patients were those with Stage III/IV Squamous Cell Carcinoma of the oropharynx or nasopharynx who had not received prior therapy, had a Karnofsky performance status ≥ 80, and for whom primary CRT was recommended. The main study objective was assessing treatment-related effects on dysphagia measures.
Details of therapy have been published elsewhere 34. In brief, intensity modulated radiotherapy (IMRT) aimed at reducing dysphagia by sparing the parts of the swallowing structures (pharyngeal constrictors, espophagus, and glottic and supraglottic larynx), as well as the major salivary glands, which were outside the targets. A dose of 70 Gy to gross disease and 56-63 Gy to subclinical disease was delivered over 35 fractions. Concurrent chemotherapy included carboplatin (AUC 1) and paclitaxel (30 mg/m2) once a week for patients with oropharyngeal cancer, and cisplatin, 100 mg/m2 every 3 weeks for patients with nasopharyngeal cancer. Percutaneous endoscopic gastrostomy feeding tubes (FTs) were inserted if weight loss during therapy approached 10%.
Objective assessment of swallowing was made by videofluoroscopy (VF), the standard objective measure for evaluation of swallowing dysfunction 32. VFs were performed pre-CRT and at 3 months and 12 months post-CRT. Detailed description of the procedure at our institution has been previously published 35. Each VF was evaluated simultaneously by two speech pathologists (T.L and M.H.), who summarized and scored the findings after reaching a consensus36. The summary score was represented on a 1-7 scale with score 1 indicating normal swallowing, scores 2-3 mild swallow dysfunction requiring dietary modifications, scores 4-5 moderate dysfunction requiring therapeutic precautions, and scores 6-7 severe dysfunction requiring supplemental/complete enteral feeding support 37.
PRD was assessed with the Head and Neck quality of life (QOL) questionnaire (HNQOLQ) 38 and the University of Washington Head and Neck-related QOL questionnaire (UWQOLQ) 39. The HNQOLQ contains an Eating Domain, which includes two dysphagia-swallowing related questions inquiring about problems in swallowing soft/solid food (q9: “HNQOLQ Solid”) or liquids (q8:“HNQOLQ Liquids”), and four additional questions which inquire about difficulties in mouth opening, dryness while eating, problems with chewing, and taste. HNQOLQ also contains a general question inquiring about the amount of disturbance or bother as a result of the patient's head and neck condition (q26: “Overall Bother”). All questions have five possible answers (“not at all”, “slightly”, “moderately”, “a lot”, and “extremely”), rated numerically from 0 through 4, respectively. The UWQOLQ includes a swallowing question (q6: “UWQOLQ Swallow”) with five possible answers: “I swallow normally”, “I cannot swallow certain solid food”, “I can only swallow soft food”, “I can only swallow liquid foods”, and “I cannot swallow”, rated 0-4, respectively. The items we used for PRD included the 3 individual swallowing questions from the two questionnaires (HNQOLQ Solid, HNQOLQ Liquids, and UWQOLQ Swallow), the HNQOLQ Overall Bother, and the HNQOLQ Eating Domain, which was calculated by summarizing the individual questions' scores and standardizing this score on a 0-4 scale, with higher scores representing worse outcome. Eating Domain scores were calculated only if responses to ≥ 4 questions were available, with adjustment for missing questions when appropriate, as detailed elsewhere 38. The questionnaires were given to patients before CRT and 3, 6 and 12 months post-CRT. The patients filled the questionnaires on each follow-up clinical visit, prior to evaluation by their physician, in order to ensure a high rate of compliance.
ORD was scored by the physician treating the patients based on Common Terminology Criteria for Adverse Events v2.0 (CTCAE) 40. Only one CTCAE item relates specifically to dysphagia: “Dysphagia (difficulty swallowing)”. It is scored 0-4 (a score of 0 denotes no dysphagia; a score of 1: symptomatic, able to eat regular diet; a score of 2: symptomatic, altered eating/swallowing, I.V. fluids indicated < 24 hours; a score of 3: symptomatic, severely altered eating/swallowing with inadequate caloric or fluid intake, I.V. fluids or FT indicated > 24 hours; and a score of 4: life-threatening, due to obstruction or perforation). Patients with FTs received CTCAE score 3 if they were FT dependent but could swallow fluids, and score 4 if they were completely incapable of swallowing. Scores were determined weekly during CRT, at 1, 3, and 4 months after CRT, and every 2 months thereafter. Scoring was made by a single physician throughout the study. The scoring physician was blinded to patients' responses to the QOL questionnaires or the results of VF.
The parameter used for reporting late toxicity in all the phase II-III studies cited in the Introduction were either FT dependency or the most severe ORD score documented ≥ 3 months post-CRT 4-33. We therefore used the most severe ORD score observed ≥ 3 months post-CRT to classify the patients into four groups (i.e. patients with maximal CTCAE score 0, 1, 2 and 3, constituted Groups 0, 1, 2 and 3, respectively), and assessed PRD and VF scores in each group.
Statistical analysis
The maximal score was determined as described by Trotti et al. 41. Patients were classified into four groups according to their most severe CTCAE-based dysphagia score (groups 0-3 for maximal scores 0-3, respectively). For each group, the mean scores for each PRD parameter were plotted over time. In order to account for repeated assessments over time we computed the area under the dysphagia-over time curve (AUC) 42-44 for each plot, and divided this value by 9 months (the overall duration of follow-up from 3 to 12 months post-therapy), with the resulting value representing the mean severity of dysphagia over time. Computation of AUC was achieved by connecting a straight line between every set of adjacent points (i.e. scores at consecutive time points) and summing up the areas beneath the curve obtained, using the trapezoid rule. Whenever data was missing the software interpolated based on the available data. We utilized the Kruskal-Wallis test to assess overall differences in scores between groups, and Mann-Whitney test to compare specific pairs of groups, if Kruskal-Wallis test was significant. All statistical tests were two sided at the 5% level. We used GraphPad prism v.5 for the statistical analysis (Graphpad Software, Inc., La Jolla, CA; www.graphpad.com).
Results
Patient Population
Sixty-two of the 79 patients who enrolled to the study had a minimum of one year post-therapy follow-up data and were included in this analysis. Median age was 56 years (range 38-78); 86% were males, 94% had oropharyngeal carcinoma (mostly tonsil or base of tongue cancers). Tumor stages were III, IVa, and IVb in 13%, 82%, and 5% of the patients, respectively.
Completeness of Data
VF scores were available for 62, 58, and 59 patients at pre-CRT and at 3 months and 12 months post-CRT, respectively. ORD scores were available for 62, 60, 59 and 62 patients and PRD scores were available for 61, 62, 56 and 62 patients, pre-CRT, 3, 6 and 12 months post-CRT, respectively.
Dysphagia according to ORD scores
Patient groups 0-3, defined according to the maximal CTCAE ORD score reported for each patient post-CRT, included 13, 34, 8, and 7 patients, respectively. We compared the pre-CRT PRD and VF scores among the four Groups and did not find any significant differences. There was a trend towards worse scores in Group 3 according to UWQOL Swallow and HNQOLQ Overall Bother (p=0.09).
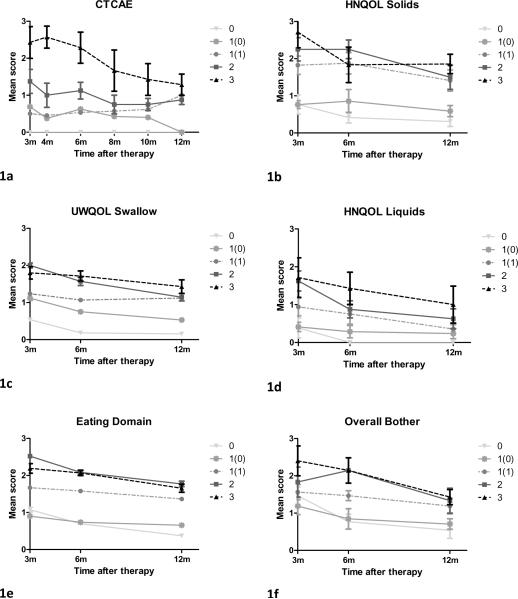
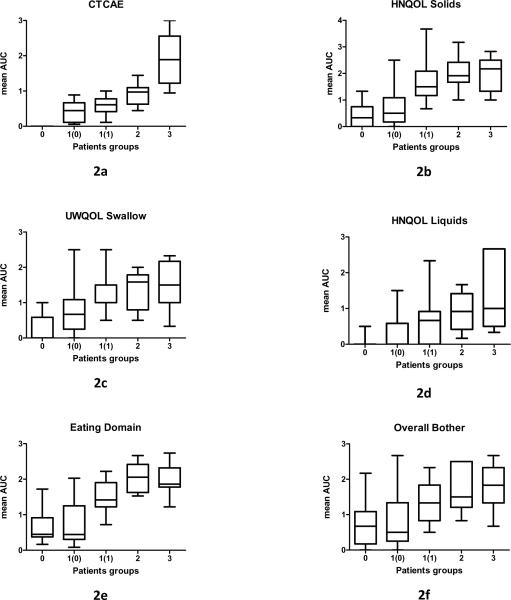
All the patients in group 0 continued (by definition) to have CTCAE score 0 at 12m, while 13 of the 15 patients from Groups 2 and 3 had a score of 1 at 12m (one patient had score 0 and one patient score 3). 50% of the patients from Group 1 continued to have a score of 1 at twelve months post-RT, and 50% improved and had CTCAE score 0. We plotted mean post-CRT CTCAE scores over time for Groups 0-3 and found a clear separation between the curves during the entire period of follow-up (Figure 1a) as well as statistically significant differences between the AUCs of the various Groups (Figure 2a). The most substantial difference was found between Group 3 and Group 2 (AUC values 0.9 and 1.9, respectively; p=0.01).
Figure 1.
Mean dysphagia scores over time according to mean observer-rated CTCAE scores (1a) and patient-reported dysphagia (1b-1f). Patients were grouped (groups 0-3) according to the most severe CTCAE dysphagia grade recorded ≥ 3 months post-therapy. Group 1 was subdivided into two groups according to the CTCAE score 12 months post-therapy [1(0) and 1(1) for CTCAE scores 0 and 1, respectively]. Bars=standard error.
Figure 2.
Area under the curve (AUC) of dysphagia scores from 3 months through 12 months post-therapy in patients' groups 0-3 (classified according to most severe dysphagia grade recorded ≥ 3 months post-therapy). Group 1 was subdivided into two groups according to 12 months post-therapy CTCAE score [1(0) and 1(1) for CTCAE scores 0 and 1, respectively]. (2a) Observer-rated dysphagia (CTCAE scores). (2b-f) Patient-reported dysphagia. Horizontal lines=median; Bars=range
Dyspagia according to PRD measures
Post-CRT mean PRD scores plotted over time for Groups 0-3 revealed that compared to the pattern observed with the ORD, the distinction between groups, particularly between Groups 3 and 2, was much less prominent (Figures 1, b-f). Comparisons of the AUC values revealed that Group 0 had significantly less dysphagia than all other groups according to all PRD measures (p ≤ 0.03) excluding Overall Bother (p=0.25); Group 1 had significantly less dysphagia than Group 2 according to HNQOL liquids (p=0.02), HNQOL solids (p=0.01) and Eating Domain (p=0.0006), and Group 2 had similar scores to Group 3 (p ≥ 0.6).
Dysphagia according to VF
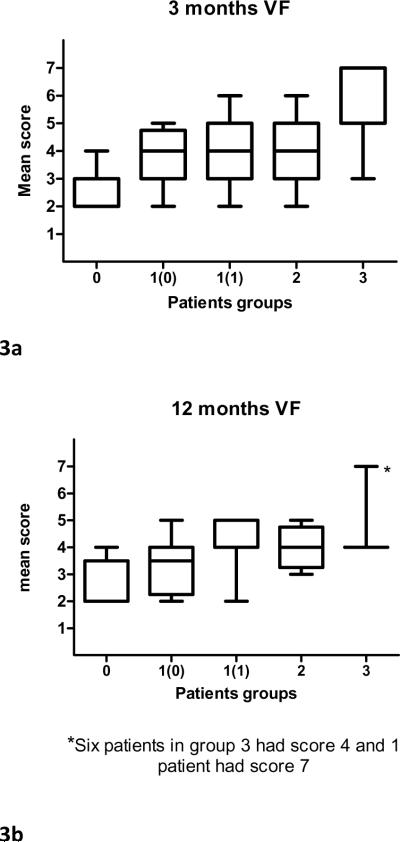
Comparison of VF summary scores at 3 months and 12 months post-CRT showed that at both time points Group 0 had significantly better scores than Group 1 (mean scores 2.5 vs. 3.9 and 2.5 vs. 3.8, respectively; p ≤ 0.0009), (Figure 3), and that there was no significant difference between Groups 1 and 2 (the mean scores for Group 2 at both time-points was 4; p=0.9). Group 3 had significantly worse scores than Group 2 at three months post-CRT (mean score 6; p=0.02), but not at 12 months post-CRT (mean scores 4.4; p=0.6, Figure 3).
Figure 3.
Videofluoroscopy (VF) summary scores in patient groups 1-3 (classified according to most severe dysphagia grade recorded ≥ 3 months post-CRT). Group 1 was subdivided into 2 groups according to 12 months post-CRT CTCAE score [1(0) and 1(1) for CTCAE scores 0 and 1, respectively]. Horizontal lines=median; Bars=range.
Taken together, the above analyses showed that according to PRD and VF scores, Group 0 did significantly better than all other groups and Group 2 was similar to Group 3. Group 1, which was the largest group, was found to be heterogeneous according to all modalities: CTCAE scores at 12 months were 0 in 50% and 1 in 50%, PRD measures had large standard deviations (Figure 1-2), and VF scores were low (2-3) in a third of the patients and moderate (4-5) in the rest. An additional exploration was therefore undertaken for Group 1. We compared patients from Group 1 whose CTCAE score improved to 0 at 12 months [1(0)] to patients from group 1 whose score persisted to be 1 at 12 months [1(1)] (Figures 1- 2). We found significant differences between the two sub-groups according to HNQOL Solids (p=0.0004), UWQOL Swallow (p=0.02), HNQOL Eating Domain (0.0003), HNQOL Overall Bother (p=0.01), and 12 months VF scores (p=0.02). The curves of the mean PRD scores were similar among groups 1(1), 2, and 3, and the AUC values for Group 1(1) were not significantly different from Groups 2 or 3 in any PRD item (p>0.14), except for Eating Domain (p=0.013). Similarly, the VF scores for Group 1(1) at 12 months were identical to those of Groups 2 and 3 (Fig 3b). In comparison, PRD or VF scores of Group 1(0) were indistinguishable from Group 0 in most items (Figs 1b-f, 2b-f, 3b).
Discussion
This study has demonstrated that patients with low maximal ORD CTCAE scores, either a score of 2 or a score of 1 which persists through 12 months and does not improve to 0, had self-reported dysphagia and objective swallowing dysfunction which were not significantly different from those with maximal CTCAE scores of 3. Patients with maximal CTCAE score of 0, or a maximal score of 1 which improved to 0 at 12 months, were the only groups with consistently low scores in all dysphagia measures. These results suggest, therefore, that reporting only the number of patients with maximal dysphagia scores ≥3 would underestimate the burden of dysphagia following therapy.
In recent years there have been contradicting trends in the research of toxicity reporting. While some studies raised concern about under-reporting of toxicity 2,41, others warned against a deluge of toxicity data 45. Since most therapies being tested in prospective studies cause lowgrade AEs in a high proportion of patients 3, it is understandable that authors limit their reporting to those AEs that are thought to be life threatening, require dose reduction, or therapeutic intervention. In the design of the CTCAE, such AEs were meant to be encompassed by Grades 3-4 46 categories, and therefore lower grades of toxicity are often not reported.
Dysphagia is a subjective symptom, and as such it is likely to be underestimated by clinicians, as reported by Basch et al. who found that for highly subjective adverse events the clinicians tended to assign a lesser grade of severity than the patients 47. Similarly, Jensen et al. found in a cross-sectional study that observer-rated [according to the Danish Head and Neck Cancer Group (DAHANCA) scale] and patient-assessed toxicities (according to EORTC H&N35 questionnaire) were correlated, but that observer-based rating under-estimated the patientscored symptoms 48. As reported by Pauloski et al., patients with complaints of dysphagia have significantly worse objective swallowing function, and more limitations and alteration of their diet, compared to patients with no complaints 49. Hence, if low grade ORD is disregarded, clinically important dysphagia assessed by PRD and objective tests may be under-reported. Similar findings have been reported for xerostomia after radiotherapy of HNC 50. To the best of our knowledge, our study is the first one evaluating the implications on CTCAE-based reporting of the under-estimation of the degree of toxicity by the observer.
In Group 3, severe dysphagia according to VF scores (6-7) was mostly found at 3 months post-CRT, but by 12 months post-CRT, there was only one patient with high VF score in this group. Similarly, ORD and PRD scores improved over time. These findings are similar to those published by Cmelak et al., who found that severe swallowing impairment after CRT was transient in the majority of patients 5. These improvements, or lack thereof, need to be taken into account in AE reporting. Alternatives to reporting only the maximal observer-rated AE scores include new proposed systems that take into account time and multiplicity of high-grade events 41, and the addition of QOL measures. In recent years, few studies have reported the results of QOL instruments in some of the participating patients 5,6,8,10,11,16,17,25 or reported them in secondary publications, while the main publication contained only observer-rated AEs 51. The best way to incorporate patient-reported QOL into reports of CRT, which QOL instruments should be used, and how much weight should be assigned to their results when different therapies are compared, are subjects of current debate. Until better knowledge and uniformity in applying QOL instruments in clinical research are gained, this study suggests that for some critical toxicities, such as dysphagia, reporting moderate observer-rated grades may improve the accuracy of the assessment of the toxicity burden. An alternative would be incorporating patient-reported scores into the clinical evaluation and reporting of adverse events, as has recently been suggested52.
Limitations of the study include follow-up of one year only, and a need for validation of its results in a larger cohort of patients. It is possible that in a larger cohort of patients, the differences we observed in PRD or VF scores between groups 1(1), 2, and 3, would have reached statistical significance; however, the magnitude of these differences appears to be small in our study, such that they are unlikely to be clinically important or change the overall conclusions.
In conclusion, dysphagia, as measured by PRD and by objective measures of swallowing dysfunction, in patients with maximal CTCAE scores ≥ 3, seems to be similar to dysphagia in patients with lesser maximal scores: a score of 2, or even a score of 1 which does not subside to 0 by one year. These findings suggest that the reporting of only grades ≥3, an almost exclusive reporting in trials of therapy of HNC in recent years, may not provide an adequate estimate of the burden of dysphagia. We believe that these findings are relevant to many other AEs in which CTCAE scoring is based on the observer estimating the severity of patients' subjective symptoms. Thus, this study provides an example of much wider problems, and will hopefully stimulate further explorations and discussions about how we should evaluate and report toxicities.
Acknowledgments
Supported in part by NIH grant PO1 CA59827
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Presented in part at the 50th Annual meeting of the American Society of Radiation Oncology (ASTRO), Boston, Sep 21–25 2008
References
- 1.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285:437–43. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 2.Scharf O, Colevas AD. Adverse event reporting in publications compared with sponsor database for cancer clinical trials. J Clin Oncol. 2006;24:3933–8. doi: 10.1200/JCO.2005.05.3959. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SJ. Some thoughts on the reporting of adverse events in phase II cancer clinical trials. J Clin Oncol. 2006;24:3821–2. doi: 10.1200/JCO.2006.06.9856. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99-14. J Clin Oncol. 2005;23:3008–15. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 5.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. Journal of clinical oncology. 2007;25:3971–7. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 6.Cohen EE, Haraf DJ, List MA, et al. High survival and organ function rates after primary chemoradiotherapy for intermediate-stage squamous cell carcinoma of the head and neck treated in a multicenter phase II trial. J Clin Oncol. 2006;24:3438–44. doi: 10.1200/JCO.2006.05.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garden AS, Harris J, Vokes EE, et al. Preliminary results of Radiation Therapy Oncology Group 97-03: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2004;22:2856–64. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Haraf DJ, Rosen FR, Stenson K, et al. Induction chemotherapy followed by concomitant TFHX chemoradiotherapy with reduced dose radiation in advanced head and neck cancer. Clin Cancer Res. 2003;9:5936–43. [PubMed] [Google Scholar]
- 9.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in carcinoma of the head and neck. J Clin Oncol. 2000;18:1458–64. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 10.Kies MS, Haraf DJ, Rosen F, et al. Concomitant paclitaxel and fluorouracil, oral hydroxyurea, and hyperfractionated radiation for locally advanced squamous head and neck cancer. J Clin Oncol. 2001;19:1961–9. doi: 10.1200/JCO.2001.19.7.1961. [DOI] [PubMed] [Google Scholar]
- 11.Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma. J Clin Oncol. 2002;20:3964–71. doi: 10.1200/JCO.2002.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Psyrri A, Kwong M, DiStasio S, et al. Cisplatin, fluorouracil, and leucovorin induction chemotherapy followed by concurrent cisplatin chemoradiotherapy for organ preservation in head and neck cancer. J Clin Oncol. 2004;22:3061–9. doi: 10.1200/JCO.2004.01.108. [DOI] [PubMed] [Google Scholar]
- 13.Rischin D, Peters L, Fisher R, et al. Tirapazamine, Cisplatin, and Radiation versus Fluorouracil, Cisplatin, and Radiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2005;23:79–87. doi: 10.1200/JCO.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Sunwoo JB, Herscher LL, Kroog GS, et al. Concurrent paclitaxel and radiation in head and neck cancer. J Clin Oncol. 2001;19:800–11. doi: 10.1200/JCO.2001.19.3.800. [DOI] [PubMed] [Google Scholar]
- 15.Tsao AS, Garden AS, Kies MS, et al. Phase I/II study of docetaxel, cisplatin, and concomitant boost radiation for head and neck cancer. J Clin Oncol. 2006;24:4163–9. doi: 10.1200/JCO.2006.05.7851. [DOI] [PubMed] [Google Scholar]
- 16.Vokes EE, Kies MS, Haraf DJ, et al. Concomitant chemoradiotherapy as primary therapy for advanced head and neck cancer. J Clin Oncol. 2000;18:1652–61. doi: 10.1200/JCO.2000.18.8.1652. [DOI] [PubMed] [Google Scholar]
- 17.Vokes EE, Stenson K, Rosen FR, et al. Weekly carboplatin and paclitaxel followed by concomitant paclitaxel, fluorouracil, and hydroxyurea chemoradiotherapy for head and neck cancer. J Clin Oncol. 2003;21:320–6. doi: 10.1200/JCO.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 20.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 21.Bourhis J, Lapeyre M, Tortochaux J, et al. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer. J Clin Oncol. 2006;24:2873–8. doi: 10.1200/JCO.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 22.Budach V, Stuschke M, Budach W, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer. J Clin Oncol. 2005;23:1125–35. doi: 10.1200/JCO.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 25.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 26.Fu KK, Pajak TF, Trotti A, et al. A RTOG phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck carcinomas. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 27.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–73. doi: 10.1200/JCO.2004.12.193. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7. Lancet, The. 2003;362:933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 29.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy. Int J Radiat Oncol Biol Phys. 2001;50:1161–71. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 30.Eisbruch A. Dysphagia and aspiration following chemo-irradiation of head and neck cancer: major obstacles to intensification of therapy. Annals of Oncology. 2004;15:363–4. doi: 10.1093/annonc/mdh117. [DOI] [PubMed] [Google Scholar]
- 31.Salama JK, Seiwert TY, Vokes EE. Chemoradiotherapy for locally advanced head and neck cancer. J Clin Oncol. 2007;25:4118–26. doi: 10.1200/JCO.2007.12.2697. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–43. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 33.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life. Journal of clinical oncology. 2008;26:3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 34.Feng FYFY, Kim HMHM, Lyden THTH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. International journal of radiation oncology, biology, physics. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 35.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after chemoradiation for head-and-neck cancer. International journal of radiation oncology, biology, physics. 2002;53:23–8. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 36.Karnell MPME. A database information storage and reporting system for videofluorographic oropharyngeal swallowing evaluation. Am J Speech Language Pathol. 1994;8:54–60. [Google Scholar]
- 37.O'Neil KH, Purdy M, Falk J, et al. The Dysphagia Outcome and Severity Scale. Dysphagia. 1999;14:139–45. doi: 10.1007/PL00009595. [DOI] [PubMed] [Google Scholar]
- 38.Terrell JE, Nanavati KA, Esclamado RM, et al. Head and neck cancer-specific quality of life: instrument validation. Archives of otolaryngology--head & neck surgery. 1997;123:1125–32. doi: 10.1001/archotol.1997.01900100101014. [DOI] [PubMed] [Google Scholar]
- 39.Hassan SJ, Weymuller EA., Jr. Assessment of quality of life in head and neck cancer. Head Neck. 1993;15:485–96. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 40.Trotti A, Colevas AD, Setser A, et al. CTCAE: development of a comprehensive grading system. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 41.Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by RTOG. Lancet Oncol. 2007;8:613–24. doi: 10.1016/S1470-2045(07)70144-4. [DOI] [PubMed] [Google Scholar]
- 42.Hollen PPJ, Gralla RRJ, Cox CC, et al. A dilemma in analysis: issues in the serial measurement of quality of life. Lung Cancer. 1997;18:119–36. doi: 10.1016/s0169-5002(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 43.Scott C. Statistical issues in studies of toxicity modifiers. Semin Radiat Oncol. 2002;12:91–6. doi: 10.1053/srao.2002.31381. [DOI] [PubMed] [Google Scholar]
- 44.Wiklund I. Assessment of patient-reported outcomes in clinical trials. Fundamental & clinical pharmacology. 2004;18:351–63. doi: 10.1111/j.1472-8206.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 45.Mahoney MR, Sargent DJ, O'Connell MJ, et al. Dealing with a deluge of data: an assessment of adverse event data. J Clin Oncol. 2005;23:9275–81. doi: 10.1200/JCO.2004.00.0588. [DOI] [PubMed] [Google Scholar]
- 46.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. Journal of clinical oncology. 2007;25:4096–103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 47.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting. Lancet Oncol. 2006;7:903–9. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 48.Jensen K, Bonde Jensen A, Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. Radiother Oncol. 2006;78:298–305. doi: 10.1016/j.radonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Pauloski BR, Rademaker AW, Logemann JA, et al. Swallow function and perception of dysphagia in patients with head and neck cancer. Head & neck. 2002;24:555–65. doi: 10.1002/hed.10092. [DOI] [PubMed] [Google Scholar]
- 50.Meirovitz A, Murdoch-Kinch CA, Schipper M, et al. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of HN cancer. Int J Radiat Oncol Biol Phys. 2006;66:445–53. doi: 10.1016/j.ijrobp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with radiotherapy alone or with cetuximab. J Clin Oncol. 2007;25:2191–7. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 52.Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and evolution of adverse reporting in oncology. Journal Clin Oncol. 2007;25:5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]