Abstract
Human apolipoprotein(a) (apo(a)), synthesized in the liver, contains oxidized phosphatidylcholine (oxPtdPC) adducts probably generated at the hepatic site. Since plasminogen (Plg), also synthesized in the liver, is genetically related and structurally homologous to apo(a), we wanted to determine whether it contains oxPtdPCs and their location. We used Plg isolated from fresh or frozen normal human plasma and several commercial preparations. Some were freed of non-covalently bound lipids by organic solvent extraction. By immunoblot analyses all products reacted against T15, a natural IgM monoclonal antibody specific for phosphorylcholine -containing oxidized phospholipids (ox-PLs). This immunoreactivity was retained in urokinase type plasminogen activator -generated plasmin and was abrogated in Plg previously digested with lipoprotein-associated phospholipase A2 (Lp-PLA2), a reaction that generated that predominantly C16:0 lysophosphatidylcholine species as determined by mass spectrometry. Lysoderivatives were also generated upon the cleavage by Lp-PLA2 of a model ox-PL chemically linked to a lysine-containing pentapeptide. From inorganic phosphorous analyses, we found 2 moles of oxPtdPC/mole of Plg distributed between the kringles 1-4 and mini-Plg domain. OxPtdPCs were also present in the Plg isolated from the serum-free medium of cultured human HepG2 cells. In conclusion, our results provide strong evidence that naturally occurring Plg contains oxPtdPC probably linked by a Schiff base and also suggest that the linkage occurs at the hepatic site. Given the emerging evidence for the cardiovascular pathogenicity of oxPtdPCs we speculate that they may impart athero-thrombogenic properties to Plg under inflammatory conditions.
Keywords: Plasminogen, Phosphatidylcholine, Lysophosphatidylcholine, Oxidation, Monoclonal antibody T15
1. Introduction
Phospholipids (PLs), normal components of cell membranes and plasma lipoproteins, readily undergo structural changes under oxidative stress and inflammatory conditions [1, 2] These modified PLs exhibit functional changes regarding their mode of interaction with cells, proteins, elements of the immune system and also play an important role in the various stages of the atherosclerotic process [3, 4]. Under oxidative conditions, the polyunsaturated fatty acid that occupies the sn2 position of the glycerol backbone is converted into an aldehyde that readily forms a Schiff base adduct with candidate epsilon amino groups of specific lysine residues of peptides and proteins [5]. In previous studies we showed that oxidized phosphatidylcholine (oxPtdPC), can link by a Schiff base to 1 or 2 lysine residues of kringle V located in the C-terminal domain of human apolipoprotein(a) (apo(a)) [6]. We also provided evidence from experiments in cultured human macrophages, that this chemical modification can impart pro-inflammatory properties to apo(a) [6]. More recently, in studies carried out on the plasma of human subjects without either clinical or laboratory evidence of ongoing inflammatory processes, we showed that the oxPtdPCs in the lipoprotein(a) (Lp(a)) particles are located in apo(a) and that these oxPtdPCs are not derived from the circulating lipoproteins and are probably of an hepatic origin [7]. In the current studies we asked whether other kringle-containing protein in the plasma may have oxPtdPC adducts and to this effect directed our attention to plasminogen (Plg) known to have a marked structural similarity to apo(a). Both proteins are genetically-related structures characterized by a multikringle domain followed by a catalytic serine protease that is only active in Plg [8]. Both proteins contain distinct classes of kringles, named 1 to 5 in the case of Plg, and in the case of apo(a), the kringle IV class is comprised of 10 subclasses of which the type 2 is repeated several times accounting for the variability in apo(a) size [9].
We studied human Glu-Plg, the native form of Plg [10] which consists of two carbohydrate variants and has Glu as its amino terminal amino acid, after its isolation from normal human plasma from various sources as well as derivatives thereof (Fig. 1). In addition, we studied cultures of human HepG2 cells in order to determine whether Plg was secreted by these cells, and whether it contained oxPtdPCs. In order to identify the potential presence of modified phospholipids we used T15, a natural IgM monoclonal antibody with specificity for the phosphorylcholine (PC) residue of PLs. This antibody was identified in the early studies by Kearney et al [11] and found later to be immunologically indistinguishable from monoclonal EO6 by Shaw et al [12]. The immunological identity between T15 and EO6 was also shown in our previous work on human apo(a) [7]. We further defined the nature of the modified PLs by subjecting Plg and its derivatives to the action of lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme with a proven specificity for oxidized phospholipids. The results of these studies are the subject of this report.
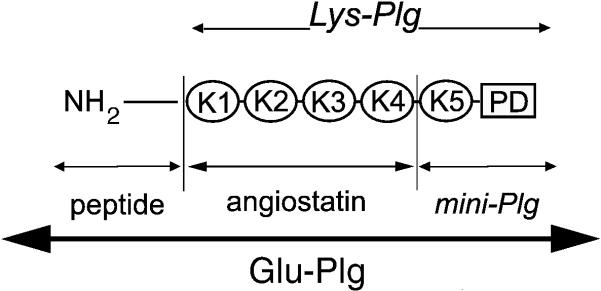
Fig. 1.
Schematic representation of Glu-Plg. The single polypeptide chain comprises the NH2-terminal peptide, 5 distinct kringle regions numbered 1-5 and a serine protease domain. The angiostatin region comprises K1-4. Lys –Plg is produced by plasmin digestion of Glu-Plg. Mini-Plg is the product of the elastase digestion of Glu-Plg. PD, protease domain.
2. Materials and Methods
2.1. Materials
The materials purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) were BSA, Tween-20, SDS, ε-amino caproic acid, (EACA), 4-(2-Aminoethyl)-benzene sulfonylfluoride (AEBSF), N-a-tosyl-L-lysine chloromethylketone hydrochloride (TLCK), D-Val-Leu-Lys- p-nitoranilide dihydrochloride (S-2251), phospholipase C (PLC) (3.1.4.3) from Bacillus cereus (2000 units/mg; P9439) (< 20 units/mg sphingomyelinase activity) and bovine intestinal mucosal alkaline phosphatase. The protease inhibitor, 1-10, phenanthroline and urokinase type plasminogen activator (uPA) (high molecular weight from human urine) were from EMD Biosciences, Inc. (La Jolla, CA). Immobilon-P membranes from Millipore Corp. (Billerica, MA. USA) and Superblock blocking buffer and the Supersignal® West Dura Extended Duration Substrate from Thermo Scientific (Rockford, IL). Coomassie staining solution (Page Blue) was from Fermentas Inc. (Glen Burnie, MD). The hepatocarcinoma cell line, HepG2 was obtained from the American Type Culture Collection (Manassas, VA), and fetal bovine serum was from Atlanta Biologicals (Lawrence, GA). Media and tissue culture reagents and precast acrylamide gels were from Invitrogen Corp. (Carlsbad, CA). All other chemicals were of reagent grade.
2.2. Antisera
The murine T15 antibody-secreting cell line, BH8 (IgM) that reacted with oxPtdPC antigens was a gift from Dr. John F. Kearney (University of Alabama) and referred to here as T15. This cell line was maintained in the Frank W. Fitch Antibody Facility of the University of Chicago. The antibodies in the supernatant medium were isotyped and contained a high titer of IgM antibodies (greater than 90%) as verified by the major Coomassie stained band on SDS-PAGE.
Goat anti-human plasminogen (affinity purified IgG) antibodies were obtained from Enzyme Research Laboratories (South Bend, IN) and anti-phosphoserine MAb were from Millipore (Temecula, CA). Peroxidase labeled (HRP) secondary antibodies, goat anti-mouse IgMμ chain specific), rabbit anti-goat IgG were from Sigma-Aldrich Chemical Co. (St. Louis, MO. USA).
2.3. POVPC-Peptide
The peptide, Ac-Ala-Ala-Lys-Ala-Tyr-OH was conjugated to POVPC (1-palmitoyl-2-(5’-oxo)valeroyl-sn-glycero-3-phosphorylcholine) by Avanti Polar Lipids Inc., (Alabaster, AL), and the structure of the conjugated product was examined by NMR and mass spectrometry and found to have the expected structure and was of high purity.
2.4. Cell culture
HepG2 cells were grown in 10 cm petri dishes containing 10 ml of Dulbecco's modified Eagle's minimal essential medium supplemented with 10% fetal bovine serum as described previously.[13]
2.5. Plg and derivatives
We utilized three sources of human Plg. In our laboratory we utilized fresh plasma from healthy donors previously studied without evidence of either acute or chronic inflammation [7]. All subjects in this study signed an informed consent document approved by the Institutional Review Board of the University of Chicago. The procedure of Plg isolation was carried out as described by Deutsch and Mertz[14] followed by a purification step with G25 Sepharose chromatography. We also used on several occasions commercial preparations of Glu-Plg from Enzyme Research Laboratories (South Bend, IN) shipped frozen in 50 mM Tris-HCl/0.1 M NaCl/pH 7.4 and from Haematologic Technologies, Inc. (Essex Junction, VT) shipped in 50% glycerol/H20 (vol/vol) in dry ice.
Human Lys-Plg, plasmin, Factor XII, and prothrombin were commercial preparations from Enzyme Research Laboratories (South Bend, IN) and from Haematologic Technologies, Inc. (Essex Junction, VT). Human angiostatin peptides K1-4 with (NH2-K1-4), and without the 78 residue amino terminus and mini-Plg (K5-protease domain) (Fig. 1) were obtained from Haematologic Technologies Inc., (Essex Junction, VT)). The peptides were derived from human Glu- and Lys-plasminogen, respectively.
2.6. Preparation of Plg from serum-free HepG2 medium
Near-confluent monolayer cultures were extensively washed with PBS and incubated with serum-free DMEM for 24h referred to as conditioned medium. Thereafter, the serum-free medium was collected, AEBSF was added at a final concentration of 1 mM and either immediately frozen at -80°C or applied on a Lysine-Sepharose column and the Plg purified as described above.
2.7. Preparation and purification of Lp-PLA2
The full-length human Lp-PLA2 cDNA was cloned into the vector pcMV6-XL4 (Origene, Rockville, MD). We sub-cloned the Lp-PLA2 cDNA from pcMV6-XL4 into pcDNA5/FRT (Invitrogen Corp., Carlsbad, CA) to facilitate stable transfection into mammalian cells. A histidine tag (six residues) was also engineered into pcDNA/5/FRT/Lp-PLA2 before the stop codon. This vector was then transfected into 293T/FRT cells (Invitrogen Corp., Carlsbad, CA). Conditioned serum-free medium was collected from cultured cells for FPLC purification of each recombinant protein. An affinity column was packed using a 20 mL Ni-NTA resin (Qiagen, Valencia, CA). The buffers used for the non-denaturing purification were Binding buffer (50mM NaH2PO4, 1M NaCl, 10mM imidazole, pH 8), Washing buffer (50mM NaH2PO4, 1M NaCl, 20mM imidazole, pH 8), and Elution buffer (50mM NaH2PO4, 1M NaCl, 1M imidazole, pH 8). The peak fractions (2-4 fractions of an imidazole gradient elution) of Lp-PLA2 from the affinity column were pooled and concentrated with Centricon Plus-70 Centrifugal Filter Units with a molecular weight cutoff of 30 kDa (Millipore, Temecula, CA) using two washes with PBS buffer.
2.8. Lp-PLA2 activity assay
Lp-PLA2 containing medium was incubated with platelet activating factor (PAF), 0.1μCi 3H-PAF (3H-acetate at the sn-2 position) (Perkin Elmer, Waltham, MA), 99.96umol/L C16-PAF (Cayman Chemical, Ann Arbor, MI) and PBS buffer in a total volume of 100 μL. The reaction was stopped by the addition of chloroform:methanol (2:1 (v/v)) after a 20 min incubation at 37°C. The mixture was centrifuged at 1500×g for 10 min after a brief vortex. An aliquot (100 μL) was removed from the upper layer for scintillation counting.
2.9. Phospholipase C (PLC)
Digestion of Glu-Plg and derivatives was conducted at 37°C in 50 mM Tris-HCl, pH 7.5, 5 mM Ca2+ for 4h. The reaction was stopped with 1 mM 1,10-phenanthroline and immediately analyzed by SDS-PAGE.
2.10. Enzymatic dephosphorylation of Plg
Glu-Plg (30-60 pmoles) was dephosphorylated with bovine intestinal mucosa alkaline phosphatase (800 units) in 25 mM NH4HCO3, pH7.9. The reaction occurred at 37°C overnight. Aliquots were then analyzed on immunoblots with anti-phosphoserine antibodies.
2.11. ESI-MS/MS LysoPtdPC analysis
An electrospray ionization-tandem mass spectrometry approach was used. The samples were lyophilized and dissolved in chloroform. An aliquot of the sample was combined with solvents, such that the ratio of chloroform/methanol/300 mM ammonium acetate in water was 300/665/35 (v/v/v). The lipid extract was introduced by continuous infusion into the ESI source on a triple quadrupole MS/MS (API 4000, Applied Biosystems, Foster City, CA) at 30 μl/min.
A precursor scan (Pre 184.1) in positive ion mode of the extracts produces a spectrum revealing lipid species ([M + H]+ ions) containing a PC head group. The collision gas pressure was set was +40 V. The declustering potential was +100 V, the entrance potential was +14 V, and the exit potential was +14 V. The mass analyzers were adjusted to a resolution of 0.7μ full width at half height. Continuum scans were averaged in multiple channel analyzer (MCA) mode. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV was applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), and the two ion source gases were set at 45 (arbitrary units).
2.12. Electrophoretic methods
SDS-PAGE, (gradients of 4–12% and 4-20% polyacrylamide) was performed on a Novex system (Novex, San Diego, CA) for 1.5 h at constant voltage (120 V) at 22°C as previously described [6]. Immediately after electrophoresis, the gels were placed onto Immobilon-P sheets (Millipore Corp., Bedford, MA), which were previously wetted with a buffer containing 48 mM Tris, 39 mM glycine, pH 8.9. Blotting was performed on a horizontal semi-dry electroblot apparatus (Amersham Biosciences) at 0.8–1 mA/cm2 for 45 min at 23°C.
2.13. Immunoblot analyses
After electroblotting, the Immobilon-P sheets were blocked in Superblock (Thermo Scientific, Inc. Rockford, IL. USA) for 1 h at 23°C followed by incubation with goat anti-human plasminogen IgG or anti-phosphoserine MAb (IgG1) or in the case of T15 by incubation in 10% Superblock for 18 h at 4°C. Proteins were visualized using secondary antibodies conjugated to HRP; rabbit anti-goat IgG (for Plg), goat anti-mouse IgG (for phosphoserine) and goat anti-mouse IgM (μ -chain specific) (for oxPtdPC). The blots were developed with Supersignal® West Dura Extended Duration Substrate from Thermo Scientific, Inc. (Rockford, IL) according to the manufacturer's instructions.
2.14. Delipidation of Glu-Plg
Lipids were extracted from purified Glu-Plg using a modified Bligh and Dyer method [15] in that chloroform was replaced by anhydrous diethyl ether in a ratio of 1:2 v/v (ether:methanol). Glu-Plg in 0.8 ml 50 mM Tris-HCl containing 0.1 M NaCl, pH 7.4 was added to the ethyl ether-methanol mixture dropwise and rotated at 4° C for 6 h. The mixture was then centrifuged and the precipitated proteinwashed 3 times with diethyl ether. The washed precipitate was dried with argon gas and dissolved in the Tris buffer. The protein recovery was close to 85% of the original starting material. An alternative delipidation technique was also carried out using a 3:2 v/v mixture of ethanol:diethyl ether as already published for apo(a) [6]. In both delipidation techniques the Glu-Plg was soluble in aqueous buffers.
2.15. Other analyses
Inorganic phosphorous was quantified using the microtechnique described by Bartlett [16] by first exhaustively dialyzing the samples to remove all extraneous inorganic phosphate and then digesting the sample with 10 N H2SO4 at 160 °C for 18 h. PL values were obtained by multiplying the inorganic phosphate results by 25. The calculations also accounted for the single phosphorylation site in the protease region [17]. Protein determinations were performed by the Bio-Rad DC protein assay using BSA as a standard.
3. Results
3.1.Studies on Glu-Plg
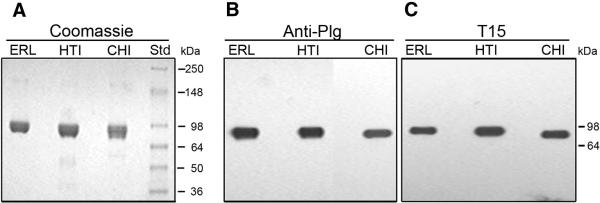
Three representative preparations, one from Enzyme Research Laboratories (ERL), one from Haematologic Technologies, Inc (HTI) and one from our own laboratory in Chicago (CHI) were examined by non-reduced 4-12% SDS-PAGE (Fig. 2). The average apparent molecular mass as determined by the Coomassie Blue stained gel (Fig. 2A) was 88 kDa, consistent with the calculated value from the protein sequence, 88.4 kDa. We attribute the small differences in mobility to carbohydrate heterogeneity. By immunoblot analysis, each preparation gave a single band when probed with anti-human Plg (Fig. 2B). In order to determine the presence of oxPtdPC in the preparations examined, the immunoblots were developed with T15, a monoclonal antibody that recognizes oxPtdPC as we showed previously in studies on apo(a) [6]. The three preparations exhibited a strong band in the expected migrating position for Glu-Plg (Fig. 2C). Based on these results we utilized the three Plg preparations interchangeably.
Fig. 2.
SDS-PAGE of purified Plg from three sources stained with Coomassie (A), anti-Plg (B) and T15 (C). Glu-Plg from different sources was analyzed by non-reduced 4-12% SDS-PAGE (A) and on Western blots (B and C). The sources were Enzyme Research Laboratories, South Bend, IN) (ER), Haematologic Technologies Inc., Essex Junction, VT (HTI) and our laboratory at the University of Chicago (CHI). A, Coomassie stained gel of purified Glu-Plg (8 μg). The size and position of molecular weight standards (Invitrogen, Corp., Carlsbad, CA) are indicated (Std). B, Probed with goat anti-human Glu-Plg antibody (1 ng sample applied). C, Probed with T15 antibody (1 μg sample applied).
3.2. T15 reactivity of Plg derivatives
To define the domain(s) of Glu-Plg containing oxPtdPC, we examined Lys-Plg, plasmin, NH2-K1-4, K1-4, and mini-Plg (Fig. 3). The immunoblot analyses show that all products reacted with T15. On the other hand, purified HSA, the kringle containing human Factor XII and prothrombin exhibited no T15 immunoreactivity.
Fig. 3.
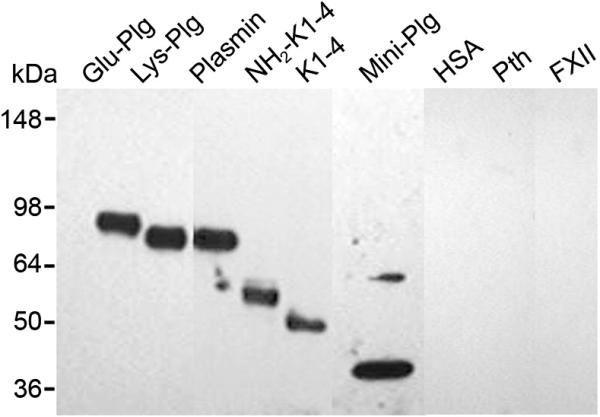
T15 reactivity to Plg and derivatives. Immunoblot analysis on a non-reduced 4-12% SDS-PAGE probed with T15 antibody. NH2-K1-4 is derived from a single cleavage within the Glu-Plg molecule resulting in a product that includes kringles 1-4 as well as the amino terminal 78 amino acids and terminates at Pro 452 with an apparent size of 50 kDa. Cleavage of NH2-K1-4 with plasmin removes the amino terminal 77 residues resulting in the first four kringles of Plg, K1-4, with an apparent size of 45 kDa. Mini-Plg has an apparent size of 38 kDa and is the product of the single cleavage of Glu-Plg with plasmin (see also Figure 1). HSA, human serum albumin; Pth, human prothrombin; FXII, human factor XII.
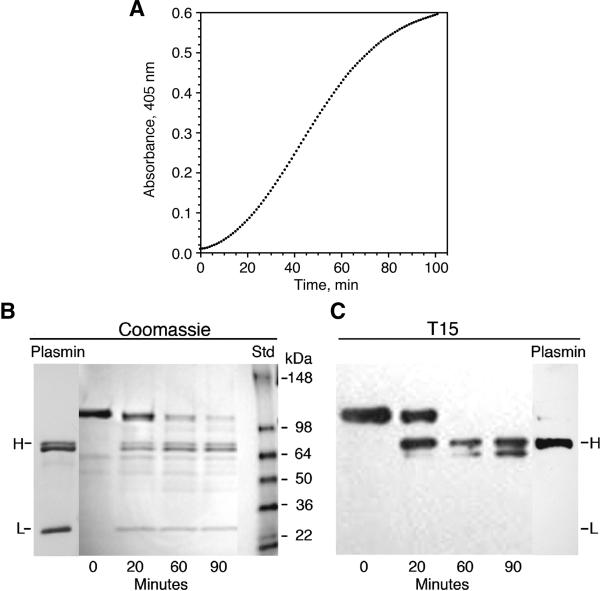
3.3. Activation of Glu-Plg by uPA
The action of the uPA-mediated conversion of Glu-Plg to plasmin is illustrated in Fig. 4. The time course of the reaction, monitored by the generation of plasmin amidolytic activity on the substrate S-2251, is shown in Fig. 4A and supported by the Coomassie stained SDS-PAGE shown in Figure 4B. These data were obtained in the presence of 10 mM TLCK, known to inhibit plasmin but not uPA [18]. Under gel reducing conditions, bands designated H and L were generated corresponding to the heavy and light chains of plasmin, respectively. In parallel, aliquots from the same reaction mixtures were probed with T15 on Western blots (Fig. 4C). The bands corresponding to Glu-Plg and the heavy chain of plasmin reacted positively with T15. In contrast, the light chain exhibited no reactivity. This finding, when coupled with those reported in Fig. 3 suggests that kringle 5 is the site of the linkage.
Fig. 4.
Activation of Plg by uPA. A, Time course of Plg activation by uPA as measured by the amidolytic activity of plasmin. The reaction mixture contained 0.28 μM Glu-Plg 0.06 units of uPA and 55μM of S-2251. B, Coomassie stained SDS-PAGE on 4-12% acrylamide gels of reduced samples from the activation of Plg by uPA in the presence of TLCK. Under reduced conditions, the electrophoretic mobilities of the kringle containing bands, such as Plg and plasmin, are slower than under non-reduced conditions; uPA cleaves the peptide bond between Arg-560 and Val-561. C, Immunoblot analysis probed with T15. The conditions for the uPA reaction are the same as for B. H, heavy chain of plasmin containing kringles 1-5; L, light chain of plasmin containing residues 561-790.
3.4. Studies with phospholipase C
As shown in supplemental Fig. 1, treatment with PLC caused abrogation of the T15 reactivity in all samples. PLC cleaves before the phosphate group of phospholipids to produce PC and the diacylglycerol moiety. Although PC reacts with T15, [5] gel electrophoresis of the digested Plg and derivatives showed no reaction with T15 due to the enzyme dependent release of the PC group further documenting the presence of the oxPtdPC adduct in the Plg samples examined.
3.5 LysoPtdPC generation by digesting an oxPtdPCl linked synthetic pentapeptide with Lp-PLA2
We tested the activity of this enzyme, known to cleave short fatty acyl chains in the sn2 position of oxidized phospholipids, on the pentapeptide (Ac-Ala-Ala-Lys-Ala-Tyr-OH) that was covalently modified by attaching the ε-amino group of lysine to the aldehyde group of POVPC. The expected generation of lysoPtdPC 16:0 was confirmed by ESI/MS/MS analysis (supplemental Fig 2).
3.6. LysoPtdPC generation by digesting Plg samples with Lp-PLA2
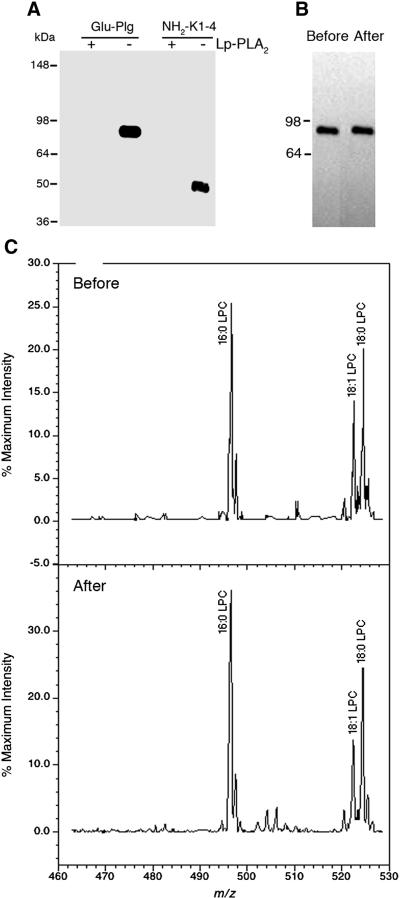
When in subsequent studies we incubated Glu-Plg and derivatives with Lp-PLA2, immunoblot analyses showed that Glu-Plg and NH2-K1-4 reacted with T15 before but not after their digestion (Fig. 5A). In preparation for gel electrophoresis, the samples routinely contained 6M urea and SDS and were boiled for 5 min, under such conditions any non-covalent associated phospholipids would migrate with mobilities significantly faster than the protein. Moreover, extensive delipidation of Glu-Plg produced a soluble protein with electrophoretic mobility identical to that of non-delipidated Glu-Plg (Fig. 5B). Thus, the retention of positive reactivity of Glu-Plg against T15 suggests that the oxPtdPC is chemically linked to Glu-Plg. The analysis by ESI/MS/MS of these non-delipidated and delipidated Lp-PLA2 digested products showed lysoPtdPC 16:0 as the major lipid released (Fig. 5C) along with minor peaks corresponding to 18:0 and 18:1 lysoPtdPC. This information was corroborated in supplemental Fig. 3A which shows the position of a standard sample of 16:0 lysoPtdPCs at 496.6 m/z. Moreover, when we infused 300μg of Glu-Plg without Lp-PLA2 digestion, the 496.6 m/z peak was absent even when the sensitivity was expanded 20 fold (supplemental Fig. 3B). In contrast, when Glu-Plg (23 μg) was digested with Lp-PLA2, a major peak at 496.6 m/z was observed along with a smaller peak at 524.4m/z (18:0 lysoPtdPC) and minor peaks at 520.3m/z and 522.4 m/z corresponding to 18:2 and 18:1 lysoPtdPCs , respectively (supplemental Fig. 3C). Taken together, the above results provide a firm documentation for the presence of oxPtdPC in Plg.
Fig. 5.
Analyses showing that Lp-PLA2 causes Plg to lose T15 immunoreactivity before and after delipidation. A, Immunoblot analysis of a non-reduced 4-12% SDS-PAGE of Glu-Plg and NH2-K1-4 probed with T15 antibody. Digestions with and without enzyme were performed at 37°C overnight. (+), with enzyme; (-), without enzyme. B, Immunoblot analysis of a non-reduced 4-12% SDS-PAGE of Glu-Plg before and after delipidation. Removal of exogeneous lipids from Glu-Plg was performed according to the method of Bligh and Dyer[15], modified as described in “Materials and Methods”. C, ESI/MS/MS before and after delipidation of Glu-Plg incubated with Lp-PLA2 . The major peak was 16:0 lysoPtdPC followed by relatively smaller peaks of 18:0 and 18:1 lysoPtdPC. LPC, lysoPtdPC.
3.7. Inorganic phosphate analysis
Glu-Plg is known to have a major phosphorylation site at Ser-578 [17] that may introduce a confounder in the analyses of the inorganic phosphate related to the oxidized phospholipid adducts. To this effect we carried out a dephosphorylation procedure using alkaline phosphatase on Glu-Plg and tested the resulting product for T15 reactivity. Before dephosphorylation, Glu-Plg reacted with anti-phosphoserine MAb on immuno blots (supplemental Fig. 4). On the other hand, when Glu-Plg underwent enzymatic dephosphorylation, the reactivity with anti-phosphoserine was abrogated. In contrast, when probed with T15, positive reactivity was exhibited irrespective of the phosphorylation state. We also determined the amounts of inorganic phosphate in Glu-Plg and its derivatives as described in “Materials and Methods”. The results showed that Glu- and Lys-Plg each contained close to 2 moles of PL/mole of protein and mini-Plg contained one mole of PL/per mole of protein after subtracting one mole of phosphate deriving from the phosphoserine 578. The smaller fragments, NH2-K1-4, K1-4 each contained approximately one mole of PL/mole of protein. The calculations of these molar ratios were based on the following apparent sizes; NH2-K1-4, 50 kDa; K1-4, 45 kDa; mini-Plg, 38 kDa; Glu-Plg, 88 kDa; Lys-Plg, 83 kDa. These data were collected on at least 4 different preparations of the proteins and carried out in triplicate.
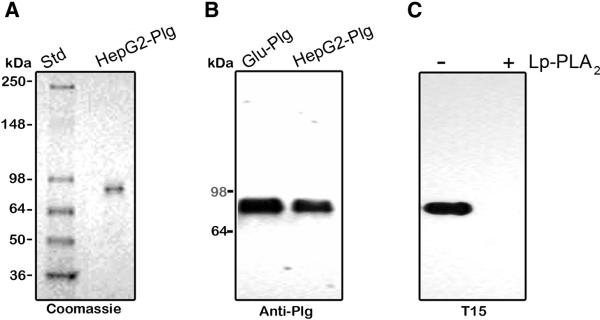
3.8. Studies on HepG2 cells
We used HepG2 cells as an experimental model based on the notion that the liver is the organ of Plg production and examined the Plg from the serum-free conditioned medium. In a typical experiment, Hep G2 cells were conditioned with serum-free medium for 24 h and the medium collected and applied on a Lysine-Sepharose column to isolate Plg, [14] as described in “Materials and Methods”. The yields of purified Plg from 100 ml of media were 50 to 100 μg. Analysis on a non-reduced 4-12% SDS-PAGE stained with Coomassie Blue showed a single band of an apparent mass of 88 kDa migrating in the same position as that of plasma derived Glu-Plg (Fig. 6A). Moreover, on immunoblots, the HepG2-secreted product reacted strongly against both anti-human Plg (Figure 6B) and T15 (Fig. 6C). In addition, this reactivity was abrogated by the action of Lp-PLA2 (Fig. 6C). Together, these results indicate that the HepG2 secreted Plg contains linked oxPtdPC.
Fig. 6.
Analysis of Plg purified from HepG2 serum-free medium. Plg isolated from the conditioned serum-free medium as described in “Materials and Methods” was analyzed on non-reduced 4-12% SDS-PAGE (A) and on immunoblots (B and C). A, 1 μg of HepG2 derived Plg were analyzed. The size and position of molecular weight standards are indicated (Std). B, 1 ng of Plg purified from HepG2 conditioned serum-free medium were analyzed and probed with goat anti-human Glu-Plg antibody. C, Plg as in B was incubated in the absence and presence of Lp-PLA2 at 37°C overnight and 1 μg of the product analyzed on immunoblots. The blot was probed with T15 antibody. (-), without enzyme; (+), with enzyme.
4. Discussion
Our studies have shown that native Glu-Plg isolated from normal human plasma from various sources either fresh or frozen, prepared either in-house or commercially, contains chemically linked oxPtdPCs. This conclusion is based on the results of combined immunochemical (T15 antibody), enzymatic (PLC and Lp-PLA2) and mass spectrometry documenting the generation of lysoPtdPC species from the Lp-PLA2-mediated cleavage of the oxPtdPCs linked to Plg, a reaction that was validated by using as a substrate a model oxPtdPC (POVPC) chemically linked to a synthetic pentapeptide containing one lysine residue (supplemental Fig 2) although in this study the exact structure of the PC adduct was not defined. Moreover, the existence of a chemical linkage was suggested by: 1) the results of inorganic phosphorous analyses, 2) the retention of oxPtdPCs in samples from which non-covalently linked lipids were removed by extraction with organic solvents, 3) the presence of T15 reactivity in both fresh and frozen samples and 4) the presence of this reactivity in boiled, denatured samples of Plg after separation on SDS gels.
Regarding the source of the Plg-oxPtdPC complex, our results point at the liver as the most probable site. This hypothesis is consistent with the notion that in vivo Plg is synthesized only in the liver, [10] that the plasma is a strong anti-oxidant milieu [19, 20] and our own current observation that the Plg isolated from the serum-free medium of HepG2 cells had linked oxPtdPC. This also implies that the liver is the site where oxPtdPCs are generated likely via a lipid peroxidation process [21] and then linked to Plg probably by an enzymatic-driven reaction involving two sites based on the results of our phosphorous analyses. In this regard, we wish to draw attention to the fact that the generation of oxPtdPCs occurred under presumed physiological conditions since the studies were conducted in the plasma of normal human subjects who we previously found to have in their plasma oxPtdPCs chemically linked to apo(a) [6] a kringle-containing structure of the Plg gene family but not in apoB100-containing lipoproteins [8].Thus, it is apparent that within a basal, minimal pro-oxidant microenvironment there is generation of oxPtdPCs that link to these two genetically and structurally related proteins probably via a Schiff base to candidate lysines.
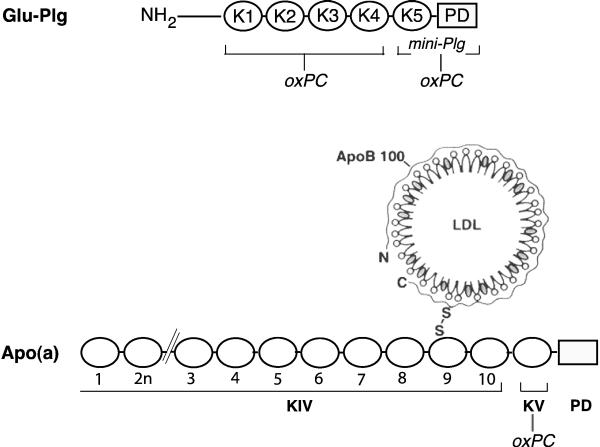
Plg isolated from human plasma has a mass of about 88 kDa and a total of 47 lysines, the candidate sites for oxPtdPC linkage, distributed among the N-terminal peptide, the kringle 5 region and the protease domain [10]. In the current study, we found two moles of oxPtdPC/mole of Plg distributed between kringles 1-4 and the mini-Plg domain taking into account in our calculations the presence in the latter region of a phosphorylation locus in serine 578 [17] and we previously reported that in human apo(a), kringle V is the site of oxPtdPC linkage [6] (Fig. 7). The results of our current studies summarized in Fig. 3 and 4 also point at kringle 5 as one of the PtdPC linkage sites in Plg.
Fig. 7.
Schematic representation of the location of oxPtdPCs in Plg and Lp(a). A, Plg, showing the NH2 terminal peptide, followed by kringles 1 to 5, and the protease domain. The kringle 1-4 fragment and mini-Plg, with the oxPtdPCs identified. B, Lp(a). Shown is the LDL component wrapped around by apoB100 linked by a disulfide bridge to apo(a) that is made of 10 classes of kringle type IV, one kringle type V and the pseudo protease domain. In previous studies, we found oxPtdPCs to be located on kringle V but not in the LDL particle [6].
The notion that both Plg and apo(a) have chemically linked oxPtdPCs deserves a comment. From a comparative standpoint, on a molar basis, both molecules isolated from normal human plasma, have about the same content of linked oxPtdPCs, but differ markedly in plasma concentration. In the case of Plg, its concentration is around 20 mg/dL and is rather stable among individuals [22]. On the other hand, the plasma Lp(a) concentration, expressed in terms of apo(a) [7] is highly variable between 0.01 and 3 mg/dL. Even in very high plasma Lp(a) subjects (an uncommon occurrence), the levels of apo(a) are well below those of Plg. Thus, under normal conditions, in the circulation the total number of molecules of Plg containing oxPtdPCs exceeds by far those of apo(a). This observation is not consistent with the proposal that in the human plasma, apo(a) is the preferential carrier of oxPLs [23] and raises important questions not just of a comparative quantitative nature but also of a cardiovascular relevance regarding the role played by oxPtdPC when bound to Plg or apo(a). The clinical evidence, reported by the Witztum laboratory [23] regarding the cardiovascular pathogenicity of oxPLs associated with Lp(a), remains to be unequivocally proven given the absence of direct measurements of ox-PLs in apo(a) and the confounding influence by ox-LDL particles. Regarding Plg, our current data are of a physiological nature and need to be extended to pathological situations. Those studies will require an assay under development in this laboratory, for the quantification of the oxPtdPC associated with plasma Plg along the lines reported for apo(a) [7].
The biological significance of Plg linked to oxPtdPCs is not readily apparent particularly in the absence of an understanding of the molecular mechanisms underlying assembly of the oxPtdPC/Plg complex, a process that is amenable to exploration in the HepG2 cell model examined in this study and approproate mouse models since we recently found that the Plg isolated from mouse plasma contains oxPtdPCs (Edelstein C. and Scanu A.M. unpublished observation, 2009). Mice do not produce apo(a) [24]. Our accessibility to this animal species will allow us to independently examine some of the unknowns regarding the biology of Plg. If, as our current data suggest, the liver is the site where Plg and oxPtdPC associate, we can explore whether Plg may serve a beneficial function by promoting the transfer into the circulation of potentially pathogenic oxPtdPCs. Along these lines, we can determine how oxPtdPCs bound to Plg are removed from the circulation and, from the cardiovascular standpoint, how they enter the artery wall, a question yet unresolved also in the case of apo(a), although in a previous study, we found oxPtdPCs linked to apo(a) in surgically derived human carotid artery plaque [25]. Of note, the development of Plg-deficient mice has opened new vistas on the involvement of Plg in various biological processes particularly those in the areas of atherosclerosis, thrombosis and chronic inflammation although the molecular mechanisms underlying these processes have not yet been clearly defined [26]. Given the athero-thrombotic potentials of oxPLs in general, it is tempting to speculate that under pro-oxidant and pro-inflammatory conditions the linked oxPtdPCs may contribute to the pathobiology of Plg as suggested for human apo(a) [23, 27].
While the results of previous and current investigations have shown that both Plg and apo(a) react against T15, we cannot rule out that this reactivity may be exhibited by other plasma components. Based on our current data, we can only state that oxPtdPC linking is not a general property of all plasma proteins since T15 reactivity was absent in albumin, the most abundant protein in the plasma, and two proteins of the blood coagulation system, Factor XII and prothrombin that contain 1 and 2 kringles, respectively [28]. A continuing investigation on this subject is warranted and is underway in this laboratory.
The presence of 1 mole of oxPtdPC in the Plg kringle 1-4 domain is of interest given its recognized angiostatic and anti-cancer properties [29] the presence of 1 mole of oxPtdPC in mini-Plg because of its recognized endostatic activity and thrombophilic potential [29, 30]. Of note, the latter region is where a major phosphorylation site is present at Ser 578 [17]. Our findings raise questions about the potential participation of oxPtdPCs in those reported activities, a question that also applies to human apo(a) fragments that have been reported to have angiostatic properties [31] comparable to those exhibited by Plg by-products. We considered an investigation on this issue beyond the scope of our current studies.
Regarding the fibrinolytic system, our results have shown that Plg with linked oxPtdPC is competent via uPA mediation, to generate plasmin that has linked oxPtdPC. In this vein, we also found oxPtdPCs in the commercial preparations of plasmin that had been also generated by the action of uPA on Plg isolated from normal human plasma. We wished to carry out studies on Plg having no chemically linked oxPtdPCs using naturally occurring products, the focus of our studies. However, all preparations that we examined, whether in-house or commercial, contained oxPtdPCs. The drastic methods required for their removal would cause major structural changes rendering Plg unsuited for meaningful functional studies. A recombinant approach needs consideration keeping in mind, however, that the products may not provestrictly comparable to naturally occurring hepatic-derived Plg, the focus of this current investigation.
Years ago, when a marked structural similarity between human apo(a) and Plg was discovered, the existing knowledge on Plg served as a guide to the ensuing studies on apo(a). Later came the observation that oxPtdPCs are linked to apo(a) and now we show that this is also true for Plg. Thus it appears that, as in the case of apo(a), oxPtdPCs are integral components of circulating Plg and some of its derivatives, leading us to presume that previous studies on Plg were conducted without the awareness of linked oxPtdPCs. We believe that this notion should be taken into account in future Plg investigations. As in the case of human apo(a), T15 provides an easy and reliable way to screen for the presence of chemically linked oxPtdPC in Plg from natural human sources.
Supplementary Material
Acknowledgments
We greatly appreciate the discussions on the MS analyses with Dr. Ruth Welti of the Kansas Lipidomics Research Center. The ESI-MS-MS studies were performed at the Kansas Lipidomics Research Center Analytical Laboratory.
The studies at the University of Chicago were supported by NHLBI, National Institutes of Health Grant HL 63209 (to AMS). Grants to J.S.H. were from the Canadian Institutes of Health Research (MOP74480) and the Heart and Stroke Foundation of British Columbia and Yukon. Instrument acquisition and method development at the Kansas Lipidomics Research Center was supported by National Science Foundation (EPS 0236913, MCB 0455318, DBI 0521587), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20RR16475), and Kansas State University.
Abbreviations
- apo(a)
Apolipoprotein(a)
- oxPtdPC
Oxidized phosphatidylcholine
- Lp-PLA2
Lipoprotein-associated phospholipase A2
- Plg
Plasminogen
- Glu-Plg
Plasminogen native form
- PL
Phospholipid
- PC
Phosphorylcholine
- PLC
Phospholipase C
- uPA
Urokinase type plasminogen activator
- POVPC
1-palmitoyl-2-(5’-oxo)valeroyl-sn-glycero-3-phosphorylcholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashraf MZ, Kar NS, Podrez EA. Oxidized phospholipids: Biomarker for cardiovascular diseases. Int. J. Biochem Cell Bio. 2009;41:1241–1244. doi: 10.1016/j.biocel.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 2009;50:S207–S212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtiss LK. Reversing atherosclerosis? N. Engl. J. Med. 2009;360:1144–1146. doi: 10.1056/NEJMcibr0810383. [DOI] [PubMed] [Google Scholar]
- 4.Hazen SL. Oxidized phospholipids as endogeneous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman P, Hörkkö S, Steinberg D, Witztum JL. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. J. Biol. Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein C, Pfaffinger D, Hinman J, Miller E, Lipkind G, Tsimikas S, Bergmark C, Getz GS, Witztum JL, Scanu AM. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J. Biol. Chem. 2003;26:52841–528447. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein C, Philips B, Pfaffinger D, Scanu AM. The oxidized phospholipids linked to human apolipoprotein(a) do not derive from circulating low-density lipoproteins and are probably of cellular origin. FASEB J. 2009;23:950–956. doi: 10.1096/fj.08-122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean JW, Tomlinson JE, Kuang W, Eaton DL, Chen EY, Fless GM, Scanu AM, Lawn RM. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 9.Scanu AM, Edelstein C. Kringle-dependent structural and functional polymorphism of apolipoprotein (a) Biochim. Biophys. Acta. 1995;1256:1–12. doi: 10.1016/0005-2760(95)00012-2. [DOI] [PubMed] [Google Scholar]
- 10.Ponting CP, Marshall JM, Cederholmwilliams SA. Plasminogen - a structural review. Blood Coagulation & Fibrinolysis. 1992;3:605–614. [PubMed] [Google Scholar]
- 11.Kearney JF, Barletta R, Quan ZS, Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the antiphosphorylcholine response in BALB/c mice. Eur. J. Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- 12.Shaw PX, Hörkkö S, Chang-Kyung M, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with theT15 idiotype may act in atherosclerosis, apopotic clerance, and protective immunity. J. Clin. Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein C, Kaiser M, Piras P, Scanu A, M Demonstration that the enzyme that converts precursor of apolipoprotein A-I to apolipoprotein A-I is secreted by the hepatocarcinoma cell line Hep G2. Arch. Biochem. Biophys. 1988;267:23–30. doi: 10.1016/0003-9861(88)90003-3. [DOI] [PubMed] [Google Scholar]
- 14.Deutsch DG, Mertz ET. Plasminogen: Purification from plasma by affinity chromatography. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 15.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett GR. Phosphorous assay in column chromatography. J. Biol. Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 17.Wang H, Prorok M, Bretthauer RK, Castallino FJ. Serine-578 is a major phosphorylation locus in human plasma plasminogen. Biochemistry. 1997;36:8100–8106. doi: 10.1021/bi970328d. [DOI] [PubMed] [Google Scholar]
- 18.Walther PJ, Steinman HM, Hill RL, McKee PA. Activation of human plasminogen by urokinase. Partial characterization of a pre-activation peptide. J. Biol. Chem. 1974;249:1173–1181. [PubMed] [Google Scholar]
- 19.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 21.Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Castellino FJ, Powell JR. Human plasminogen. Methods Enzymol. 1981;80:365–378. doi: 10.1016/s0076-6879(81)80031-6. [DOI] [PubMed] [Google Scholar]
- 23.Tsimikas S, Witztum JL. The role of oxidized [hospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 2008;19:369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 24.Scanu AM, Nakajima K, Edelstein C. Apolipoprotein(a): structure and biology. Frontiers in Bioscience. 2001;6:546–554. doi: 10.2741/scanu. [DOI] [PubMed] [Google Scholar]
- 25.Joy ST, Patel PN, Pfaffinger D, Edelstein C, Scanu AM. Apolipoprotein(a) in the carotid artery plaque: Evidence for proteolytic and pro-inflammatory modifications. Vasc. Dis. Prev. 2008;5:1–8. [Google Scholar]
- 26.Plow EF, Hoover-Plow J. The functions of plasminogen in cardiovascular disease. Trends Cardiovasc. Med. 2004;14:180–186. doi: 10.1016/j.tcm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Philips B, Scanu A, M Viewing the cardiovascular pathogenicity of Lp(a) from the pro-inflammatory side. Vasc. Dis. Prev. 2008;5:150–155. [Google Scholar]
- 28.Cao Y, Xue L. Angiostatin. Sem. Thromb. Hem. 2004;30:83–93. doi: 10.1055/s-2004-822973. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y. Antiangiogeni cancer therapy. Sem. Cancer Bio. 2004;14:139–145. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Ribatti D. Endogeneous inhibitors of angiogenesis. A historical review. Leukemia Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Lippi G, Franchini M, Salvagno GL, Guido GC. Lipoprotein(a) and cancer: Anti-neoplastic effect besides its cardiovascular potency. Cancer Treatment Rev. 2007;33:427–436. doi: 10.1016/j.ctrv.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.