Abstract
LAT-1 (L-type amino acid transporter 1) is a system L, Na+-independent amino acid transporter responsible for transport of large neutral amino acids. Dysregulated expression of LAT-1 is characteristic of many primary human cancers and it’s over expression is related to tumor invasion. LAT-1 is highly expressed in the trophoblast giant cells (TGCs) at the time of implantation. Since trophoblast giant cells are highly invasive during the process of endometrial implantation and placentation, LAT-1 may play a role in the invasive phenotype. Our objectives were to identify the effects of increased and decreased LAT-1 expression on mouse trophoblast invasion. We therefore examined the role of amino acid deprivation, pharmacologic blockade specific to leucine transport and gene silencing (siRNA) on LAT-1 expression and trophoblast cell invasion. We utilized mouse primary trophoblast stem (TS) cells. LAT-1 mRNA expression was quantified by real-time qPCR, protein by Western blotting and cell invasion was measured in Transwell plates through Matrigel. Amino acid transport using uptake of tritiated leucine. Under limited leucine availability and/or pharmacologic blockage, LAT-1 gene expression was significantly increased, p<0.05. This was associated with a 3-fold increase in cell invasion, p<0.05. In contrast, following siRNA-mediated gene silencing decreased LAT-1 expression (both mRNA and protein) was associated with decreased cell invasion and decreased leucine uptake, p<0.05. Upregulation of LAT-1 gene expression via limited amino acid availability or following pharmacologic blockade of transport leads to an increase in mouse trophoblast stem cell invasiveness. Downregulation of LAT-1 expression via genetic silencing leads to inhibition of invasiveness. These results demonstrate that LAT-1 plays an important role in trophoblast invasion.
Keywords: trophoblast stem cell, L amino acid transporter, LAT-1, Slc7a5, invasion, implantation
INTRODUCTION
Amino acids are critical for pre and post Implantation placenta and embryo development [1]. However, the amino acids may act at different times and by different mechanisms. Mouse blastocyst outgrowth in vitro and implantation in vivo require amino acid signaling via the target of rapamycin (TOR) pathway [2]. Amino acid dependent signaling does not simply support protein synthesis and trophoblast differentiation. Rather, it regulates development of trophoblast protrusive activity and may act as a developmental checkpoint for implantation [2]. Moreover, intracellular amino acids per se are insufficient to elicit TOR signaling. Instead de novo transport of amino acids, and particularly of leucine and arginine, stimulates the mammalian target of rapamycin (mTOR) activity at the blastocyst stage, and stimulates trophoblast outgrowth during implantation [3]. Thus, amino acids exert important signaling functions and their transport may help to regulate their intracellular abundance. These observations demonstrate that amino acids play a critical role in the process of implantation.
System L is a major nutrient transport system responsible for the transport of large branched chain, aromatic, and neutral amino acids including several essential amino acids [4,5]. It involves Na+- independent uptake of amino acids via heterodimers of a catalytic light chain subunit, that are covalently associated with the 4F2/CD98 heavy chain glycoprotein [6]. 4F2hc is widely expressed [6], whereas LAT-1 (Slc7a5) expression is highly tissue specific. It is expressed in restricted sites such as brain, fetal liver, bone marrow, spleen, testis, ovary and placenta [6,7]. Investigations have shown that LAT-1 may play an important role in carcinogenesis. Over expression of LAT-1 is characteristic of many primary human cancers and may be related to tumor progression [8]. Studies of rat primary hepatocyte cultures demonstrated that TA1/LAT-1 RNA levels are increased in response to amino acid depletion [9]. Upregulation of LAT-1 following amino acid restriction is associated with enhanced protein synthesis, shortened cell cycle progression and enhanced proliferation, suggesting adaptive nutrient regulation. In contrast, LAT-1 expression in transformed hepatic cell lines is not responsive to media amino acid concentrations. In transformed cells LAT-1 is constitutively expressed [9]. These observations indicate that LAT-1 expression may confer a growth and survival advantage under the limited amino acid availability that accompanies rapid tumor growth. Since trophoblast cells are highly proliferativeand highly invasive, studies of the mechanism(s) regulating placental development have been suggested as a model for tumor invasion. Therefore, the mechanism(s) of LAT-1 regulation during placentation will provide important insights into its developmental role and may provide clues to its role in tumor progression and invasion.
Our recently reported expression studies of LAT-1 in the pre-implantation and post-implantation mouse embryos show increased LAT-1 expression (mRNA) as the embryo developed from zygote to blastocyst and increased LAT-1 expression in placenta from gestation day 7.5 to 13.5 [1]. Moreover, expression studies of LAT-1 using Laser Capture Microdissected samples from developing mouse placenta showed the highest levels of LAT-1 mRNA in the trophoblast giant cells at 7.5 days post coitum (dpc) [1]. Since trophoblast giant cells are highly invasive during the process of implantation and placentation, LAT-1 may play a role in the invasive phenotypeleading to implantation.
In the present study we sought to determine whether trophoblast LAT-1 expression was modified by nutrient availability as in primary hepatocytes. Since amino acid availability can alter gene expression in other cell lines, e.g. primary hepatocytes [9], we investigated the role of decreased leucine availability on LAT-1 gene expression in trophoblast stem (TS) cells. We also sought to determine whether altered expression of LAT-1 in trophoblast cells was associated with changes in trophoblast invasion. We used TS cells as they have been shown to differentiate in vitro into trophoblast giant cells (TGCs) under differentiating conditions and have been described as an excellent model for measuring trophoblast invasiveness [10,11]. TGCs are large, polyploid cells that form through the process of endoreduplication. They are invasive as well as phagocytic and they mediate the invasion of the implanted conceptus into the maternal decidua [12]. We examined changes in LAT-1 gene expression in trophoblast stem (TS) cells in three different ways: 1) by limiting Leucine availability (amino acid deprivation), 2) by blocking transport activity with pharmacologic blockade specific to leucine transport using bicyclic amino acid, 2-aminobicyclo[2.2.1] heptane-2-carboxylic acid (BCH) and 3) by gene silencing (siRNAs). We then tested the biological importance of these findings by correlating them with changes in transport activity and invasion.
MATERIALS AND METHODS
Cell culture and transfection
All of the experiments were carried out on the Rs26 TS cell line [10,11]. TS cells were maintained and propagated in RPMI 1640 medium supplemented with 10% FBS, 1mM sodium pyruvate, 50 microg/ml penicillin/streptomycin (Invitrogen), 2mM L-glutamine (Invitrogen). Additional additives included 5.5 × 10−5 M β-mercaptoethanol, 25ng/mL of FGF4 ), and 1microg/mL Heparin (Sigma). We included 10 ng/mL of Activin A (R&D Systems). In all experiments TS cells were grown in custom RPMI 1640 medium lacking arginine and leucine. For “complete” culture medium L-arginine and L-leucine (Sigma) were added to the media at a final concentration of 50 mg/L and 200mg/L, respectively. To achieve different leucine concentrations, media with 50 mg/L of L-arginine was supplemented with leucine to achieve final concentration from 20 to 100 microM. All culture media was further supplemented with 10% FBS, 1mM sodium pyruvate, 50 ug/ml penicillin/streptomycin, 5.5 × 10 −5 M β-mercaptoethanol, 2mM L-glutamine, 25ng/mL of FGF4, 1microg/mL Heparin and 10 ng/mL of Activin A. In cell invasion assays differentiating medium was used which contained all of the above reagents except FGF4, Heparin and Activin A. BCH medium was prepared by adding BCH to a final concentration of 10 microM in complete medium (4). Rapamycin was added at 20 nM concentration to both low leucine medium and complete medium for invasion assays.
To investigate the role of LAT-1 gene expression in trophoblast invasion, we targeted gene disruption with siRNAs specific for LAT-1. We tested three different siRNAs (designated numbers 1 through 3), alone and in combination (Ambion, Inc). Cells were transfected with siRNAs by Amaxa Nucleofector using Nucleofector Solution L and program L-029 (Lonza Cologne AG). The procedure was otherwise carried out according to the manufacturer’s recommendations.
RNA Extraction and Reverse Transcription
RNA was isolated using the Micro RNA Isolation Kit (Stratagene, La Jolla, CA) that uses guanidinium isothiocyanate to lyse cells and inactivate nucleases. This method was carried out according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) synthesis was performed using random hexamers and the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Real Time PCR
Samples were amplified with real-time PCR TaqMan gene expression assays on demand (LAT-1 Assay Cat.# Mm00441516_m1 and GAPDH Cat. # 4352339E-0506006). LAT-1 expression was normalized to endogenous GAPDH. The Delta Delta CT method was used for quantitation of mRNA (1).
Western blotting
At 24 h cells were harvested by trypsinization and pellets were resuspended in buffer F( 10 mM Tris pH 7.0, 50 mM NaCl, 30 mM sodium pyrophosphate, 50mM NaF, 5mM ZnCl2, 0.1 mM NaVO4, 1% Triton-X 100, 10−2 M AcBSF, 2.5U/mL leupeptin and 2.8ug/mL aprotinin), incubated for 10 min on ice, and centrifuged at 20,000 × g for 10 min at 4C. Supernatants were quantitated with Pierce assay and aliquots of protein extracts (50 micrograms of total protein/sample) were resolved on the precast gradient Midi gels (Invitrogen) and electro-transfered to Milipore PVDF Immobilon-P membranes (0.45 µm)using a Bio-Rad semi dry transfer apparatus. Membranes were blocked overnight in Tris-buffered saline at pH 7.6 with 0.05% Tween-20 (TBS-T) and 5% dehydrated with non-fat milk. After rinsing with TBS-T membranes were immunoblotted with a rabbit polyclonal antibodyraised against a polypeptide corresponding to the c-terminal region of human LAT-1 (Cosmo Bio, Japan) and rabbit polycloncal antibody raised against full-length GAPDH (loading control, Santa Cruz Biotechnology, Inc., USA). While not tested by the manufacturer, we have previously shown the rabbit polyclonal anti-LAT-1 to cross react with mouse LAT-1 and to display appropriate specificity for both human and mouse applications [1]. The immunodetected isofroms were visualized using ECL reagents (Amersham Pharmacia biotech, Inc., Canada) and quantified using a Bio Rad ChemiDoc XRSTm detection system equipped with a super cooled CCD camera and the manufacturers Quantity OneTm analysis software. All LAT-1 signals were normalized to GAPDH.
Cell Invasion Assay
For invasion assays we used BD BioCoat™ Matrigel™ Invasion Chambers (Cat# 354480) that consist of BD Falcon Cell Culture Inserts containing an 8 micron pore size PET Membrane coated with Matrigel Matrix. On the day of each experiment inserts were removed from −20°C storage, allowed to equilibrate to RT and rehydrated with 500 microL of culture medium (appropriate for each condition ) for 2 h at 37°C under 95% humidity and 5% CO2. Confluent TS cells, in100-mm dishes, cultured either in complete medium, low leucine medium or medium supplemented with BCH were trypsinized with 1mL trypsin-EDTA (Invitrogen) and resuspended in 4 mL of appropriate culture medium. Cells were counted using a hemacytometer. 1×104 cells were resuspended in 300 microL of appropriate differentiating medium and added to the top chamber, and the bottom chamber was filled with 800microL of corresponding culture medium. After the 24 h incubation time, Transwell inserts were washed with 1XPBS and fixed for 5 min in ice cold MeOH. Any cells that remained on top of the filters as well as the Matrigel coating were scraped off with Q-tips and the TS cells attached to the Transwell inserts were stained overnight with hematoxylin. The next day the filters were washed in tap water and allowed to air dry. Using a scalpel filters were cut out from the Transwell inserts and mounted on the glass slide with Cytoseal mounting medium. Sections were viewed and photographed on a Nikon Eclipse 80i microscope. The number of cells that had traversed the filter was quantified by visual counting of five selected areas (middle, top, bottom, left and right) of each triplicate. The identification of invasive cells as trophoblast giant cells was based on nuclear and cell sizes. Each experiment was repeated three separate times.
Amino Acid Transport Measurement
The uptake of radiolabled leucine (3H-Leu, Perkin Elmer) by TS cell monolayers was performed using the method of Gazzola et al [13]. Cells were seeded in 24-well culture dishes so that after settling overnight the plates were near confluent at the time of the transport assay. Before the transport assays, TS cells were rinsed with warm Na+-free Krebs-Ringer phosphate buffer (cholKRP), in which the content of sodium has been osmotically replaced with choline, to remove extracellular Na+ and amino acids. Cells were incubated in prewarmed (37oC) cholKRP for 10 min to deplete intracellular amino acids. The uptake of radiolabled Leucine (5microCi of 3H-Leu/mL) at a final concentration of 100 microM in either 200 microL of cholKRP or NaKRP was measured after 30 seconds at 37°C. Cold L-leucine was added to uptake buffers at 10 mM final concentration. Uptake was quenched by washing the cells rapidly four times with 1mL/well of ice-cold cholKRP buffer. Cells were then incubated for 1h with 0.2 mL/well of 0.2% (w/v) SDS with 0.2 N NaOH to release intracellular radioactivity. A 50 microL aliquot from each well was neutralized with 50 microL of 0.2 n HCL and quantified on the liquid scintillation counter (Delta 300, TM Analytic). Another two 50 microL aliquots from each well were analyzed for protein content using the bicinchoninic acid (BCA) Protein Assay(Pierce). Transport velocities were calculated from radioactive counts, specific activities of uptake mixes, and protein absorbance values and expressed as pmol amino acid transported per milligram of protein per minute.
Statistical Analysis
The values are given as the mean ± SEM of three independent experiments. Data were analyzed by analysis of variance (ANOVA) and Newman-Keuls multiple comparison tests. The significance level was set as p<0.05.
RESULTS
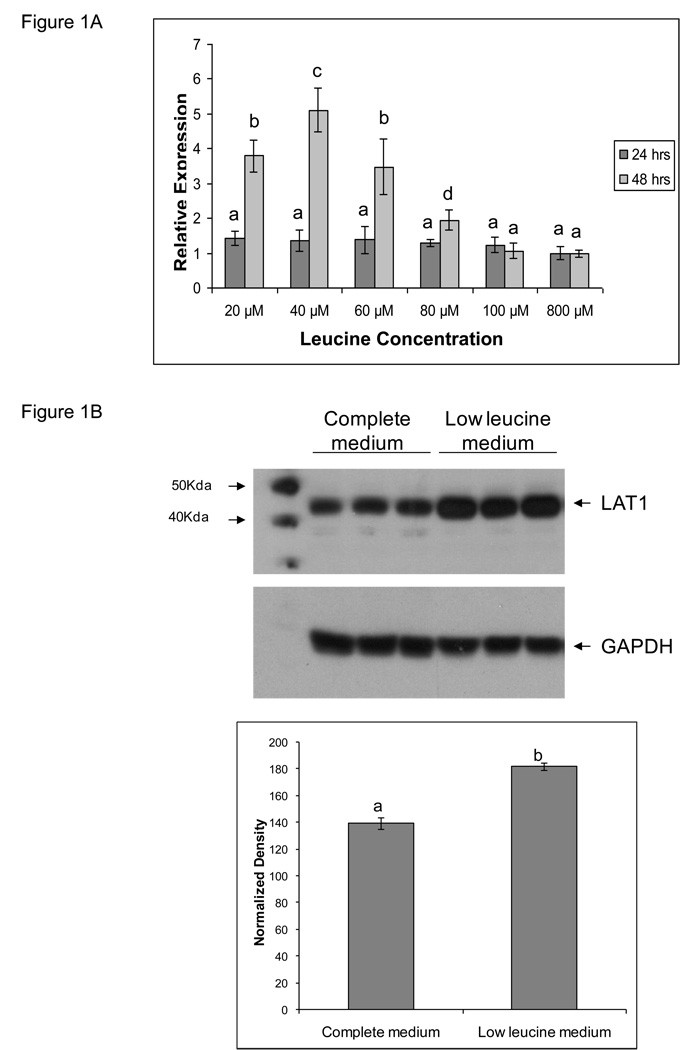
We cultured TS cells in customized media containing graded concentrations of leucine, 20microM, 40microM, 60microM, 80microM, 100microM and 800microM for a period of 24 and 48 hrs. The 800microM concentration is found in complete medium with fetal calf serum. The effect on LAT-1 gene expression by quantitative real time PCR, shown in Figure 1A, revealed upregulation of LAT-1 mRNA message in the three lowest concentrations of leucine (20microM, 40microM and 60microM) with highest upregulation (~3-fold) of LAT-1 in cells cultured in presence of 40microM of leucine, p<0.05. This maximal upregulation of LAT-1 message was seen at 48 hours of culture in low leucine media. Based on these results, all further experiments where we altered LAT-1 gene expression by reducing leucine concentration were carried out in media containing 40microM of leucine (now referred to as low leucine medium) with a 48 hr culture period. Immunoblots of TS cell extracts, shown in Figure 1B, reveal comparable upregulation (~2-fold) of LAT-1 protein in cells cultured in low leucine medium compared to complete medium, p<0.05.
Figure 1. LAT1 gene expression in TS cells cultured in media containing various concentrations of leucine.
A) The effect of leucine availability on LAT1 mRNA expression was measured by real time PCR. Trophoblast stem (TS) cells were cultured in media containing final leucine concentrations of 20, 40, 60, 80, and 100microM. 800 microM is found in complete medium. Cells were cultured for 24 or 48 hrs. LAT-1 is expressed relative to GAPDH by the Delta Delta CT method. LAT1 mRNA expression was highest in TS cells cultured in 40 µM leucine medium. B) Protein expression was measured by Western blot. Protein expression in TS cells cultured in 40 microM leucine compared to complete medium containing 800 microM leucine. All experiments were carried out at least three independent times with similar results. Values are the means ± SEM. Values from a representative experiment are shown in B. Bars with different letters represent significant differences in LAT1 gene expression among the experimental conditions (p≤0.05).
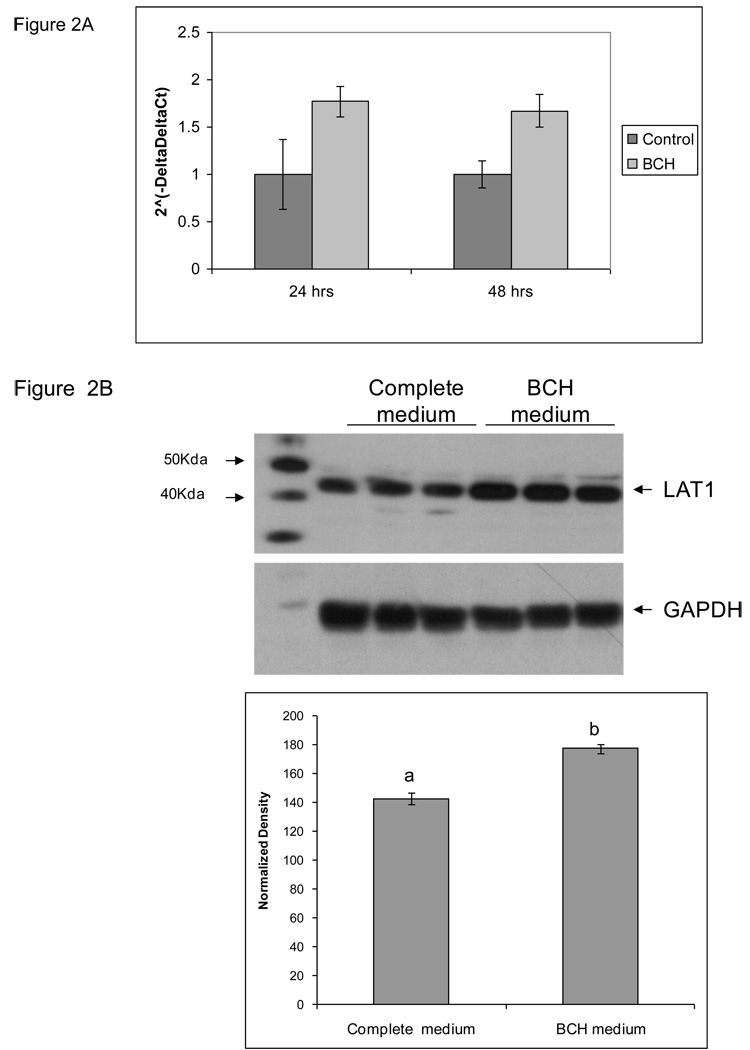
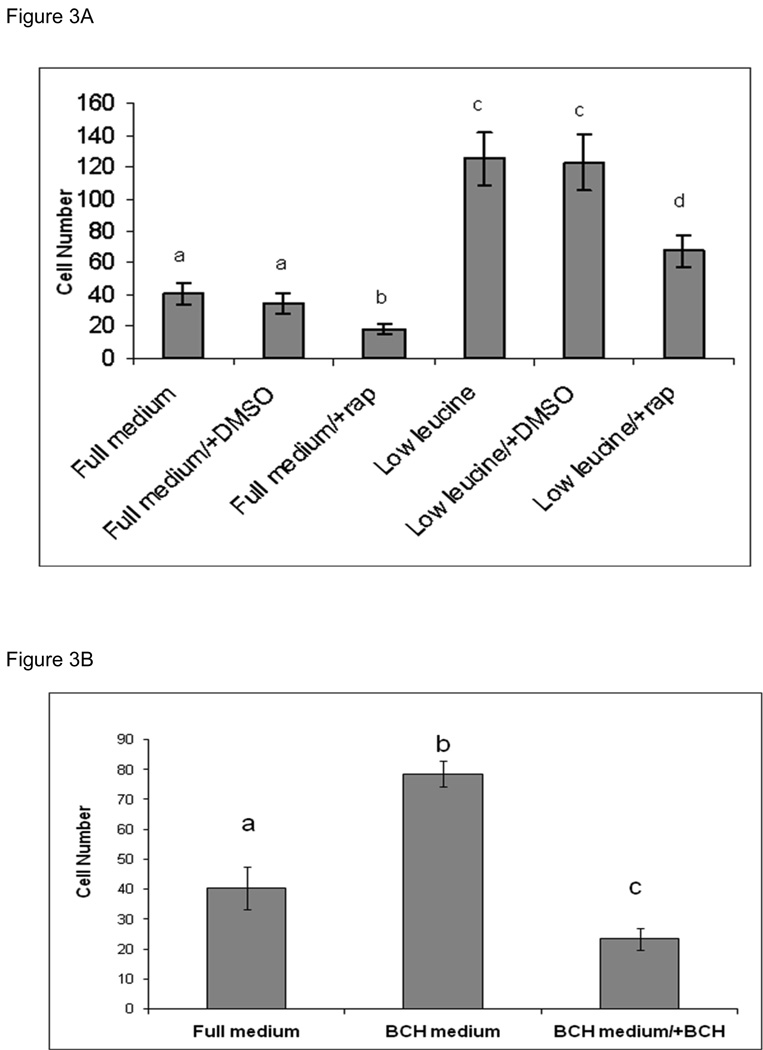
Treatment of TS cells with BCH, a competitive inhibitor of Leucine transport, at 10microM concentration also resulted in a comparable magnitude of upregulation at 24 and 48 hrs of culture (Figure 2A). Immunoblots of TS cell extracts from cells cultured for 24 hrs in presence and absence of BCH are shown in Figure 2B. BCH treatment also led to upregulation of LAT-1 protein. We examined the effect of these treatments in cell invasion assays (Figure 3). TS cells cultured in low leucine medium show 3-fold increase in cell invasion compared to cells cultured in complete medium (Figure 3A). Rapamycin, inhibited the motility of these cells, but did not diminish the increase in cell invasion completely. In cells cultured in complete media as well as in cells cultured in the low leucine media, rapamycin decreased cell invasion by ~2-fold. Treatment with BCH for 24 hrs prior to invasion assay also resulted in a 2-fold increase in cell invasion, however continuous treatment of cells with BCH during the invasion assay led to a 2-fold decrease in cell invasion (Figure 3B).
Figure 2. LAT1 gene expression in TS cells cultured in medium containing 10 microM BCH.
The effect of BCH treatment on LAT1 A) mRNA expression was measured by real time PCR, and B) protein expression was measured by western blots. For mRNA analysis TS cells were cultured in medium containing 10 microM concentration of BCH for 24 and 48 hrs, whereas for protein analysis cells were cultured in the above condition for 24 hrs only. All experiments were carried out three independent times with similar results. Values are the means ± SEM of three measurements. Values from a representative experiment are shown. Bars with different letters represent significant differences in LAT1 gene expression among the experimental conditions (p≤0.05).
Figure 3. Cell invasion assay in TS cells cultured in low leucine vs. complete medium and in presence of BCH.
A) The effect of leucine availability and treatment with BCH on TS cells ability to invade the Matrigel was measured as the number of cells that migrated through the Matrigel barrier to the other side of the filter at 24 hr time point. TS cells were cultured in low leucine medium and complete medium for 48 hrs prior to the invasion assay. Upon seeding of the cells in the invasion chambers, cells were treated with DMSO (vehicle for rapamycin) and/or rapamycin. B) TS cells were cultured in medium containing 10 microM BCH and complete medium for 24hrs prior to invasion assay. Cells cultured in 10microM BCH were seeded with and without BCH in the culture media during invasion assay. All experiments were carried out at least three independent times with similar results. Values are the means ± SEM Bars with different letters represent significant differences in TS cell invasion among the experimental conditions (p≤0.05).
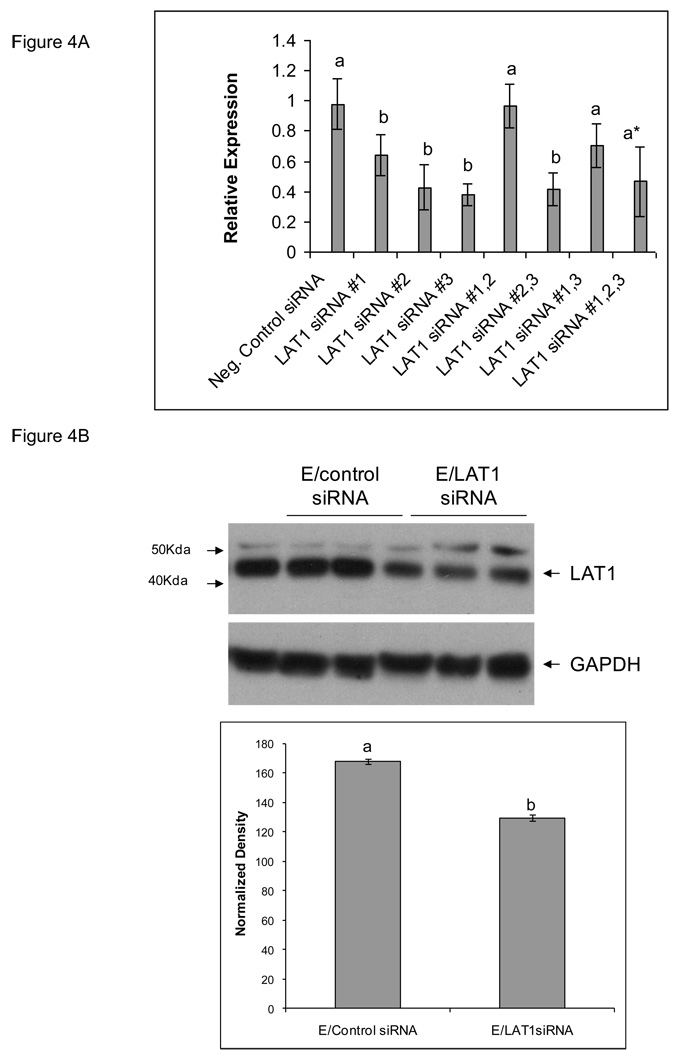
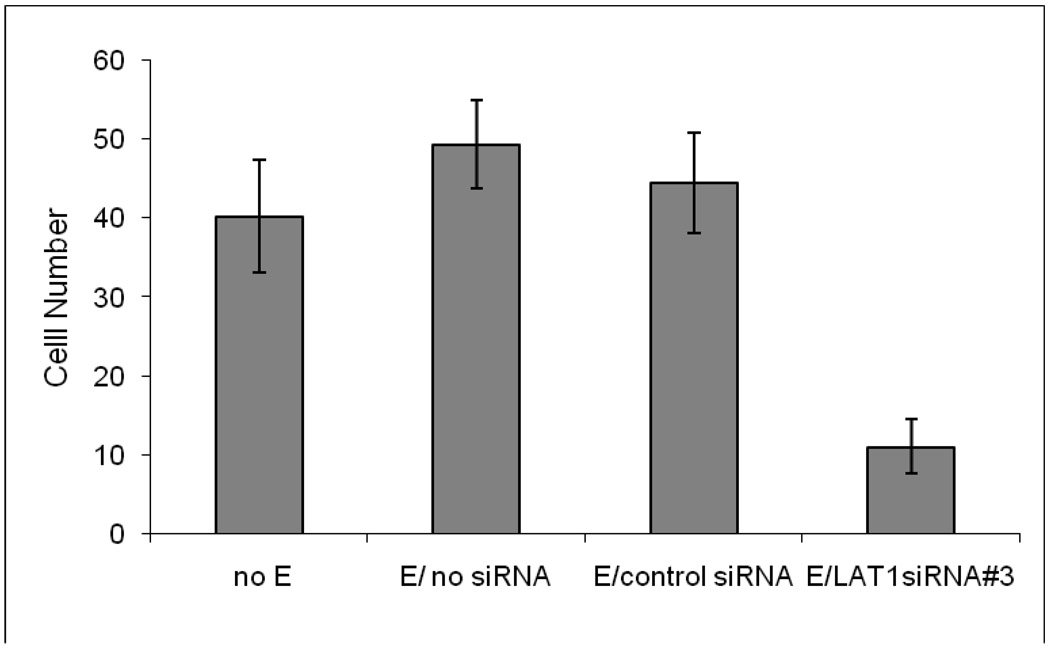
As shown in Figure 4), the most effective silencing result was achieved with siRNA#3 where we were able to knockdown LAT-1 mRNA expression by 60% and protein expression by 50% (Figures 4A and 4B, respectively). Invasion studies on TS cells transfected with siRNA#3 compared to no transfection or transfection with a negative control siRNA with a similar nucleotide content but scrambled sequence are shown in Figure 5. As can be seen, there was no effect of electroporation but there was a significant inhibition of cell invasion following transfection with LAT-1 siRNA#3 (Figure 5).
Figure 4. LAT1 gene expression in TS cells transfected with LAT1 siRNA.
A) The effect of LAT1 siRNAs on LAT1 A) mRNA expression was measured by real time PCR, and B) protein expression was measured by Western blot. TS cells transfected with scrambled siRNA vs. LAT1 siRNA are shown. All experiments were carried out three independent times with similar results. Values are the means ± SEM of three measurements. Bars with different letters represent significant differences in LAT1 gene expression among the experimental conditions (p≤0.05).
Figure 5. Cell invasion assay of TS cells transfected with LAT1 siRNA.
The effect of LAT1 gene silencing cell invasion was measured by # of cells that migrated through the Matrigel barrier to the other side of the filter at the 24hr time point. TS cells were electroporated (E) with siRNAs 24hrs prior to invasion assay. All experiments were carried out three independent times with similar results. Values are the means ± SEM of three measurements. Bars with different letters represent significant differences in TS cell invasion among the experimental conditions (p≤0.05).
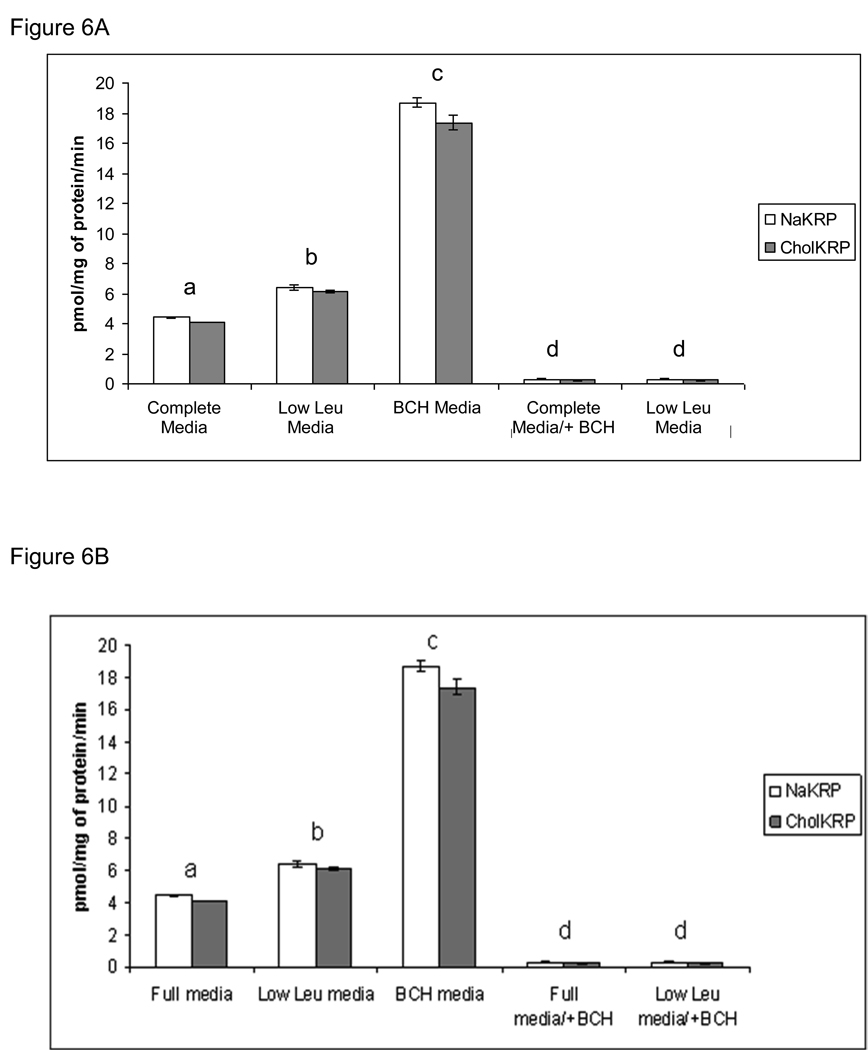
The amino acid uptake assays showed a small increase in Leucine uptake in TS cells cultured in low leucine media (~1.5-fold). There was a larger increase in leucine uptake in TS cells which had been treated with BCH, perhaps due to depletion and restoration of amino acid pool size (Figure 6A). The uptake of leucine in TS cells transfected with siRNA#3 showed a modest but significant decrease in uptake (Figure 6B).
Figure 6. Amino acid transport activity of leucine in TS cells.
A) The uptake of 50 microM of radiolabled leucine in two uptake buffers (NaKRP and CholKRP) was measured in TS cells cultured in complete media containing 800 microM Leu, low Leu media containing 40 microM Leu, and BCH media containing 10 microM of BCH. B) The uptake of radiolabled leucine was measured in TS cells electroporated (E) with no siRNA, scrambled siRNA and LAT1 siRNA#3. Transport activity was measured at 24 hrs of culture with exception of TS cells cultured in low leucine media, which was measured at 48 hrs. Leucine uptake was inhibited with BCH marked as +BCH. All transport values are expressed as pmol/mg of protein/min. Each experiment was repeated three times with similar results. Values are the means ± SEM of three measurements from three independent experiments. Bars with different letters represent significant differences in leucine uptake among the experimental conditions (p≤0.05). No difference was observed between two buffers used (NaKRP-open bars, CholKRP-shaded bars).
DISCUSSION
In this study, we have investigated the role of the LAT-1 transporter in trophoblast stem cell invasion. Our data demonstrates that LAT-1 gene expression is upregulated in mouse TS cells in response to limited amino acid availability (by culture in low leucine medium or by treatment with BCH). The upregulation in trophoblast stem cells was associated with an increase in invasion. In contrast, LAT-1 silencing experiments showed a decrease in an in vitro cell invasion assay, demonstrating that LAT-1 plays a key role in trophoblast invasion.
The placenta is composed of many specialized, trophoblast cell types, each having a particular function and pattern of gene expression. All of these trophoblast cell types are derived from the trophectoderm, which forms an outer shell of cells surrounding the inner cell mass (ICM) at the blastocyst stage of development. The cells of the mural trophectoderm, not in direct contact with ICM, stop dividing and differentiate into trophoblast giant cells (TGC’s) that line the implantation site and invade the maternal decidua. In contrast, the cells in direct contact with ICM, the polar trophectoderm, continue to proliferate and give rise to the trophoblast cell types that form the placenta [10–12]. We utilized mouse trophoblast stem (TS) cells to study trophoblast invasion. These cells were established from 3.5 dpcblastocysts or the extraembryonic ectoderm of 6.5 dpc embryos [10]. They can be maintained in a proliferative state or be induced to differentiate into a somewhat homogenous population of giant cells. Removal of factors such as FGF4, heparin and Activin A from the culture media as well as contact with ECM leads to differentiation of these cells into GCs [10,11].
In this study, we were able to upregulate LAT-1 gene expression (both mRNA and protein) in two ways: 1) by limiting the availability of leucine in the culture medium and 2) by treating the cells with a competitive inhibitor of leucine transport. The deprivation of cells from leucine led to an increase in LAT-1 mRNA. Treatment with BCH also increased LAT-1 mRNA, but by a lesser magnitude. Even though we found a modest differential expression of LAT-1 mRNA under these experimental conditions,analysis of expression by Western blotting revealed similar upregulation following leucine deprivation or blockade of leucine transport. Likewise, both experimental conditions led to a similar increase in trophoblast invasion. In contrast, treatment of cells with BCH continuously during the invasion assay led to a decrease in invasion. This observation is consistent with previous findings that active transport of amino acids is crucial for motility and invasive phenotype of trophoblast cells [2].
Over expression of LAT-1 is characteristic of many primary human cancers and may be related to tumor progression [8,9,14,15]. In human primary astrocytic tumors it has been demonstrated that high LAT-1 expression correlated with poor survival for the study group as a whole (p<0.0001) and for those with glioblastoma multiforme in particular (p=0.0001) [14]. Moreover, LAT-1 expression was one of the most significant predictors of outcome, independent of all other variables. BCH mediated blockade of C6 glioma cells in vitro and in vivo using a rat C6 glioma model was associated with a significant increase in survival. These findings suggest that LAT-1 could be one of the molecular targets in glioma therapy. Similarly, LAT-1 may play a significant role in cellular proliferation, lymph node metastasis, and poor outcome in patients with neuroendocrine tumors of the lung [15].
Studies of the placenta and placental cell lines have been employed by many investigators as a valuable experimental model for cancer biology. Like cancer, trophoblast cells are highly proliferative and can express an invasive phenotype. We have shown that LAT-1 is one of the earliest amino acid transporter genes expressed in the pre-implantation stages of mouse embryo development [22] and, in the early stages of post-implantation at 7.5 dpc its highest expression is in trophoblast giant cells [1]. Trophoblast giant cells, which arise from the mural trophectoderm cells of the blastocyst, transform after implantation into invasive cells that displace uterine epithelial cells, penetrate the uterine stroma, and make vascular connections with the maternal blood supply. Our findings thus suggested that LAT-1 might have a role in trophoblast GCs’ migratory/invasive phenotype. LAT-1 and 4F2hc must dimerize. However, whereas 4F2hc is ubiquitously expressed, LAT-1 expression is restricted [5–7]. This has been the rationale for many studies where the expression of LAT-1 has been used to infer its biological and pathophysiological importance. This has been corroborated in studies by our collaborators in which they demonstrated that LAT-1 but not 4F2 responds to nutrient availability in rat hepatic cells [9]. Thus, we chose to focus on LAT-1, the light chain of the CD98 complex in TS cell motility and invasion.
Amino acid dependent regulation of intracellular signaling pathways has been described in many systems [16]. Amino acid signaling activates the serine-threonine kinase mTOR, which then phosphorylates at least two proteins involved in regulation of translation initiation, p70S6K and PHAS-I [17]. mTOR is an intracellular signaling molecule that is essential for cell growth and proliferation. The initiation of trophoblast cell motility also depends on amino acid signaling through mTOR. In vitro studies of blastocysts cultured in the absence of amino acids or rapamycin treatment revealed inhibition of trophoblast protrusion and spreading behavior via mTOR signaling [2,3,25]. Under conditions of amino acid deprivation or rapamycin treatment, p70S6K remains un-phosphorylated, confirming that mTOR activation is inhibited in both cases [2]. Disruption of the mTOR gene is associated with a limited level of trophoblast outgrowth in vitro and early post implantation lethality in vivo [26]. [2]. In our study, we treated TS cells cultured in complete medium vs. low leucine medium with rapamycin to see whether rapamycin inhibited the invasion that is stimulated by low leucine availability and subsequent LAT-1 upregulation. In both experimental conditions (complete medium vs. low leucine medium), while rapamycin did not inhibit cell invasion completely, it led to a significant decrease in cell invasion. These results confirm that amino acid-dependent mTOR signaling is involved in the development of trophoblast cell motility and invasive phenotype. We showed here that leucine availability alters LAT-1 expression and ability of TS cells to invade extracellular matrix. Our gene silencing experiments provided complementary data addressing the role of LAT-1 in invasion. While we were not able to completely knockdown LAT-1 protein expression, we showed a significant impact of LAT-1 downregulation on amino acid uptake and an associated decrease in trophoblast invasiveness, which correlated with the decrease LAT-1 protein expression.
Amino acids have been shown to be critical not only as nutrients for the mammalian embryo but also as regulators of cell motility during implantation and continued development [18–21]. Trophoblast invasion is controlled by very sophisticated systems that specifically regulate motility, independently of many aspects of trophectoderm differentiation. Regulated motility confers the ability of developing trophoblast cells to initiate invasion. Amino acid signaling in the embryo is regulated in part through the ambient amino acid concentration in uterine environment and by the temporal expression of amino acid transporters [22]. LAT-1 is one of the earliest transporters expressed. Unlike most other transport systems known to be important in later embryonic development [22], LAT-1 is expressed in preimplantation stages [1]. Amino acid uptake and signaling thus provides one way for the cross talk between developmental changes in uterine and embryo development to be coordinated to the local environmental milieu. Since intracellular amino acids are not sufficient to activate mTOR signaling, de novo transport of amino acids, and particularly of leucine, is necessary for this activation. It has been proposed that the activity of the broad-scope and yet leucine- selective amino acid transport system B0,+ can also produce such increases in intracellular amino acid concentrations [2,3]. System B0,+ uses a Na+ gradient to drive amino acid uptake, and the Na+ concentration in uterine secretions increases by nearly two-fold about 18hrs before implantation. The resultant mTOR signaling may trigger polyamine, insulin-like growth factor II, and nitric oxide production in blastocysts and the increased cell motility which is sometimes associated with synthesis of these bioactive molecules [2, 19]. We suggest that B0,+ and LAT-1, working in synergy, supply the amino acids needed to activate the signaling cascade(s) leading to initiation of motility. Downregulation in activity of placental amino acid transporters is characteristic of intrauterine growth restriction (IUGR). System L and TAUT are both reduced in microvillous membrane and basal plasma membrane isolated form IUGR placentas [23,24]. A recent study in our lab, has also demonstrated an inverse correlation of LAT-1 expression with placental disorders associated with abnormalities in implantation, such as spontaneous abortion, intrauterine growth restriction and preeclampsia (unpublished observations).
Amino acids that stimulate mTOR signaling pathway are transported into cells through several plasma membrane transporter systems, including L amino acid transporters [29]. A recent study on a head and neck squamous cell carcinoma cell line (Hep-2) demonstrated that treatment of these cells with BCH (specific inhibitor of leucine transport) leads to decrease in phosphorylation of mTOR, p70S6K and 4EBP1 [30]. Other groups have reported that the expression of LAT-1 in vascular smooth muscle cells is stimulated by PDGF in a mTOR-dependent manner [31] and treatment with rapamycin, reduces system L activity, but not activities of system A or the taurine transporter (TAUT), in the primary villous fragments from the human placenta [32]. mTOR inhibition studies in primary trophoblast cells revealed downregulation of few amino acid transporters [33]. Activities of system A, system L and taurine were reduced by 17%, 28%, and 40%, respectively. Real time PCR revealed downregulation of LAT-1 and the taurine transporter (TAUT) mRNA, by 13% and 50%, respectively and no change in mRNA of system A isoforms (1,2 and 3), LAT2 or 4F2hc. There was no change in protein expression for all transporters. Therefore the authors concluded that mTOR regulates placental amino acid transporters by either posttranslational modifications or by affecting trafficking of the transporter to the plasma membrane [33].
Future studies that demonstrate the effects of the modifications of LAT-1 expression on the signaling pathways will be very valuable. They may reveal unique aspects of regulation of mTOR activation and/or unique activation of downstream signaling. Continuing evaluation of amino acid signaling, mTOR activation, LAT-1 regulation and trophoblast cell invasiveness may provide possible targets for better understanding of implantation disorders and/or cancer interventions.
ACKNOWLEDGEMENTS
We acknowledge Dr. Nancy L. Thompson’s lab for training in some of the techniques and for insightful scientific discussions. This study was supported by NIEHS T32ESOO7272 Training in Environmental Pathology Grant, the Departments of Pathobiology and Pediatrics at Brown University and by a Center of Biomedical Research Excellence (COBRE) grant NCRR P20 RR018728., The rs26 TS cell lines were kindly provided by Dr. James Cross (University of Calgary, Calgary) and originated in the laboratory of Dr. Janet Rossant (University of Toronto).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIEHS T32ESOO7272 Training in Environmental Pathology Grant, the Graduate Program in Pathobiology, Department of Pediatrics at Brown University and by a Center of Biomedical Research Excellence (COBRE) grant NCRR P20 RR018728.
REFERENCES
- 1.Chrostowski MK, McGonnigal BG, Stabila JP, Padbury JF. LAT-1 expression and pre- and post- implantation embryos and placenta. Placenta. 2009;30:270–276. doi: 10.1016/j.placenta.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PM, Sutherland AE. Exogenous Amino Acids Regulate Trophectoderm Differentiation Through an mTOR-dependent pathway. Dev. Biol. 2001;240:182–193. doi: 10.1006/dbio.2001.0461. [DOI] [PubMed] [Google Scholar]
- 3.Martin PM, Sutherland AE, Von Winkle LJ. Amino Acid Transport Regulates Blastocyst Implantation. Biol. Reprod. 2003;69:1101–1108. doi: 10.1095/biolreprod.103.018010. [DOI] [PubMed] [Google Scholar]
- 4.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression Cloning and Characterization of a Transporter for Large Neutral Amino Acids Activated by the Heavy Chain of 4F2 Antigen (CD98) J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 5.Segawa H, Fukasawa K, Miyamoto E, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, et al. 4F2 (CD98) Heavy Chain Is Associated Covalently with an Amino Acid Transporter and Controls Intracellular Trafficking and Membrane Topology of 4F2 Heterodimer. J. Biol. Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 7.Kim DK, Ahn SG, Park JC, Kanai Y, Endou H, Yoon JH. Expression of L-type Amino Acid Transporter 1 (LAT-1) and 4F2 Heavy Chain (4F2hc) in Oral Squamous Cell Carcinoma and its Precursor Lesions. Anticancer Res. 2004;24:1671–1675. [PubMed] [Google Scholar]
- 8.Campbell WA, Sah DE, Medina MM, Albina JE, Coleman WB, Thompson NL. TA1/LAT-1/CD98 Light Chain and System L Activity, but not 4F2 /CD98 Heavy Chain, Respond to Arginine Availability in Rat Hepatic Cells. Loss of Response in Tumor Cells. J. Biol. Chem. 2000;275:5347–5354. doi: 10.1074/jbc.275.8.5347. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 10.Hemberger M, Hughes M, Cross J. Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev Bio. 2004;271:362–371. doi: 10.1016/j.ydbio.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Zybina EV, Zybina TG, Stein GI. Trophoblast cell invasiveness and capability for the cell and genome reproduction in rat placenta. Early Pregnancy. 2000;4:39–57. [PubMed] [Google Scholar]
- 12.Gazzola GC, Asta VD, Franchi-Gazzola R, White M. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Analy Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- 13.Nawashiro H, Otani N, Shinomiya N, Fukui S, Ooigawa H, Shima K, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer. 2006;119:484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 14.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. Expression of L-type amino acid transporter 1 (LAT-1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204:553–561. doi: 10.1016/j.prp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2001;4:39–43. doi: 10.1097/00075197-200101000-00008. Review. [DOI] [PubMed] [Google Scholar]
- 16.Volarevic S, Thomas G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog Nucleic Acid Res Mol Biol. 2001;65:101–127. doi: 10.1016/s0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- 17.Gardner DK, Lane M. Amino Acids and Ammonium Regulate Mouse Embryo Development in Culture. Biol. Reprod. 1993;48:377–385. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- 18.Devreker F, Hardy K, Van den Bergh M, Vannin AS, Emiliani S, Englert Y. Amino Acids Promote Human Blastocyst Development in Vitro. Hum Reprod. 2001;16:749–756. doi: 10.1093/humrep/16.4.749. [DOI] [PubMed] [Google Scholar]
- 19.Lane M, Gardner DK. Nonessential Amino Acids and Glutamine Decrease the Time of the First Three Cleavage Divisions and Increase Compaction of Mouse Zygotes in vitro. J Assist Reprod Genet. 1997;14:398–403. doi: 10.1007/BF02766148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane M, Gardner DK. Differential Regulation of Mouse Embryo Development and Viability by Amino Acids. J Reprod Fertil Dev. 1997;109:153–164. doi: 10.1530/jrf.0.1090153. [DOI] [PubMed] [Google Scholar]
- 21.Van Winkle LJ. Amino Acid Transport Regulation and Early Embryo Development. Biol. Reprod. 2001;64:1–12. doi: 10.1095/biolreprod64.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Jansson T, Scholtbach B, Pawell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland AE. Mechanisms of implantation in the mouse: differential and functional importance of trophoblast giant cell behavior. Dev Biol. 2003;258:241–251. doi: 10.1016/s0012-1606(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 25.Christensen HN. Role of amino acid transport and counter transport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi K, Sakurai H, Kimura T, Wiriyasermkul P, Nagamori S, Kanai Y, et al. System L amino acid transporter inhibitor enhances anti-tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett. 2009;276:95–101. doi: 10.1016/j.canlet.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, et al. Platelet-derived growth factor stimulates LAT-1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:768–770. doi: 10.1096/fj.03-0886fje. [DOI] [PubMed] [Google Scholar]
- 28.Roos S, Jansson NL, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down regulated in restricted fetal growth. J Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol. 2009;296:C142–C150. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]