Abstract
Activation of phospholipases A2 (PLA2s) leads to the generation of biologically active lipid mediators that can affect numerous cellular events. The Group VIA Ca2+-independent PLA2, designated iPLA2®, is active in the absence of Ca2+, activated by ATP, and inhibited by the bromoenol lactone suicide inhibitor (BEL). Over the past 10–15 years, studies using BEL have demonstrated that iPLA2β participates in various biological processes and the recent availability of mice in which iPLA2β expression levels have been genetically-modified are extending these findings. Work in our laboratory suggests that iPLA2® activates a unique signaling cascade that promotes β-cell apoptosis. This pathway involves iPLA2® dependent induction of neutral sphingomyelinase, production of ceramide, and activation of the intrinsic pathway of apoptosis. There is a growing body of literature supporting β-cell apoptosis as a major contributor to the loss of β-cell mass associated with the onset and progression of Type 1 and Type 2 diabetes mellitus. This underscores a need to gain a better understanding of the molecular mechanisms underlying β-cell apoptosis so that improved treatments can be developed to prevent or delay the onset and progression of diabetes mellitus. Herein, we offer a general review of Group VIA Ca2+-independent PLA2 (iPLA2β) followed by a more focused discussion of its participation in β-cell apoptosis. We suggest that iPLA2β-derived products trigger pathways which can lead to β-cell apoptosis during the development of diabetes.
Keywords: iPLA2β, apoptosis, β-cell, ceramides, mitochondria
A. The Biology of Group VIA Phospholipase A2 (iPLA2β)
1. Classification of phospholipases A2 (PLA2s)
In pancreatic islet subcellular organelles, similar to brain tissue [1], arachidonic acid is a major sn-2 substituent of membrane phospholipids [2, 3]. Arachidonic acid and its oxygenated metabolites and the lysophospholipids are potent bioactive mediators that regulate a myriad of physiological and pathophysiological processes [4, 5]. Phospholipases A2 (PLA2s) are a diverse group of enzymes that catalyze hydrolysis of arachidonic acid (and other sn-2 substituents) from glycerophospholipid substrates. [6]. For more detailed descriptions of the biology of the PLA2 enzymes, the reader is referred to a number of comprehensive reviews [7–17].
To date, 15 distinct groups of PLA2s are recognized [7, 8] and among them, the Ca2+-independent PLA2s (iPLA2s) are the most recently described and the least well characterized. The iPLA2 was first purified from macrophages in 1994 [18] and was subsequently cloned from multiple sources between 1997 and 1999 [19–21]. A brief review of the biology and properties of iPLA2 is provided here and the reader is referred to other, more comprehensive reviews for more detailed discussions of the iPLA2 enzymes [15–17].
2. Sub-classification, activity, and localization of Group VI PLA2s (iPLA2s)
The iPLA2s have a conserved C-terminal lipase consensus motif (GXSXG) and manifest catalytic activity in the absence of Ca2+. According to the current classification, iPLA2 products of different genes are designated as follows: VIA or iPLA2β (Table 1), VIB or iPLA2γ [22–26], VIC or iPLA2δ [27, 28], and VID or iPLA2ε, VIE or iPLA2ζ, and VIF or iPLA2η [29].
Table 1.
Isoforms of Group VIA PLA2
| VIA | aa | kDa | Ankyrin Repeats | ATP Binding (GXGXXG) | Lipase Activity (GXSXG) | Origin | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 752 | 84–85 | 8 | Yes | Yes | Alt Spl | [18, 20, 21, 30] |
| 2 | 806 | 88–90 | 7 | Yes | Yes | Alt Spl | [21, 31] |
| 3 | 640 | ~70 | 7–8 | Yes | Yes | Alt Spl | [31] |
| Ank-1 | 479 | ~53 | 7 | No | No | Alt Spl | [31] |
| Ank-2 | 427 | ~47 | 7 | No | No | Alt Spl | [31] |
| “4” | 623 | ~70 | 7 | Yes | Yes | PTM | [32, 33] |
| “5” | ~640 | ~70 | 8 | Yes | Yes | PTM | [34] |
A unique characteristic of the group VIA iPLA2s is the presence of 7–8 ankyrin N-terminal repeats that are not found in the other iPLA2s. The VIA-1 and VIA-2 are designated as the short and long form iPLA2β, where the long form is a product of alternatively spliced exon 8 that generates a protein containing a 54 amino acid insertion in the eighth ankyrin repeat. The Ank-1 and Ank-2 are truncated iPLA2β proteins that interact with full-length iPLA2β and suppress catalytic activity in a dominant negative fashion [31, 35, 36]. The VIA isoforms arising from post-translational modification (PTM), designated here as groups VIA-4 and VIA-5, are generated via cleavage of iPLA2β at the N-terminal by caspase-3 (VIA-4) [32, 33] and at the C-terminal by an unknown mechanism (VIA-5) [34] and are catalytically active.
In addition to a PLA2 activity, the iPLA2s exhibit lysophopholipase and transacylase activities [37] and the iPLA2β also expresses an acyl-CoA thioesterase activity [38, 39]. The group VIA iPLA2β is the most extensively studied iPLA2 and under basal conditions is predominantly localized in the cytosol but upon certain stimulation translocates to the nucleus [33, 40], ER [41–43], Golgi [42, 43], and mitochondria [44].
3. Structural Features of iPLA2β
The iPLA2β is encoded by mRNA species that yield a protein with an expected molecular mass of 84–88 kDa. Full-length iPLA2β protein consists of the lipase motif preceded by the eight N-terminal ankyrin-repeats [20, 21, 30]. Other salient features of the iPLA2β amino acid sequence include a caspase-3 cleavage site (DVTD*), an ATP-binding domain (GGGVKG), a serine lipase consensus sequence (GTSGT), a putative bipartite nuclear localization sequence (KREFGEHTKMTDVKKPK), a C- terminal 1-9-14 calmodulin-binding motif (IRKGQGNKVKKLSI), and a calmodulin-binding peptide (AWSEMVGIQYFR) [45–47]. The recent findings that certain stimuli promote translocation of iPLA2β to the ER and mitochondria [41, 44] suggest that additional targeting sequences, not yet identified, also reside within the iPLA2β.
4. Alternative splicing and post-translational modifications of iPLA2β
The iPLA2β gene undergoes a variety of alternative splicing events, generating variants that differ in their subcellular localization, catalytic activity, and likely cellular function [31, 36, 48]. Group VIA-1 iPLA2β is the “classic” 84–85 kDa isoform. The 88 kDa iPLA2β isoform is a product of an mRNA species that arises from an exon-skipping mechanism of alternate splicing [31] and contains a 54-amino acid sequence that interrupts the eighth ankyrin repeat. Two additional splice variants (Ank-1 and Ank-2) encode premature stop codons due to alternatively spliced exon 10a. The proteins encoded by these splice variants, group VIA Ank-1 and group VIA Ank-2, terminate after the ankyrin repeat domain but before the active site.
In addition to the 70 kDa isoforms that result from proteolytic cleavage of full-length iPLA2β, mass spectrometry analyses reveal that iPLA2β is a candidate for N-terminal modification and truncation [43, 49] (Song et al, under review). Though the mechanisms responsible for generating the iPLA2β protein variants or their role in biological processes have yet to be determined, these observations indicate that iPLA2β is a candidate for post-translational modification by NH2-terminal processing and that this might represent a means to regulate its activity, subcellular location, or interaction with other proteins.
5. Chemical and biological modulation of iPLA2β Activity
Chemical Inhibitors
Hazen et al. (1991) reported the synthesis of (E)-6-(bromomethylene) tetrahydro-3-(1-naphthalenyl)- 2H-pyran-2-one, designated initially as haloenol lactone suicide substrate (HELSS) [50] but referred to now as bromoenol lactone (BEL). This suicide inhibitor selectively targets iPLA2β and other group VI PLA2 enzymes and has little or no effect on cPLA2 or sPLA2 activity [29, 50, 51]. Although BEL treatment results in covalent modification of iPLA2β [50, 52], the modified residues are cysteines, and not the active site, likely due to a diffusible bromoketomethyl acid that is generated when iPLA2β acts on the inhibitor [53].
iPLA2β is also targeted by arachidonyl trifluoromethyl ketone (AACOCF3), methyl arachidonyl fluorophosphonate (MAFP), and palmitoyl trifluoromethyl ketone (PACOCF3), inhibitors that are sometimes used for “selective” inhibition of cPLA2 [52, 54]. This underscores the importance of pairing AACOCF3, PACOCF3, and MAFP experiments with BEL treatments to accurately assess involvement of specific PLA2s a given system.
Over the years, BEL has been used to discern the involvement of iPLA2 in biological processes and is still considered the only available specific irreversible inhibitor of iPLA2. Recently, the S- and R-enantiomers of BEL have been demonstrated to exhibit specific inhibition of iPLA2β and iPLA2γ, respectively [55]. However, several examples of inhibition of non-iPLA2 enzymes by BEL have been described [28, 29, 56–58] and the mechanism of inhibition does not appear to involve the active site of iPLA2 [53, 59]. In view of this, the now available mice genetically-modified to be deficient in or overexpress iPLA2β [60–62] are expected to significantly promote studies leading to further understanding of iPLA2 participation in various biological processes.
Biological regulators
ATP
The iPLA2β contains a consensus nucleotide binding motif (GXGXXG) that is homologous to those of the protein kinases [22, 46]. This feature likely mediates the well-established regulation and stabilization of iPLA2 activity by ATP [18, 37, 63, 64], which is independent of phosphorylation of the enzyme [18, 63, 64]. Although the ATP binding domain is not disrupted by the 54 amino acid insertion that distinguishes group VIA-1 from group VIA-2 iPLA2β, only VIA-2 is activated by ATP [21]. This is likely because the insertion is highly enriched in proline residues that can change conformation of the adjacent ATP binding site.
Phosphorylation
Several investigators have proposed that iPLA2 is activated downstream of serine-threonine protein kinases, including PKC and p38 kinases [26, 65–70]. However, there is little direct evidence for iPLA2β activation in response to phosphorylation. Although iPLA2 activity increases when the enzyme binds Ca2+/calmodulin dependent protein kinase βII (CaMKII) in pancreatic β-cells [71], there is no evidence for CaMKII-dependent phosphorylation of the enzyme.
Ca2+-Calmodulin
The iPLA2β is bound by calmodulin affinity columns [72], suggesting that the enzyme may be regulated by calcium-calmodulin complexes. Indeed, iPLA2 activity increases in Ca2+-depleted cells [73–75], consistent with the observation that calmodulin suppresses catalytic activity [76]. Negative regulation of iPLA2β by Ca2+/calmodulin complexes has important implications for activation of store-operated Ca2+ channels [74] and neutrophil activation in response to stress [77].
Caspase-mediated proteolysis
The iPLA2β has been implicated in apoptosis induced through both the intrinsic and extrinsic pathways [32, 33, 41, 44, 78, 79]. The executioner caspase, caspase-3, is common to both pathways and can cleave iPLA2β at Asp183, producing a 62–70 kDa-truncated enzyme that has enhanced iPLA2β catalytic activity [32, 33, 78]. Another group has suggested that the highly active iPLA2β is a 26 kDa protein, generated after processing at an additional caspase-3 cleavage site at Asp513, just upstream of the active site [80]. The truncated iPLA2β is responsible for the arachidonic acid release that occurs during apoptosis of U937 monocytes, in response to TNFα or anti-Fas [32, 78]. Caspase-processed iPLA2 also generates lysophosphatidylcholine (LPC), a chemoattractant that recruits monocytes and thereby promotes phagocytosis and clearance of apoptotic cells and lysophosphatidic acid (LPA), a survival factor that protects the cells against apoptosis [79, 80].
Oligomerization
Radiation inactivation studies indicate that active iPLA2β is a homotetramer [18], which indicates oligomerization is another mechanism for the regulation of iPLA2 activity. This is most likely due to the N-terminal ankyrin repeats, which can facilitate protein-protein interactions [81] and mediate oligomerization of iPLA2β into active tetramers [31, 48]. The truncated iPLA2β proteins encoded by VIA Ank-1 and group VIA Ank-2, terminate after the ankyrin repeat domain but before the active site. These proteins retain the ability to oligomerize with full-length monomers but are catalytically inactive. As a result, the proteins encoded by VIA Ank-1 and group VIA Ank-2 are endogenous dominant-negative proteins that oligomerize with full-length monomers and prevent the monomers from assembling into active oligomers [31, 36, 48].
It was reported earlier that oxidants inactivate iPLA2 by a mechanism involving oxidation of sulfhydryl groups within the iPLA2 [82]. Subsequently, Song et al. identified oligomerization of iPLA2β in INS-1 cells in response to oxidative stress [59]. Oxidant-induced oligomerization alters the subcellular localization of iPLA2β and results in reduced release of arachidonic acid, suggesting inhibition of iPLA2β catalytic activity. These non-productive oligomers are DTT-sensitive and therefore likely generated through intermolecular disulfide bonds. Like iPLA2β, iPLA2γ activity is also suppressed by oxidants, but restored when oxidant-inhibited enzyme is treated with reducing agent [82, 83]. Together, these studies indicate that iPLA2β monomers are capable of assembling into both productive and non-productive monomers. The productive oligomerization is mediated through the N-terminal ankyrin repeat domain while inactive oligomers are formed through intramolecular disulfide bonds.
Regulation of iPLA2β expression
While iPLA2β was once thought to be constitutively expressed, there is mounting evidence that its expression is regulated by a variety of stimuli. For example, iPLA2β expression is regulated by lipids and in response to changes in systemic lipid metabolism. The human iPLA2β gene contains a sterol response element. Sterol response element binding protein-2 (SREBP-2) binds this element and is likely the mechanism for iPLA2β induction in lipid-depleted cells [84]. Both iPLA2β and iPLA2γ expression increase during adipocyte differentiation, and these responses are required for adipocyte development [85]. Although the molecular mechanism for iPLA2β induction during adipogenesis is not yet certain, it may be mediated through PPARγ or FOXO4 transcription factors which have been linked to adipogenesis [86, 87] and have putative binding sites in the 5′ flanking region of the iPLA2β gene (unpublished observation). In addition, iPLA2β expression is regulated in the retina [88, 89], in the cerebral cortex and hippocampus [90], and in the myocardium of rats undergoing congestive heart failure [91] through mechanisms that have not yet been elucidated.
6. iPLA2β in cell survival versus apoptosis
A significant number of reports over the years from various laboratories, including ours [42, 43, 71, 92–99] indicate that iPLA2β has a prominent role in phospholipid remodeling, the maintenance of phosphatidylcholine (PC) mass, and signal transduction [78, 100–107]. Studies describing this function have escalated in the past five years and are now greatly facilitated by the availability of iPLA2β knockout [61, 108] and iPLA2β transgenic mice [60]. A list of biological processes in which iPLA2β participation has been described is provided in Table 2. For more details regarding the role of iPLA2β in these and other biological processes, the reader is referred to other recent reviews [15–17]. As the focus of this review is the role of iPLA2β in ®-cell apoptosis, we will focus on only one biological process in this section: the role of iPLA2β in cell survival vs. apoptosis.
Table 2.
Biological Processes in Which iPLA2β Participates
| Biological Process | Citation |
|---|---|
| Nerve Degeneration | [109, 110] |
| Insulin Secretion | [60, 111, 112] |
| Onset of Acute Pleurisy | [113, 114] |
| Neurotransmission in Hippocampus | [114, 115] |
| Diabetes- and Ischemia-Induced Arrhythmias | [116] |
| Impairment in Memory Acquisition | [54, 117] |
| Schizophrenia | [118, 119] |
| Muscle Degeneration | [120] |
| Skeletal Muscle Contractility | [121, 122] |
| HIV-Induced Cardiomyopathy | [122] |
| Drip Formation in Muscle | [123] |
| Photoreceptor Cell Renewal | [89] |
| Exfoliation Glaucoma | [124] |
| Bipolar Disorder and Neuroinflammation | [90, 125] |
| Infantile Neuroaxonal Dystrophy | [126–128] |
| Bone Formation | [129] |
| Skeletal Muscle Fatty Acid Oxidation | [38] |
| Chemotaxis | [130–132] |
| Store-Operated Ca2+ Entry | [133, 134] |
| Neurodegeneration Associated with Brain Iron Accumulation | [135] |
| Vascular smooth muscle cell contraction | [136] |
iPLA2β involvement in cell proliferation
Although iPLA2 has important roles in a variety of apoptotic responses in some systems [26, 32, 40, 41, 78, 79, 137–140], studies utilizing BEL have led to identification of a role for iPLA2β in proliferation in other systems. Addition of BEL to culture media decreases cell proliferation [141–144] and this is reversed by addition of arachidonic acid [143, 145]. Consistent with these observations, knock-down of iPLA2β suppresses and overexpression of iPLA2β accelerates proliferation of insulinoma cells [94, 99] and proliferation of vascular smooth muscle cells from iPLA2β-null mice is severely impaired but is reversed by addition of arachidonic acid or PGE2 [146]. Other studies suggest that iPLA2β is required for cell cycle progression [109, 140, 144, 145, 147], through both p53-dependent and independent mechanisms. The molecular mechanism whereby iPLA2β promotes cell cycle progression and proliferation remains unclear, but is likely to be related to bioactive lipid mediators that are generated by the enzyme. For example, the products of iPLA2β activity may activate genes involved in cell division [30, 144, 148–151]. Arachidonic acid and eicosanoids have been connected to iPLA2-dependent proliferation [143, 145]. Ovarian cancer cells produce lysophosphatidic acid (LPA) in an iPLA2-dependent manner and this potent mitogen acts in an autocrine fashion to induce proliferation and migration [144, 148]. The effect of iPLA2β on cell survival vs. apoptosis and its mechanism of action are likely to be cell type-specific and dependent on the spectrum of bioactive lipids that are generated by the enzyme.
iPLA2β involvement in apoptosis
Another recognized role of iPLA2β is its participation in programmed cell death (i.e. apoptosis; recently reviewed in [152]. An early indication for this was provided by Dr. Kenneth Polonsky’s group [153] who reported that apoptosis of mouse insulinoma cell line MIN6 due to ER stress induced by sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) inhibitors occurred via a mechanism that does not require an increase in the cytosolic Ca2+ concentration but that does require the generation of arachidonic acid 12-lipoxygenase products. These observations were soon followed by a report from the group of the late Dr. Ichiro Kudo that Fas-induced death of U937 cells was unaffected by inhibition of cPLA2 or sPLA2 but was delayed by inhibition of iPLA2β [78]. The same group subsequently demonstrated that induction of U937 cell apoptosis was associated with caspase-3-mediated cleavage of iPLA2β in the N-terminal region (DVTD183) that generated a more active truncated iPLA2β product [32]. These latter reports arising from Dr. Kudo’s group are the first demonstrations of a link between iPLA2β activation during the apoptotic process. Subsequently, several laboratories demonstrated that iPLA2β activation contributes to apoptosis of various cell system and their studies are summarized in Table 3.
Table 3.
Evidence for iPLA2β involvement in apoptosis
| Year | Citation | Stimulus | System |
|---|---|---|---|
| 1998 | [78] | Fas | Human leukemic monocyte lymphoma U937 cells |
| 2000 | [32] | Fas & TNF/CHX | Human leukemic monocyte lymphoma U937 cells |
| 2000 | [154] | Thapsigargin | T cell lymphoma S49 cells |
| 2002 | [155] | Polychlorinated biphenyls | Rat pheochromocytoma PC12 cells |
| 2002 | [139] | Polycyclic aromatic hydrocarbons | Human coronary artery endothelial cells |
| 2003 | [40] | Hypoxia | Rat pheochromocytoma PC12 cells & Mouse cerebellar granule neurons |
| 2004 | [138] | H2O2 | Human leukemic monocyte lymphoma U937 cells |
| 2005 | [156] | Chemotherapeutic drugs | Human renal cell models |
| 2005 | [157] | Cancer | Human cancer cells |
| 2005 | [158] | ROS generation | Non-synaptosomal brain mitochondria |
| 2006 | [159] | iPLA2 siRNA | Human epithelial cells (HEK 293 and Caki-1) |
| 2006 | [160] | Depolarization and Ca2+ accumulation | Rat liver mitochondria |
| 2006 | [161] | iPLA2β overexpression | Human leukemic monocyte lymphoma U937 cells |
| 2006–2008 | [140, 144, 162–164] | p53/p21 mediated cell cycle arrest and cell growth | Cancer cell lines |
| 2007 | [163] | Virus | Human hepatoma (Huh7) and breast adenocarcinoma (MCF7) |
| 2008 | [137] | Free cholesterol loading | Murine macrophages |
| 2009 | [165] | Extracellular ATP | Murine macrophages |
In contrast to these observations, other studies suggest that iPLA2β involvement is not crucial for the execution of apoptosis. For instance, while arachidonic release or iPLA2β activation occurred during apoptosis of S49 caused by thapsigargin [154], of human macrophages by Mycobacterium tuberculosis [166], or of cultured epithelial cells and fibroblasts by Pseudomonas aeruginosa [167], inhibition of iPLA2β with BEL did not suppress the apoptosis. In fact, some studies suggest that BEL treatment can actually induce apoptosis [57, 154]. However, in these studies the cells were exposed to BEL for up to 24h, which may allow its inhibition of non-iPLA2 proteins to come into play. Further, a recent study reported that androgen receptor activation of iPLA2 upregulates prostate specific antigen (PSA) expression and secretion and PSA via activation of the PI3K/Akt pathway provides a survival signal in prostate cancer cells [168]. It has also been reported that mitochondrial abnormalities promoted by increased generation of ROS and subsequent apoptosis are prevented by expression of iPLA2β, which facilitates repair of membrane phospholipids, in particular cardiolipins, which are susceptible to damage by ROS-mediated peroxidation [169].
Though a more active truncated iPLA2β generated by caspase-3-mediated cleavage of iPLA2β at the N-terminal region is proposed to amplify apoptosis [32], it has been reported that nuclear shrinkage and PC12 cell death due to hypoxia requires activation of iPLA2β but occurs via a caspase-independent pathway [40]. As noted above, caspase-cleaved iPLA2 generates LPC, arachidonic acid, and LPA [79, 80]. These bioactive lipids not only promote safe clearance of dying cells but are also potent mitogens that may protect against apoptosis [79, 80, 134, 170]. It is suggested that a 32 kDa product generated by caspase-mediated cleavage of iPLA2β at a site proximal to the lipase site (DLFD513) or 25/26 kDa fragments generated by truncation of the 32 kDa product at other putative caspase-consensus sequences in the C-terminal region (MVVD733, DCTD737, or RAVD744) facilitate generation of the “attraction signals” [79, 80, 134].
B. iPLA2β role in ®-cell apoptosis
1. ER Stress and β-cell apoptosis
The work by Polonsky and co-workers [153] demonstrated that insulinoma cells were sensitive to SERCA inhibitors. These agents deplete ER Ca2+ stores and this can lead to ER stress. Being a site for Ca2+ storage, the ER responds to various stimuli to release Ca2+ and is therefore extremely sensitive to changes in cellular Ca2+ homeostasis. In addition to being a storage site for cellular Ca2+, the ER is also the site where secretory proteins are synthesized, assembled, folded, and post-translationally modified. Interruption of these functions can lead to production of malfolded proteins that require rapid degradation. ER stress ensues when an imbalance occurs between the load of client proteins on the ER and the ER’s ability to process the load occurs, as when ER Ca2+ is depleted [171, 172]. Prolonged ER stress promotes induction of stress factors and activation of caspase-12, localized in ER [173–176], and can subsequently lead to downstream activation of caspase-3, a protease that is central to the execution of apoptosis [177].
The secretory function of β-cells endows them with a highly developed ER and heightens their susceptibility to ER stress. Thapsigargin, a widely used SERCA inhibitor [178] induces ER stress and promotes caspase-12 cleavage [175, 179] and apoptosis of neurons and insulin-releasing BRIN-BID11 cells [175] and Apaf-1 null cells [176]. While SERCA inhibitors promote loss of ER Ca2+ stores, induction of MIN-6 insulinoma cell apoptosis by these agents occurs by a pathway that does not require an increase in [Ca2+]i but instead requires the generation of arachidonic acid metabolites [153]. These findings were an early indication that ER stress-induced apoptosis may involve Ca2+-independent generation of arachidonic acid.
The likelihood that this process occurs in β-cells is enhanced by the fact that glucose-responsive insulinoma cells, pancreatic islets, and β-cells express iPLA2β and also contain an abundance of arachidonate-containing membrane phospholipids [2, 3, 20, 21, 34, 98]. Consistent with these features, thapsigargin-induced ER stress in pancreatic islets leads to hydrolysis of arachidonic acid from membrane phospholipids by a Ca2+-independent mechanism that is suppressed by BEL [73], supporting the possibility that ER stress in β-cells promotes iPLA2β activation.
2. iPLA2β involvement in β-cell apoptosis
Our lab has used the INS-1 insulinoma cell line, which behaves similarly to pancreatic islet β-cells [180], to address this possibility and elucidate the mechanism by which iPLA2β participates in β-cell apoptosis [33, 41, 44]. We found that thapsigargin induces ER stress in INS-1 cells, as evidenced by increases in ER stress factors GRP78/BiP, pPERK, and peIF2a. Prolonged ER stress activated the apoptotic process and this was associated with induction of ER stress apoptotic factor CHOP, activation of ER caspase-12, activation of apoptosis executioner caspase-3, and cleavage of PARP, which facilitates cellular disassembly. These events led to apoptotic INS-1 cell death, as reflected by DNA laddering and increased TUNEL staining. Pre-treatment of the cells with BEL suppressed apoptosis, suggesting that iPLA2β activation was involved in ER stress-induced apoptosis. A direct role of iPLA2β was examined next by overexpressing in INS-1 cells. Exposure of the iPLA2β-overexpressing INS-1 cells to thapsigargin significantly amplified the various outcomes described above, including apoptosis, and DNA laddering and TUNEL-positivity in these cells were inhibited by BEL.
To verify that the development of ER stress and subsequent apoptosis was not a unique property of insulinoma cells, we exposed human islets to thapsigargin and found that they also exhibit ER stress-induced apoptosis and that pretreatment of the human islets with BEL suppresses islet β-cell death (Lei et al. in review). These findings are taken to indicate that native pancreatic islets are susceptible to ER stress and that the process in islets also involves iPLA2β activation. Consistent with this, islets prepared from iPLA2β-KO mice were resistant and islets prepared from iPLA2β-Tg mice were more susceptible to ER stress-induced apoptosis (Lei et al., in preparation). That this was a specific effect in β-cells is suggested by the fact that the iPLA2β-Tg mice were generated using the Rat Insulin I Promoter (RIP) to drive iPLA2β overexpression and as such, iPLA2β expression was only increased in the β-cells [60].
The above-described findings were made in insulinoma cell and islet preparations that were treated with a chemical agent to induce ER stress or in which iPLA2β expression was genetically-modified. We therefore considered whether iPLA2β participation was necessary under conditions where ER stress developed in the β-cell in the absence of chemical intervention. To address this, we compared β-cell lines generated from wild type (WT) and Akita mice. The Akita mouse contains a spontaneous mutation in the insulin-2 gene that results in insulin misfolding and leads to development of diabetes due to ER stress-induced β-cell apoptosis [181, 182]. Consistent with their pre-disposition to developing ER stress, basal pPERK and activated caspase-3 are higher in the Akita cells [183]. Interestingly, basal iPLA2β is higher in the Akita cells, relative to WT cells and exposure to thapsigargin induces expression in both the Akita and WT cells. These findings are taken as further proof of iPLA2β involvement during the development and progression of ER stress in the β-cell. In support of these observations in the cultured Akita cell lines, expression of iPLA2β is markedly increased in islets from Akita mice, relative to WT mouse islets. Collectively, these findings suggest that increases in iPLA2β expression and activity during ER stress are not unique to INS-1 cells or artifacts of overexpressing iPLA2β in INS-1 cells or native pancreatic islet β-cells but that they are indeed evident in spontaneous models of ER stress.
3. Evidence for iPLA2β-induced ceramide generation in β-cell apoptosis
An unexpected finding associated with induction of ER stress in INS-1 cell apoptosis was an increase in ceramide generation that was significantly amplified in iPLA2β-overexpressing INS-1 cells [33]. Ceramides are lipid messengers that can suppress cell growth and induce apoptosis [184–186] and they can be generated via multiple pathways. Interestingly, ceramide accumulation in INS-1 cells during ER stress was not associated with changes in mRNA levels of serine palmitoyl-transferase, the rate limiting enzyme in de novo synthesis of ceramides [41]. However, both message and protein levels of neutral sphingomyelinase (NSMase), which hydrolyzes sphingomyelins to generate ceramides, were temporally increased in the INS-1 cells. This was reflected by increased hydrolysis of sphingomyelins and increased generation of ceramides in the INS-1 cells undergoing prolonged ER stress. The increases in NSMase expression in the ER-stressed INS-1 cells were associated with corresponding temporal elevations in ER-associated iPLA2β protein and catalytic activity and pretreatment with BEL prevented induction of NSMase message and protein.
Relative to control INS-1 cells, the effects of ER stress were accelerated and/or amplified in iPLA2β overexpressing INS-1 cells [41]. However, inhibition of iPLA2β or NSMase (chemically or with siRNA) suppressed induction of NSMase message, ceramide generation, sphingomyelin hydrolysis, and apoptosis in both control and iPLA2β-overexpressing INS-1 cells during ER stress. In contrast, inhibition of serine palmitoyltransferase did not suppress ceramide generation or apoptosis in either control or iPLA2β overexpressing INS-1 cells. These findings indicate that iPLA2β activation participates in ER stress-induced INS-1 cell apoptosis by promoting ceramide generation via NSMase-catalyzed hydrolysis of sphingomyelins, raising the possibility that this pathway contributes to β-cell apoptosis due to ER stress. This is in contrast to the contribution of the de novo pathway to lipoapoptosis of β-cells in ZDF rats [187] or of pancreatic islets exposed to free fatty acids [188, 189]. Ongoing studies indicate that NSMase expression is increased in the Akita islet β-cells and thapsigargin-treated iPLA2β-Tg mouse or native human pancreatic islets, relative to corresponding controls.
To determine if the same ceramide-generating mechanism is expressed and is activated in the ER or mitochondria during ER stress, ER and mitochondrial fractions prepared from INS-1 cells were analyzed by mass spectrometry. Such analyses revealed that ER stress induces ceramide generation and sphingomyelin hydrolysis in both the ER and mitochondrial fractions of INS-1 cells. Although the ER and mitochondrial fractions were not completely free of plasma membrane contamination, it is not totally unexpected that both the ER and the mitochondria are capable of generating ceramides via sphingomyelin hydrolysis. The membranes of the nucleus and ER are contiguous [190, 191] and they both express NSMase [192, 193]. Purified mitochondria from cells exposed to various agents have increased ceramide levels [194] and the contribution of mitochondrial sphingomyelin hydrolysis to ceramide generation and apoptosis has been demonstrated in other studies [195, 196]. These observations suggest that the ER and mitochondria express components of sphingolipid metabolism and raise the possibility that these organelles may also contribute to the generation of ceramides via sphingomyelin hydrolysis during apoptosis.
4. Evidence for iPLA2β-induced ceramide generation leading to the mitochondrial pathway of apoptosis
Although ER stress alone can induce the necessary factors to cause apoptosis, it is becoming increasingly apparent that the mitochondria, as an organelle that sequesters Ca2+ released from the ER, plays an important role in supporting the apoptosis process initiated by ER stress [197, 198]. Recent studies in our lab [44] reveal that both caspase-12 and caspase-3 are activated in INS-1 cells following induction of ER stress with thapsigargin, but only caspase-3 cleavage is amplified in iPLA2β overexpressing INS-1 cells, relative to control cells, and is suppressed by iPLA2β inhibition. Unexpectedly, ER stress also promoted the release of cytochrome c and Smac and their accumulation in the cytosol is amplified in iPLA2β-overexpressing cells. These findings raise the likelihood that iPLA2β participates in ER stress-induced apoptosis by activating the intrinsic apoptotic pathway.
Several lines of study support a link between iPLA2β and mitochondria during apoptosis. The work of Brustovetsky et al. [158] raised the possibility that truncated BID and BAX activate ROS generation, leading to iPLA2β activation in the mitochondria, which promotes changes in the outer mitochondrial membrane (OMM) and release of mitochondrial apoptotic factors into the cytosol [158]. Similarly, mitochondrial-associated iPLA2β was suggested to be activated during energy-dependent Ca2+ accumulation leading to opening of the permeability transition pore with sustained activation of iPLA2β leading to rupture of the OMM and release of cytochorome c into the cytosol [160]. While a precise mechanism connecting iPLA2β activation with mitochondrial abnormalities was not elaborated in these studies, it has been suggested that iPLA2β-mediated generation of AA causes disruption of membrane integrity [157].
Consistent with this possibility, we find that ER stress promotes iPLA2β accumulation in the mitochondria, opening of mitochondrial permeability transition pore, and loss in mitochondrial membrane potential in INS-1 cells and that these changes are amplified in iPLA2β overexpressing cells. These ER stress-induced mitochondrial abnormalities and apoptosis are suppressed by inactivation of iPLA2β or NSMase. These data suggest that iPLA2β triggers mitochondrial abnormalities through the generation of ceramides via sphingomyelin hydrolysis during ER stress. In support, inhibition of iPLA2β or NSMase prevents cytochrome c release. Taken together, our findings indicate that the iPLA2β-ceramide axis plays a critical role in activating the mitochondrial apoptotic pathway in insulin-secreting cells during ER stress (summarized in Fig. 1). Interestingly, in contrast to our findings and those of the others [157, 158, 160], Seleznev et al. reported that staurosporine-induced generation of ROS in the mitochondria and apoptosis of INS-1 cells is suppressed by overexpression of iPLA2β. They suggest that staurosporine-mediated down-regulation of iPLA2β results in the loss of mitochondrial membrane repair and that this leads to mitochondrial failure and apoptosis. These contrasting findings suggest that different stimuli may activate different apoptotic pathways in the same or different cell systems.
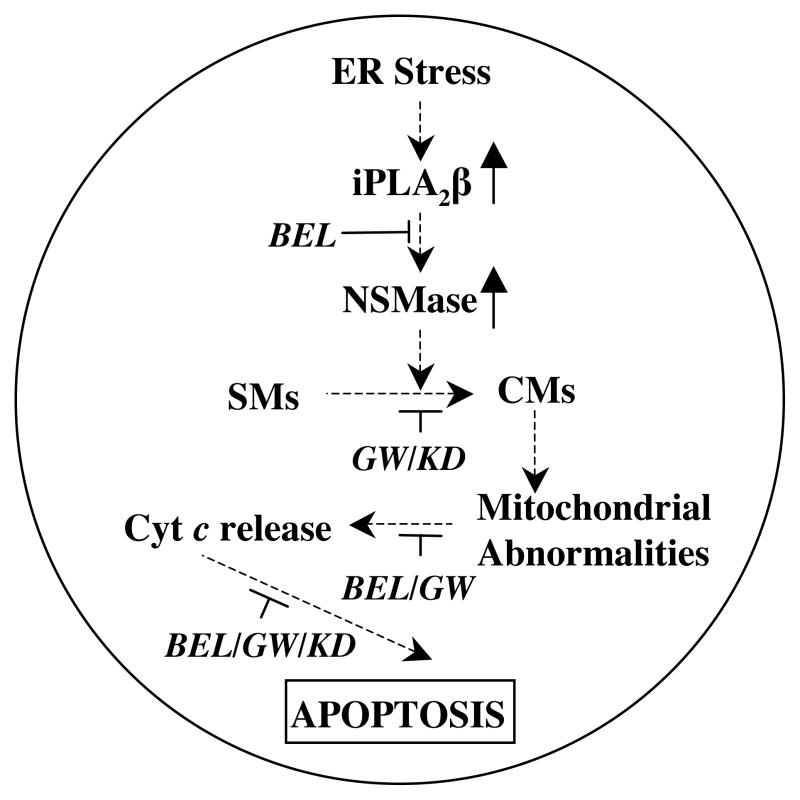
Figure 1. Role of iPLA2β and ceramides in ER stress-induced β-cell apoptosis.
Proposed mechanism of iPLA2β and ceramide involvement in ER stress-induced β-cell apoptosis. ER stress in β-cells leads to activation of iPLA2β and this induces neutral sphingomyelinase (NSMase), which promotes generation of ceramides (CMs) via hydrolysis of sphingomyelins (SMs). The ceramides cause mitochondrial membrane depolarization, opening of mitochondrial permeability transition pore, and release of cytochrome c. Accumulation of cytochrome c in the cytosol leads to activation of caspases and causes apoptosis of the β-cell. (BEL, bromoenol lactone suicide inhibitor of iPLA2β; GW, GW4869 inhibitor of NSMase; KD, knock-down of NSMase with siRNA.)
C. Summary and conclusions
Diabetes mellitus is the most prevalent human metabolic disease, and it results from loss and/or dysfunction of β-cells in pancreatic islets. Type 2 diabetes mellitus (T2DM) results from a progressive decline of β-cell function and chronic insulin resistance. Autopsy studies indicate that β-cell mass in obese T2DM patients is smaller than in obese non-diabetic subjects and that the decrease is not due to reduced β-cell proliferation or neogenesis but to increased β-cell apoptosis [199]. Type 1 diabetes mellitus (T1DM) is caused by autoimmune β-cell destruction and apoptosis plays a prominent role in β-cells loss during its development and cytokine-mediated β-cell apoptosis is a recognized contributor to β-cell death during the development of T1DM [200]. It is therefore important to understand the mechanisms underlying β-cell apoptosis if this process is to be prevented or delayed.
β-cell apoptosis can be mediated not only via death receptors residing in the plasma membrane and/or mitochondrial signaling but as a consequence of prolonged ER stress. A third organelle gaining recognition as a participant in apoptosis is the endoplasmic reticulum (ER) [179]. A number of factors can induce ER stress and this process is thought to cause various diseases, including Alzheimer’s and Parkinson’s [201]. β-cell death in the Akita [202] and NOD.k iHEL nonimmune [203] diabetic mouse models are reported to be due to ER stress. Further, mutations in genes encoding the ER-stress transducer pancreatic ER kinase (PERK) [204] and the ER resident protein involved in degradation of malfolded ER proteins have been linked to diminished β-cell health clinically [205, 206]. As the secretory function of β-cells endows them with a highly developed ER and the β-cell is one of the most sensitive cells to nitric oxide (NO) [207], it is not unexpected that β-cells exhibit a heightened susceptibility to autoimmune-mediated ER stress. In support of this, Wolfram syndrome, which is associated with juvenile-onset diabetes mellitus, is proposed to be a consequence of chronic ER stress in pancreatic β-cells [208].
Phospholipases A2 serve an important function in cells by providing lipid mediators (i.e. arachidonic acid) that subsequently participate in a variety of biological processes, including influencing cell survival. Among the PLA2s is the iPLA2β, and our work has revealed that prolonged activation of this enzyme triggers mitochondrial abnormalities that subsequently cause β-cell apoptosis and that this occurs by a novel mechanism involving iPLA2β-dependent ceramide generation via sphingomyelin hydrolysis. Continued studies to understand the role of iPLA2β in β-cell apoptosis will enable us to more precisely define its contribution to the onset and progression of diabetes. Findings from such studies will further our knowledge of factors that influence β-cell health in diabetes mellitus and identify potential targets for future therapeutic interventions to prevent β-cell death.
Acknowledgments
The authors would like to thank the expert technical assistance of Ms. Sheng Zhang, Dr. Mary Mueller, and Mr. Alan Bohrer for contributing to the work presented here. We would also like to thank Dr. John Turk (Washington University School of Medicine, St. Louis, MO) for providing the iPLA2β-null and iPLA2β-Tg mice and the ICR Basic Science Islet Distribution Program and Washington University/Juvenile Diabetes Research Foundation (Award no. 31-2008-382 to Dr. Thalachallour Mohanakumar) for providing (to approved user SR) the human islets used in the studies. The work was supported, in whole or in part, by grants from the National Institutes of Health (R01-DK69455, R37-DK34388, P41-RR00954, P60-DK20579, P30-DK56341), and The American Diabetes association (to SR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freysz L, Bieth R, Judes C, Sensenbrenner J, Jacob M, Mandel P. Quantitative distribution of phospholipids in neurons and glial cells isolated from rat cerebral cortex. J Neurochem. 1968;15:307–313. doi: 10.1111/j.1471-4159.1968.tb11615.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramanadham S, Bohrer A, Gross RW, Turk J. Mass spectrometric characterization of arachidonate-containing plasmalogens in human pancreatic islets and in rat islet beta-cells and subcellular membranes. Biochemistry. 1993;32:13499–13509. doi: 10.1021/bi00212a015. [DOI] [PubMed] [Google Scholar]
- 3.Ramanadham S, Bohrer A, Mueller M, Jett P, Gross RW, Turk J. Mass spectrometric identification and quantitation of arachidonate-containing phospholipids in pancreatic islets: prominence of plasmenylethanolamine molecular species. Biochemistry. 1993;32:5339–5351. doi: 10.1021/bi00071a009. [DOI] [PubMed] [Google Scholar]
- 4.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 5.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 6.Gijon MA, Leslie CC. Phospholipases A2. Semin Cell Dev Biol. 1997;8:297–303. doi: 10.1006/scdb.1997.0151. [DOI] [PubMed] [Google Scholar]
- 7.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system, Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Ann Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prgr Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kramer R, Hession C, Johansen B, Hayes G, McGray P, Chow E, Tizard R, Pepinsky R. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 12.Seilhamer J, Pruzanski W, Vadas P, Plant S, Miller J, Kloss J, Johnson L. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 13.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 14.Kramer R, Roberts E, Manetta J, Putnam J. The Ca2+-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991;266:5268–5272. [PubMed] [Google Scholar]
- 15.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hooks SB, Cummings BS. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol. 2008;76:1059–1067. doi: 10.1016/j.bcp.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkins WP, 3rd, Barbour SE. Group VI phospholipases A2: homeostatic phospholipases with significant potential as targets for novel therapeutics. Curr Dr Targets. 2008;9:683–697. doi: 10.2174/138945008785132385. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann E, Kempner E, Dennis E. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 19.Balboa MA, Balsinde J, Jones SS, Dennis EA. Identity between the Ca2+-independent phospholipase A2 enzymes from P388D1 macrophages and Chinese hamster ovary cells. J Biol Chem. 1997;272:8576–8580. doi: 10.1074/jbc.272.13.8576. [DOI] [PubMed] [Google Scholar]
- 20.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J Biol Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 21.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Takeya R, Sumimoto H. A novel intracellular membrane-bound calcium-independent phospholipase A2. Biochemical and Biophysical Research Communications. 2000;272:320–326. doi: 10.1006/bbrc.2000.2776. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Han X, Gross RW. Identification of hepatic peroxisomal phospholipase A2 and characterization of arachidonic acid-containing choline glycerophospholipids in hepatic peroxisomes. FEBS Letters. 2003;546:247–250. doi: 10.1016/s0014-5793(03)00581-7. [DOI] [PubMed] [Google Scholar]
- 25.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in oxidant-induced renal cell death. Am J Physiol Renal Physiol. 2002;283:F492–498. doi: 10.1152/ajprenal.00022.2002. [DOI] [PubMed] [Google Scholar]
- 26.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther. 2004;308:921–928. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- 27.Glynn P. Neuropathy target esterase and phospholipid deacylation, Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2005;1736:87–93. doi: 10.1016/j.bbalip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.van Tienhoven M, Atkins J, Li Y, Glynn P. Human neuropathy target esterase catalyzes hydrolysis of membrane lipids. J Biol Chem. 2002;277:20942–20948. doi: 10.1074/jbc.M200330200. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 30.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 31.Larsson PKA, Claesson HE, Kennedy BP. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Atsumi G, Murakami M, Kojima K, Hadano A, Tajima M, Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2α inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J Biol Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 33.Ramanadham S, Hsu FF, Zhang S, Jin C, Bohrer A, Song H, Bao S, Ma Z, Turk J. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2β) and suppressed by inhibition of iPLA2β. Biochemistry. 2004;43:918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramanadham S, Song H, Hsu FF, Zhang S, Crankshaw M, Grant GA, Newgard CB, Bao S, Ma Z, Turk J. Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2 (iPLA2β) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2β transcript. Biochemistry. 2003;42:13929–13940. doi: 10.1021/bi034843p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poulsen KA, Pedersen SF, Kolko M, Lambert IH. Induction of group VIA phospholipase A2 activity during in vitro ischemia in C2C12 myotubes is associated with changes in the level of its splice variants. Am J Physiol Cell Physiol. 2007;293:C1605–1615. doi: 10.1152/ajpcell.00012.2007. [DOI] [PubMed] [Google Scholar]
- 36.Manguikian AD, Barbour SE. Cell Cycle Dependence of Group VIA Calcium-independent Phospholipase A2 Activity. J Biol Chem. 2004;279:52881–52892. doi: 10.1074/jbc.M410659200. [DOI] [PubMed] [Google Scholar]
- 37.Lio YC, Dennis EA. Interfacial activation, lysophospholipase and transacylase activity of group VI Ca2+-independent phospholipase A2. Biochim Biophys Acta. 1998;1392:320–332. doi: 10.1016/s0005-2760(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 38.Carper MJ, Zhang S, Turk J, Ramanadham S. Skeletal muscle group VIA phospholipase A2 (iPLA2β): Expression and role in fatty acid oxidation. Biochemistry. 2008;47:12241–12249. doi: 10.1021/bi800923s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2β: Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J Biol Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 40.Shinzawa K, Tsujimoto Y. PLA2 activity is required for nuclear shrinkage in caspase-independent cell death. J Cell Biol. 2003;163:1219–1230. doi: 10.1083/jcb.200306159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao S, Jin C, Zhang S, Turk J, Ma Z, Ramanadham S. {beta}-Cell calcium-independent group VIA phospholipase A2 (iPLA2β): Tracking iPLA2β movements in response to stimulation with insulin secretagogues in INS-1 cells. Diabetes. 2004;53:S186–189. doi: 10.2337/diabetes.53.2007.s186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramanadham S, Song H, Bao S, Hsu FF, Zhang S, Ma Z, Jin C, Turk J. Islet complex lipids: Involvement in the actions of group VIA calcium-independent phospholipase A2 in β-cells. Diabetes. 2004;53:S179–185. doi: 10.2337/diabetes.53.2007.s179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2β. Implications for structure and function. J Biol Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 46.Ma Z, Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog Nucleic Acid Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 47.Turk J, Ramanadham S. The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2β) in beta-cells. Can J Physiol Pharmacol. 2004;82:824–832. doi: 10.1139/y04-064. [DOI] [PubMed] [Google Scholar]
- 48.Forsell PKAL, Kennedy BP, Claesson HE. The human calcium-independent phospholipase A2 gene. Eur J Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 49.Song H, Hecimovic S, Goate A, Hsu FF, Bao S, Vidavsky I, Ramanadham S, Turk J. Characterization of N-Terminal processing of group VIA phospholipase A2 and of potential cleavage sites of amyloid precursor protein constructs by automated identification of signature peptides in LC/MS/MS analyses of proteolytic digests. J Amer Soc Mass Spec. 2004;15:1780–1793. doi: 10.1016/j.jasms.2004.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazen S, Zupan L, Weiss R, Getman D, Gross R. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium- dependent and -independent phospholipases A2. J Biol Chem. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 51.Ma Z, Ramanadham S, Hu Z, Turk J. Cloning and expression of a group IV cytosolic Ca2+-dependent phospholipase A2 from rat pancreatic islets. Comparison of the expressed activity with that of an islet group VI cytosolic Ca2+-independent phospholipase A2. Biochim Biophys Acta. 1998;1391:384–400. doi: 10.1016/s0005-2760(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 52.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 53.Song H, Ramanadham S, Bao S, Hsu FF, Turk J. A bromoenol lactone suicide substrate inactivates group VIA phospholipase A2 by generating a diffiusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry. 2006;45:1061–1073. doi: 10.1021/bi052065q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaeffer EL, Gattaz WF. Inhibition of calcium-independent phospholipase A2 activity in rat hippocampus impairs acquisition of short- and long-term memory. Psychopharmacology (Berl) 2005;181:392–400. doi: 10.1007/s00213-005-2256-9. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2β), and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J Biol Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- 56.Daniels S, Cooney E, Sofia M, Chakravarty P, Katzenellenbogen J. Haloenol lactones. Potent enzyme-activated irreversible inhibitors for alpha-chymotrypsin. J Biol Chem. 1983;258:15046–15053. [PubMed] [Google Scholar]
- 57.Fuentes L, Perez R, Nieto ML, Balsinde J, Balboa MA. Bromoenol lactone promotes cell death by a mechanism involving phosphatidate phosphohydrolase-1 rather than calcium-independent phospholipase A2. J Biol Chem. 2003;278:44683–44690. doi: 10.1074/jbc.M307209200. [DOI] [PubMed] [Google Scholar]
- 58.Balsinde J, Dennis EA. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J Biol Chem. 1996;271:31937–31941. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- 59.Song H, Bao S, Ramanadham S, Turk J. Effects of biological oxidants on the catalytic activity and structure of group VIA phospholipase A2. Biochemistry. 2006;45:6392–6406. doi: 10.1021/bi060502a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao S, Jacobson DA, Wohltmann M, Bohrer A, Jin W, Philipson LH, Turk J. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic β-cells and in iPLA2β-null mice. Am J Physiol Endocrinol Metab. 2008;294:E217–229. doi: 10.1152/ajpendo.00474.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, Yang J, Yan W, Yang K, Green K, Abendschein DR, Saffitz JE, Gross RW. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J Biol Chem. 2007;282:9216–9227. doi: 10.1074/jbc.M607307200. [DOI] [PubMed] [Google Scholar]
- 63.Hazen S, Gross R. ATP-dependent regulation of rabbit myocardial cytosolic calcium- independent phospholipase A2. J Biol Chem. 1991;266:14526–14534. [PubMed] [Google Scholar]
- 64.Hazen SL, Gross RW. Human myocardial cytosolic Ca2+-independent phospholipase A2 is modulated by ATP. Concordant ATP-induced alterations in enzyme kinetics and mechanism-based inhibition. Biochem J. 1991;280:581–587. doi: 10.1042/bj2800581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akiba S, Ohno S, Chiba M, Kume K, Hayama M, Sato T. Protein kinase Cα-dependent increase in Ca2+-independent phospholipase A2 in membranes and arachidonic acid liberation in zymosan-stimulated macrophage-like P388D1 cells. Biochem Pharmacol. 2002;63:1969–1977. doi: 10.1016/s0006-2952(02)00988-7. [DOI] [PubMed] [Google Scholar]
- 66.Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2005;288:C475–482. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- 67.Steer SA, Wirsig KC, Creer MH, Ford DA, McHowat J. Regulation of membrane-associated iPLA2 activity by a novel PKC isoform in ventricular myocytes. Am J Physiol Cell Physiol. 2002;283:C1621–1626. doi: 10.1152/ajpcell.00109.2002. [DOI] [PubMed] [Google Scholar]
- 68.Tay HK, Melendez AJ. Fc{gamma}RI-triggered Generation of Arachidonic Acid and Eicosanoids Requires iPLA2 but Not cPLA2 in Human Monocytic Cells. J Biol Chem. 2004;279:22505–22513. doi: 10.1074/jbc.M308788200. [DOI] [PubMed] [Google Scholar]
- 69.Yellaturu CR, Rao GN. A Requirement for Calcium-independent Phospholipase A2 in Thrombin-induced Arachidonic Acid Release and Growth in Vascular Smooth Muscle Cells. J Biol Chem. 2003;278:43831–43837. doi: 10.1074/jbc.M301472200. [DOI] [PubMed] [Google Scholar]
- 70.Aoto M, Shinzawa K, Suzuki Y, Ohkubo N, Mitsuda N, Tsujimoto Y. Essential role of p38 MAPK in caspase-independent, iPLA2-dependent cell death under hypoxia/low glucose conditions. FEBS Letters. 2009;583:1611–1618. doi: 10.1016/j.febslet.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Ramanadham S, Ma ZA, Bao S, Mancuso DJ, Gross RW, Turk J. Group VIA phospholipase A2 forms a signaling complex with the calcium/calmodulin-dependent protein kinase IIβ expressed in pancreatic islet β-cells. J Biol Chem. 2005;280:6840–6849. doi: 10.1074/jbc.M405287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf MJ, Gross RW. Expression, Purification, and Kinetic Characterization of a Recombinant 80-kDa Intracellular Calcium-independent Phospholipase A2. J Biol Chem. 1996;271:30879–30885. doi: 10.1074/jbc.271.48.30879. [DOI] [PubMed] [Google Scholar]
- 73.Nowatzke W, Ramanadham S, Ma Z, Hsu FF, Bohrer A, Turk J. Mass spectrometric evidence that agents that cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology. 1998;139:4073–4085. doi: 10.1210/endo.139.10.6225. [DOI] [PubMed] [Google Scholar]
- 74.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 75.Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- 76.Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. Implications for cardiac cycle-dependent alterations in phospholipolysis. J Biol Chem. 1996;271:20989–20992. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- 77.Zhang D, Shooshtarizadeh P, Laventie BJ, Colin DA, Chich JF, Vidic J, de Barry J, Chasserot-Golaz S, Delalande F, Van Dorsselaer A, Schneider F, Helle K, Aunis D, Prévost G, Metz-Boutigue MH. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One. 2009;4:e4501. doi: 10.1371/journal.pone.0004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, Wang D, Zhao Z, Xiao Y, Sengupta S, Zhang R, Lauber K, Wesselborg S, Feng L, Rose TM, Shen Y, Zhang J, Prestwich G, Xu Y. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J Biol Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 80.Lauber K, Bohn E, K̂ber SM, Xiao Y-j, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 81.Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Tr Biochem, Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- 82.Cummings BS, Gelasco AK, Kinsey GR, Mchowat J, Schnellmann RG. Inactivation of Endoplasmic Reticulum Bound Ca2+-Independent Phospholipase A2 in Renal Cells during Oxidative Stress. J, Am, Soc, Nephrol. 2004;15:1441–1451. doi: 10.1097/01.asn.0000127923.57438.ec. [DOI] [PubMed] [Google Scholar]
- 83.McHowat J, Swift LM, Arutunyan A, Sarvazyan N. Clinical Concentrations of Doxorubicin Inhibit Activity of Myocardial Membrane-associated, Calcium-independent Phospholipase A2. Cancer Res. 2001;61:4024–4029. [PubMed] [Google Scholar]
- 84.Seashols SJ, del Castillo Olivares A, Gil G, Barbour SE. Regulation of group VIA phospholipase A2 expression by sterol availability, Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2004;1684:29–37. doi: 10.1016/j.bbalip.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Su X, Mancuso DJ, Bickel PE, Jenkins CM, Gross RW. Small Interfering RNA Knockdown of Calcium-independent Phospholipases A2β or γ Inhibits the Hormone-induced Differentiation of 3T3-L1 Preadipocytes. J Biol Chem. 2004;279:21740–21748. doi: 10.1074/jbc.M314166200. [DOI] [PubMed] [Google Scholar]
- 86.Wang B, Zhu J, Mounzih K, Chehab EF, Ke Y, Chehab FF. Overexpression of the transcription factor foxo4 is associated with rapid glucose clearance. Mol Cell Endocrinol. 2009;307:217–223. doi: 10.1016/j.mce.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 87.Park KW, Halperin DS, Tontonoz P. Before they were fat: Adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Anfuso CD, Lupo G, Romeo L, Giurdanella G, Motta C, Pascale A, Tirolo C, Marchetti B, Alberghina M. Endothelial cell-pericyte cocultures induce PLA2 protein expression through activation of PKCα and the MAPK/ERK cascade. J Lipid Res. 2007;48:782–793. doi: 10.1194/jlr.M600489-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Kolko M, Wang J, Zhan C, Poulsen KA, Prause JU, Nissen MH, Heegaard S, Bazan NG. Identification of intracellular phospholipases A2 in the human eye: Involvement in phagocytosis of photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2007;48:1401–1409. doi: 10.1167/iovs.06-0865. [DOI] [PubMed] [Google Scholar]
- 90.Aid S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca2+-independent phospholipase A2 is altered in rat hippocampus during normal aging. Br Res Bull. 2007;73:108–113. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McHowat J, Tappia PS, Liu SY, McCrory R, Panagia V. Redistribution and abnormal activity of phospholipase A2 isoenzymes in postinfarct congestive heart failure. Am J Physiol Cell Physiol. 2001;280:C573–580. doi: 10.1152/ajpcell.2001.280.3.C573. [DOI] [PubMed] [Google Scholar]
- 92.Gross RW, Ramanadham S, Kruszka KK, Han X, Turk J. Rat and human pancreatic islet cells contain a calcium ion independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by adenosine triphosphate and is specifically localized to islet beta-cells. Biochemistry. 1993;32:327–336. doi: 10.1021/bi00052a041. [DOI] [PubMed] [Google Scholar]
- 93.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2β) indicate a signaling rather than a housekeeping role for iPLA2β. J Biol Chem. 2001;276:13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- 94.Ma Z, Bohrer A, Wohltmann M, Ramanadham S, Hsu FF, Turk J. Studies of phospholipid metabolism, proliferation, and secretion of stably transfected insulinoma cells that overexpress group VIA phospholipase A2. Lipids. 2001;36:689–700. doi: 10.1007/s11745-001-0774-9. [DOI] [PubMed] [Google Scholar]
- 95.Ma Z, Zhang S, Turk J, Ramanadham S. Stimulation of insulin secretion and associated nuclear accumulation of iPLA2β in INS-1 insulinoma cells. Am J Physiol Endocrinol Metab. 2002;282:E820–833. doi: 10.1152/ajpendo.00165.2001. [DOI] [PubMed] [Google Scholar]
- 96.Ramanadham S, Gross RW, Han X, Turk J. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta-cell cytosolic calcium ion concentration. Biochemistry. 1993;32:337–346. doi: 10.1021/bi00052a042. [DOI] [PubMed] [Google Scholar]
- 97.Ramanadham S, Hsu FF, Bohrer A, Ma Z, Turk J. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J Biol Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- 98.Ramanadham S, Wolf MJ, Li B, Bohrer A, Turk J. Glucose-responsitivity and expression of an ATP-stimulatable, Ca2+-independent phospholipase A2 enzyme in clonal insulinoma cell lines. Biochim Biophys Acta. 1997;1344:153–164. doi: 10.1016/s0005-2760(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 99.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem. 2006;281:187–198. doi: 10.1074/jbc.M509105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Isenovic E, LaPointe MC. Role of Ca2+-independent phospholipase A2 in the regulation of inducible nitric oxide synthase in cardiac myocytes. Hypertension. 2000;35:249–254. doi: 10.1161/01.hyp.35.1.249. [DOI] [PubMed] [Google Scholar]
- 102.Maggi LB, Jr, Moran JM, Scarim AL, Ford DA, Yoon JW, McHowat J, Buller RML, Corbett JA. Novel role for calcium-independent phospholipase A2 in the macrophage antiviral response of inducible nitric-oxide synthase expression. J Biol Chem. 2002;277:38449–38455. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 103.Moran JM, Buller RML, McHowat J, Turk J, Wohltmann M, Gross RW, Corbett JA. Genetic and pharmacologic evidence that calcium-independent phospholipase A2β regulates virus-induced inducible nitric-oxide synthase expression by macrophages. J Biol Chem. 2005;280:28162–28168. doi: 10.1074/jbc.M500013200. [DOI] [PubMed] [Google Scholar]
- 104.Tithof PK, Olivero J, Ruehle K, Ganey PE. Activation of neutrophil calcium-dependent and -independent phospholipases A2 by organochlorine compounds. Toxicol Sci. 2000;53:40–47. doi: 10.1093/toxsci/53.1.40. [DOI] [PubMed] [Google Scholar]
- 105.Williams SD, Ford DA. Calcium-independent phospholipase A2 mediates CREB phosphorylation and c-fos expression during ischemia. Am J Physiol Heart Circ Physiol. 2001;281:H168–176. doi: 10.1152/ajpheart.2001.281.1.H168. [DOI] [PubMed] [Google Scholar]
- 106.Tithof PK, Peters-Golden M, Ganey PE. Distinct phospholipases A2 regulate the release of arachidonic acid for eicosanoid production and superoxide anion generation in neutrophils. J Immunol. 1998;160:953–960. [PubMed] [Google Scholar]
- 107.Derrickson BH, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through Gq/G11 and the calcium-independent phospholipase A2. Am J Physiol Renal Physiol. 1997;272:F781–788. doi: 10.1152/ajprenal.1997.272.6.F781. [DOI] [PubMed] [Google Scholar]
- 108.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group via phospholipase A2 deficiency in mice: A model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berti-Mattera LN, Harwalkar S, Hughes B, Wilkins PL, Almhanna K. Proliferative and morphological effects of endothelins in Schwann cells: roles of p38 mitogen-activated protein kinase and Ca2+-independent phospholipase A2. J Neurochem. 2001;79:1136–1148. doi: 10.1046/j.1471-4159.2001.00642.x. [DOI] [PubMed] [Google Scholar]
- 110.Mendes CT, Gattaz WF, Schaeffer EL, Forlenza OV. Modulation of phospholipase A2 activity in primary cultures of rat cortical neurons. J Neural Transm. 2005;112:1297–1308. doi: 10.1007/s00702-004-0271-3. [DOI] [PubMed] [Google Scholar]
- 111.Bao S, Song H, Wohltmann M, Ramanadham S, Jin W, Bohrer A, Turk J. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis. J Biol Chem. 2006;281:20958–20973. doi: 10.1074/jbc.M600075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem. 2007;282:7442–7449. doi: 10.1074/jbc.M607858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 114.St-Gelais F, Menard C, Congar P, Trudeau LE, Massicotte G. Postsynaptic injection of calcium-independent phospholipase A2 inhibitors selectively increases AMPA receptor-mediated synaptic transmission. Hippocampus. 2004;14:319–325. doi: 10.1002/hipo.10176. [DOI] [PubMed] [Google Scholar]
- 115.Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J Lipid Res. 2008;49:939–944. doi: 10.1194/jlr.R700017-JLR200. [DOI] [PubMed] [Google Scholar]
- 116.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005;44:5234–5245. doi: 10.1021/bi047773a. [DOI] [PubMed] [Google Scholar]
- 117.Schaeffer E, Forlenza O, Gattaz W. Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology. 2009;202:37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- 118.Junqueira R, Cordeiro Q, Meira-Lima I, Gattaz WF, Vallada H. Allelic association analysis of phospholipase A2 genes with schizophrenia. Psychiatr Genet. 2004;14:157–160. doi: 10.1097/00041444-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Smesny S, Kinder D, Willhardt I, Rosburg T, Lasch J, Berger G, Sauer H. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psych. 2005;57:399–405. doi: 10.1016/j.biopsych.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 120.Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg UT. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119:3733–3742. doi: 10.1242/jcs.03184. [DOI] [PubMed] [Google Scholar]
- 121.Gong MC, Arbogast S, Guo Z, Mathenia J, Su W, Reid MB. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol. 2006;100:399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]
- 122.Kan H, Xie Z, Finkel MS. iPLA2 inhibitor blocks negative inotropic effect of HIV gp120 on cardiac myocytes. J Mol Cell Cardiol. 2006;40:131–137. doi: 10.1016/j.yjmcc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 123.Poulsen KA, Young JF, Theil P, Kolko M, Oksbjerg N, Lambert IH. Role of phospholipase A2 in the induction of drip loss in porcine muscle. J Agr Food Chem. 2007;55:1970–1976. doi: 10.1021/jf062341n. [DOI] [PubMed] [Google Scholar]
- 124.Ronkko S, Rekonen P, Kaarniranta K, Puustjarvi T, Terasvirta M, Uusitalo H. Phospholipase A2 in chamber angle of normal eyes and patients with primary open angle glaucoma and exfoliation glaucoma. Mol Vis. 2007;13:408–417. [PMC free article] [PubMed] [Google Scholar]
- 125.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psych. 2006;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 126.Biancheri R, Rossi A, Alpigiani G, Filocamo M, Gandolfo C, Lorini R, Minetti C. Cerebellar atrophy without cerebellar cortex hyperintensity in infantile neuroaxonal dystrophy (INAD) due to PLA2G6 mutation. Eur J Paediatr Neurol. 2007;11:175–177. doi: 10.1016/j.ejpn.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 127.Westaway SK, Gregory A, Hayflick SJ. Mutations in PLA2G6 and the riddle of Schindler disease. J Med Genet. 2007;44:e64. doi: 10.1136/jmg.2006.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Novack DV, Christiansen B, Tu X, Zhang S, Lei X, Turk J. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2β)-null mice. Am J Pathol. 2008;172:868–881. doi: 10.2353/ajpath.2008.070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cathcart MK. Signal-activated phospholipase regulation of leukocyte chemotaxis. J Lip Res. 2009;50(Suppl):S231–236. doi: 10.1194/jlr.R800096-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carnevale KA, Cathcart MK. Calcium-independent phospholipase A(2) is required for human monocyte chemotaxis to monocyte chemoattractant protein 1. J Immunol. 2001;167:3414–3421. doi: 10.4049/jimmunol.167.6.3414. [DOI] [PubMed] [Google Scholar]
- 132.Mishra RS, Carnevale KA, Cathcart MK. iPLA2β: Front and center in human monocyte chemotaxis to MCP-1. J Exp Med. 2008;205:347–359. doi: 10.1084/jem.20071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bolotina VM. Orai, STIM1 and iPLA2β: A view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park KM, Trucillo M, Serban N, Cohen RA, Bolotina VM. Role of iPLA2 and store-operated channels in agonist-induced Ca2+ influx and constriction in cerebral, mesenteric, and carotid arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1183–1187. doi: 10.1152/ajpheart.01148.2007. [DOI] [PubMed] [Google Scholar]
- 135.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JEV, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 136.Ratz PH, Miner AS, Barbour SE. Calcium-independent phospholipase A2 participates in KCl-induced calcium sensitization of vascular smooth muscle. Cell Calc. 2009;46:65–72. doi: 10.1016/j.ceca.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bao S, Li Y, Lei X, Wohltmann M, Jin W, Bohrer A, Semenkovich CF, Ramanadham S, Tabas I, Turk J. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group VIA phospholipase A2. J Biol Chem. 2007;282:27100–27114. doi: 10.1074/jbc.M701316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perez R, Melero R, Balboa MA, Balsinde J. Role of group VIA calcium-independent phospholipase A2 in arachidonic acid release, phospholipid fatty acid incorporation, and apoptosis in U937 cells responding to hydrogen peroxide. J Biol Chem. 2004;279:40385–40391. doi: 10.1074/jbc.M402562200. [DOI] [PubMed] [Google Scholar]
- 139.Tithof PK, Elgayyar M, Cho Y, Guan W, Fisher AB, Peters-Golden M. Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. FASEB J. 2002;16:1463–1464. doi: 10.1096/fj.02-0092fje. [DOI] [PubMed] [Google Scholar]
- 140.Zhang XH, Zhao C, Seleznev K, Song K, Manfredi JJ, Ma ZA. Disruption of G1-phase phospholipid turnover by inhibition of Ca2+-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J Cell Sci. 2006;119:1005–1015. doi: 10.1242/jcs.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balboa MA, Perez R, Balsinde J. Calcium-independent phospholipase A2 mediates proliferation of human promonocytic U937 cells. FEBS J. 2008;275:1915–1924. doi: 10.1111/j.1742-4658.2008.06350.x. [DOI] [PubMed] [Google Scholar]
- 142.Roshak AK, Capper EA, Stevenson C, Eichman C, Marshall LA. Human calcium-independent phospholipase A2 mediates lymphocyte proliferation. J Biol Chem. 2000;275:35692–35698. doi: 10.1074/jbc.M002273200. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez T, Moreno JJ. Calcium-independent phospholipase A2 through arachidonic acid mobilization is involved in Caco-2 cell growth. J Cell Physiol. 2002;193:293–298. doi: 10.1002/jcp.10162. [DOI] [PubMed] [Google Scholar]
- 144.Song Y, Wilkins P, Hu W, Murthy KS, Chen J, Lee Z, Oyesanya R, Wu J, Barbour SE, Fang X. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem J. 2007;406:427–436. doi: 10.1042/BJ20070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herbert SP, Walker JH. Group VIA calcium-independent phospholipase A2 mediates endothelial cell S phase progression. J Biol Chem. 2006;281:35709–35716. doi: 10.1074/jbc.M600699200. [DOI] [PubMed] [Google Scholar]
- 146.Moon SH, Jenkins CM, Mancuso DJ, Turk J, Gross RW. Smooth muscle cell arachidonic acid release, migration, and proliferation are markedly attenuated in mice null for calcium-independent phospholipase A2β. J Biol Chem. 2008;283:33975–33987. doi: 10.1074/jbc.M805817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sanchez T, Moreno JJ. The effect of high molecular phospholipase A2 inhibitors on 3T6 fibroblast proliferation. Biochem l Pharmacol. 2001;61:811–816. doi: 10.1016/s0006-2952(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 148.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced lysophosphatidic acid autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 149.Sellmayer A, Danesch U, Weber PC. Effects of different polyunsaturated fatty acids on growth-related early gene expression and cell growth. Lipids. 1996;31:S37–40. doi: 10.1007/BF02637048. [DOI] [PubMed] [Google Scholar]
- 150.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lip Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Turk J, Gross RW, Ramanadham S. Amplification of insulin secretion by lipid messengers. Diabetes. 1993;42:367–374. doi: 10.2337/diab.42.3.367. [DOI] [PubMed] [Google Scholar]
- 152.Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochim Biophys Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 153.Zhou YP, Teng D, Dralyuk F, Ostrega D, Roe MW, Philipson L, Polonsky KS. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wilson HA, Allred DV, O’Neill K, Bell JD. Activities and interactions among phospholipases A2 during thapsigargin-induced S49 cell death. Apoptosis. 2000;5:389–396. doi: 10.1023/a:1009647912056. [DOI] [PubMed] [Google Scholar]
- 155.Shin KJ, Chung C, Hwang YA, Kim SH, Han MS, Ryu SH, Suh PG. Phospholipase A2-mediated Ca2+ influx by 2,2′,4,6-tetrachlorobiphenyl in PC12 cells. Toxicol Appl Pharmacol. 2002;178:37–43. doi: 10.1006/taap.2001.9317. [DOI] [PubMed] [Google Scholar]
- 156.Zhang L, Peterson BL, Cummings BS. The effect of inhibition of Ca2+-independent phospholipase A2 on chemotherapeutic-induced death and phospholipid profiles in renal cells. Biochem Pharmacol. 2005;70:1697–1706. doi: 10.1016/j.bcp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 157.Liou JY, Aleksic N, Chen SF, Han TJ, Shyue SK, Wu KK. Mitochondrial localization of cyclooxygenase-2 and calcium-independent phospholipase A2 in human cancer cells: implication in apoptosis resistance. Exp Cell Res. 2005;306:75–84. doi: 10.1016/j.yexcr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 158.Brustovetsky T, Antonsson B, Jemmerson R, Dubinsky JM, Brustovetsky N. Activation of calcium-independent phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J Neurochem. 2005;94:980–994. doi: 10.1111/j.1471-4159.2005.03248.x. [DOI] [PubMed] [Google Scholar]
- 159.Saavedra G, Zhang W, Peterson B, Cummings BS. Differential roles for cytosolic and microsomal Ca2+-independent phospholipase A2 in cell growth and maintenance of phospholipids. J Pharmacol Exp Ther. 2006;318:1211–1219. doi: 10.1124/jpet.106.105650. [DOI] [PubMed] [Google Scholar]
- 160.Gadd ME, Broekemeier KM, Crouser ED, Kumar J, Graff G, Pfeiffer DR. Mitochondrial iPLA2 activity modulates the release of cytochrome c from mitochondria and influences the permeability transition. J Biol Chem. 2006;281:6931–6939. doi: 10.1074/jbc.M510845200. [DOI] [PubMed] [Google Scholar]
- 161.Perez R, Matabosch X, Llebaria A, Balboa MA, Balsinde J. Blockade of arachidonic acid incorporation into phospholipids induces apoptosis in U937 promonocytic cells. J Lip Res. 2006;47:484–491. doi: 10.1194/jlr.M500397-JLR200. [DOI] [PubMed] [Google Scholar]
- 162.Kim SY, Jo HY, Kim MH, Cha YY, Choi SW, Shim JH, Kim TJ, Lee KY. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J Biol Chem. 2008;283:33563–33568. doi: 10.1074/jbc.M806578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lallemand C, Blanchard B, Palmieri M, Lebon P, May E, Tovey MG. Single-stranded RNA viruses inactivate the transcriptional activity of p53 but induce NOXA-dependent apoptosis via post-translational modifications of IRF-1, IRF-3 and CREB. Oncogene. 2007;26:328–338. doi: 10.1038/sj.onc.1209795. [DOI] [PubMed] [Google Scholar]
- 164.Zhang XH, Zhao C, Ma ZA. The increase of cell-membranous phosphatidylcholines containing polyunsaturated fatty acid residues induces phosphorylation of p53 through activation of ATR. J Cell Sci. 2007;120:4134–4143. doi: 10.1242/jcs.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Costa-Junior HM, Mendes AN, Davis GH, da Cruz CM, Ventura AL, Serezani CH, Faccioli LH, Nomizo A, Freire-de-Lima CG, Bisaggio Rda C, Persechini PM. ATP-induced apoptosis involves a Ca2+-independent phospholipase A2 and 5-lipoxygenase in macrophages. Prost Lip Mediat. 2009;88:51–61. doi: 10.1016/j.prostaglandins.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 166.Duan L, Gan H, Arm J, Remold HG. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J Immunol. 2001;166:7469–7476. doi: 10.4049/jimmunol.166.12.7469. [DOI] [PubMed] [Google Scholar]
- 167.Kirschnek S, Gulbins E. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infect Immun. 2006;74:850–860. doi: 10.1128/IAI.74.2.850-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nicotera TM, Schuster DP, Bourhim M, Chadha K, Klaich G, Corral DA. Regulation of PSA secretion and survival signaling by calcium-independent phopholipase A2β in prostate cancer cells. The Prostate. 2009;69:1270–1280. doi: 10.1002/pros.20968. [DOI] [PubMed] [Google Scholar]
- 169.Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA. Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J Biol Chem. 2006;281:22275–22288. doi: 10.1074/jbc.M604330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA2 activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aridor M, Balch WE. Integration of endoplasmic reticulum signaling in health and disease. Nat Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- 172.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes. 2002;51:S455–461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 174.Mehmet H. Caspases find a new place to hide. Nature. 2000;403:29–30. doi: 10.1038/47377. [DOI] [PubMed] [Google Scholar]
- 175.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner B, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 176.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. AN Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 177.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. 1989. Ag Act. 1994;43:187–193. doi: 10.1007/BF01986687. [DOI] [PubMed] [Google Scholar]
- 179.Diaz-Horta O, Kamagate A, Herchuelz A, Van Eylen F. Na/Ca exchanger overexpression induces endoplasmic reticulum-related apoptosis and caspase-12 activation in insulin-releasing BRIN-BD11 cells. Diabetes. 2002;51:1815–1824. doi: 10.2337/diabetes.51.6.1815. [DOI] [PubMed] [Google Scholar]
- 180.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin- secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 181.Kayo T, Koizumi A. Mapping of murine diabetogenic gene mody on chromosome 7 at D7Mit258 and its involvement in pancreatic islet and beta cell development during the perinatal period. J Clin Invest. 1988;101:2112–2118. doi: 10.1172/JCI1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 183.Lei X, Zhang S, Barbour SE, Bohrer A, Ford EL, Koizumi A, Papa FR, Ramanadham S. Spontaneous development of ER stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 (iPLA2β) expression: A role for regulation by SREBP-1. J Biol Chem. 2009 Dec 23; doi: 10.1074/jbc.M109.084293. [Epub ahead of print] (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva MY, Obeid LM, Hannun YA. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 185.Obeid LM, Hannun YA. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995;58:191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- 186.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 187.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 188.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–30021. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 189.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 190.Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 192.Fensome AC, Josephs M, Katan M, Rodrigues-Lima F. Biochemical identification of a neutral sphingomyelinase 1 (NSM1)-like enzyme as the major NSM activity in the DT40 B-cell line: absence of a role in the apoptotic response to endoplasmic reticulum stress. Biochem J. 2002;365:69–77. doi: 10.1042/BJ20020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tamiya-Koizumi K, Umekawa H, Yoshida S, Kojima K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J Biochem (Tokyo) 1989;106:593–598. doi: 10.1093/oxfordjournals.jbchem.a122901. [DOI] [PubMed] [Google Scholar]
- 194.Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- 195.Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 196.Morales MC, Pérez-Yarza G, Rementería NN, Boyano MD, Apraiz A, Gómez-Muñoz A, Pérez-Andrés E, Asumendi A. 4-HPR-mediated leukemia cell cytotoxicity is triggered by ceramide-induced mitochondrial oxidative stress and is regulated downstream by Bcl-2. Fr Rad Res. 2007;41:591–601. doi: 10.1080/10715760701218558. [DOI] [PubMed] [Google Scholar]
- 197.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calc. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 198.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol (Lond) 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 200.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 202.Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 203.Socha L, Silva D, Lesage S, Goodnow C, Petrovsky N. The role of endoplasmic reticulum stress in nonimmune diabetes: NOD.k iHEL, a novel model of β-cell death. Ann NY Acad Sci. 2003;1005:178–183. doi: 10.1196/annals.1288.022. [DOI] [PubMed] [Google Scholar]
- 204.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Cell Pr. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 205.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 206.Takeda K, Inoue H, Tanizawa Y, Matsuzaki Y, Oba J, Watanabe Y, Shinoda K, Oka Y. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10:477–484. doi: 10.1093/hmg/10.5.477. [DOI] [PubMed] [Google Scholar]
- 207.Kroncke KD, Brenner HH, Rodriguez ML, Etzkorn K, Noack EA, Kolb H, Kolb-Bachofen V. Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 1993;1182:221–229. doi: 10.1016/0925-4439(93)90144-p. [DOI] [PubMed] [Google Scholar]
- 208.Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H, Aburatani H, Miyazaki J-i, Oka Y. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet. 2006;15:1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]