Abstract
The effectiveness of cell-based therapy to treat muscle disease has been hampered by difficulties in isolating, maintaining and propagating the stem cells that are needed for treatment. Here we report the isolation of muscle-derived stem cells from both young and old mice and their propagation over extended periods of time in culture as “free-floating” myospheres. Analysis of these sphere-forming cells showed that they express stem cell antigen-1 (Sca-1), β1 integrin (CD29), Thy-1 (CD90), and CD34, but did not express CD45, CD31, or myogenic markers (Pax7, Myf5, and MyoD). We found that cells derived from myospheres and then grown adherently (MDACs) behaved similar to primary myoblasts, in that these cells expressed myogenic markers and were able to easily form multinucleated myotubes. Unlike the parental myopsheres but analogous to primary myoblasts, MDACs expressed Pax7, Myf5, and MyoD, indicating that the parent myosphere cells were a more primitive type of cell. In support of this we demonstrated that myopsheres were also able to differentiate into adipogenic and osteogenic cells in culture, as well as being able to contribute to injured muscle in vivo. In summary, we report that primitive adult muscle stem cells can be easily isolated and sustained in culture as myospheres.
Keywords: Stem cell, myosphere, myogenic, non-adherent, multipotent
Introduction
One of the greatest challenges in using cell therapy for the treatment of muscle disease is the ability to isolate, expand and deliver suitable donor cells needed for transplantation. Recently there has been a large body of literature focused on the isolation of prospective stem cells for muscle regeneration [1–7]. Each reported cell type has slightly different characteristics and varying potential origins. Here we choose to keep these stem cells and their properties in mind while developing a simple effective approach to isolate and maintain muscle stem cells in culture. Properties we considered to be important included ease of isolation, the ability to be maintained and expanded in culture without loss of “stemness”, the availability of cells from young as well as old donors, and myogenic potential.
The most common marker associated with stem cells is the presence of stem cell antigen-1 (Sca-1). This marker is frequently used in conjunction with other markers to identify different stem cell populations. The expression of Sca-1 has been hypothesized to play a role in maintaining the stem cell pool by influencing signaling involved in proliferation versus differentiation [8, 9]. Many of the muscle stem cell populations with the exception of satellite cells [10–12], have been shown to express Sca-1. These include muscle-derived stem cells (MDSCs) [13, 14], mesoangioblasts [15], and side population (SP) cells [16, 17]. Another marker that is specific for muscle and associated with satellite stem cells is the transcription factor Pax7 [18]. The expression of Pax7 has been shown to be critical for satellite cell survival and is the upstream regulator of myogenesis [18–21]. Cells expressing Pax7 have the potential for a myogenic fate but are not necessarily committed to that fate [22, 23]. Muscle stem cells that do not express Pax7, such as SP cells and pericytes have been shown to be “pre-myogenic” in nature, that is, although these cells are initially Pax7 negative, they later become Pax7 positive and are able to contribute to the satellite cell pool [1, 10]. In addition to the expression of Sca-1 and Pax7, many muscle stem cells have been shown to have characteristics similar to mesenchymal stem cells. For example, muscle SP cells [24], perivascular cells [25], and pericytes [1] all express CD90 (Thy 1), which is a mesenchymal stem cell surface marker [26, 27]. Additionally, muscle stem cells have also been shown to exhibit the mesenchymal-like property of multipotency, in that they (satellite cells [28], mesoangioblasts [29], and muscle SP cells [30]) have the ability to differentiate into adipogenic and osteogenic cells.
After examining the markers expressed, whether they are multipotent, and the locations that the various muscle stem cells have been isolated from, we noted that there was one common characteristic among these cell populations. All of these cells, regardless of how they were isolated, go through an initial phase as non-adherent cells; whether it be the preplate technique used in the isolation of MDSCs, [3, 14, 31], Hoechst dye exclusion to isolate muscle SP cells [16, 32], or through a sphere forming intermediate which occurs with perivascular cells [25]. This concept of non-adhering cells maintaining a more primitive state is not new, for years Qu-Peterson and Huard have touted that the slowly adhering cells isolated from muscle are associated with more “stem-cell” like populations [3, 14]. Recently, Sarig, et al. isolated a population of cells derived from muscle, as free-floating spheres termed myospheres, however the cells composing these spheres were shown to express MyoD indicating that these were not stem cells but more committed cells already leaning toward a myogenic fate [33]. Others have also grown cells isolated from muscle as spheres, yet the cells derived from these spheres were shown to be more endothelial than myogenic in nature [34, 35].
In the current study, based on a report in JCB [36] we show that we were able to isolate and maintain muscle stem cells in culture over long periods of time in a “pre-myogenic” state by propagating them as non-adherent free-floating myospheres. We found that the cells composing myospheres expressed both Sca-1 and CD90, but did not express satellite cell marker Pax7 or other myogenic markers such as MyoD. Here we demonstrate that like other muscle stem cells, myospheres are multipotent, in that they are able to differentiate into adipocytes and osteogenic cells. In addition, we found that myospheres are relatively easy to generate from cells isolated from both young and old mice, and upon differentiation these cells were found to be myogenic in nature as they were able to easily form multinucleated myotubes in vitro as well as contribute to regenerating muscle in vivo.
Materials and Methods
Animals
Wild type C57BL/6 (Charles River Laboratories), C57BL/10 (Jackson Laboratory), and 129X1/SVJ (Source/Jackson) were used to generate and analyze myospheres in the in vitro studies. MDX mice (C57BL/10ScSn-Dmd mdx, transplant recipient) and wild type (C57BL/10, transplant donor) were used in the in vivo studies (both from Jackson Laboratory). All experimental procedures were conducted in accordance with the Harvard Medical School Standing Committee on Animals.
Cell Culture
Myoblast isolation
Primary myoblasts were isolated from the hindlimb muscles of newborn (3–12 days) and adult (4–8 week) mice using the protocol described by Richler and Yaffe [37, 38]. Muscle tissue was minced and enzymatically dissociated by a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A (both from Roche) for 1–1½ hours at 37 °C. After digestion 10 ml of F10 media (GIBCO) containing 20% FCS (Hyclone) was added to inactivate dispase/collagenase. The slurry was passed through a 70 μm cell strainer (BD Falcon) and centrifuged for 10 minutes at 156 × g. The pellet was resuspended in 10 ml of F10 media containing 20% FCS, 100 U/ml Pen/Strep (GIBCO), and 5 ng/ml bFGF (PeproTech) in a 10 cm uncoated dish. Primary myoblasts were obtained using the pre-plating technique described by Qu-Petersen, et al. [14] (primary myoblasts were isolated from pre-plates 3 and 4).
Myosphere isolation
Myospheres were isolated based on the methods used to isolate spore-like cells [36]. Myosphere cultures were generated from the hindlimb muscles of mice at various ages: newborn (3–12 days), adult (4–8 weeks), and old (16–20 months). Muscle tissue was minced and enzymatically dissociated by a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A for 1 hour at 37°C. After digestion the slurry was dissociated further using a scalpel and then F10 media containing 20% FCS was added to inactivate dispase/collagenase. The slurry was passed through a 70 μm cell strainer and centrifuged for 10 minutes at 156 × g. Cell pellets were resuspended in 1 ml of red blood cell lysis buffer (0.15 M ammonium chloride/0.01M potassium bicarbonate solution, pH 7.4) for 2½ minutes on ice. After lysis 20 ml of DMEM:F12 (1:1) was added and the cells were pelleted and then resuspended in 5 ml of B27 media (DMEM:F12 containing B27 supplement and 100 U/ml Pen/Strep (all from GIBCO)), triturated through progressively narrowed pasteur pipets (1 mm–10 μm), filtered through a 40 μm cell strainer (BD Falcon), and then brought up to a total volume of 12 ml of B27 media with 20 ng/ml hEGF, 20 ng/ml bFGF (both from PeproTech), and 2 μg/ml heparin (StemCell Tech). Fresh growth factors were added every 2–3 days and once a week 3–4 ml of fresh B27 media was added after allowing the spheres to settle so some old media could be removed. Myospheres were passaged by collecting spheres greater than 100 μm (using a cell strainer), dissociating the spheres with a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A for 20–30 minutes, and then plating at a density of 1–10 × 105 cells/ml in B27 media with fresh growth factors.
Myosphere Derived Adherent Cells (MDACs)
Myospheres were collected in a 70 μm cell strainer, dissociated using 0.25% Trypsin-EDTA, resuspended in DMEM containing 10% FCS, 100 U/ml Pen/Strep, and 5 ng/ml bFGF, and then plated on an uncoated 10 cm dish. After 1–2 hours the supernatant, which contains floating cells, was removed and replated on an ECL (Upstate) coated dish (replating is usually done 2 to 3 times). MDAC colonies formed within 1–2 weeks. Once colonies started to form the cells were trypsinized and plated on collagen-coated dishes (Upstate).
Bone marrow derived cells
Bone marrow was flushed through femurs and tibias of 6–8 week old mice using a 21 gauge needle with a 10 ml syringe filled with MEM media (GIBCO). Flushed bone marrow was filtered through 100 μm cell strainer (BD Falcon) and then centrifuged for 10 minutes at 156 × g. The pellet was resuspended in MEM media, filtered a second time, overlaid onto Lympholyte-M solution (Cedarlane), and centrifuged at room temperature for 20 minutes at 1000 × g. The layer just above the boundary was removed and washed 3X with MEM media containing 15% FCS (Hyclone) and 100 U/ml Pen/Strep. Red blood cells were lysed using 1–2 ml of 0.15 M ammonium chloride/0.01 M potassium bicarbonate solution (pH 7.4) for 2½ minutes on ice. After lysis the pelleted bone marrow cells were placed in MEM media containing 15% FCS at a density of 1 × 106 cells/ml.
Flow cytometry
The percentage of cells expressing: Sca-1 (PE conjugated), CD45 (PE conjugated), CD90.2 (FITC conjugated), all rat anti-mouse from BD Pharmingen, CD31 (PE conjugated, rat anti mouse, e-Bioscience), β1 integrin (FITC conjugated, hamster anti-mouse, Santa Cruz), and CD34 (FITC conjugated rat anti-mouse, eBioscience) were analyzed by FACS-Vantage SE (Becton-Dickinson). Cells analyzed include myospheres, MDAC, myoblasts, and bone marrow derived cells. The myospheres were dissociated by a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A, MDAC and myoblasts were lifted from the plate using PBS containing 0.5% FCS and 2 mM EDTA. All cells were washed once with HANKS containing 2% FCS (HF), and then incubated with conjugated antibodies for 1 hour on ice. Bone marrow cells were incubated with rat anti-mouse Fc Block (CD16/CD32, BD Pharmingen) 5 minutes prior to the addition of all antibodies. Cells were washed twice in HF after antibody incubation and then resuspended in 200 μl HF for analysis. Unstained cells were used as a negative control. Sorted cells were stained with CD31-PE, CD45-PE, and Sca-1-FITC (all from BD Pharmingen) and then sorted into individual wells using a BD FACS Aria.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 20 minutes at 20 °C and blocked and permeabilized in PBS containing 10% Normal Goat Serum (NGS) and 0.2% Triton-X-100 for 45 minutes at 20 °C. Cells were washed 1X with PBS containing 0.1% Triton-X-100 and then incubated for 2 hours at 20 °C with the following primary antibodies: Pax7 (1:50, Developmental Studies Hybridoma Bank), MyoD (1:50, Santa Cruz), and Desmin (1:100, DakoCytomation). Cells were then washed 2X with PBS containing 0.1% Triton-X-100 and incubated for 30 minutes at 20 °C with secondary antibodies (1:200 Alexa 488 goat anti-mouse (Invitrogen) or 1:5000 Cy3 goat anti-mouse (Jackson ImmunoResearch). Results were viewed using an Olympus 1×70 fluorescent microscope. Pax7 pictures were taken with a digital camera using Spot Advantage software version 4.5. MyoD, Desmin, and Sca-1 pictures were taken with a Kodak DC290 camera using 1D acquisition software (Kodak). Overlays were done using the Spot advantage software.
RNA Isolation, RT-PCR
Total RNA was extracted from myospheres, myoblasts, MDAC, and bone marrow derived cells using the RNeasy Plus RNA isolation kit (Qiagen). GeneAMP RNA PCR kit (Applied Biosystems) was used for reverse transcription of RNA and amplification of cDNA. Primers were as follows: Pax-7: GAA AGC CAA ACA CAG CAT CGA/ACC CTG ATG CAT GGT TGA TGG (466bp), Myf5: GTC AAC CAA GCT TTC GAG ACG/CGG AGC TTT TAT CTG CAG CAC (304bp), MyoD: GGA GGA GCA CGC ACA CTT CC/CGC TGT AAT CCA TCA TGC CA (464bp), and Sca-1: CTC TGA GGA TGG ACA CTT CT/GGT CTG CAG GAG GAC TGA GC (404bp). β-Actin: GCT GGT CGT CGA CAA CGG CTC/GTT GAA GGT CTC AAA CAT (364bp) was used as a control. Samples were denatured at 95 °C for 1 minute, followed by amplification: 94 °C for 30 seconds (denature), 60 °C for 30 seconds (anneal), and 72 °C for 1 minute (extension) for 35 cycles, followed by 72 °C 10 minutes.
Lineage differentiation
Myogenic differentiation
MDAC cells were plated in F10 media containing 20% FCS and 100 U/ml Pen/Strep in 24 well dishes. Media was changed the following day to DMEM media containing 2% heat inactivated horse serum and 100 U/ml Pen/Strep. Myotubes formed within 3–5 days. Myotubes were fixed with 4% paraformaldehyde at 20 °C for 20 minutes and permeablized for 1 hour with 10% NGS and 0.2% triton in PBS. Nuclear staining was done using 20 μg/ml DAPI (4′, 6-diamidino-2-phenylindole dihydrochloride) for 5 minutes at 20 °C.
Adipogenic differentiation
Myospheres (>70 μm) were collected in a cell strainer, dissociated using 0.25% Trypsin-EDTA, and then cultured in DMEM containing 10% FCS, 0.5 mM isobutyl-methyl xanthine (Sigma), 1 μM dexamethasone (Sigma), 10 μg/ml insulin (Sigma), and 100 U/ml Pen/Strep in 24 well dishes at a density of 1×105 cells/ml. Cells were cultured in adipogenic media for 1–2 weeks. For Oil Red O staining, cells were fixed with 4% paraformaldehyde at 20 °C for 20 minutes and stained for 15 minutes with fresh 0.3% Oil Red O solution (Sigma) in 60% isopropyl alcohol.
Osteogenic differentiation
Myospheres (>70 μm) were collected in a cell strainer, dissociated using 0.25% Trypsin-EDTA, and then cultured in DMEM containing 10% FCS, 100 ng/ml BMP-2 (R+D Systems), 10 mM β-glcerophosphate (Sigma), 50 nM dexamethasone (Sigma), 50 μg/ml ascorbic acid (Sigma), and 100 U Pen/Strep in 24 well dishes at a density of 2.5 ×105 to 1 ×106 cells/ml. Cells were cultured in osteogenic media for 2–3 weeks. For alkaline phosphatase staining, cells were fixed with 4% paraformaldehyde at 20 °C for 15 minutes, washed with 10 mM Tris buffer (pH 7.5), and stained for 30 minutes with alkaline phosphatase solution [1 mg/ml naphthol AS-MX (Sigma) and 5 mg/ml fast blue BB (Fluka), 2 mM magnesium chloride (Sigma), and 0.5% dimethylformamide (Sigma) in 0.2 M Tris Buffer pH 8.5].
Lentiviral transduction
Virus was produced by transient transfection of 293T cells with 3.2 μg of GFP expressing lentiviral vector, 4.0 μg of Gag-Pol-Vif plasmid, and 0.4 μg of each Rev, Tat, and VSV-G using Fugene (Roche) as described earlier in [39]. Supernatants were collected and centrifuged at 24,000 RPM for 1½ hours to concentrate viral stocks. Myosphere cultures were transduced immediately after isolation at a MOI of 1:50 in the presence of 6 μg/ml protamine sulfate for 3 hours. Transduced cells could be monitored for GFP expression within 48 hours of transduction by fluorescence microscopy.
Muscle regeneration
Injury was induced in the tibialis anterior (TA) muscle of (6 week old) MDX mice by injecting 30 μl of 10 μM cardiotoxin (Accurate Chemical) 3 days prior to transplantion of donor cells. Donor cells were obtained from myospheres whose initial cultures had been transduced with a lentiviral vector expressing GFP. These myosphere isolations had remained in culture for 17–30 days and were generated from wild type (6 week old) C57BL/10 mice. Myospheres >100 μm were collected, dissociated into individual cells using a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A, and then washed 2X in HANKS buffer prior to injection. The TA muscles of recipient mice were injected with 4 ×105 cells in a total volume of 18 μl of HANKS buffer, as a control, some of the mice were injected in the contralateral TA muscle with 18 μl of HANKS buffer alone. All injections were done using a 26-guage needle. Mice were sacrificed 2 weeks after transplantation.
Immunohistochemistry
Recipient mice were perfused with 4% paraformaldehyde and the TA muscles were removed and immersed in 4% paraformaldehyde overnight. The following day samples were transferred to 30% sucrose solution for 24–48 hours. Samples were embedded in OCT compound (Sakura), flash frozen in isopentane, and then stored at −80 °C. Serial 12 μm thick cross-sections were cut and mounted on gelatin-coated slides. Slides were washed 3X with PBS, blocked with PBS containing 5% NGS, 2% BSA, and 0.2% triton at 20 °C for 1 hour, incubated first at 4 °C overnight and then 20 °C for 2 hours with either rabbit anti-GFP (1:100, Chemicon) or rabbit anti-laminin (1:500, Sigma) diluted in 2% NGS. Slides were washed 3X with PBS containing 0.1% triton and then incubated at 20°C for 1 hour with goat anti-rabbit CY3 (1:300, Jackson ImmunoResearch) or Alexa 647 (1:400, Invitrogen), respectively, which were diluted in PBS containing 2% NGS and 0.1% triton. Slides were washed 3X with PBS, 2X with H2O, and then stained with DAPI for 5 minutes. Coverslips were mounted on slides with vectashield solution (Vector Laboratories). Slides were viewed using an Olympus 1X70 fluorescent microscope. Pictures were taken with a digital camera using Spot Advantage software version 4.5.
Results
Isolation of Free-Floating Spheres from Muscle
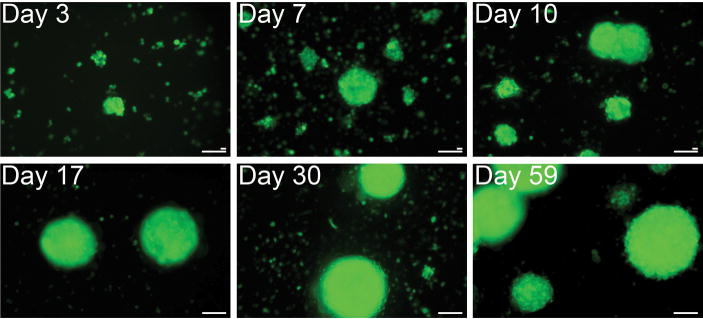
To generate free-floating myospheres mouse hindlimb muscles were dissociated enzymatically and then manually triturated though a series of finer and finer pipettes to a final bore of 10 μm in diameter to assure that all the mature cells had been destroyed. Due to the degree of trituration the initial myosphere cultures contain a great deal of debris and very few adherent cells (usually no adherent cells are seen for the first 4–5 days after isolation). In order to track viable cells among the debris the initial myosphere cultures were transduced with a lentiviral vector expressing green fluorescent protein (GFP) so that the formation and growth of the myospheres could be monitored over time (Fig. 1). In the first few days after isolation small round cells were seen floating among the debris. Over the next few days the single cells become small clusters of 3–4 cells, then larger clusters of 8–16 cells, later forming rounded sphere-like structures. The sphere-like structures were initially 25–50 μm in size and continue to grow over time reaching sizes as large as 300–400 μm.
Figure 1. Formation and growth of myospheres over time.
Immediately after isolation myosphere cultures were transduced with a lentiviral vector expressing GFP, the formation of myospheres were monitored at various time points after isolation: 3, 7, 10, 17, 30, and at 59 days. The size of the myospheres formed ranged from 50 μm to over 300 μm. Larger scale bar represents 100 μm, and the smaller bar, 20 μm.
To determine if myospheres could be isolated from both young and old animals, myosphere cultures were generated from the hindlimb muscles of pups (3–12 days old), adult mice (4–8 weeks old), and old mice (17–20 months old), and then these cultures were monitored over time. We found that cultures isolated from pups formed spheres earlier than those isolated from adult and old mice, which appeared to form spheres at the same pace. The myosphere cultures generated from pups formed small spheres (50–100 μm) in 3 to 6 days, medium spheres (100–300 μm) in 5–10 days, and large spheres (>300 μm) 13 days after isolation. Cultures generated from adult and old mice formed clusters in 3–8 days, small spheres (50–100 μm) in 4–16 days, medium spheres (100–300 μm) in 11–20 days, and large spheres (>300 μm) 23 days after isolation. We observed that myosphere cultures grow in stages. During the first stage there is slow cell growth, which lasts about 10 days and encompasses the time from the initial isolation when small round cells are surrounded by debris until the spheres begin to form and reach about 50 μm in size. The second stage is a rapidly growing phase that lasts about 10–15 days and encompasses the time when the spheres range in size from 100–300 μm. During this stage the number of spheres and size of the spheres increase rapidly, this is also the optimal time to passage the myospheres. Myospheres can be passaged every 20–30 days by trapping spheres that are >100 μm, dissociating them with dispase/collagenase and then plating the cells at a density of 1–10×105 cells/ml. The newly replated myosphere cells will over a period of several days form new free-floating spheres. In addition to the newly passaged spheres, the original plate from which the spheres were removed will continue to generate new spheres. Myosphere cells cultured in this manner could be maintained as free-floating spheres for as long as 6 months. The final stage of myosphere growth occurs when the spheres approach 300 μm or larger, cell growth slows and if the spheres are not passaged they will slowly darken, drop down and the cells composing the spheres will begin to mature and migrate away from the spheres (Supplementary Fig. 1).
Clonal Isolation of Myospheres
A common question that arises in culturing sphere-forming cells is whether the spheres are clonal or do they form via cellular aggregation. To address this question, individual cells were plated in single wells of 96 well plates using FACS sorting. Due to the amount of debris present in the freshly isolated cultures we sorted for viable cells that were Sca-1+/CD31−/CD45−. We found that out of the 192 wells plated a majority of the cells did not survive. Two weeks after sorting 31.7% of the wells contained between 1–4 small adherent cells, 1.0% of the wells contained a small colony of rounded adherent cells, and 6.3% of the wells contained small adherent spheres (<100μm). At 36 days after sorting there was little change in the number of cells per well or in the growth of the spheres, indicating that perhaps an essential factor needed for the propagation of these cells is missing. Because the clonally derived spheres generated were no longer non-adherent, these spheres could not be used to further characterize non-adherent myospheres. As will be shown later, once myosphere cells become adherent their phenotype, as defined by cell surface and myogenic marker expression, changes. To determine if the sorting had an affect on the growth of the plated single myosphere cells we sorted for pools of Sca-1+/CD31−/CD45− cells (Supplementary Fig. 2). We found that pooled cells remained non-adherent, but required about two weeks after sorting for new myospheres to form. The spheres that were generated from the pooled sorted cells ranged in size from 50–150μm, appeared healthy (Supplementary Fig. 2), and could be passaged to generate new spheres, however these cultures generated less cells and less spheres than cultures that were not sorted. These results suggest that like sphere-forming cells from other tissues, such as neurospheres, it is difficult to demonstrate the clonal nature of sphere-forming cells from skeletal muscle. In the case of neurospheres the clonal nature of these cells was demonstrated through mixing experiments in which sphere forming cells were labeled with two different markers (such as yellow and red fluorescent proteins) and then mixed together. These experiments showed that the majority of the spheres generated after mixing contained only one of the two fluorescent labels, indicating that that the primary spheres that form after isolation grew clonally [40]. This has also been shown by Sairg, et al. to be the case for the growth of myospheres [33].
Characterization of Myospheres
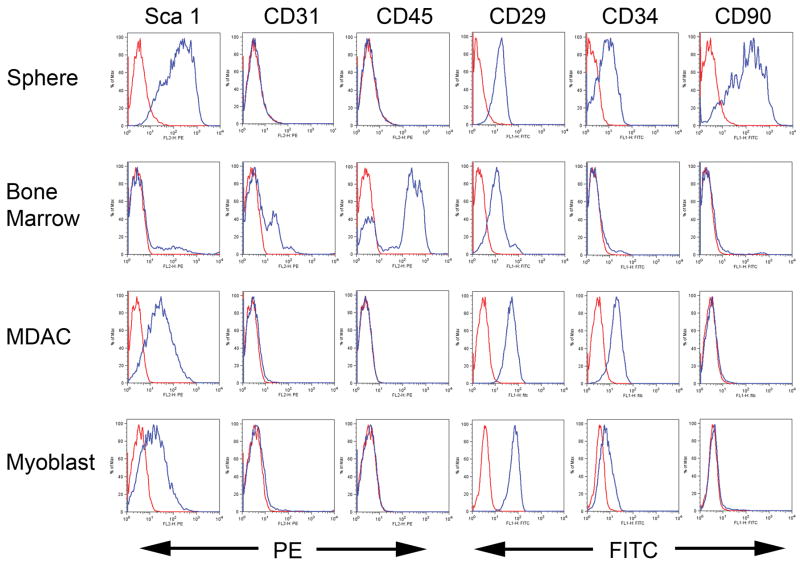
In order to better determine the composition of the myospheres, cells derived from dissociated myospheres were analyzed by FACS for expression of several cell surface markers. To show that myosphere cells were not derived from hematopoietic or endothelial cells, FACS analysis was also done on mouse bone marrow cells as a control. These markers included stem cell (Sca-1, CD34, and β1 integrin), hematopoietic (CD45), endothelial (CD31), and mesenchymal stem cell (CD90) markers. We found that myosphere cells were highly positive for the expression of Sca-1 (>80%), β1 integrin (>90 %), and CD34 (~40%) indicating that the cells composing myospheres were possibly stem cells (Fig. 2). Myosphere cells also express the mesenchymal marker CD90 (~59%, Fig. 2). Myospheres did not express CD45 or CD31, indicating that the myospheres were not derived from hematopoietic or endothelial cells. In addition, we examined myosphere cells for the ability to exclude Hoechst dye. We found the myosphere cells do not exclude Hoechst dye, indicating that myospheres are not composed of muscle SP cells (Supplementary Fig. 3).
Figure 2. Comparison of FACS profiles.
The expression of various cell surface markers were determined by FACS analysis for: myospheres (N=10), bone marrow cells (N=4), MDACs (N=3), and primary myoblasts (N=4). A representative profile is shown for each. Antibodies for Sca-1, CD31, and CD45 were all directly conjugated to PE. Antibodies for CD29, CD34, and CD90 were all directly conjugated to FITC. Red lines represent the negative control (unstained) and the blue lines represent cells with antibody. The y-axis indicates percentage of cells, and the x-axis the intensity of fluorescence.
Isolation and Characterization of Myosphere Derived Adherent Cells (MDACs)
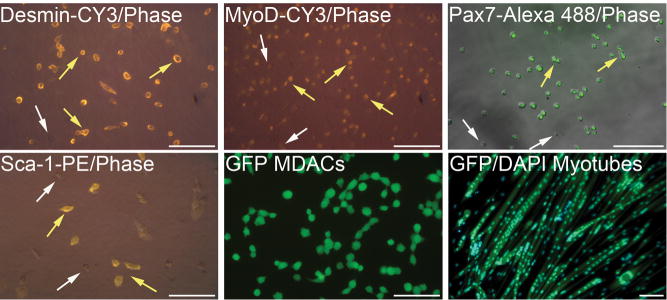
To determine if myospheres could be used to generate myogenic cells, myospheres were removed from their serum free defined media, dissociated using trypsin, and the resulting cells plated on ECL coated dishes in media containing 20% FCS so that the cells would grow adherently, one to two weeks later colonies formed. Colonies were dissociated, plated on collagen-coated plates and then grown adherently like primary myoblasts. These “myoblast-like” cells derived from myospheres were called “myosphere-derived adherent cells” or MDACs. To determine if the MDAC surface markers differed from the their myosphere predecessors, FACS analysis was done comparing the markers expressed by the MDACs to those expressed by myosphere cells and primary myoblasts (Fig. 2). The resulting FACS profiles showed that MDACs continued to express Sca-1 (~73%), however at a slightly lower level than the myosphere cells. We also found that MDACs, like primary myoblasts, expressed β1 integrin (99%), CD34 (~88%) but did not express CD90. Overall, MDACs were found to be similar to primary myoblasts. MDACs were also found by immunostaining to express Pax 7 (>90%), MyoD (60–80%), and desmin (>90%), indicating that these cells are myogenic (Fig. 3). To determine if MDACs would form multinucleated myotubes, GFP labeled MDACs were plated densely on ECL coated dishes, the media was changed from myoblast media containing 20% FCS to myotube media containing 2% HS, and then the cells were monitored for the formation of myotubes. MDACs were able to form multinucleated myotubes within 3–6 days of plating (Fig. 3).
Figure 3. MDACs express myogenic markers.
Immunostaining of MDACs by the antibodies and stains indicated. The staining for Desmin (cytoplasmic), MyoD (nuclear), and Sca-1 (surface) are shown in yellow-red. The staining for Pax7 (nuclear) is shown in green. Yellow arrows indicate positive staining, white arrows negative staining. MDACs express Desmin, MyoD, Pax7, and Sca-1. The MDACs shown in the bottom middle and right panels express GFP. The morphology of MDACs is shown in the bottom middle panel and the myotubes formed from the MDACs is shown in the bottom right panel. Nuclear staining in the myotubes is indicated by DAPI, showing MDACs form multinucleated myotubes. Scale bars for all pictures represent 100 μm.
Myospheres are Pre-myogenic, MDACs are Myogenic
To determine if myosphere cells, which are the predecessors to the MDACs, expressed myogenic markers, we analyzed myospheres and MDACs for expression of Pax7, Myf5, and MyoD by reverse transcriptase PCR. RNA was extracted from myospheres (>40 μm in size), MDACs, primary myoblasts (as a positive control), and bone marrow derived cells (as a negative control). The myosphere preps shown were obtained from four independent cultures isolated from the hindlimb muscles of adult mice (4–8 weeks) and old mice (20 months), prior to RNA isolation these myospheres had remained in culture for a period of time ranging from 63–101 days. We found that unlike MDACs, myosphere cells did not express Pax7, Myf5, or MyoD, even when isolated from old mice (Fig. 4) indicating that the cells composing the myospheres are not yet myogenic, but lean toward a myogenic fate. The RT-PCR profile generated by the MDACs was found to be similar to the profile for primary myoblasts, both cell types expressed Pax7, Myf5, and MyoD, further demonstrating that MDACs are myogenic. Additionally, we confirmed the expression of Sca-1 by RT-PCR, once again showing myospheres express Sca-1 and that MDACs and primary myoblasts also express Sca-1 (Fig. 4).
Figure 4. Reverse transcriptase PCR for myogenic markers.

RT-PCR was done for the expression of myogenic markers Pax7, Myf5, and MyoD, and for the expression of Sca-1. RNA was isolated from myospheres >40 μm in size. Myosphere profiles shown were obtained from 4 different isolations (three from animals 4–8 weeks old, one 20 months old). RNA was isolated from the myospheres at the time points indicated. Profiles for the MDACs, which were derived from the myospheres, as well as primary myoblasts (positive control) and bone marrow cells (negative control) are also shown. β-actin with and without RT was used as an internal control. Myospheres are pre-myogenic they do not express Pax7, Myf5, or MyoD. MDACs are myogenic they express Pax7, Myf5, and MyoD.
The Presence of Serum Alters Myosphere Phenotype
To determine why our myospheres differed from previously reported myospheres, we cultured freshly isolated myospheres in the B27 growth media alone or B27 growth media containing 15% FCS. The presence of serum caused the following differences in phenotype: 1.) During the first 4 days after isolation there are no adherent cells present in myosphere cultures grown without serum, however those grown in the presence of serum contain many adherent cells. 2.) Myospheres isolated from adult mice begin to form around 10 days in the cultures maintained without serum and these spheres remain non-adherent. In cultures containing serum myospheres formed within 3 days and by 5 days most of these spheres had become adherent (Supplementary Fig. 4). 3.) Myospheres generated without serum express high levels of Sca-1, whereas those with serum expressed high levels of Sca-1 for the first 4 days and then by day 8 expressed very little Sca-1. 4.) Myospheres cultured without serum are Pax7−/Myf5−/MyoD− while cultured non-adherently and become Pax7+/Myf5+/MyoD+ when grown adherently, however myospheres generated in the presence of serum were Pax7+/Myf5+/MyoD+ (determined by RT-PCR). These results suggest that in the presence of serum spheres quickly became adherent and committed to the myogenic lineage.
Myosphere Cells Differentiate into Adipogenic and Osteogenic Cells in vitro
To determine if myosphere cells could differentiate into other mesenchymal cell lineages, myospheres >100 μm were collected and dissociated by trypsin, and then plated in media favoring the growth of either adipocytes or osteogenic cells. We found that myosphere cells plated in adipogenic media formed adipocytes within 1 to 2 weeks, which was detected by Oil Red O staining (Fig. 5A) and the corresponding cells plated in osteogenic media containing BMP-2, formed osteogenic cells within 2 to 3 weeks of plating, these cells were positive for alkaline phosphatase staining (Fig. 5B). In addition, because we were unable to generate enough clonally derived non-adherent myosphere cells, we isolated individual spheres, dissociated these spheres and then plated them in parallel into adipogenic, ostegenic, and myogenic media. It was found that 84% of the individual myospheres (N=18) were able to differentiate into all three of these lineages (the remaining 16% did not contain enough cells to determine if they could differentiate into osteogenic cells). This data indicates that myosphere cells are multipotent, in that they are capable of giving rise to myogenic, adipogenic, and osteogenic cells.
Figure 5. Multipotent potential of myospheres.
Myospheres >100 μm were dissociated and plated at high density in either adipogenic media or osteogenic media. (A) Adipogenic cells derived from myospheres as detected by Oil Red O staining. (B) Osteogenic cells derived from myospheres as detected by alkaline phosphatase staining. Scale bars in both pictures represent 100 μm.
Myosphere Cells can Contribute to Regenerating Muscle
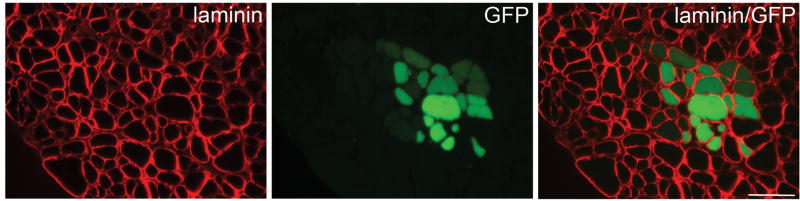
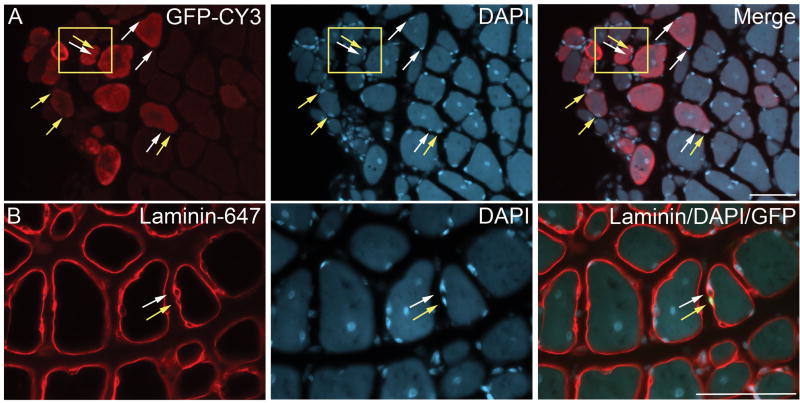
To determine if myospheres have the capacity to regenerate injured muscle in vivo, myosphere cells (4 × 105 cells/muscle) were injected into the TA muscle of cardiotoxin injured MDX mice. The myosphere donor cells were isolated from C57BL/10 mice, transduced with a GFP lentivirus, and then cultured until medium sized spheres formed (17–30 days). Myospheres >100 μm in size were trapped using a 100 μm cell strainer, dissociated by dispase/collagenase, and then resuspended in HANKS and injected into the TA muscle of the recipient mice. As a control the contralateral TA muscles of four mice were injected with HANKS alone. Two weeks after injection the mice were sacrificed, the TA muscle was fixed with paraformaldehyde, sectioned, and then stained by immunohistochemistry for the expression of GFP. Clusters of GFP expressing fibers were seen in all the TA muscles that received GFP expressing donor cells with a mean of 61.8 ± 11.1 (N=11) GFP positive fibers/TA cross section, demonstrating that myosphere cells can contribute to injured muscle in vivo (Figs. 6 and 7). In addition, we also observed the presence of GFP positive myonuclei (Fig. 7A) as well as GFP positive mononuclear cells, which were found immediately adjacent to, but within the basal lamina of the myofibers (Fig. 7B). There was no GFP expression seen in the contralateral TA muscles, which received only HANKS.
Figure 6. Myospheres contribute to regeneration of muscle.
Donor myosphere cells expressing GFP were injected into the TA muscle of CTX injured mdx mice. Two weeks after injection the mice were sacrificed. Cross-sections of the injected TA muscles were stained for laminin (detected by Alexa 647) and GFP positive fibers (indicating incorporation of donor cells) were detected using a narrow bandpass GFP filter, which greatly minimizes autofluorescence [44]. Laminin is shown in red (left panel), GFP in green (center panel), and the merging of the laminin and GFP (right panel). Scale bar represents 50 μm.
Figure 7. Myospheres contribute to mononuclear cells in regenerating muscle.
Donor myosphere cells expressing GFP were injected into the TA muscle of CTX injured mdx mice. Two weeks after injection the mice were sacrificed. Cross-sections of the injected TA muscles are shown. (A) GFP staining detected by CY3 shown in red (left panel), DAPI staining (nuclear, center panel), and the merger of GFP and DAPI (right panel). Yellow arrows indicate GFP positive nuclei from the donor cells and white arrows indicate GFP negative nuclei of the recipient mouse. (B) Laminin staining detected by Alexa 647 shown in red (left panel), DAPI staining (nuclear, center panel), and a merger of laminin and DAPI staining with GFP (right panel). The yellow arrow shows a donor mononuclear cell found within the basal lamina (GFP positive, shown in green) and the white arrow shows a mononuclear cell from the recipient mouse (GFP negative). Scale bars represents 50 μm.
Discussion
Here we present evidence demonstrating that muscle stem cells can be reproducibly isolated and maintained in culture for several months as free-floating myospheres. Furthermore, we were able to isolate myospheres not only from the muscles of young mice but also from very old mice. When we examined the cells composing myospheres for surface markers, we found that these cells were highly positive for the expression of stem cell marker Sca-1 as well as for a mesenchymal stem cell marker CD90, however they did not express the hematopoietic marker CD45 or the endothelial marker CD31. Since our myosphere cells expressed β1 integrin and CD34, which are also expressed by muscle satellite cells [11, 41, 42], we examined them for expression of myogenic markers to determine if myospheres were composed of resident muscle stem cells and found that myosphere cells did not express satellite cell markers Pax7 or Myf5. Given that Pax7 is the upstream regulator of myogenesis and that the expression of Pax7 is required for satellite cells to generate committed myogenic precursors [18, 19, 22], we next speculated that the cells composing myospheres may be “pre-myogenic” because they did not express Pax7 and Myf5, but may have the potential to express Pax7 and Myf5 upon maturation. This theory was confirmed by examining the progeny of myosphere cells, which were allowed to differentiate by culturing dissociated myospheres adherently in media containing serum. The adherently grown cells, which we referred to as MDACs, were found to be similar to primary myoblasts, and like primary myoblasts expressed myogenic markers Pax7, Myf5, MyoD, and desmin demonstrating that myospheres, which are composed of Pax7 negative cells, can upon maturation generate Pax7 positive cells that are myogenic (MDACs). We also found that the MDACs and primary myoblasts exhibited similar FACS profiles where both expressed β1 integrin, CD34 and Sca-1, while neither expressed hematopoietic (CD45), endothelial (CD31), or mesenchymal (CD90) markers. In addition, when MDACs were plated densely and allowed to differentiate further, they easily formed multinucleated myotubes. We further confirmed the pre-myogenic nature of the myosphere cells by in vivo studies, in which GFP labeled myosphere cells were injected into the TA muscle of cardiotoxin injured mdx mice. The presence of GFP positive fibers and myonuclei demonstrate that myosphere cells were able to contribute to regenerating muscle. In addition, some GFP positive mononuclear cells were observed within the basal lamina immediately adjacent to the myofibers, indicating that myosphere cells may also contribute to the satellite cell niche.
To better understand the makeup of the myospheres, we compared our myosphere cells to other reported sphere forming cells derived from muscle. These cells fell into two categories. The first category of muscle sphere forming cells, are those that lean towards a myogenic fate [31, 33]. In comparing our myosphere cells to those presented recently by Sarig, et al. [33] and Arsic, et al. [31] we found that our myosphere cells were more primitive, given that our cells not only express higher levels of Sca-1 (>80% versus 4–20% or 65%) but also have not yet begun to express myogenic markers such as Pax7, Myf5, or MyoD. It is interesting to note that some of the differences between our myosphere cells and these other cells may be due to differences in culture conditions such as the fact that our myospheres are grown only in serum free media whereas these other sphere-forming cells were grown in media containing fetal calf serum. Another striking difference we found between our myosphere cells and these other cells is that when our myosphere cells are grown adherently as MDACs they were able to form multinucleated myotubes more readily than the progeny of either of these previously mentioned sphere-forming cells. The second category of muscle derived spheres are quite different from the myospheres presented here in that these spheres are derived from vessel walls found within the muscle and as such instead of leaning toward a myogenic fate these cells lean towards an endothelial fate [34, 35].
Interestingly, we found that myospheres appeared to have a lot in common with muscle SP cells. For example both myosphere and muscle SP cells are cultured non-adherently [16, 43] and both are highly positive for the expression of Sca-1 [16, 17]. Additionally, myosphere and muscle SP cells both exhibit some mesenchymal stem cell properties such as the expression of CD90 [30] and the ability to differentiate into adipogenic and osteogenic cells [30]. However when taking a closer look we determined that myosphere cells were not SP cells in that unlike muscle SP cells the cells composing myospheres do not exclude Hoechst dye [6, 16]. We also noted that even though myosphere cells have mesenchymal-like properties they are not mesenchymal stem cells because myosphere cells express CD34, which is not normally expressed by mesenchymal stem cells [26, 27]. The question that remains is what is the origin of these cells that grow as non-adherent spheres and to answer that question more information will be needed.
In conclusion, here we present evidence that a unique population of muscle stem cells can be reproducibly isolated from skeletal muscle of mice. It is our belief that because myospheres can be easily isolated and propagated from mice of all ages that these cells represent an important new frontier in our ability to isolate and maintain muscle stem cells in culture, which in turn could extend the possibilities of cell-based therapies for muscular diseases.
Supplementary Material
These images show adherent myospheres in which the cells composing the myospheres are maturing and migrating away from the sphere. Scale bars in both pictures represent 100 μm.
These images show the growth of clonally derived myospheres after sorting for Sca-1+/CD31−/CD45− cells. In the first 3 panels cells were plated at a density of one cell per well, in the last panel cells were sorted and plated at a density of 1×105 cells/ml. The time these cells remained in culture after the sort is indicated on each panel. Scale bars represent 100μm.
FACS profiles of myosphere cells showing these cells do not exclude Hoechst dye. Profiles show Hoechst staining on the y-axis versus PI on the x-axis. (A) Staining in the presence of verapamil (which inhibits Hoechst efflux) and (B) without verapamil. Cells found within the boxed region exclude Hoechst dye.
These images show the growth of myospheres over time from pooled cultures isolated at the same time from the same adult mice. The upper panels show cultures that were maintained in B27 media containing 15% FCS while the lower panels show cultures maintained in serum free B27 media. Although spheres formed more quickly in serum containing media, these spheres quickly became adherent and the cells composing the spheres began to differentiate. Myospheres that were formed in serum free media formed more slowly and could be maintained for longer periods of time in a more primitive state as non-adherent spheres (shown here at 1 month). The time in which these the cells remained in culture after isolation is indicated on each panel. Scale bars represent 100μm.
Acknowledgments
We thank Emanuela Gussoni for her advice and critical reading of the manuscript. This work was supported by grants from NIH-NIAMS (5KO1AR52372) to KAW and (2R01AR43140, and 1P01AR044750) to PDA.
Role of the Funding Source
The funding sources include the NIH-NIAMS (KO1, RO1, and a PO1) and Brigham and Women’s Department of Anesthesia. These funding sources had no involvement with the design, research, or publication of this paper.
Footnotes
Disclosure Statement
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen A. Westerman, Email: kwest@zeus.bwh.harvard.edu.
Ashley Penvose, Email: apenvose@partners.org.
Zhong Yang, Email: zyang@zeus.bwh.harvard.edu.
Paul D. Allen, Email: allen@zeus.bwh.harvard.edu.
Charles A. Vacanti, Email: cvacanti@partners.org.
References
- 1.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 4.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 5.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;Chapter 2(Unit 2B):1. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 6.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 9.Epting CL, Lopez JE, Pedersen A, Brown C, Spitz P, Ursell PC, Bernstein HS. Stem cell antigen-1 regulates the tempo of muscle repair through effects on proliferation of alpha7 integrin-expressing myoblasts. Exp Cell Res. 2008;314:1125–1135. doi: 10.1016/j.yexcr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell PO, Mills T, O’Connor RS, Kline ER, Graubert T, Dzierzak E, Pavlath GK. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol. 2005;283:240–252. doi: 10.1016/j.ydbio.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Jankowski RJ, Deasy BM, Cao B, Gates C, Huard J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 14.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 19.McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 22.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Motohashi N, Uezumi A, Yada E, Fukada S, Fukushima K, Imaizumi K, Miyagoe-Suzuki Y, Takeda S. Muscle CD31(−) CD45(−) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am J Pathol. 2008;173:781–791. doi: 10.2353/ajpath.2008.070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 29.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 30.Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, Takeda S. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun. 2006;341:864–873. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Arsic N, Mamaeva D, Lamb NJ, Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarig R, Baruchi Z, Fuchs O, Nudel U, Yaffe D. Regeneration and transdifferentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells. 2006;24:1769–1778. doi: 10.1634/stemcells.2005-0547. [DOI] [PubMed] [Google Scholar]
- 34.Nomura T, Ashihara E, Tateishi K, Asada S, Ueyama T, Takahashi T, Matsubara H, Oh H. Skeletal myosphere-derived progenitor cell transplantation promotes neovascularization in delta-sarcoglycan knockdown cardiomyopathy. Biochem Biophys Res Commun. 2007;352:668–674. doi: 10.1016/j.bbrc.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 35.Grenier G, Scime A, Le Grand F, Asakura A, Perez-Iratxeta C, Andrade-Navarro MA, Labosky PA, Rudnicki MA. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis. Stem Cells. 2007;25:3101–3110. doi: 10.1634/stemcells.2006-0795. [DOI] [PubMed] [Google Scholar]
- 36.Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455–460. [PubMed] [Google Scholar]
- 37.Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerman KA, Ao Z, Cohen EA, Leboulch P. Design of a trans protease lentiviral packaging system that produces high titer virus. Retrovirology. 2007;4:96. doi: 10.1186/1742-4690-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, van der Kooy D. Don’t look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26:2938–2944. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liadaki K, Luth ES, Kunkell LM. Co-detection of GFP and dystrophin in skeletal muscle tissue sections. Biotechniques. 2007;42:699–700. doi: 10.2144/000112494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These images show adherent myospheres in which the cells composing the myospheres are maturing and migrating away from the sphere. Scale bars in both pictures represent 100 μm.
These images show the growth of clonally derived myospheres after sorting for Sca-1+/CD31−/CD45− cells. In the first 3 panels cells were plated at a density of one cell per well, in the last panel cells were sorted and plated at a density of 1×105 cells/ml. The time these cells remained in culture after the sort is indicated on each panel. Scale bars represent 100μm.
FACS profiles of myosphere cells showing these cells do not exclude Hoechst dye. Profiles show Hoechst staining on the y-axis versus PI on the x-axis. (A) Staining in the presence of verapamil (which inhibits Hoechst efflux) and (B) without verapamil. Cells found within the boxed region exclude Hoechst dye.
These images show the growth of myospheres over time from pooled cultures isolated at the same time from the same adult mice. The upper panels show cultures that were maintained in B27 media containing 15% FCS while the lower panels show cultures maintained in serum free B27 media. Although spheres formed more quickly in serum containing media, these spheres quickly became adherent and the cells composing the spheres began to differentiate. Myospheres that were formed in serum free media formed more slowly and could be maintained for longer periods of time in a more primitive state as non-adherent spheres (shown here at 1 month). The time in which these the cells remained in culture after isolation is indicated on each panel. Scale bars represent 100μm.