Abstract
Objective
A dysfunctional neural reward system has been shown to be associated with alcoholism. The current study aims to examine reward processing in male alcoholics by using event related potentials (ERPs) as well as behavioral measures of impulsivity and risk-taking.
Methods
Outcome related negativity (ORN/N2) and positivity (ORP/P3) derived from a single outcome gambling task were analyzed using a mixed model procedure. Current density was compared across groups and outcomes using standardized low resolution electromagnetic tomography (sLORETA). Behavioral scores were also compared across groups. Correlations of ERP factors with behavioral and impulsivity factors were also analyzed.
Results
Alcoholics showed significantly lower amplitude than controls during all outcome conditions for the ORP component and decreased amplitude during the loss conditions for the ORN component. Within conditions, Gain produced higher amplitudes than Loss conditions. Topographically, both groups had an anterior focus during Loss conditions and posterior maxima during Gain conditions, especially for the ORN component. Decreased ORP current density at cingulate gyrus and less negative ORN current density at sensory and motor areas characterized the alcoholics. Alcoholics had higher levels of impulsivity and risk-taking features than controls.
Conclusions
Deficient outcome/reward processing and increased impulsivity and risk-taking observed in alcoholics may be at least partly due to reward deficiency and/or dysfunctional reward circuitry in the brain, suggesting that alcoholism can be considered as part of the cluster of the reward deficiency syndrome (RDS).
Keywords: Outcome-related negativity, Outcome-related positivity, Error-related negativity, Medial Frontal Negativity, N2, P3, impulsivity, alcoholism, Reward Deficiency Syndrome
1. Introduction
Alcoholism has been considered to be a complex neuropsychiatric condition with multifactorial etiology, and understanding of this disorder has warranted studies of diverse neurobiological methods. Event-related potentials (ERPs), derived from the scalp-recorded electroencephalogram (EEG) during a task condition, is considered to be one of the most effective and useful measure to understand the neurocognitive dysfunctions in alcoholism (Begleiter et al., 1980, 1984; Porjesz et al., 2005b). The amplitude reduction of the P3 component of the ERPs, a robust positivity around 300 ms, has been considered to be a marker for alcoholism and risk (Begleiter et al., 1984; Porjesz and Begleiter, 1990; Porjesz et al., 1998). Further, this ‘P3-amplitude reduction’ (P3-AR) was also found to be common for a host of similar disorders called externalizing/disinhibitory disorders that often coexist with alcoholism (Patrick et al., 2006; Carlson et al., 2007).
Recent imaging studies have examined the brain reward system in alcoholics and suggested that alcoholism may be a part of a spectrum of disorders subsumed under a Reward Deficiency Syndrome (RDS), as alcoholics showed abnormalities in the brain structures related to the reward network (Wrase et al., 2007; Makris et al., 2008; de Greck et al., 2009; Tanabe et al., 2009). ERP studies can further characterize the “millisecond-specific” brain dynamics of reward processing as well as deficiency in alcoholics. Although several ERP studies on reward processing (during monetary reward conditions) in healthy human subjects have been previously documented since the early 1980’s (e.g., Homberg et al., 1980, 1981; Begleiter et al., 1983; Otten et al., 1995; Ramsey and Finn, 1997; Gehring and Willoughby, 2002; Yeung and Sanfey, 2004; Hajcak et al., 2006; Kamarajan et al., 2009), there have been very few ERP studies on the reward/outcome processing in individuals diagnosed with alcohol dependence. To our knowledge, there have been only two ERP studies carried out on alcoholics during reward processing. Probably the first study of this kind was done by Porjesz et al. (1987a), who reported decreased P3 amplitude in response to incentive stimuli in abstinent alcoholics. More recently, using the Balloon Analogue Risk Task (BART) which measures risk-taking propensity, Fein and Chang (2008) reported smaller amplitude in feedback negativity in treatment-naive alcoholics with a greater family history density of alcohol problems compared to controls. Although these findings lend support to the notion that alcoholics may have a specific deficiency in reward processing, the nature of these deficits are still not very clear due to the paucity of such studies in alcoholics. Further, there have been as yet no studies of reward processing in alcoholics using a gambling paradigm, and the present study is the first of its kind.
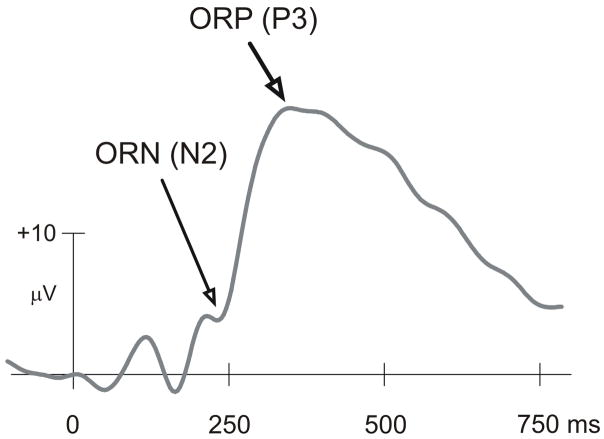
In recent years, a predominant electrophysiological task paradigm has been used to study reward processing, namely the “Gambling Paradigm” and the two ERP components that have been reported to occur during outcome processing are the negativity analogous to N2 (between 200–300 ms) and the positivity analogous to P3 (between 300–600 ms) as shown in Fig. 1 (Gehring and Willoughby, 2002; Luu et al., 2004; Nieuwenhuis et al., 2004, 2005b; Yeung and Sanfey, 2004; Cohen et al., 2007; Mennes et al., 2008; Kamarajan et al., 2009). While these components have been referred to by different names, we introduced and justified the terms ORN and ORP in our earlier works on outcome processing (Kamarajan et al., 2008; Kamarajan et al., 2009) respectively in the gambling task. In our earlier study, we analyzed the ERP waveforms, topography and functional significance of the ORN and ORP components in healthy individuals using a Single Outcome Gambling (SOG) task that involved monetary losses and gains (Kamarajan et al., 2009). To our knowledge, only very few studies have examined the ERP components of monetary reward processing in alcoholics, and the current study is the first study on alcoholics using a typical “gambling paradigm”.
Fig. 1.
Typical ERP waveform at the CZ electrode as produced by the single outcome gambling task. The ORN component that occurs approximately between 200 ms and 275 ms and the ORP component that lies approximately between 275 ms and 700 ms are considered to be important in the evaluative processes during loss and gain.
The main goal of the present study is to examine reward/outcome processing in alcohol dependent individuals as compared to healthy controls while they subjectively experience monetary loss and gain during the performance of a gambling task. Since impulsivity is an important component of alcoholism as depicted both by models of disinhibition (Gorenstein and Newman, 1980; Begleiter and Porjesz, 1999; Krueger et al., 2002) as well as models of reward deficiency syndrome (Blum et al., 2000; Bowirrat and Oscar-Berman, 2005), we studied impulsivity in detail, both as a state measure (i.e. the task performance) and as a trait measure using Barratt impulsiveness scale (BIS) (Barratt, 1985; Patton et al., 1995). Although previous findings reported that ORN was localized to medial frontal areas (Gehring and Willoughby, 2002; Nieuwenhuis et al., 2004; Masaki et al., 2006), our earlier study using sLORETA (standardized low resolution electromagnetic tomography) (Pascual-Marqui, 2002) in healthy individuals showed that ORN during the loss conditions had a medial frontal source while the ORN for the gain conditions primarily had a medial posterior source (Kamarajan et al., 2009). Therefore, in the current study, we have used the sLORETA to further understand the possible alterations in the current density and/or source activities of ORN and ORP in alcoholic individuals. In addition, since there were distinct gender differences observed in the electrophysiological indices of reward processing (Kamarajan et al., 2008, 2009), it was decided to analyze each gender separately. Therefore, the present study has been designed to examine the ERP components only in male alcoholics (as there were too few female alcoholics to have a combined sample at the time of the study). Our hypotheses were the following: 1) alcoholics will show decreased amplitude in both ORN and ORP components; 2) the source activity of the reward processing will be localized (by the sLORETA) to regions of frontal lobes and reward circuitry, 3) alcoholics will have higher impulsivity, increased risk-taking and decreased cognitive control on the behavioral measures; and 4) lower amplitude in ORN and ORP components will be correlated with increased impulsivity and risk-taking. It is expected that alcoholics will demonstrate deficient reward processing in terms of decreased amplitude in both ORN and ORP components, apart from significant differences in topography, current density, and in behavioral measures of impulsivity and risk-taking.
2. Methods
2.1. Participants
A sample of 40 male alcoholics with an age range of 24–46 years (Mean = 38.28, SD = 6.44) and 40 healthy male controls aged between 18 and 35 years (Mean = 21.07, SD = 3.36) were selected. Control subjects were recruited through newspaper advertisements and notices. The alcoholic group consisted of alcohol dependent individuals who completed the de-addiction program in the treatment centers, and were abstinent from alcohol intake at least for 28 days before the EEG recording. The Bard/Porjesz Adult Alcoholism Battery (BAAB), a semi-structured clinical assessment schedule based on DSM IV, was used to obtain the clinical data related to alcohol dependence and alcohol-related medical problems. The patients who were receiving treatment medication, such as antabuse and/or psychoactive drugs, were excluded from the study to avoid the possible interaction of drugs with the EEG profile. The participants did not have any other personal and/or family history of major medical or psychiatric disorders and substance-related addictive illnesses. Subjects who had positive findings (for their recent drug use within 48 hours) in the urine screen and Breathalyzer test were excluded from the study. Subjects with hearing or visual impairment, liver disease, or head injury were also excluded from the study. The individuals who scored less than 21 on the mini mental state examination (MMSE) (Folstein et al., 1975) were excluded from the study in order to rule out possible cognitive deficits due to an organic pathology. The MMSE scores in the final sample ranged from 21 to 30 with a mean of 27.77 for alcoholics while controls had a score range of 23–30 with a mean of 27.88. Experimental procedures and ethical guidelines were in accordance with approval of the institutional review board (IRB).
2.2. The gambling task
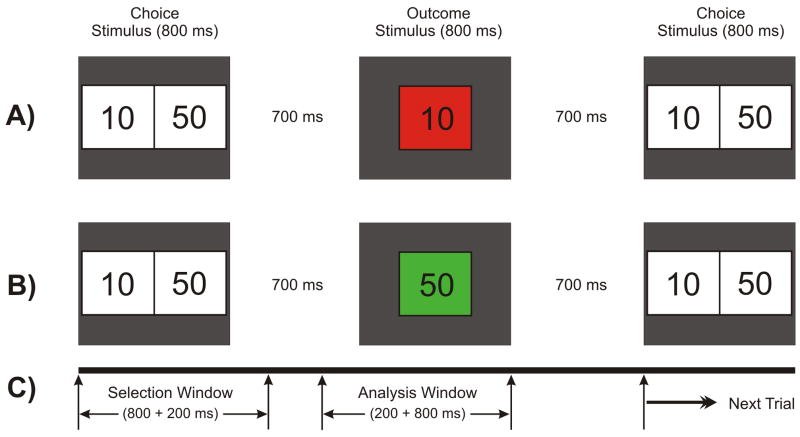
The Single Outcome Gambling (SOG) task used in the study is illustrated in Fig. 2. At the start of each trial, a choice stimulus (CS) with two numbers 10 (left box) and 50 (right box), representing the monetary value in US cents, was displayed for 800 ms. The subject was instructed to select one number by pressing the left button for ‘10’ or the right button for ‘50’. The outcome stimulus (OS) appeared 700 ms after the CS offset and lasted 800 ms. The OS comprised the selected number inside a green box (to indicate a gain) or a red box (to indicate a loss). Thus, there were four possible outcomes: gain 50 (+50), loss 50 (−50), gain 10 (+10), and loss 10 (−10). The subject had to respond by selecting either 10¢ or 50¢ within 1000 ms of CS onset. The OS would not appear if the subject did not respond/select within the specified time (1000 ms), and the next trial would commence. While the occurrence of loss (in red) or gain (in green) in the OS was maintained at equal probability (50%), the order of appearance was pseudo-randomized. Each subject had the identical presentation (i.e. there was no counter-balancing of trials). The subjects were not made aware of the probability of loss/gain or sequence of the task prior to the experiment. There were a total of 172 trials and the inter-trial interval was 3000 ms throughout the experiment. The task was presented in two blocks with each block (86 trials) lasting for 4 minutes; the procedure was identical in both blocks. At the end of each block, the status of overall ‘loss’ or ‘gain’ for the entire block was displayed on the monitor screen. The next block was started by the operator when the subject was ready. The instruction to the subject was as follows: “This task is a gambling type task in which you will be playing with 10 and 50 cents in each trial of the task. Two boxes with the numbers 10 and 50 will appear on the screen. Button #1 corresponds to the number 10 and button #4 corresponds to the number 50. When you see the numbers, select one of them quickly by pressing the appropriate button. Following your selection, the number you choose will reappear in a red box or a green box. If the number you selected is in a green box you gained that amount of money. If it is in a red box you lost that amount. The experiment is conducted in two blocks, and you will see a message telling you whether you’re winning or losing money after each block. Please try not to blink and sit as still as possible.” At the end of the experiment, all the participants received the monetary reward of the total amount they had accrued during the gambling trials.
Fig. 2.
Schematic illustration of the single outcome gambling task used in this experiment. One of the two numbers (10 or 50) in the choice stimulus (800 ms) is to be selected by the subject. The selected amount appears as the outcome stimulus (800 ms) either in red (to indicate a loss) or in green (to indicate a gain). A) a typical trial showing a loss of 10 in red box; B) another trial having a gain of 50 in green box; and C) the time duration for the task events: the selection window (1000 ms) wherein the subject selects either of the numbers and the analysis window (200 ms prestimulus + 800 ms poststimulus) represents the time segment that was used for the ERP analysis.
2.3. Measures of impulsivity
There were two types of impulsivity measures used in the study: 1) Barratt impulsiveness scale, version 11 (BIS-11) (Barratt, 1985; Patton et al., 1995), a self-rated measure that assesses trait-related impulsivity, and 2) task-related behavioral (TRB) scores as derived from the performance of the gambling task. The BIS-11 consists of thirty items yielding a total score, and additional scores for three subcategories: motor impulsivity (acting without thinking), cognitive impulsivity (making decisions quickly), and non-planning (lack of prior planning or of future orientation). The TRB scores were of 3 categories: 1) reaction time (RT) for the task conditions and responses, 2) selection frequency (SF)—number of times a particular amount (10 or 50) was chosen—following a single trial of loss and following two consecutive trials of loss (based on the absolute score), and 3) SF followed by a losing or gaining trend (based on the cumulative score) in the previous 2 to 4 trials. The gaining and losing trends were computed based on the resultant outcome of the cumulative account of the preceding outcomes. For example, if the previous three outcomes were −10, −10, and +50, then the trend was considered to be a gain (of 30¢), whereas if the previous three outcomes were +10, +10, and −50 then the trend would be considered as a loss (of 30¢).
2.4. EEG data acquisition and signal analysis
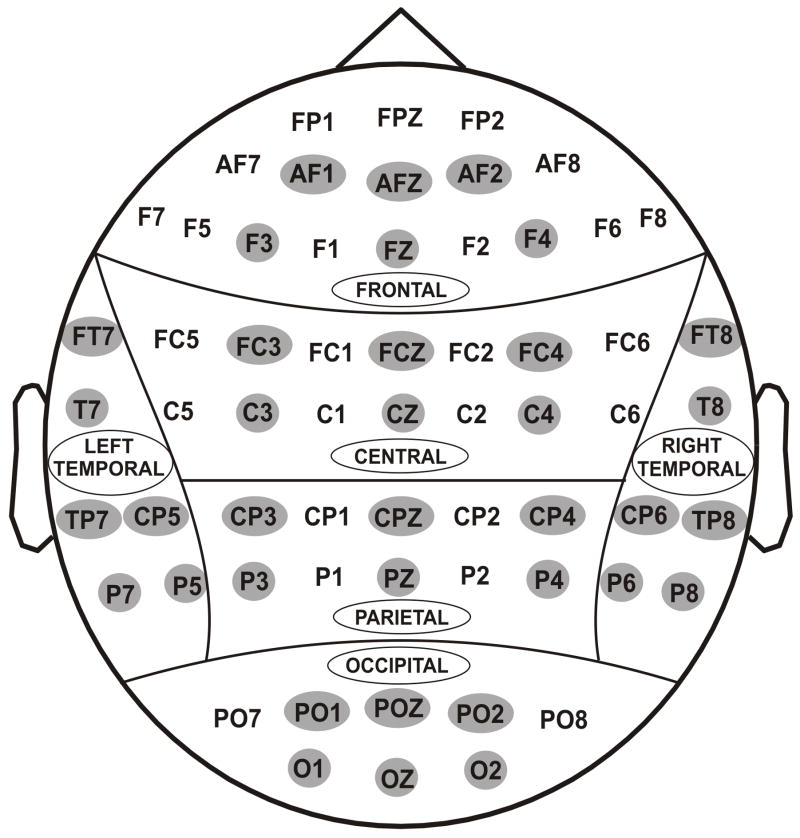
EEG was recorded on a Neuroscan system (Version 4.1) using a 61-channel electrode cap (see Fig. 3), referenced to the tip of the nose with a ground electrode at the forehead. A supraorbital vertical lead and a horizontal lead on the external canthus of the left eye recorded the electro-oculogram (EOG). Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02–100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). EEG segments that exceeded ±75 μV threshold were rejected as artifacts. The grand averaged ERPs of each individual were also screened visually for further artifact rejection. Although the entire experiment consisted of two identical blocks, the analysis was done on all trials by combining the trials from both blocks. The statistical analyses were performed on the amplitude and latency data of ORN and ORP components.
Fig. 3.
Sixty one electrodes as recorded from the surface of the scalp. For statistical analyses, 36 electrodes (as highlighted) were selected to represent 6 electrodes in 6 regions of the brain viz., frontal, central, parietal, occipital, left-temporal and right-temporal.
The “outcome window” (1500 ms) began with the onset of OS, as the objective of the study was to analyze the outcome-related potentials of the ERPs (see Fig. 2). The ORP amplitude was measured as the voltage difference from the pre-stimulus baseline (200 ms) to the largest positive going peak in the waveforms filtered at 0.25–32.0 Hz in the latency window 275–700 ms after the stimulus onset, whereas the ORN was measured as a baseline-trough in the waveforms filtered at 2.0–16.0 Hz within post-stimulus 200–275 ms (Fig. 1). Since the ORP is very robust and prominent (compared to the ORN) and also involves slow wave activity (less than 2 Hz), the ORN component, which is often small and subtle, gets subsumed by the ORP component and is not apparent in the ERP signal. A filter setting of 2.0 – 16.0 Hz makes the ORN component relatively more prominent than with the regular filter setting of P3/ORP component. This approach of removing the slow wave activity has already been employed by several studies on error-related negativity (ERN) paradigms. For example, Luu et al. (2004) filtered the ERPs within a 4–12 Hz bandpass, while Trujillo and Allen (2007) used 3–13 Hz bandpass filter in order to optimize ERN component. In our previous study in healthy normals, we used a filter at 2.0 – 16.0 Hz for plotting the ORN topography (Kamarajan et al., 2009).
2.5. Current density analysis using sLORETA
Current density and source activity during reward processing were examined using sLORETA to identify brain regions involved and also to localize the brain deficits in alcoholics. The sLORETA is considered to be a successful solution to the inverse problem, i.e. the problem of localization of brain activity in the EEG and EMG data (Pascual-Marqui, 2002; Pascual-Marqui, 1999). Detailed description and technical information on sLORETA have been provided by its author, R.D. Pascual-Marqui, at: http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm. In the present study, the current density maps were created for the grand mean data of each condition in alcoholics and controls separately. Then, the time frames that represented the peak ORN and ORP activity were compared across groups and across conditions using the independent and paired t-tests respectively. Statistical analyses involved a voxel-wise Statistical non-Parametric Mapping (SnPM) with 5000 permutations. The t-values in all 2394 voxels were plotted and the t-values above the critical threshold (i.e. significance level) were highlighted (based on the color scale) to show the areas of significance. These values are calculated via a randomization method (Nichols and Holmes, 2002), and the SnPM approach controls for Type I error (Flor-Henry et al., 2004).
2.6. Statistical analysis of ERP data
Thirty six electrodes, as indicated in Figure 3, were grouped in to 6 scalp regions for the statistical analyses. The ERP data were analyzed by performing a linear mixed model of the Analysis of Variance (ANOVA) using the Statistical Analysis System (SAS, version 9.2) (SAS Institute Inc., NC 27513, USA). The application of mixed effects model of the Analysis of Variance (ANOVA) in our study is due to its several advantages over the traditional methods of ANOVA (Gueorguieva and Krystal, 2004), and has been successfully implemented by researchers to analyze EEG data (Bachman et al., 2008). The covariance structure used in the model was ‘Compound Symmetry’ which has a constant variance and constant covariance. The model included five factors as fixed effects: Valence (loss, gain), Amount (50¢, 10¢), Region (frontal, central, parietal, occipital, left-temporal, and right-temporal), Electrode (6 electrodes) as within-subjects factors, and Group (control, alcoholic) as a between-subjects factor. In order to keep the number of electrodes equal across each region, only 6 electrodes from each region were selected (as some regions had only 6 electrodes). We treated electrodes as nested within region, as we were interested in the “region” effects but not in the individual “electrode” effects. Age was included as a covariate in the ANOVA model as age as a factor is known to have a significant influence on the ERP measures (Walhovd et al., 2008).
The BIS and TRB variables were compared across groups using t-tests. Based on the analyses done in our previous study (Kamarajan et al., 2009), the correlations between ERP variables and Behavioral measures were analyzed in a 2-step procedure: 1) Factor analysis was performed in order to reduce the ERP variables (N = 144) as well as the TRB variables (N = 24) into a few specific factors; and 2) Pearson (bivariate) correlations were performed to analyze the relationship between behavioral factors and ERP factors. ERP variables for the factor analysis comprised 9 electrodes (F3, FZ, F4, C3, CZ, C4, P3, PZ, P4), 4 outcomes (+50, +10, −50, −10), 2 components (ORN, ORP), and 2 measures (amplitude, latency). For factor analysis, only 9 electrodes were selected for two reasons: 1) we expected that factors comprising fewer but representative electrodes of maximum amplitude would facilitate the interpretation of the components and their correlation with other factors (due to data reduction), and 2) the topography of ORN and ORP components suggested 3 major regions, viz., frontal, central, and parietal areas. The factors were extracted using principal component analysis (PCA), and varimax rotation with Kaiser normalization was performed. The optimal number of factors was determined based on the shape of the scree plots (i.e. components lie on the steep slope). However, factor analysis was not done on BIS scores as they were already categorized into three distinct factors (Barratt, 1985; Patton et al., 1995).
3. Results
3.1. ERP waveforms and topography
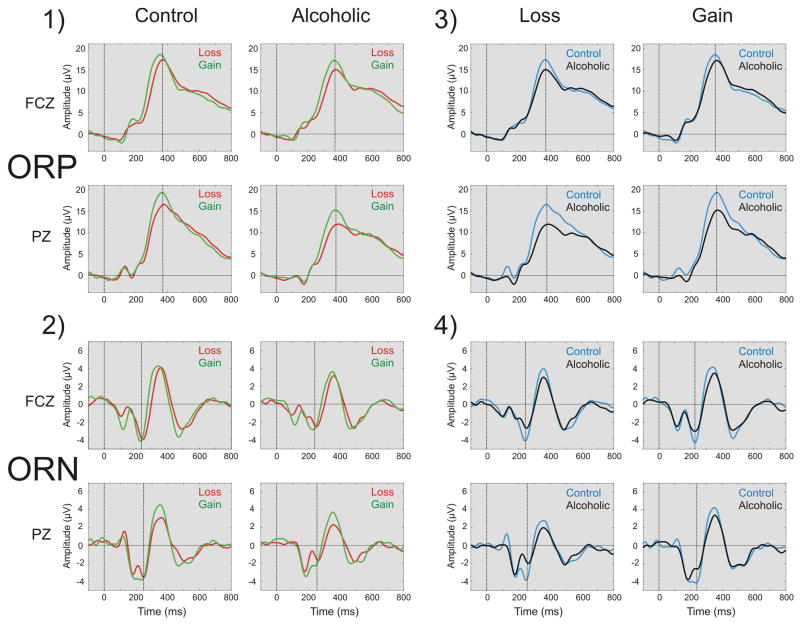
The ERP waveforms of comparisons among conditions in each group and between groups in each condition have been illustrated in Fig. 4. The Gain conditions (+50 and +10) had higher ORP amplitude than Loss conditions in both groups. Alcoholics showed decreased ORP amplitude compared to Controls in both Loss and Gain conditions. Although there were no significant condition differences, the ORN component showed a prominent group differences wherein the alcoholic group had a smaller ORN component than the control group.
Fig. 4.
The ERP waveforms compared across conditions (loss vs. gain) in each group (panels 1 & 2) and across groups (control vs. alcoholic) in each condition (panels 3 & 4) at FCZ and PZ electrodes at filter setting for ORP (0.25 – 16.0 Hz) shown in panels 1 & 3) and ORN (2.0 – 16.0 Hz) as shown in panels 2 & 4) respectively. ERPs during the Gain condition (+50) have higher ORP amplitudes than the Loss condition (−50) in both groups, more robust in the PZ electrode. Alcoholics have decreased ORP amplitude compared to Controls in both Loss and Gain conditions, especially at PZ electrode. ORN component showed only a group difference wherein alcoholics had a smaller ORN component than controls.
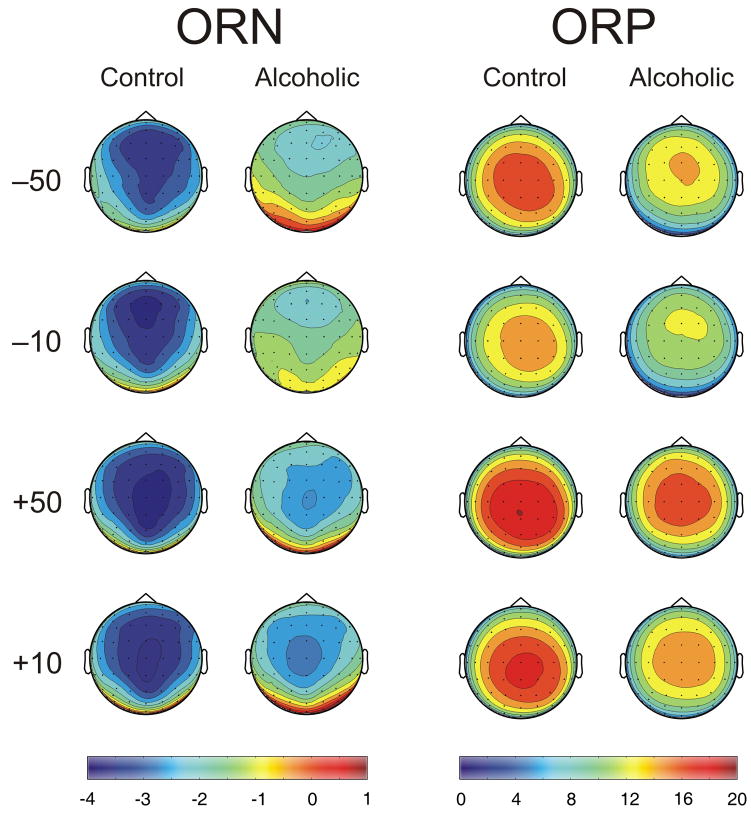
On the other hand, the topographic maps of both ORN and ORP, derived from the waveforms filtered at 2.0–16.0 Hz and 0.25–16.0, respectively, showed significant group as well as condition differences. The loss conditions had anterior maxima while the gain conditions had posterior maxima, especially for the ORN component. Alcoholics showed decreased amplitude in both ORN and ORP components in all conditions. While the amplitude differences were robust, topographic differences were not prominent.
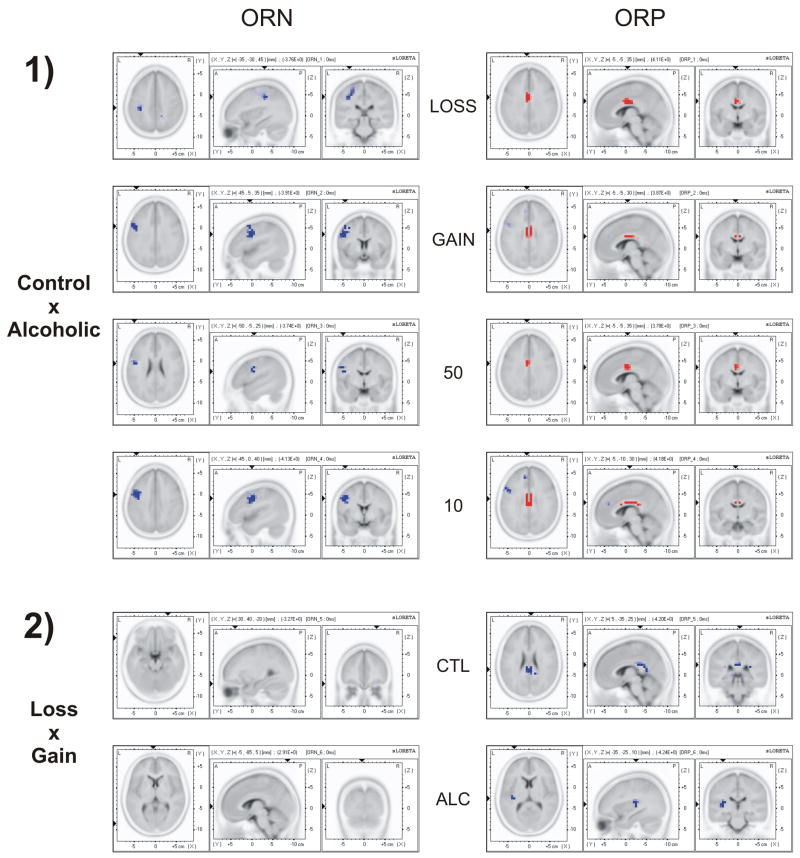
3.2. Current density across groups and conditions
The results of the voxel-by-voxel comparison of current density across groups and conditions are shown in Fig. 6 and Table 1. For the ORN component, alcoholics showed less negative current density compared to controls at postcentral gyrus, inferior frontal gyrus and precentral gyrus. In the ORP component, alcoholics showed decreased current density at anterior cingulate gyrus (BA-24). Differences between valences were significant only in the ORP component, wherein Gain showed more activity than Loss at posterior cingulate area (BA-23) in controls and at the insula region in alcoholics. However, current density differences were not significant for amount.
Fig. 6.
The sLORETA (current density) images of voxel-by-voxel t-statistics comparisons of ORN (left-side panels) and ORP (right-side panels) between Control and Alcoholic groups (panel set 1), and between Loss and Gain conditions (panel set 2). Alcoholics showed decreased ORP activity at cingulate gyrus (as marked in red in top-right panels) and less negative ORN current density (as marked in blue in top-left panels) related activity at postcentral gyrus, inferior frontal gyrus and precentral gyrus. Gain showed more ORP activity than Loss at posterior cingulate area in controls and at insula region in alcoholics (as marked in blue in bottom-right panels). Differences between valences were not significant in the ORN component. Neither ORN nor ORP was significant between the amounts 50 and 10 (not shown). The blue color in the images indicates significant negative t-values while the red color indicates significant positive t-values.
Table 1.
The voxel-by-voxel comparison of current density across groups and conditions. For ORN, alcoholics showed less negative current density than controls at postcentral gyrus, inferior frontal gyrus and precentral gyrus. For ORP, alcoholics showed decreased current density at cingulate gyrus. Differences between valences were significant only in the ORP component. Neither ORN nor ORP was significant between the amounts 50 and 10.
| ERP component | Comparison between | Factor compared | Direction of Significance | MNI Coordinates [BA] | Brain Area | Lobe |
|---|---|---|---|---|---|---|
| ORN | Ctl × Alc | Loss | Ctl < Alc | −35, −30,−45 [2] | Postcentral Gyrus | Parietal Lobe |
| Gain | Ctl < Alc | −45,−5, −5 [9] | Inferior Frontal Gyrus | Frontal Lobe | ||
| 50 | Ctl < Alc | −50, −5,−25 [6] | Precentral Gyrus | Frontal Lobe | ||
| 10 | Ctl < Alc | −45,−0,−40 [6] | Precentral Gyrus | Frontal Lobe | ||
| Loss × Gain | Ctl | None | – | – | – | |
| Alc | None | – | – | – | ||
| 50 × 10 | Ctl | None | – | – | – | |
| Alc | None | – | – | – | ||
| ORP | Ctl × Alc | Loss | Alc < Ctl | −5, −5, 35 [24] | Anterior Cingulate Gyrus | Limbic system |
| Gain | Alc < Ctl | −5, −5, 30 [24] | Anterior Cingulate Gyrus | Limbic system | ||
| 50 | Alc < Ctl | −5, −5, 35 [24] | Anterior Cingulate Gyrus | Limbic system | ||
| 10 | Alc < Ctl | −5, −10, 30 [24] | Anterior Cingulate Gyrus | Limbic system | ||
| Loss × Gain | Ctl | Loss < Gain | −5, −35, 25 [23] | Posterior Cingulate Gyrus | Limbic system | |
| Alc | Loss < Gain | −35, −25, 10 [13] | Insula | Sub-lobar | ||
| 50 × 10 | Ctl | None | – | – | – | |
| Alc | None | – | – | – |
Ctl – Control, Alc – Alcoholic, BA – Brodmann Area, None – Not Significant.
3.3. Mixed model ANOVA
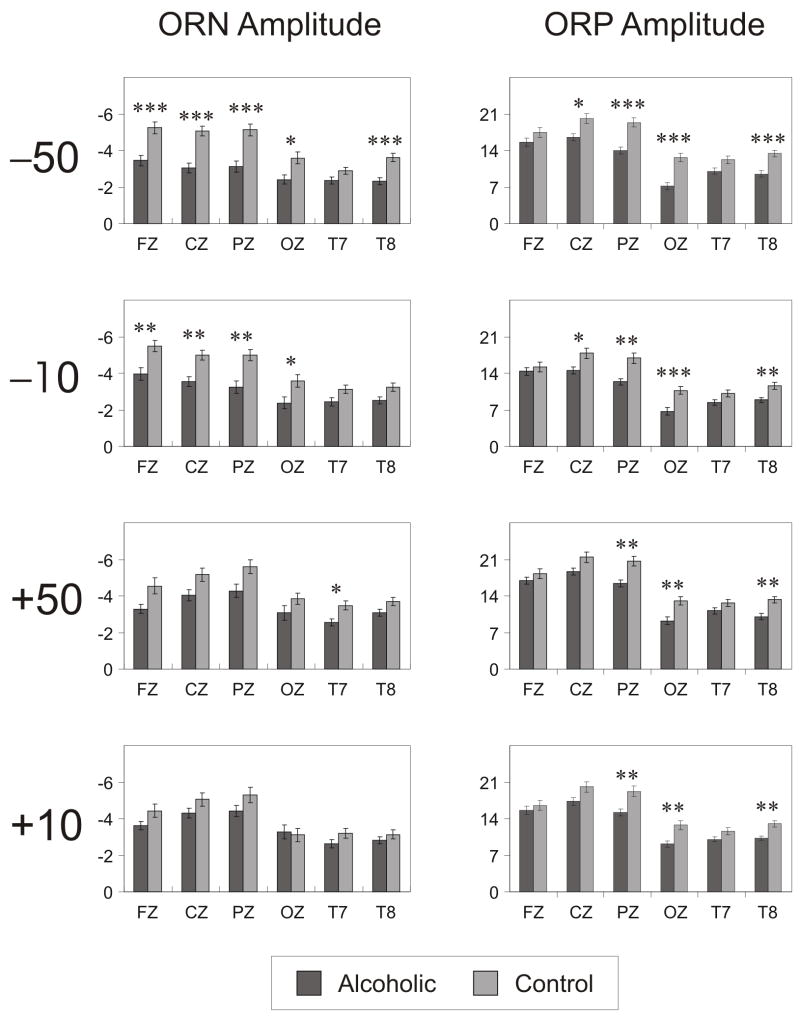
Results of the mixed model ANOVA have been tabulated in Table 2. Group as a main effect was not significant. Main effects of Valence, Amount and Region were significant. Gain condition as well as large amount (50) had higher amplitude and shorter latencies than Loss condition and small amount (10), respectively. Frontal, central and parietal areas had higher amplitudes and shorter latencies than occipital, left temporal and right temporal areas. Group × Outcome interaction was significant in all four measures while Group × Amount interaction was significant only for the amplitudes of ORN and ORP. Group × Valence × Region was significant only for the ORN measures, and the Group × Amount × Region was not significant in any of the ERP measures. Pairwise comparisons were done to explain these findings (see Figs. 7 and 8). Comparisons of ORN and ORP amplitudes across groups in each outcome condition at different electrode sites are illustrated in Fig. 7 along with Bonferroni adjusted significance levels. Alcoholics showed significantly lower ORP amplitude during all outcome conditions and decreased ORN amplitude during loss conditions (−50 and −10). Latencies showed no group differences (not shown).
Table 2.
Results of the mixed model ANOVA, showing the main and interaction effects (rows) in terms of F-values and p-values for ORN and ORP measures (columns). Group × Valence interaction was significant in all four measures while Group × Amount interaction was significant only for the amplitudes of ORN and ORP.
| ORN Amplitude | ORN Latency | ORP Amplitude | ORP Latency | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Group | 3.83 | 0.0541 | 1.98 | 0.1636 | 0.19 | 0.6630 | 0.00 | 0.9731 |
| Valence | 87.60 | <0.0001*** | 880.46 | <0.0001*** | 599.67 | <0.0001*** | 875.46 | <0.0001*** |
| Amount | 1.11 | 0.2963 | 1.25 | 0.2661 | 380.42 | <0.0001*** | 22.90 | <0.0001*** |
| Region | 119.37 | <0.0001*** | 61.67 | <0.0001*** | 786.77 | <0.0001*** | 164.43 | <0.0001*** |
| Group × Valence | 96.54 | <0.0001*** | 13.51 | 0.0004*** | 23.90 | <0.0001*** | 19.87 | <0.0001*** |
| Group × Amount | 44.93 | <0.0001*** | 2.32 | 0.1316 | 18.15 | <0.0001*** | 0.18 | 0.6766 |
| Group × Region | 10.36 | <0.0001*** | 1.36 | 0.2378 | 63.75 | <0.0001*** | 6.13 | <0.0001*** |
| Valence × Amount | 14.58 | 0.0003*** | 39.06 | <0.0001*** | 41.52 | <0.0001*** | 36.96 | <0.0001*** |
| Valence × Region | 24.98 | <0.0001*** | 3.05 | 0.0103* | 6.56 | <0.0001*** | 3.67 | 0.0029** |
| Amount × Region | 4.00 | 0.0015** | 1.61 | 0.1558 | 3.94 | 0.0017** | 0.80 | 0.5475 |
| Group × Valence × Amount | 12.48 | 0.0007*** | 9.44 | 0.0029** | 7.21 | 0.0089** | 0.09 | 0.7646 |
| Group × Valence × Region | 3.95 | 0.0017** | 3.20 | 0.0076** | 0.16 | 0.9781 | 0.94 | 0.4545 |
| Group × Amount × Region | 0.44 | 0.8212 | 1.35 | 0.2437 | 0.21 | 0.9580 | 1.63 | 0.1499 |
| Valence × Amount× Region | 0.66 | 0.6529 | 1.06 | 0.3833 | 0.68 | 0.6407 | 0.08 | 0.9952 |
| Electrode (Region) | 11.59 | <0.0001*** | 2.55 | <0.0001*** | 49.57 | <0.0001*** | 6.67 | <0.0001*** |
p < 0.05
p < 0.01
p < 0.001
Fig. 7.
The bar graphs show the comparison of ORN (left-side panels) and ORP (right-side panels) amplitudes between alcoholic and control groups during each of four outcomes. Six electrode sites are represented in the x-axis and the amplitude is scaled in the y-axis. Alcoholics showed significantly lower ORP amplitude during all outcome conditions and decreased ORN amplitude during loss conditions (−50 and −10). Latencies showed no group differences (not shown). Bonferroni adjusted significance level is marked with asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001). The error bars represent 1 Standard Error.
Fig. 8.
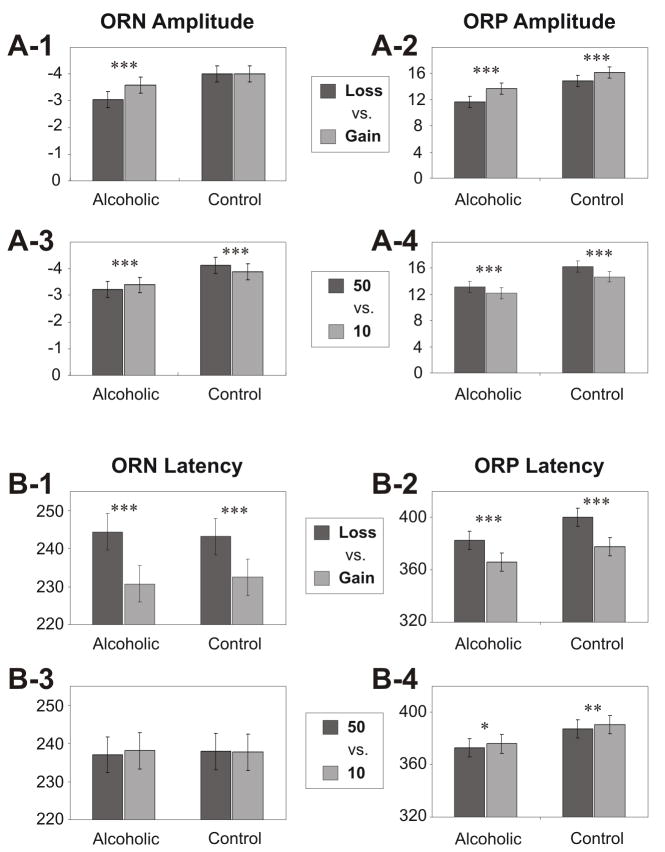
The bar graphs showing pair-wise comparisons of least squares means of ORN (left-side panels) and ORP (right-side panels) between different outcomes in control and alcoholic groups. In the ORP measures, Gain (compared to Loss) conditions and Large amount (compared to Small amount) conditions showed significantly higher amplitude and shorter latency in control as well as alcoholic groups. The findings on the ORN measures are: 1) amplitude differences between valences (loss vs. gain) were obvious only in alcoholic group (panel A-1), whereas latency differences were explicit in both groups (panel B-1); and 2) Alcoholics, in contrast to controls, showed more ORN amplitude for the small amount ‘10’ than for the large amount ‘50’ (panel A-3). Bonferroni adjusted significance level is marked with asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001). The error bars represent 1 Standard Error.
Comparisons across valences (loss vs. gain) and across amounts (50 vs. 10) are illustrated in Fig. 8 along with Bonferroni adjusted significance levels. For the ORP measures, both Gain and Large amount ‘50’ conditions showed significantly higher amplitude and shorter latency than Loss and Small amount ‘10’ conditions respectively, in control as well as alcoholic groups. The findings in ORN measures are: 1) amplitude differences between valences (loss vs. gain) were obvious only in the alcoholic group (panel A-1), whereas latency differences were explicit in both groups (panel B-1); and 2) Alcoholics, in contrast to controls, showed more ORN amplitude for small amount ‘10’ than for Large amount ‘50’ (panel A-3).
3.4. Group differences in behavioral variables
The statistical comparison of behavioral variables between controls and alcoholics is shown Table 3. Alcoholics scored significantly higher than controls in non-planning and total score of BIS. There was also a trend towards significance in motor impulsivity (i.e. loss of significance due to Bonferroni correction), and in selection frequency for ‘10’ after a Loss trend of previous 2 trials and for ‘50’ after a Loss trend of previous 3 trials.
Table 3.
Comparison of impulsivity scores between control (N=40) and alcoholic (N=40) groups. Mean, standard deviation (SD), t-value and p-value are shown. Alcoholics had significantly higher scores than controls in non-planning and total score of BIS. There was also a trend towards significance in motor impulsivity and in two of the selection frequency scores.
| Variable | Control | Alcoholic | t | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| BIS Total score | 60.67 | 9.75 | 68.26 | 9.36 | 3.42 | 0.0010** |
| BIS Non-planning | 22.39 | 3.89 | 26.00 | 4.33 | 3.77 | 0.0000*** |
| BIS Motor Impulsivity | 23.44 | 6.42 | 26.21 | 4.63 | 2.14 | 0.0360β |
| BIS Cognitive Impulsivity | 14.83 | 3.53 | 16.08 | 3.68 | 1.48 | 0.1420 |
| SF for ‘50’ after a single Loss trial | 38.62 | 3.08 | 38.58 | 3.63 | 0.05 | 0.9580 |
| SF for ‘50’ after two consecutive Loss trials | 19.79 | 4.00 | 21.10 | 4.92 | 1.29 | 0.2000 |
| SF for ‘50’ after a Loss trend of previous 2 trials | 29.77 | 6.43 | 31.40 | 5.94 | 1.17 | 0.2450 |
| SF for ‘10’ after a Loss trend of previous 2 trials | 28.59 | 5.92 | 25.22 | 7.70 | 2.17 | 0.0330β |
| SF for ‘50’ after a Gain trend of previous 2 trials | 53.54 | 9.34 | 56.25 | 9.45 | 1.28 | 0.2040 |
| SF for ‘10’ after a Gain trend of previous 2 trials | 46.28 | 9.01 | 47.22 | 8.86 | 0.47 | 0.6400 |
| SF for ‘50’ after a Loss trend of previous 3 trials | 38.59 | 8.04 | 43.02 | 9.51 | 2.24 | 0.0280β |
| SF for ‘10’ after a Loss trend of previous 3 trials | 36.97 | 7.84 | 34.45 | 9.25 | 1.31 | 0.1950 |
| SF for ‘50’ after a Gain trend of previous 3 trials | 44.36 | 8.30 | 44.18 | 6.95 | 0.11 | 0.9150 |
| SF for ’10’ after a Gain trend of previous 3 trials | 37.26 | 7.83 | 37.45 | 7.74 | 0.11 | 0.9120 |
| SF for ‘50’ after a Loss trend of previous 4 trials | 33.36 | 7.98 | 35.95 | 7.58 | 1.48 | 0.1430 |
| SF for ‘10’ after a Loss trend of previous 4 trials | 30.79 | 7.39 | 28.82 | 8.29 | 1.11 | 0.2690 |
| SF for ‘50’ after a Gain trend of previous 4 trials | 49.03 | 8.49 | 50.80 | 8.71 | 0.92 | 0.3620 |
| SF for ‘10’ after a Gain trend of previous 4 trials | 43.00 | 9.67 | 42.52 | 9.25 | 0.22 | 0.8240 |
| RT following −50 trials | 340.40 | 90.37 | 341.48 | 76.81 | 0.06 | 0.9540 |
| RT following −10 trials | 330.71 | 79.68 | 338.71 | 72.16 | 0.47 | 0.6410 |
| RT following +50 trials | 340.56 | 83.78 | 351.05 | 82.72 | 0.56 | 0.5770 |
| RT following +10 trials | 333.83 | 85.38 | 339.63 | 70.21 | 0.33 | 0.7420 |
| RT following loss trials (−50 and −10) | 335.12 | 82.46 | 339.68 | 72.66 | 0.26 | 0.7950 |
| RT following gain trials (+50 and +10) | 338.08 | 81.43 | 344.50 | 73.37 | 0.37 | 0.7140 |
| RT for trials of higher amount (−50 and +50) | 332.23 | 81.00 | 339.18 | 70.22 | 0.41 | 0.6850 |
| RT for trials of lower amount (−10 and +10) | 340.34 | 86.09 | 345.70 | 77.22 | 0.29 | 0.7710 |
p < 0.01;
p < 0.001
The significance was lost after Bonferronni correction for multiple testing; BIS, Barratt Impulsivity Scale; IR-1, Impulsive response-1 (selecting 50 after a single event of loss); IR-2, Impulsive response-1 (selecting 50 after two consecutive events of loss); RT, Reaction time; SF, Selection frequency (refers to the number of times a particular amount was selected).
3.5. Factor extraction and correlation among factors
The PCA based factors obtained from TRB as well as ERP variables are explained in Table 4. Total variance accounted for the TRB and ERP factors were 77.34 % and 52.49 %, respectively. The three TRB factors were: 1) Eight variables representing reaction times; 2) six variables of selection frequencies following Loss trends/trials; and 3) three variables of selection frequencies following Gain trends. The four ERP factors were: 1) Twelve variables representing ORN amplitudes during Loss (−50 and −10) conditions; 2) Nine variables of ORP amplitudes during −10 condition; 3) Nine variables of ORP amplitudes during +10 condition; and 4) Nine variables of ORN latencies during −10 condition. Table 5 shows the correlation between ERP factors and behavioral (TRB and BIS) factors. In the total sample, non-planning and motor impulsivity of BIS showed significant correlations with ERP factors 3 and 4 respectively. There was no correlation between ERP and TRB factors. In the control group, TRB factor 1 (reaction times) correlated with ERP factor 4 (ORN latencies during −10) and non-planning (BIS) correlated with ERP factor 3 (ORP amplitudes during +10). In the alcoholic group, TRB factor 1 (reaction times) correlated with ERP factor 1 (ORN amplitudes during loss conditions).
Table 4.
Description of PCA based factors that were extracted from the set of TRB and ERP variables. Eigen value, percentage of variance accounted for, and the detail and the number (N) of variables that had significantly high positive (r ≥ +0.5) and negative (r ≤ −0.5) loadings with the factor have been listed.
| Factors | Eigen Value | Accounted variance in % | Variables with significantly high positive loadings [N] | Variables with significantly high negative loadings [N] |
|---|---|---|---|---|
| ERP Factor 1 | 34.92 | 24.25 | ORN amplitudes during Loss (−50 and −10) conditions [12] | None |
| ERP Factor 2 | 19.09 | 13.26 | ORP amplitudes during −10 condition [9] | None |
| ERP Factor 3 | 12.23 | 8.49 | ORP amplitudes during +10 condition [9] | None |
| ERP Factor 4 | 9.34 | 6.49 | ORN latencies during −10 condition [9] | None |
| TRB Factor 1 | 7.98 | 36.25 | All the RT variables [8] | None |
| TRB Factor 2 | 5.98 | 27.18 | SF for 50 after two consecutive Loss trials; SF for 50 after a Loss trend of previous 2 trials, 3 trials and 4 trials [4] | SF for 10 after a Loss trend of previous 2 trials and 3 trials [2] |
| TRB Factor 3 | 3.06 | 13.91 | SF for 50 after a Gain trend of previous 2 trials, 3 trials and 4 trials [3] | None |
RT, Reaction time; SF, Selection frequency (refers to the number of times a particular amount was selected); N, number of variables; TRB, Task-related behavioral variables
Table 5.
Correlation between ERP factors and Impulsivity (TRB and BIS) factors in each group and in total sample. Correlation coefficient (r, top number within the cell) and the level of significance (p, bottom number within the cell) are shown. The significant correlations (highlighted in bold font) have been indicated with asterisks. The minus sign (−) indicates a negative correlation.
| TRB-1 | TRB-2 | TRB-3 | BIS-NP | BIS-MI | BIS-CI | ||
|---|---|---|---|---|---|---|---|
| Control (N=40) | ERP-1 | 0.214 | 0.038 | 0.037 | 0.159 | 0.030 | 0.055 |
| 0.1912 | 0.8190 | 0.8238 | 0.3532 | 0.8613 | 0.7496 | ||
| ERP-2 | −0.225 | 0.026 | 0.080 | −0.158 | −0.125 | 0.168 | |
| 0.1677 | 0.8751 | 0.6294 | 0.3565 | 0.4690 | 0.3269 | ||
| ERP-3 | −0.266 | −0.089 | −0.009 | −0.400* | −0.113 | −0.224 | |
| 0.1021 | 0.5897 | 0.9552 | 0.0157 | 0.5120 | 0.1899 | ||
| ERP-4 | 0.420** | 0.038 | 0.023 | 0.042 | −0.278 | 0.045 | |
| 0.0078 | 0.8180 | 0.8887 | 0.8086 | 0.1011 | 0.7929 | ||
| Alcoholic (N=40) | ERP-1 | −0.339* | −0.131 | −0.123 | 0.114 | −0.016 | 0.115 |
| 0.0321 | 0.4199 | 0.4512 | 0.4962 | 0.9242 | 0.4913 | ||
| ERP-2 | 0.027 | 0.128 | 0.169 | −0.053 | 0.011 | −0.016 | |
| 0.8663 | 0.4301 | 0.2965 | 0.7505 | 0.9457 | 0.9239 | ||
| ERP-3 | 0.038 | −0.182 | −0.004 | −0.208 | 0.068 | 0.112 | |
| 0.8152 | 0.2613 | 0.9799 | 0.2104 | 0.6836 | 0.5019 | ||
| ERP-4 | −0.231 | −0.213 | −0.277 | −0.202 | −0.271 | 0.084 | |
| 0.1520 | 0.1862 | 0.0839 | 0.2228 | 0.1005 | 0.6176 | ||
| Total (N=80) | ERP-1 | −0.037 | 0.033 | −0.050 | 0.173 | 0.114 | 0.109 |
| 0.7468 | 0.7708 | 0.6623 | 0.1408 | 0.3315 | 0.3558 | ||
| ERP-2 | −0.131 | 0.035 | 0.105 | −0.168 | −0.116 | 0.052 | |
| 0.2508 | 0.7601 | 0.3589 | 0.1516 | 0.3265 | 0.6621 | ||
| ERP-3 | −0.137 | −0.143 | −0.010 | −0.317** | −0.067 | −0.080 | |
| 0.2275 | 0.2099 | 0.9277 | 0.0058 | 0.5704 | 0.5003 | ||
| ERP-4 | 0.027 | −0.118 | −0.159 | −0.102 | −0.240* | 0.070 | |
| 0.8101 | 0.2999 | 0.1610 | 0.3886 | 0.0398 | 0.5529 | ||
p < 0.05
p < 0.01
4. Discussion
The aim of the present study was to analyze the ERP as well as behavioral measures of reward/outcome processing in alcoholics (as compared to healthy controls) during the feedback of monetary outcomes (losses and gains) during a gambling task. The results revealed several key findings: 1) alcoholics had significantly lower ORP amplitudes than controls during both loss and gain conditions and decreased ORN amplitudes during loss conditions (Fig. 7); 2) in both alcoholic and control groups, Gain (+50 and +10) conditions had higher ORP amplitudes and shorter ORP latencies compared to Loss (−50 and −10); similarly, the Large amount (+50 and −50) had higher ORP amplitudes and shorter latencies compared to the Small amount (+10 and −10) conditions (Fig. 8, right-side panels); 3) in terms of topography, in both groups, the loss conditions had anterior maxima while the gain conditions had posterior maxima, especially in the ORN component (Fig. 5); 4) sLORETA analysis showed that alcoholics, compared to controls, had a significantly decreased ORP current density at cingulate gyrus and less negative ORN current density at primary sensory and motor areas (Fig. 6); 5) comparison of behavioral measures indicated that alcoholics showed a significantly higher impulsivity non-planning category of BIS impulsivity, and there was also a tendency towards higher motor impulsivity and behavioral risk-taking features among alcoholics; and 6) Some of the behavioral/impulsivity dimensions appeared to have high correlations with ERP measures, although alcoholics and controls differed in the nature of these correlations.
Fig. 5.
The topographic maps of ORN (left-side plots) and ORP (right-side plots) in control and alcoholic groups at the peak/trough in the respective waveforms. Alcoholics show decreased amplitude (in μV) in both ORN and ORP components in all conditions. The loss conditions had anterior maxima while the gain conditions had posterior maxima in both groups, especially in the ORN component. The headplots of ORN and ORP were derived from waveforms filtered at 2.0–16.0 Hz and 0.25–16.0 Hz respectively. While the amplitude differences are robust, topographic differences are not prominent.
4.1. Deficient reward processing in alcoholics
The key finding is that alcoholics had significantly lower amplitudes during outcome processing characterized by: i) markedly decreased ORP amplitude during all outcome conditions and ii) suppressed ORN amplitude during loss conditions (see Fig. 7). This decrease in amplitudes could be suggestive of a dysfunctional reward processing (system) in alcoholics. Further, deficits in both earlier (ORN/N2) and later (ORP/P3) components may also indicate that cognitive resources necessary for different levels of reward processing are impaired in alcoholics. It is well-known in the literature that alcoholics show deficits in N2 and P3 in a variety of cognitive tasks (Porjesz et al., 1987b, 1996, 2005a). However, it is still unclear whether the deficits observed in ORN and ORP components are reflective of generic cognitive deficits or a specific dysfunction in reward processing. Our previous findings from a single outcome gambling paradigm suggested that ORN and ORP could involve both evaluative/cognitive and emotional/affective processing (Kamarajan et al., 2009). Although it was argued that the ORN is functionally similar to the generic N2 component and does not have task-specific functions (Holroyd et al., 2008), our previous finding in a gambling paradigm indicated that ORN amplitude differed as a function of valence and amount (Kamarajan et al., 2009; Kamarajan et al., 2008), suggesting that ORN is functionally distinct from the generic N2 component observed in signal processing. Several studies of gambling tasks showed that the amplitude of the negative component analogous to N2 reflects activity that codes the ongoing evaluation of events in terms of favorable (i.e. gain, correct) or unfavorable (i.e. loss, error) outcomes (Gehring and Willoughby, 2002; Holroyd and Coles, 2002; Hajcak et al., 2006). Thus, it may be stated that the ORN may indicate the detection of a particular outcome and the ORP may reflect the conscious recognition/awareness for the valence or magnitude of the outcome. In a similar vein, the positive component analogous to P3, was found to be sensitive to both the quality (loss/gain) and quantity (larger/smaller) of the outcomes (Toyomaki and Murohashi, 2005; Kamarajan et al., 2009), which is very distinct from the generic P3 component observed in stimulus discrimination tasks. Although the ORN and ORP components may share common features of signal processing as indexed by N2 and P3 respectively, they indicate very specific levels of signal processing during outcome evaluation. Therefore, it is suggested that the dysfunctional outcome processing, as evidenced by decreased ORN and ORP amplitudes in alcoholics, could be due to a combination of generic signal processing deficits and a specific dysfunction in evaluative processing. Thus, the decreased amplitude observed in alcoholics in the earlier negative component (ORN) and in the later positive component (ORP) may indicate neurocognitive dysfunctions in both early detection of different outcomes and subsequent evaluation of quality (loss vs. gain) and quantity (10 vs. 50) of outcomes.
Despite these possible dysfunctions in alcoholics as compared to controls, a noteworthy inference is that the neural mechanisms for reward processing are similar in both alcoholic and control groups (as shown by the within-group analysis): i) in both groups, Gain (+50 and +10) conditions had higher ORP amplitudes and shorter ORP latencies compared to Loss (−50 and −10) as did the Large amount (+50 and −50) conditions compared to Small amount (+10 and −10) conditions (Fig. 8, right-side panels); ii) in terms of topography, in both groups, the loss conditions had anterior maxima while the gain conditions had posterior (central/parietal) maxima, especially in the ORN component. It should also be mentioned that an unusual finding in this study indicated that alcoholics showed more ORN amplitude for the small amount ‘10’ than for the Large amount ‘50’ (Fig. 8, panel A-3). This finding of increased resource allocation for the small amount in alcoholics during the early processing (at ORN) is difficult to explain and poses an interesting question for further exploration.
4.2. Frontal network dysfunction in alcoholics as revealed by sLORETA
A noteworthy finding of sLORETA analysis in this study was that alcoholics, as compared to controls, showed significantly decreased current density in the ORP time frame at cingulate gyrus during each of the reward/outcome conditions. On the other hand, alcoholics demonstrated a significantly reduced negative ORN current density than controls at postcentral gyrus, inferior frontal gyrus and precentral gyrus. This finding is very much characteristic of the observation that alcoholics have a weaker and/or a dysfunctional activation in the cingulate cortex during the evaluative processing of monetary outcome, as evidenced by the decreased current density during the late processing of the ORP component. Further, alcoholics do show more activity during the early processing (of ORN component) associated with discrimination of outcomes at the sensory (postcentral area), motor (precentral area) and dorsolateral prefrontal (brodmann area 9) areas of the brain. This differential effect of possible “hyper-excitability” during early processing at primary sensory and motor areas and prefrontal areas on the one hand, and diminished response to outcome evaluation during the later processing at anterior as well as posterior cingulate gyrus on the other, differentiate alcoholic individuals from healthy controls both in terms of intensity and time course of neural activity associated with ORN and ORP components during outcome processing.
In our earlier ERP study using the gambling task in controls (Kamarajan et al., 2009), we found that the maximum current density (i.e. the focus) involved specific brain regions: i) the ORN activity involved medial frontal (including anterior cingulate) areas during the loss conditions and medial posterior (including posterior cingulate) areas for the gain conditions in both males and females; ii) the ORP activity was concentrated at the medial frontal (including anterior cingulate) areas in females and at the medial posterior (including posterior cingulate) areas in males. This finding showed the importance of both anterior and posterior cingulate cortex for the processing of monetary outcomes. In this context, a weaker activation as indicated by decreased current density in the cingulate cortex in alcoholics during ORP processing (i.e. the awareness to loss/gain event) could either indicate a localized dysfunction in the particular region of anterior cingulate cortex (ACC) or a generalized malfunction in the entire neural network of reward processing (due to the central and influential role of ACC in the reward network). Further, our findings also showed that the cingulate area and a related structure in the reward network (i.e.. insula) showed prominence in the within-group differences between valences (i.e. loss vs. gain): in the ORP component, the gain conditions (+50 and +10) showed more current density activity (than the loss conditions) at the posterior cingulate area in controls and at the insula region in alcoholics. It is also to be noted that although amplitude of ERPs were different between the amounts (see Fig. 8), current density profiles could not differentiate between amounts. This finding suggests that differentiation in terms of current density of amount plays a less significant role than that of valence (loss vs. gain) in the reward network of the human brain.
Our finding that decreased current density in the ORP time frame at cingulate gyrus can be further supported and explained in the light of relevant findings in the literature. For example, neuroimaging studies of reward processing have identified a number of brain areas that are activated by the delivery of primary reinforcers such as appetitive stimuli (Berns et al., 2001; McClure et al., 2003; O’Doherty et al., 2001), as well as secondary reinforcement such as monetary gains and losses (Breiter et al., 2001; Delgado et al., 2004; Elliott et al., 2000; Holroyd et al., 2004; Thut et al., 1997, as cited from Nieuwenhuis et al., 2005a). Increasing evidence supports the integral role of the ACC in performance monitoring, especially involving predictability of an outcome (Paulus et al., 2002, 2003, 2004), which are critical in regulating various relevant social behaviors. Subdivisions within the ACC have specialized functions: i) the dorsal (pregenual) region of the ACC may be involved in cognitive aspects of decision making, including reward based decision making, error monitoring, anticipation, working memory, motor response, and novelty detection (Bush et al., 2000, 2002; Forman et al., 2004), and ii) the rostral (infragenual) ACC has been implicated in emotional processing (Bush et al., 2000) and error monitoring (Forman et al., 2004), perhaps due to its interconnections with the orbitofrontal cortex, limbic structures, motor cortex and autonomic and endocrine systems (Bush et al., 2000). Thus, both regions of the ACC may play a role in risky decision making and also involve cognitive (probability) as well as emotive (reward/penalty) functions. These regions are all interconnected with each other and with the prefrontal cortex (Brutus et al., 1986; Bush et al., 2000; Elliott, 1992), and appear to play a role in modulation of disinhibitory behaviors, error monitoring, reward sensitivity, and emotional valence (cf. Fishbein et al., 2005). In essence, activity within this network functions to attach emotional context and valence to cognitive cues. Neurocognitive tasks such as the gambling task used in our study which, apart from the cognitive evaluation of outcomes, also include a feeling or emotional component (e.g., loss, gain) could invoke specific interconnected regions of the PFC, ACC, and limbic structures, suggesting that when task demands modify emotional responses, neural responses occur within this network (Elliott et al., 2000; Liberzon et al., 2000). Further, activity throughout this circuit has been implicated in behavioral dispositions/disorders involving addictive-impulsive-compulsive spectrum, including alcohol/drug abuse, gambling, and risk-taking (Bechara, 2001; Cavedini et al., 2002; Mitchell et al., 2002; Rogers et al., 1999; Harris et al., 2008).
4.3. Behavioral and trait impulsivity in alcoholism
Impulsivity is a complex multidimensional construct that has been frequently implicated in the pathogenesis of addictive disorders (Dom et al., 2007). The findings of the present study, which involved both trait impulsivity (using BIS) and behavioral impulsivity (derived from task performance) measures, showed that alcoholics had higher levels of impulsivity in non-planning and total scores of BIS, while there was also a tendency towards higher risk-taking features (i.e. selecting ‘50’ more often and ‘10’ less often than controls following losing trends) among alcoholics. These findings on impulsivity in alcoholics tends to offer validity to the neurocognitive models of addiction disorders that implicate impulsivity as a major component. For example, Chambers et al. (2003) proposed that the primary motivation circuitry involving cortical–striatal–thalamic-cortical loops were putatively involved in impulsivity, decision-making and the disorders of alcohol/drug addiction and pathological gambling. Goldstein and Volkow (2002) conceptualized alcohol/drug addiction as a syndrome of impaired response inhibition and salience attribution, and summarized the involvement of the frontosubcortical circuits in addiction disorders.. Earlier studies done in our lab have consistently found that disinhibition and impulsivity were the key aspects in alcoholism (Cohen et al., 1997; Kamarajan et al., 2005a; Chen et al., 2007). Further, many researchers have considered impulsivity as the key vulnerability marker for substance-use disorders, especially alcoholism (see Verdejo-Garcia, 2008 for a review).
Further, since alcoholics do show anomalies in both neurophysiological and behavioral measures, the findings on the relationship between impulsivity and ERP factors suggest possible causal links: 1) both factors could be causally linked with each other, or 2) both could have been caused/linked by other common etiological factor(s). However, the claim for causal links may not be strong enough, considering the finding that no task-related performance variables showed a strong correlation with ERP factors, although the self-report measure (i.e. BIS) showed a high correlation with ERP factors. Although the lack of correlation between performance variables of impulsivity and ERP factors poses a difficult question, this can be possibly explained in two ways: 1) earlier studies on alcohol dependent individuals suggested that correlations between behavioral measures and self-report measures were weak, suggesting that they both tap into different aspects of impulsivity (for detail, see Dom et al. (2007), and 2) age as a factor could have moderated the performance-related impulsivity (more than the trait impulsivity) resulting in a differential effect of younger controls with higher impulsivity as equated with relatively lower impulsivity in older alcoholics (i.e. higher the age lower the impulsivity). Future studies may address this issue of age effects on impulsivity. Finally, since the concepts of impulsivity, disinhibition and risk propensity forms the vulnerability not only for substance use disorders but the entire rubric of disinhibitory or externalizing psychopathology (Krueger et al., 2002; Iacono et al., 2008), ERP studies on reward processing using a gambling paradigm on a wide spectum of disorders may help integrate the relationship among these concepts and yield a comprehensive model for disease propensity.
4.4. Is alcoholism a reward deficiency syndrome?
Alcoholism has been described as part of the Reward Deficiency Syndrome (RDS) (Bowirrat and Oscar-Berman, 2005). Recently, Makris et al (2008) showed that there was a decrease in total reward-network volume in the brains of alcoholic subjects. Findings also supported the RDS model of alcoholism by identifying dysfunctions in the brain reward circuits of alcoholics (Wrase et al., 2007; Makris et al., 2008; de Greck et al., 2009; Tanabe et al., 2009). In this context, the present study is the first electrophysiological study to examine reward processing deficiencies in alcoholics using a gambling task, although several recent studies have examined the related concept of ‘decision making’ in gambling tasks in alcohol/drug dependent individuals (Bechara, 2005; Dom et al., 2006; Verdejo-Garcia et al., 2007; Cantrell et al., 2008). Since the concept of RDS involves a cluster of impulsive-additive-compulsive behaviors/disorders (Blum et al., 1995), our study has included several measures of impulsivity along with ERP measures. The findings of the current study indicate that alcoholics manifest deficient reward processing in neurocognitive measures (in terms of amplitude and current density of ORN and ORP) as well as in behavioral measures of impulsivity and risk-taking, as compared to healthy controls. These findings lend support to the notion that alcoholism can be construed as part of a reward deficiency syndrome.
According to the RDS model, a person with dysfunction in the brain reward cascade, especially in the dopaminergic system causing a hypodopaminergic trait, requires additional dopamine to feel good (Blum et al., 2000). This trait leads to multiple drug-seeking behaviors as the drugs, such as alcohol, cocaine, heroin, marijuana, nicotine, cause activation and neuronal release of dopamine, which could heal the abnormal cravings. The RDS model explains not just alcoholism but several common disorders such as pathological gambling, attention-deficit hyperactivity disorder (ADHD), Tourett’s syndrome, PTSD, conduct disorder, and antisocial behavior (Blum et al., 2000; Comings and Blum, 2000; Bowirrat and Oscar-Berman, 2005).
Another dominant model to consider is the ‘disinhibitory/externalizing spectrum’ (DES) model (Gorenstein and Newman, 1980; Krueger et al., 2002; Iacono et al., 2003) which appears to have a similar cluster of disorders and similar etiological and neurocognitive explanations. Although, in many of our earlier studies, we have supported the DES model for alcoholism, especially to explain the neurocognitive disinhibition (Begleiter and Porjesz, 1999; Kamarajan et al., 2004, 2005a, 2005b, 2006; Porjesz et al., 2005a; Porjesz and Rangaswamy, 2007; Chen et al., 2007; Rangaswamy et al., 2007), the scope of the present study appears to fit well with the RDS model, as it specifically deals with reward processing deficiency in alcoholism which is not explicitly part of the DES model. Interestingly, a common underlying aspect to both models is the ability to identify and integrate the spectrum of disorders that share a common etiology, pathophysiology, biological markers and brain circuitry. In this regard, these models may be complementary to each other in explaining different dimensions of the spectrum rather than assumed being competitive. As a final note, it should be mentioned that genetic vulnerability underlies these spectrum disorders including alcoholism, as both models illustrate (Blum et al., 1996; Porjesz and Begleiter, 1998; Comings and Blum, 2000; Hicks et al., 2004, 2007; Begleiter and Porjesz, 2006; Porjesz and Rangaswamy, 2007; Dick et al., 2008; Rangaswamy and Porjesz, 2008a, 2008b), as many of these markers and dysfunctions related to these disorders are also present in their naïve offspring (Begleiter et al., 1984; Porjesz and Begleiter, 1997, 1998; Polich et al., 1994), and these specific dysfunctions in reward processing could be mainly due to certain inherited aspects of abnormal emotional traits in alcoholics (Oscar-Berman and Bowirrat, 2005).
In conclusion, our study has shown that alcoholics demonstrate dysfunctional outcome processing, high impulsivity and risk-taking (as observed in the behavioral scores), and possibly a compromised neural reward network. A possible limitation that could compromise the validity of the present study is that the alcoholic group is significantly older than the control group, although age has been treated as a covariate in the statistical analysis. Since age as a factor may have had an impact on the impulsivity variables, we suggest that future studies try to replicate our findings in an age-matched sample of controls and alcoholics. Converging evidence, including the ‘reward deficiency’ observed in the study, suggest that alcoholism and a host of externalizing and impulse control disorders may fall into the rubric of RDS. Future studies should focus on the application of gambling paradigms to other clinical conditions of RDS in order to confirm and validate this model. Application of sophisticated methods of signal processing including event-related brain oscillations (EROs), synchrony, and componential analysis during outcome processing may further help understand the neurocognitive phenomena and the disorders, and these studies are underway. Since the present study has included only the male participants, further studies on clinical samples may be attempted to include both genders and analyze the effects in each gender separately. Further, imaging studies supplemented with simultaneous ERP recordings during the performance of a gambling task might shed more light on the exact source activity along with the time course of outcome processing.
Acknowledgments
In memory of Dr. Henri Begleiter, founder and longtime mentor of the Neurodynamics Laboratory, we acknowledge with great admiration his seminal scientific contributions to the field. We are indebted to his charismatic leadership and luminous guidance, and are truly inspired by his vision to carry forward the work he fondly cherished.
This study was supported by the National Institutes of Health (NIH) Grants #5 RO1 AA005524, AA02686 and AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). We are grateful for the valuable technical assistance of Carlene Haynes, Joyce Alonzia, Chamion Thomas, Tracy Crippen, Glenn Murawski, Eric Talbert, Patrick Harvey, Cindy Lipper, GabrielWurzel, Irina Kushnir and Aleksandr Razran.
Abbreviations
- ORN
Outcome-related Negativity
- ORP
Outcome-related Positivity
- ERN
Error-related Negativity
- MFN
Medial Frontal Negativity
- SOG task
Single Outcome Gambling task
- BIS
Barratt Impulsivity Scale
- TRB scores
Task Related Behavioral scores
- sLORETA
Standardized Low Resolution Electromagnetic Tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachman P, Kim J, Yee CM, Therman S, Manninen M, Lonnqvist J, et al. Abnormally high EEG alpha synchrony during working memory maintenance in twins discordant for schizophrenia. Schizophrenia Research. 2008;103(1–3):293–7. doi: 10.1016/j.schres.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion and Personality. New York: Elsevier; 1985. pp. 137–46. [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Seminars in Clinical Neuropsychiatry. 2001;6(3):205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism, Clinical and Experimental Research. 1999;23(7):1125–35. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. International Journal of Psychophysiology. 2006;60(2):162–71. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493–6. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou CL, Aunon JI. P3 and stimulus incentive value. Psychophysiology. 1983;20(1):95–101. doi: 10.1111/j.1469-8986.1983.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Tenner M. Neuroradiological and neurophysiological evidence of brain deficits in chronic alcoholics. Acta Psychiatrica Scandinavica Supplementum. 1980;286:3–13. doi: 10.1111/j.1600-0447.1980.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21(8):2793–8. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(Suppl):i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5(3):121–41. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brutus M, Shaikh MB, Edinger H, Siegel A. Effects of experimental temporal lobe seizures upon hypothalamically elicited aggressive behavior in the cat. Brain Research. 1986;366(1–2):53–63. doi: 10.1016/0006-8993(86)91280-1. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J. Decision Making in Alcohol Dependence: Insensitivity to Future Consequences and Comorbid Disinhibitory Psychopathology. Alcoholism, Clinical and Experimental Research. 2008 doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. Journal of Abnormal Psychology. 2007;116(3):565–77. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biological Psychiatry. 2002;51(4):334–41. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcoholism, Clinical and Experimental Research. 2007;31(1):156–65. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcoholism, Clinical and Experimental Research. 1997;21(8):1398–406. [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–78. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–41. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, et al. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics--a fMRI study. Human Brain Mapping. 2009;30(5):1691–704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14(9):1022–30. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, et al. Using dimensional models of externalizing psychopathology to aid in gene identification. Archives of General Psychiatry. 2008;65(3):310–8. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Sabbe B. Dimensions of impulsive behaviour in abstinent alcoholics. Personality and Individual Differences. 2007;42(3):465–76. [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcoholism, Clinical and Experimental Research. 2006;30(10):1670–7. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Elliott FA. Violence. The neurologic contribution: an overview. Archives of Neurology. 1992;49(6):595–603. doi: 10.1001/archneur.1992.00530300027006. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20(16):6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug and Alcohol Dependence. 2008;92(1–3):141–8. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, et al. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Research Cognitive Brain Research. 2005;23(1):119–36. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P, Lind JC, Koles ZJ. A source-imaging (low-resolution electromagnetic tomography) study of the EEGs from unmedicated males with depression. Psychiatry Research. 2004;130(2):191–207. doi: 10.1016/j.pscychresns.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55(5):531–7. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–82. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychological Review. 1980;87(3):301–15. [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry. 2004;61(3):310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–54. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism, Clinical and Experimental Research. 2008;32(6):1001–13. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, et al. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44(1):98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61(9):922–8. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, et al. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7(5):497–8. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald-Zuberbier E. The incentive value of stimuli and the P300 component of cerebral evoked potentials. Progress in Brain Research. 1980;54:629–33. doi: 10.1016/s0079-6123(08)61682-9. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald-Zuberbier E. The variation of p300 amplitude in a money-winning paradigm in children. Psychophysiology. 1981;18(3):258–62. doi: 10.1111/j.1469-8986.1981.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48(2):147–78. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral Disinhibition and the Development of Early-Onset Addiction: Common and Specific Influences. Annu Rev Clin Psychol. 2008;4(1):325–48. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, et al. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological Psychiatry. 2006;59(7):625–34. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, et al. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. International Journal of Psychophysiology. 2004;51(2):155–80. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological Psychology. 2005a;69(3):353–73. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, et al. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clinical Neurophysiology. 2005b;116(5):1049–61. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Rangaswamy M, Tang Y, Chorlian DB, Padmanabhapillai A, et al. Brain signatures of monetary loss and gain: outcome-related potentials in a single outcome gambling task. Behavioural Brain Research. 2009;197(1):62–76. doi: 10.1016/j.bbr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, et al. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain Research. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–24. [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23(5):508–16. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115(8):1821–35. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H, Takeuchi S, Gehring WJ, Takasawa N, Yamazaki K. Affective-motivational influences on feedback-related ERPs in a gambling task. Brain Research. 2006;1105(1):110–21. doi: 10.1016/j.brainres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Mennes M, Wouters H, van den Bergh B, Lagae L, Stiers P. ERP correlates of complex human decision making in a gambling paradigm: Detection and resolution of conflict. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40(12):2013–22. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005a;25(4):1302–9. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005b;21(11):3161–8. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cerebral Cortex. 2004;14(7):741–7. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatr Dis Treat. 2005;1(3):211–29. [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Gaillard AW, Wientjes CJ. The relation between event-related brain potential, heart rate, and blood pressure responses in an S1-S2 paradigm. Biological Psychology. 1995;39(2–3):81–102. doi: 10.1016/0301-0511(94)00969-5. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Review of methods for solving the EEG inverse problem. Int J Bio-chromatogr. 1999;1:75–86. [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods and Findings in Experimental and Clinical Pharmacology. 2002;24 (Suppl D):5–12. [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43(1):84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biological Psychiatry. 2004;55(12):1179–87. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG. Error rate and outcome predictability affect neural activation in prefrontal cortex and anterior cingulate during decision-making. Neuroimage. 2002;15(4):836–46. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry. 2003;53(1):65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115(1):55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7(5):465–9. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in COA’s. Alcohol Health and Research World. 1997;21(3):236–40. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Genetic basis of event-related potentials and their relationship to alcoholism and alcohol use. Journal of Clinical Neurophysiology. 1998;15(1):44–57. doi: 10.1097/00004691-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987a;4(4):283–7. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. The N2 component of the event-related brain potential in abstinent alcoholics. Electroencephalography and Clinical Neurophysiology. 1987b;66(2):121–31. doi: 10.1016/0013-4694(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Litke A, Bauer LO, Kuperman S, O’Connor SJ, et al. Visual P3 as a potential phenotypic marker for alcoholism: Evidence from the COGA national project. In: Ogura C, Koga Y, Shimokochi M, editors. Recent Advances in Event-Related Brain Potential Research. Holland: Elsevier Science; 1996. pp. 539–49. [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, et al. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcoholism, Clinical and Experimental Research. 1998;22(6):1317–23. [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific WorldJournal. 2007;7:131–41. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005a;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005b;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ramsey SE, Finn PR. P300 from men with a family history of alcoholism under different incentive conditions. Journal of Studies on Alcohol. 1997;58(6):606–16. doi: 10.15288/jsa.1997.58.606. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. International Journal of Psychophysiology. 2007;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. From event-related potential to oscillations: genetic diathesis in brain (dys)function and alcohol dependence. Alcohol Research & Health. 2008a;31(3):238–42. [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Research. 2008b;1235:153–71. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19(20):9029–38. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry. 2009;65(2):160–4. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8(5):1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. Discrepancy between feedback negativity and subjective evaluation in gambling. Neuroreport. 2005;16(16):1865–8. doi: 10.1097/01.wnr.0000185962.96217.36. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118(3):645–68. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Vilar-Lopez R, Perez-Garcia M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug and Alcohol Dependence. 2007;86(2–3):139–46. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Rosquist H, Fjell AM. P300 amplitude age reductions are not caused by latency jitter. Psychophysiology. 2008;45(4):545–53. doi: 10.1111/j.1469-8986.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–94. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24(28):6258–64. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]