Abstract
Objective
To compare respiratory compliance (Crs) and functional residual capacity (FRC) in infants randomized to a rescue course of antenatal steroids (AS) versus placebo.
Study Design
Randomized, double-blinded trial. Pregnant women ≥ 14 days after initial AS were randomized to rescue AS or placebo. The primary outcomes were measurements of Crs and FRC. This study is registered with clinicaltrials.gov [NCT00669383].
Results
44 mothers (56 babies) received rescue AS and 41 mothers (57 babies) received placebo. There was no significant difference in birth weight, or head circumference. Infants in the rescue group had an increased Crs (1.21 versus 1.01 mL/cm H2O/kg; adjusted 95% CI 0.01, 0.49; p =0.0433) compared to placebo. 13% in the rescue versus 29% in the placebo group required ≥ 30% oxygen (p <0.05). Patients delivered at ≤ 34 weeks had greater pulmonary benefits.
Conclusion
Infants randomized to rescue AS have a significantly increased Crs compared to placebo.
Keywords: Betamethasone, functional residual capacity, premature infants, pulmonary function, rescue antenatal corticosteroids, respiratory compliance
Introduction
Respiratory distress syndrome (RDS) is a common complication of preterm delivery and increases neonatal mortality and morbidity.1 A single course of antenatal steroids (AS) remains the standard of care for women at risk for preterm delivery between 24 and 34 weeks of gestation,2,3 and significantly decreases mortality and RDS. The optimal response to a course of AS occurs if it is given at least 24 hours before but within 7 days of delivery.2,3 The duration of the clinical effect of a course of AS remains uncertain, and the efficacy and safety of repeat courses of AS remains a topic of continuing debate and study.4-7
Recent randomized data show weekly courses of AS in preterm infants may improve lung function outcomes.5,6 Crowther et al randomized 982 women at < 32 weeks of gestation to weekly AS versus placebo.5 They demonstrated a significant decrease in RDS and severe lung disease in the AS group.5 The NICHD Maternal Fetal Medicine Units Network also reported improved pulmonary outcomes in preterm infants randomized to weekly courses of AS versus placebo.6 Despite the suggestion of pulmonary benefits after repeat courses of AS, concern remains of possible harm after weekly AS therapy, particularly in terms of reduced in-utero growth and long-term neurodevelopmental outcome.7 However, a recent trial by Garite et al of one rescue course of AS versus placebo showed improved neonatal outcome without apparent increased short-term risk.8
We have used measurements of pulmonary function, specifically passive respiratory compliance (Crs) and functional residual capacity (FRC), as an objective and reproducible way of quantifying the effects of AS on pulmonary function.9-11 These physiologic measurements correlate with clinical respiratory outcomes.9,12 We recently reported that infants ≤ 32 weeks of gestation treated with a single course of AS > 7 days before delivery had a significantly lower Crs compared with matched infants treated with a course of AS 1-7 days before delivery.12 The difference in Crs was more striking if the infants received AS >14 days prior to delivery.12 Our objective was to compare pulmonary function (Crs and FRC) in infants who received a course of AS, remained undelivered for at least 14 days, and were randomized to a single rescue course of AS versus placebo. We hypothesized that infants who received a rescue course of AS would have a significantly increased Crs compared to those who received placebo.
Materials and Methods
Eligibility Criteria
This prospective randomized trial was conducted at the Neonatal Intensive Care Unit at Oregon Health & Science University (Portland, Oregon) and at Sacred Heart Hospital (Pensacola, Florida). The study was approved by the Institutional Review Boards at each institution and informed consent obtained for each enrolled patient. This study is registered with clinicaltrials.gov [registration number NCT00669383]. Inclusion criteria included: 1) pregnant women 26 to < 34 weeks of gestation; 2) at least 14 days after first course of AS (93% received betamethasone); 3) continued risk of preterm delivery as determined by their care provider; 4) informed consent.
We excluded patients with: 1) multiple gestations greater than twins; 2) insulin dependent diabetes; 3) clinical chorioamnionitis; 4) major documented fetal or chromosomal abnormalities; 5) first course of AS given at < 24 weeks of gestation; 6) chronic steroid use during pregnancy for clinical care.
Study Design
This was a prospective, randomized, double-blinded trial stratified by gestational age (≤ 28 versus > 28 weeks' gestation) and multiple gestation (twins versus singletons). After obtaining informed consent patients were randomized to rescue AS or placebo only if there were recurrent signs of preterm delivery as determined by the mother's clinician. Patients assigned to the “rescue AS” group received a course of antenatal steroids (two 12 mg intramuscular injections of betamethasone [Celestone Soluspan, Schering-Plough Corporation, Kenilworth, NJ] 24 hours apart). Those assigned to the “placebo” group received 2 doses of placebo that were identical in appearance to the betamethasone and consisted of 25 mg of cortisone acetate, an inactive steroid.10 Group assignment was done using a randomization table. A staff pharmacist performed randomization and study drug preparation at each institution, and all patients, investigators and care providers were unaware of treatment allocation. Pulmonary function was measured within 72 hours of birth and before surfactant therapy, when required. Infants were studied in the supine position while quietly asleep; no sedation was used.
Comparisons of Crs and FRC between groups were our primary end points. Other pertinent clinical outcome measures between groups were also monitored. All outcomes were assessed blinded to treatment group allocation.
Measurements
Respiratory mechanics and lung volume were measured with a computerized infant pulmonary function cart (SensorMedics 2600, SensorMedics Inc., Yorba Linda, CA). Crs was obtained with the single-breath occlusion technique and FRC with the nitrogen washout method.9-12 In intubated patients including those requiring surfactant, testing was performed by connecting the infant's endotracheal tube into the system via a three-way valve that also connected to the ventilator. In non-intubated patients, a face mask was used.9-11
For the single-breath occlusion technique,9, 12-15 the airway was briefly occluded at end inspiration until an airway pressure plateau was observed and the Hering Breuer reflex invoked. The linear portion of the passive flow-volume curve was identified, and a regression line drawn to obtain the best fit. From the intercepts on the flow and volume axes, respiratory system compliance and resistance were calculated. Acceptance criteria included: 1) stable end expiratory baseline; 2) plateau pressure lasting >100 msec; 3) plateau pressure varying by < ± 0.125 cm H2O; 4) acceptable flow-volume curve by visual inspection, with linear data segment identified; 5) at least ten breaths accepted with a coefficient of variation of <20%.16
For the nitrogen washout technique,9-11, 17,18 calibration was done with 2 known volumes, and a calibration line was constructed for the system at the specific flow rate. The calibration curve was then used to correlate the nitrogen washed out to the infant's FRC. The system corrected for dead space present and corrected the FRC to body temperature, pressure, and water-saturated conditions. Total FRC was related to body weight. Acceptance criteria included: 1) infant supine and quietly asleep; 2) test initiated at end expiration; 3) no evidence of leak on tracing of the washout; 4) consistent tracings; 5) at least 3 measurements with a coefficient of variation of < 10%.16
Growth measurements (weight, head circumference and length) at birth and hospital discharge were compared between groups, and corresponding Z-scores for these measurements were calculated.19,20 Other clinical outcome parameters including surfactant administration, diagnosis of RDS (defined as clinical signs of respiratory distress with radiographic appearance and needing supplemental oxygen with FiO2 >0.21), respiratory distress with FiO2 requirement ≥ 0.30 and ≥ 0.40 at 24 hours of age, days on mechanical ventilation, and days on supplemental oxygen were monitored.
Statistical Methods
Differences in continuous variables between the two groups were first analyzed by two-tailed, independent samples Student t-tests or the Mann-Whitney U test where appropriate (for data not normally distributed). Categorical variables were evaluated with Chi-Square tests and Fisher's exact test where appropriate. Data are presented as mean ± SD, unless otherwise indicated.
The statistical analyses were on intention-to-treat. Comparisons of the primary outcomes between groups were done using linear mixed modeling.21,22 This approach accounts for the correlation (non-independence) between twins, and allows for adjustment of additional covariates and potential confounders.21,22 We began with a model including rescue dose, gestational age (GA) at birth, multiple gestations, maternal smoking history, rupture of membranes, and gestational diabetes, as these were the initial predictors of interest. The remaining covariates: GA at first AS dosing, GA at study dosing, race, gender, and mode of delivery were introduced to the model one at a time, and examined for their statistical significance and/or their effect on the relationship between rescue dose and each outcome. We also planned a subgroup analysis for infants who delivered at ≤ 34 weeks of gestation. We used SPSS for Windows, Version 15, Chicago, IL and SAS 9.1.3, SAS Institute Inc., Cary, NC for analyses.
Power calculations were based on previous studies. We reported a 50% increase in Crs in preterm infants treated with a single course of AS 1-7 days before delivery when compared with matched untreated infants.9 We also conducted a randomized trial, in which infants undelivered one week after their initial AS course were randomized to weekly AS versus placebo.10 Infants randomized to placebo delivered an average of 24 days after AS dosing and had Crs measurements that were on average 20% lower than in infants randomized to weekly AS. These findings were not significantly different perhaps due to small sample size, as the study was stopped early due to concerns of possible adverse effects of weekly AS therapy.
For our present study, we hypothesized that infants in the rescue AS group would have a significantly increased Crs than those in the placebo group. We estimated that to show about a 30% difference in Crs between the groups, we would need to study approximately 40 pregnancies in each group to reject the null hypothesis with a type I error of 0.05 and a power of 80%. Data were reviewed once by the independent, data safety monitoring committee, who were not aware of treatment-group allocation. An interim analysis was conducted when 49.6% (56/113) of babies were delivered.
Results
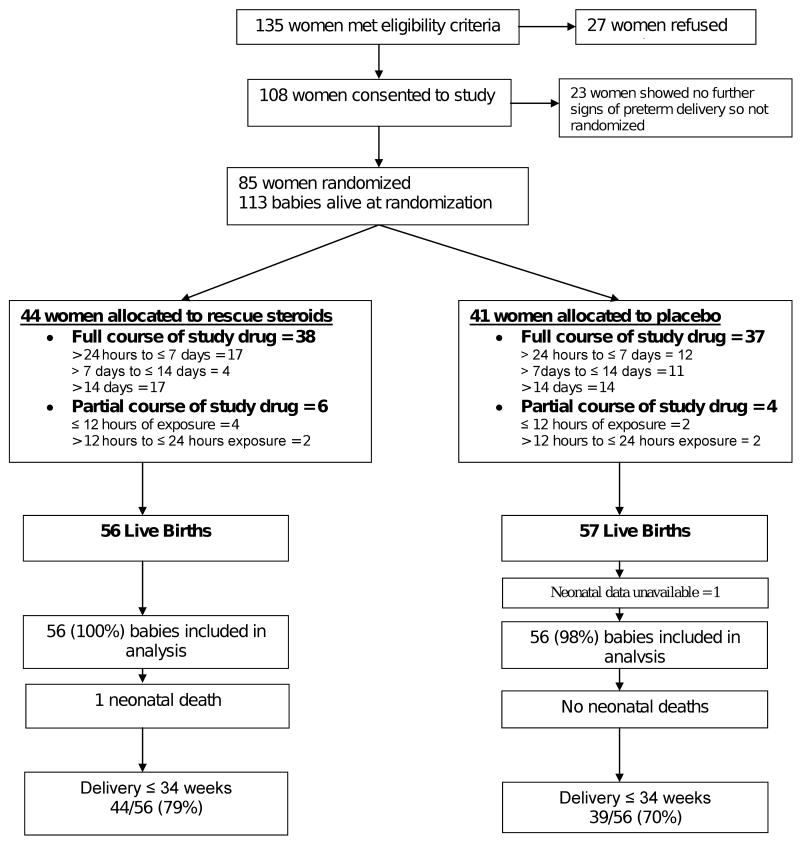
Patients were recruited from June 2001 to May 2007. The study profile is shown in Figure 1. A total of 44 mothers with 56 babies were randomized to a rescue course of AS and 41 mothers with 57 babies to placebo. The first 8 patients were recruited at Sacred Heart Hospital, with the remaining patients recruited at Oregon Health & Science University. Eighty-six percent of the rescue and 90% of the placebo group received a full course of the study medication. Neonatal data was not available for one baby in the placebo group, resulting in 56 versus 56 babies available for analysis (Figure 1).
Figure 1. Trial Profile.
Outline of enrollment and randomization of the study participants.
Maternal and infant characteristics were comparable between the groups (Table 1A and 1B), except for significantly more smokers by history in the rescue group. Both groups of mothers received their first course of AS at about 27 weeks; and received their study dose at about 30 weeks of gestation (Table 1A). Patients in the rescue AS group delivered 8 (2 – 24) days after receiving rescue/ study dosing versus 11 (3 – 20) days in the placebo group (median [25th-75th percentiles], p =0.66). Eighty-three of a total of 113 infants (73.5%) were delivered at ≤ 34 weeks. There were no significant differences in birth weight, GA at birth, admission head circumference, birth length, or incidence of small for gestational age infants (birth weight < 10th percentile) between groups. There was no difference in Z-scores for birth weight, head circumference or length between groups (Table 1B).
Table 1. A. Baseline Characteristics of the Randomized Mothers.
|
Rescue AS (n = 44) |
Placebo (n = 41) |
P | |
|---|---|---|---|
| Maternal age (years)* | 26.9 ± 7.5 | 28.6 ± 6.4 | NS |
| Caucasian (%) | 30 (68%) | 27 (66%) | NS |
| Maternal smoking (%) | 11 (25%) | 2 (5%) | <0.05 |
| Twin gestation (%) | 12 (27%) | 16 (39%) | NS |
| Rupture of membranes (hr)** | 0.5 (0 – 9) | 0 (0 – 8) | NS |
| Rupture of membranes >24 hr (%) | 9 (20%) | 6 (15%) | NS |
| Gestational diabetes (%) | 2 (5%) | 5 (12%) | NS |
| Cesarean section (%) | 24 (55%) | 24 (59%) | NS |
| Gestational age at 1st AS dosing (wks)* | 26.6 ± 1.9 | 27.1 ± 2.2 | NS |
| Gestational age at study dosing (wks)* | 29.8 ± 1.9 | 30.3 ± 2.1 | NS |
| Preterm labor (%) | 35 (80%) | 29 (71%) | NS |
| Preeclampsia (%) | 3 (7%) | 2 (5%) | NS |
| Antepartum hemorrhage (%) | 7 (16%) | 11 (27%) | NS |
| Table 1B. Demographic Characteristics of the Two Groups of Infants | |||
|
Rescue AS (n = 56) |
Placebo (n = 56) |
P | |
| Gestational age at birth (wks)* | 31.9 ± 3.3 | 32.3 ± 2.9 | NS |
| Caucasian (%) | 39 (70%) | 38 (68%) | NS |
| Female (%) | 28 (50%) | 29 (52%) | NS |
| Apgar score 1, 5 min** | 7, 8 | 8, 9 | NS |
| Birth weight (g)* | 1806 ± 778 | 1830 ± 657 | NS |
| Birth weight Z score* | -0.14 ± 0.86 | -0.14 ± 0.98 | NS |
| Small for gestational age (%) | 6 (11%) | 8 (14%) | NS |
| Birth head circumference (cm)*,a | 28.7 ± 3.0 | 29.2 ± 2.9 | NS |
| Birth head circumference Z score* | -0.19 ± 0.90 | -0.18 ± 0.93 | NS |
| Birth length (cm)* | 41.9 ± 4.7 | 42.4 ± 4.4 | NS |
| Birth length Z score* | -0.11 ± 0.99 | -0.06 ± 0.96 | NS |
Mean ± SD;
Median (25th – 75th percentiles).
Median values.
Abbreviations are: AS, antenatal steroids; NS, not significant.
Data available for 53 versus 54 patients.
Pulmonary function tests were performed at a median postnatal age of 22 hours in the rescue AS and 21 hours in the placebo group. Successful measurements of Crs were obtained in 49 rescue and 49 placebo patients. Accounting for the correlation between twins and using linear mixed modeling, there was a significant difference in Crs with the rescue AS group having a mean Crs of 1.21 mL/cm H2O/kg versus 1.01 mL/cm H2O/kg in the placebo group, adjusted 95% CI for difference (0.01 to 0.49), p =0.0433 (Table 2). We did not demonstrate a significant difference in FRC between groups: 24.8 mL/kg in the rescue versus 22.0 mL/kg in the placebo group, adjusted 95% CI for difference (-1.40 to 6.62), p =0.19 (Table 2).
Table 2. Primary Outcomes: Measurements of Pulmonary Function.
| All Infants |
Rescue AS (n = 49) |
Placebo (n = 49) |
95% CI* | P Value* |
|---|---|---|---|---|
| Crs (mL/cm H2O/kg) | 1.21 ± 0.53 | 1.01 ± 0.51 | (0.01, 0.49) | 0.0433 |
| FRC (mL/kg)** | 24.8 ± 8.8 | 22.0 ± 7.9 | (-1.40, 6.62) | NS |
| Infants ≤ 34 Weeks' Gestation |
Rescue AS (n = 40) |
Placebo (n = 34) |
95% CI* | P Value* |
| Crs (mL/cm H2O/kg) | 1.17 ± 0.55 | 0.90 ± 0.52 | (0.02, 0.60) | 0.0395 |
| FRC (mL/kg)† | 24.3 ± 9.3 | 21.1 ± 8.5 | (-0.13, 8.53) | NS |
Values are mean ± SD and (95% confidence intervals).
Adjusted for gestational age at birth, twin gestation, maternal smoking, rupture of membranes, and gestational diabetes (linear mixed modeling).
FRC obtained in 46 and 47 patients
FRC obtained in 38 and 33 patients, respectively.
Abbreviations are: AS, antenatal steroids; Crs, respiratory compliance; FRC, functional residual capacity; NS, not significant.
Although not one of the primary outcome variables of our study, fewer patients in the rescue group required surfactant or had RDS compared to the placebo group, but this was not significantly different. However, significantly fewer patients in the rescue group required ≥ 30% oxygen (13% versus 29%) or ≥ 40% oxygen (9% versus 23%) than those in the placebo group (p <0.05; Table 3). There was no significant difference in discharge weight, head circumference, or length; or in Z-scores for these growth measurements between groups (Table 3). From the total study population, there was one neonatal death due to sepsis in the rescue group.
Table 3. Secondary Outcomes in the Two Groups of Infants.
|
Rescue AS (n = 56) |
Placebo (n = 56) |
P Value | |
|---|---|---|---|
| Surfactant (%) | 15 (27%) | 22 (39%) | NS |
| Mechanical ventilation (%) | 15 (27%) | 18 (32%) | NS |
| RDS (%) | 15 (27%) | 23 (41%) | NS |
| FiO2 ≥ 0.30 (%) | 7 (13%) | 16 (29%) | <0.05 |
| FiO2 ≥ 0.40 (%) | 5 (9%) | 13 (23%) | <0.05 |
| Days of mechanical ventilation* | 0 (0 – 0.4) | 0 (0 – 1) | NS |
| Days on oxygen* | 0 (0 – 13.9) | 1.7 (0 – 25) | NS |
| Length of stay (days)*,a | 27.0 (15 – 55) | 25.0 (11 – 49) | NS |
| Neonatal survival (%) | 55 (98%) | 56 (100%) | NS |
| Discharge weight (g)*,b | 2474 (2196–2856) | 2380 (2092–2675) | NS |
| Discharge weight Z score** | -0.80 ± 0.88 | -0.77 ± 1.03 | NS |
| Discharge OFC (cm)**,b | 32.6 ± 1.7 | 32.3 ± 1.6 | NS |
| Discharge OFC Z score** | -0.26 ± 0.90 | -0.34 ± 0.94 | NS |
| Discharge length (cm)**,c | 46.5 ± 2.9 | 46.4 ± 2.8 | NS |
| Discharge length Z score** | -0.63 ± 1.14 | -0.37 ± 1.09 | NS |
Median (25th – 75th percentiles) in survivors;
Mean ± SD.
AS, antenatal steroids; NS, not significant; RDS, respiratory distress syndrome; OFC, head circumference.
51 versus 55 patients;
50 versus 55 patients;
49 versus 50 patients.
In the planned subgroup analysis of infants born at ≤ 34 weeks (44 rescue and 39 placebo patients), there were no significant differences in growth measurements or corresponding Z-scores at birth (Table 4). This subgroup demonstrated more pronounced pulmonary benefits: Crs in the rescue group was 1.17 mL/cm H2O/kg versus 0.90 mL/cm H2O/kg in the placebo group, adjusted 95% CI for difference (0.02 to 0.60), p =0.0395. There was no significant difference in FRC measurements (Table 2). Fewer patients in the rescue group had any RDS, required ≥ 30% oxygen, or ≥ 40% oxygen than patients in the placebo group (p <0.05), suggesting an enhanced benefit of rescue AS in those at highest risk for respiratory distress. There were no significant differences in discharge growth measurements or corresponding Z-scores (Table 5).
Table 4. Demographics of Subgroup of Infants ≤ 34 Weeks of Gestation.
|
Rescue AS (n = 44) |
Placebo (n = 39) |
P | |
|---|---|---|---|
| Gestational age at birth (wks)* | 30.5 ± 2.0 | 30.8 ± 2.0 | NS |
| Caucasian (%) | 30 (68%) | 25 (64%) | NS |
| Female (%) | 26 (59%) | 19 (49%) | NS |
| Apgar score 1, 5 min** | 7, 8 | 7, 8 | NS |
| Birth weight (g)* | 1467 ± 398 | 1541 ± 476 | NS |
| Birth weight (g)** | 1416 (1170-1848) | 1460 (1185-1865) | NS |
| Birth weight Z score* | -0.15 ± 0.84 | 0.01 ± 0.89 | NS |
| Small for gestational age (%) | 6 (14%) | 5 (13%) | NS |
| Birth head circumference (cm)* | 27.8 ± 2.3 | 28.0 ± 2.3 | NS |
| Birth head circumference Z score* | -0.19 ± 0.94 | -0.17 ± 0.90 | NS |
| Birth length (cm)* | 40.0 ± 3.0 | 40.3 ± 3.3 | NS |
| Birth length Z score* | -0.20 ± 0.97 | -0.15 ± 1.01 | NS |
Mean ± SD;
Median (25th – 75th percentiles).
Abbreviations are: AS, antenatal steroids; NS, not significant.
Table 5. Secondary Outcomes for Subgroup of Infants ≤ 34 Weeks of Gestation.
|
Rescue AS (n = 44) |
Placebo (n = 39) |
P Value | |
|---|---|---|---|
| Surfactant (%) | 15 (34%) | 21 (54%) | NS |
| Mechanical ventilation (%) | 15 (34%) | 18 (46%) | NS |
| RDS (%) | 15 (34%) | 22 (56%) | <0.05 |
| FiO2 ≥ 0.30 (%) | 7 (16%) | 16 (41%) | <0.05 |
| FiO2 ≥ 0.40 (%) | 5 (11%) | 13 (33%) | <0.05 |
| Days of mechanical ventilation* | 0 (0 – 1) | 0.1 (0 – 5) | NS |
| Days on oxygen* | 1.7 (0 – 18) | 4.7 (0 – 32) | NS |
| Neonatal survival (%) | 43 (98%) | 39 (100%) | NS |
| Discharge weight (g)**,a | 2424 ± 467 | 2376 ± 427 | NS |
| Discharge weight Z score** | -0.90 ± 0.81 | -0.68 ± 1.04 | NS |
| Discharge OFC (cm)**,a | 32.4 ± 1.7 | 31.9 ± 1.6 | NS |
| Discharge OFC Z score** | -0.29 ± 0.93 | -0.39 ± 1.01 | NS |
| Discharge length (cm)**,b | 45.7 ± 2.5 | 45.5 ± 2.7 | NS |
| Discharge length Z score** | -0.84 ± 1.09 | -0.55 ± 1.11 | NS |
Median (25th – 75th percentiles) in survivors;
Mean ± SD.
AS, antenatal steroids; NS, not significant; RDS, respiratory distress syndrome; OFC, head circumference.
38 versus 38 patients;
37 versus 34 patients.
Results using linear mixed models for multivariable modeling are shown in Table 6 and 7. For the entire study population, when adding GA at first AS therapy, GA at study dosing, race, gender, or mode of delivery to the model, the relationship between rescue AS dose and Crs was relatively unaffected. In addition, none of these variables were statistically significant (Table 6). Similarly, when adding these 5 covariates to the model, the relationship between rescue dose and FRC was relatively unaffected. For the subgroup of patients who were delivered at ≤ 34 weeks of gestation, when adding the same 5 covariates to the model, the relationship between rescue dose and Crs (Table 7) or rescue dose and FRC was also relatively unaffected.
Table 6. Effect of Individual Covariates on Respiratory Compliance (All Patients).
| Model* |
Estimated Difference in Crs (Between two Covariate Group) |
Rescue AS Effect (Rescue-Placebo) |
||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Effect | P Value | |
| Rescue AS* | 0.25 | 0.0433 | ||
| AS* + GA at 1st AS dosing† | 0.00 (-0.07 to 0.07) | 0.92 | 0.25 | 0.0452 |
| AS* + GA at study dosing† | 0.00 (-0.07 to 0.07) | 0.93 | 0.25 | 0.0448 |
| AS* + race | 0.16 (-0.11 to 0.42) | 0.23 | 0.25 | 0.0453 |
| AS* + gender | 0.04 (-0.17 to 0.24) | 0.71 | 0.25 | 0.0422 |
| AS* + mode of delivery | -0.08 (-0.31 to 0.16) | 0.52 | 0.25 | 0.0417 |
Indicates block of covariates included in all models, including: gestational age at birth, twin gestation, maternal smoking, rupture of membranes, and gestational diabetes.
This is the estimated difference in Crs corresponding to a 1-week increase in gestational age.
Abbreviations are: Crs, respiratory compliance; AS, antenatal steroids; GA, gestational age.
Table 7. Effect of Individual Covariates on Respiratory Compliance (Infants ≤ 34 Weeks of Gestation).
| Model* |
Estimated Difference in Crs (Between two Covariate Group) |
Rescue AS Effect (Rescue-Placebo) |
||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Effect | P Value | |
| Rescue AS* | 0.31 | 0.0395 | ||
| AS* + GA at 1st AS dosing† | 0.05 (-0.06 to 0.15) | 0.37 | 0.28 | 0.0605 |
| AS* + GA at study dosing† | 0.13 (-0.02 to 0.28) | 0.08 | 0.30 | 0.0408 |
| AS* + race | 0.13 (-0.19 to 0.45) | 0.42 | 0.30 | 0.0448 |
| AS* + gender | 0.04 (-0.22 to 0.29) | 0.76 | 0.30 | 0.0424 |
| AS* + mode of delivery | -0.14 (-0.43 to 0.15) | 0.32 | 0.32 | 0.0354 |
Indicates block of covariates included in all models, including: gestational age at birth, twin gestation, maternal smoking, rupture of membranes, and gestational diabetes.
This is the estimated difference in Crs corresponding to a 1-week increase in gestational age.
Abbreviations are: Crs, respiratory compliance; AS, antenatal steroids; GA, gestational age.
Comment
Infants randomized to a rescue course of AS have a significantly increased respiratory compliance (about a 20% increase) than patients randomized to placebo. The subgroup of infants who delivered at ≤ 34 weeks of gestation demonstrates greater pulmonary function benefits (about 30% increase in Crs). Our findings suggest that improvement in Crs translates into improved clinical respiratory outcomes, with less oxygen requirements, and a trend towards less RDS.
Although our study was a randomized, double-blind, placebo-controlled trial, we acknowledge some limitations. Preterm delivery is difficult to predict and therefore treatment with a rescue course of AS may be difficult to time. However, 73.5% of our patients delivered at ≤ 34 weeks and about 90% received the full course of study medication. Our study was powered to detect differences in pulmonary function tests (Crs), thus assessment of other clinical outcomes will need a larger sample size. Maternal and delivery factors can stimulate endogenous steroid secretion and impact the measurement of newborn pulmonary function. We limited potential confounding factors by using a randomized, placebo-controlled study design. All outcomes were assessed blinded to treatment group assignment, eliminating any possibility of expectation bias. Also our pulmonary function findings were adjusted for correlation between twins and possible confounding by GA at birth, multiple gestations, maternal smoking, rupture of membranes, and gestational diabetes.21,22 Finally, our patients were about 32 weeks of gestation at birth; therefore, our findings may not apply to an exclusive population of infants delivered at < 28 weeks.
Our findings are consistent with our previous study, demonstrating that infants ≤ 32 weeks of gestation treated with a single course of AS 1-7 days before delivery had improved Crs compared with infants treated > 7 days before delivery.12 Our findings of increased Crs in the rescue AS group also are consistent with a multicenter study,5 reporting a decrease in RDS and severe lung disease in patients randomized to weekly AS versus placebo. This suggests that the enzyme system responsible for surfactant production can be repetitively induced despite prior treatment with AS. Measurements of pulmonary function have been used to quantify the newborn infant's response to different treatments,12,15,23,24 including surfactant and AS therapy.12,15 Studies in humans and animal models demonstrated significant improvements in respiratory compliance and lung volumes after dosing with AS.9,12,25,26 A single course of AS is one of the most effective therapies available to improve the outcome of babies born prematurely, and when given within one week of delivery significantly increases Crs and FRC about 50%.2,9,12 This is the context for the significant increase in Crs of 20-30% that we are demonstrating in this study. However, there are insufficient data on the risks and benefits of repeat / weekly courses of AS,4,7,10,27 and there are no randomized trials examining the effects of a single repeat course of AS on Crs and FRC in preterm infants.
Several large randomized trials of weekly betamethasone versus placebo,5,6,28 have shown repeat courses of AS have pulmonary benefits. Wapner et al reported no difference in the primary outcome (composite morbidity) between groups.6 In the entire cohort, and particularly in infants < 32 weeks, there was a significant difference in a number of pulmonary outcomes, with the weekly AS group (24 mg of betamethasone) requiring less surfactant and less mechanical ventilation. There was no significant difference in birth weight or head circumference overall, but infants receiving ≥ 4 courses of AS had decreased birth weight.6 In addition, two studies using lower doses of betamethasone after an initial course of AS have reported conflicting results.5,29 In a large study, 982 women were randomized to weekly betamethasone (11.4 mg) versus placebo.5 Repeat doses of AS significantly decreased the primary outcomes of the study (RDS and severe lung disease) compared to placebo.5 The second study reported no difference in the primary outcome (survival without RDS or severe intraventricular hemorrhage) between patients randomized to a single dose of betamethasone (12 mg) versus placebo.29 However, 79% of the study population was born within 24 hours of dosing.29
The two most recently published studies used different regimens of repeat AS.8,30 Murphy et al reported no difference in the primary outcome (composite morbidity and mortality) between 1858 patients receiving multiple courses of AS (24 mg of betamethasone, every 14 days) or placebo,30 but documented significant decreases in birth weight, length, and head circumference in the repeat AS group. The mean GA at birth were 34.5 and 34.9 weeks, with 35% of pregnancies delivering at 33-36 weeks and 32% at ≥ 37 weeks.30 In contrast, Garite et al recently reported the first large study (n=437) evaluating a single rescue course of AS on neonatal outcome.8 They documented a significant reduction in the primary outcome, composite neonatal morbidity at <34 weeks, in patients randomized to a rescue course of AS (24 mg of betamethasone) versus placebo (43.9% versus 63.6%, p =0.002). There was also a significant decrease in RDS, ventilator support, and surfactant use in the rescue AS group; but there were no differences in perinatal mortality or other morbidities, birth weight and head circumference.8 A reduction of composite morbidity in the rescue group was still observed (32.1% versus 42.6%, p =0.034) as well as decreased respiratory morbidities, when all 558 newborns were included, regardless of GA at delivery. In this study, 55% of patients in each group delivered at <34 weeks and the mean GA at birth was 33.0 weeks for all patients.8 Our study design of dosing with a rescue course of AS was very similar to the design of the Garite et al trial. Our findings of improved respiratory compliance in the rescue AS group (about 20% increase in the total population and 30% in patients ≤ 34 weeks) are consistent with the latter study, and provide a physiological basis for the improved respiratory outcomes observed after a rescue course of AS in the Garite et al trial.8
A recent meta-analysis confirmed the pulmonary benefits of repeat/ weekly AS,31 but concern remains of possible adverse effects.4-7 Crowther et al32 and Wapner et al33 reported neurodevelopmental and growth outcomes at 24-36 months of age in infants randomized to weekly AS versus placebo.5,6 These studies offer some reassurance that limited weekly AS do not significantly increase major adverse neurodevelopmental outcomes or growth delay,7,32,33 although one of the studies reported a non-significant trend for increased cerebral palsy after weekly AS.33 Therefore, additional studies of neurodevelopmental performance and growth are warranted.7 These studies32,33 also raise questions about AS dosing and whether higher doses of prenatal corticosteroids (weekly doses of 24 mg versus 11.4 mg) may increase adverse outcomes.
AS therapy accelerates the structural and biochemical maturation of the fetal lung, reducing the incidence of RDS in preterm infants.2,3 As described by Caughey and Parer,34 to maximize the benefits while minimizing fetal exposure to AS, we randomized patients to a single rescue course of AS versus placebo. Although it has been difficult to define the duration of the pulmonary effects of a single course of AS, data from randomized trials,2,3 our recent prospective cohort study,12 and a retrospective study evaluating a rescue dose of AS35 indicate that it may decrease after 7-14 days. We found an increased Crs in infants assigned to the rescue AS group, with greater changes in infants who delivered at ≤ 34 weeks. In vitro studies with human lung explants have shown that the biochemical effects of AS begin to decrease after 7 days if AS is removed,36 although the corresponding structural changes persist. We speculate that the lower Crs in the placebo group in our study may reflect the dissipation of the beneficial effects of AS on induction of the surfactant system. FRC was only slightly increased in the rescue AS group, although not significantly different, suggesting that the structural changes following a course of AS persist longer than the biochemical changes.10,36
Conclusions
Infants randomized to a single rescue course of AS have a significantly increased respiratory compliance compared to those randomized to placebo. The subgroup of patients who delivered at ≤ 34 weeks demonstrates greater pulmonary function benefits. Our findings suggest that improvement in Crs translates into improved clinical respiratory outcomes. Long-term follow up of our patients is underway. We speculate that if treatment with a course of AS has occurred >14 days before delivery, a rescue course of AS may decrease pulmonary morbidity. This should be studied in a large placebo-controlled trial of high risk patients, and risks and benefits including long-term pulmonary, growth, and neurodevelopmental outcomes should be monitored. The recent study by Garite et al is an important step towards this goal.8
Acknowledgments
Data Safety Monitoring Board members: Robert Steiner, MD and Melanie Gillingham, PhD.
Our study was supported in part by Oregon Health & Science University, General Clinical Research Center / PHS Grant 5 M01 RR000334; by the Oregon Clinical and Translational Research Institute (OCTRI), UL1 RR024140 01 from the National Center for Research Resources (NCRR); and by the American Lung Association.
The authors would like to thank the neonatologists, obstetricians, neonatal fellows, and the staff of our Newborn Intensive Care Units for their cooperation with the study. We also thank the parents and infants who participated in this study.
Abbreviations
- AS
Antenatal steroids
- Crs
Passive respiratory system compliance
- FiO2
Fractional inspired oxygen concentration
- FRC
Functional residual capacity
- PFT
Pulmonary function test
- RDS
Respiratory distress syndrome
Footnotes
Presented at the Annual Meeting of the Pediatric Academic Societies, Honolulu, HI, May 2008 and the Society for Maternal-Fetal Medicine, San Diego, CA, January 2009.
Conflict of interest disclosure: None
CONDENSATION: A placebo-controlled trial of a rescue course of antenatal steroids demonstrates improved newborn pulmonary function (respiratory compliance).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jobe AH, Bancalari E. NICHD/NHLBI/ORD Workshop Summary. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Conference Statement. Effect of corticosteroids for fetal maturation on perinatal outcomes, February 28-March 2, 1994. Am J Obstet Gynecol. 1995;173:246–252. [Google Scholar]
- 3.Crowley PA. Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–334. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: Repeat courses-NIH Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol. 2001;98:144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- 5.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: A randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 6.Wapner RJ, Sorokin Y, Thom EA, et al. NICHD Maternal Fetal Medicine Units Network. Single versus weekly courses of antenatal corticosteroids: Evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 7.Stiles AD. Prenatal corticosteroids: Early gain, long-term questions. N Engl J Med. 2007;357:1248–1250. doi: 10.1056/NEJMe078161. [DOI] [PubMed] [Google Scholar]
- 8.Garite TJ, Kurtzman J, Maurel K, Clark R Obstetrix Collaborative Research Network. Impact of a rescue course of antenatal corticosteroids: A multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248.e1–248.e9. doi: 10.1016/j.ajog.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy C, Bowling S, Williamson K, Stewart M, Durand M. Functional residual capacity and passive compliance measurements after antenatal steroid therapy in preterm infants. Pediatr Pulmonol. 2001;31:425–430. doi: 10.1002/ppul.1070. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy C, Bowling S, Williamson K, et al. The effect of a single remote course versus weekly courses of antenatal corticosteroids on functional residual capacity in preterm infants: A randomized trial. Pediatrics. 2002;110:280–284. doi: 10.1542/peds.110.2.280. [DOI] [PubMed] [Google Scholar]
- 11.McEvoy C, Bowling S, Williamson K, Collins J, Tolaymat L, Maher J. Timing of antenatal corticosteroids and neonatal pulmonary mechanics. Am J Obstet Gynecol. 2000;183:895–899. doi: 10.1067/mob.2000.108876. [DOI] [PubMed] [Google Scholar]
- 12.McEvoy C, Schilling D, Spitale P, Peters D, O'Malley J, Durand M. Decreased respiratory compliance in infants less than or equal to 32 weeks' gestation, delivered more than 7 days after antenatal steroid therapy. Pediatrics. 2008;121:e1032–e1038. doi: 10.1542/peds.2007-2608. [DOI] [PubMed] [Google Scholar]
- 13.Haland G, Lodrup Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 14.LeSouef PN, England SJ, Bryan AC. Passive respiratory mechanics in newborns and children. Am Rev Respir Dis. 1984;129:552–556. [PubMed] [Google Scholar]
- 15.Kelly E, Bryan H, Possmayer F, Frndova H, Bryan C. Compliance of the respiratory system in newborn infants pre- and postsurfactant replacement therapy. Pediatr Pulmonol. 1993;15:225–230. doi: 10.1002/ppul.1950150408. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society/European Respiratory Society. Respiratory mechanics in infants: Physiologic evaluation in health and disease. Am Rev Respir Dis. 1993;147:474–496. doi: 10.1164/ajrccm/147.2.474. [DOI] [PubMed] [Google Scholar]
- 17.Yuksel B, Greenough A, Chan V, Russell RR. Comparison of helium dilution and nitrogen washout measurements of functional residual capacity in premature infants. Pediatr Pulmonol. 1993;16:197–200. doi: 10.1002/ppul.1950160310. [DOI] [PubMed] [Google Scholar]
- 18.Cotton RB, Olsson T, Law AB, et al. The physiologic effects of surfactant treatment on gas exchange in newborn premature infants with hyaline membrane disease. Pediatr Res. 1993;34:495–501. doi: 10.1203/00006450-199310000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61:1380–1385. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics, Centers for Disease Control and Prevention, US Department of Health and Human Services. Available at www.cdc.gov/nchs/about/major/nhanes/growthcharts/zscore/zscore.htm.
- 21.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, United Kingdom: John Wiley and Sons; 2003. [Google Scholar]
- 22.Chow SC, Liu JP. Design and Analysis of Clinical Trials: Concepts and Methodologies. New York, NY: John Wiley and Sons; 1998. [Google Scholar]
- 23.McEvoy C, Sardesai S, Macri C, Paul R, Durand M. Neonatal pulmonary mechanics and oxygenation after prophylactic amnioinfusion in labor: A randomized clinical trial. Pediatrics. 1995;95:688–692. [PubMed] [Google Scholar]
- 24.Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: Dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. 2002;109:262–268. doi: 10.1542/peds.109.2.262. [DOI] [PubMed] [Google Scholar]
- 25.Ikegami M, Polk DH, Jobe AH, et al. Effect of interval from fetal corticosteroid treatment to delivery on postnatal lung function of preterm lambs. J Appl Physiol. 1996;80:591–597. doi: 10.1152/jappl.1996.80.2.591. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami M, Polk D, Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol. 1996;174:1408–1413. doi: 10.1016/s0002-9378(96)70581-1. [DOI] [PubMed] [Google Scholar]
- 27.Banks BA, Cnaan A, Morgan MA, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. Am J Obstet Gynecol. 1999;181:709–717. doi: 10.1016/s0002-9378(99)70517-x. [DOI] [PubMed] [Google Scholar]
- 28.Guinn DA, Atkinson MW, Sullivan L, et al. Single versus weekly courses of antenatal corticosteroids for women at risk of preterm delivery: A randomized controlled trial. JAMA. 2001;286:1581–1587. doi: 10.1001/jama.286.13.1581. [DOI] [PubMed] [Google Scholar]
- 29.Peltoniemi OM, Kari MA, Tammela O, et al. Repeat Antenatal Betamethasone Study Group. Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics. 2007;119:290–298. doi: 10.1542/peds.2006-1549. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KE, Hannah ME, Willan AR, et al. MACS Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth (MACS): A randomised controlled trial. Lancet. 2008;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 31.Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev. 2007;3:CD003935. doi: 10.1002/14651858.CD003935.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS. Outcome at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 33.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 34.Caughey AB, Parer JT. Recommendations for repeat courses of antenatal corticosteroids: A decision analysis. Am J Obstet Gynecol. 2002;186:1221–1229. doi: 10.1067/mob.2002.123742. [DOI] [PubMed] [Google Scholar]
- 35.Vermillion ST, Bland ML, Soper DE. Effectiveness of a rescue dose of antenatal betamethasone after an initial single dose. Am J Obstet Gynecol. 2001;185:1086–1089. doi: 10.1067/mob.2001.117633. [DOI] [PubMed] [Google Scholar]
- 36.Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]