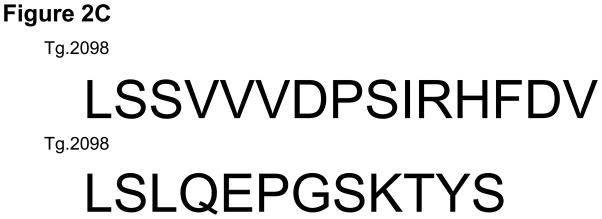Abstract
An HLA-DR variant containing Arginine at position 74 of the DRβ1 chain confers a strong genetic susceptibility to autoimmune thyroid diseases (AITD), Graves’ disease (GD) and Hashimoto’s thyroiditis (HT), while Glutamine at position DRβ1-74 is protective. We hypothesized that the DRβ1-Arg74 variant is able to present pathogenic thyroglobulin (Tg) peptides to T-cells more efficiently, thereby triggering thyroid autoimmunity. Indeed, we have previously identified 4 human Tg (hTg) peptides that bind specifically to DRβ1-Arg74 with much weaker binding to the protective variant DRβ1-Gln74. The aim of our study was to examine in vivo whether an hTg peptide that binds strongly and specifically to DRβ1-Arg74 is capable of stimulating T-cells during the induction of thyroiditis in a “humanized” mouse expressing human DR3, and in patients positive for Tg antibodies. Sequencing of exon 2 of the DR transgene in the DR3-mice, null for endogenous MHC II molecules, confirmed that they expressed the disease associated DRβ1-Arg74 variant, thus making them an ideal in vivo model to test the presentation of hTg peptides by DRβ1-Arg74 HLA-DR. Induction of EAT in the DR3 mice lead to T-cell stimulation and proliferation to Tg.2098, a strong and specific DRβ1-Arg74 binder, while a non-binding control peptide, Tg.2766 did not induce this response. Moreover, Tg.2098 stimulated T-cells from 4 individuals who were positive for thyroglobulin antibodies, demonstrating that Tg.2098 is an immunogenic peptide capable of being presented in vivo and activating T-cells in EAT and AITD. Energetic analysis of the complex formed by Tg.2098 and DRβ-Arg74 has shown that the origin of the affinity was determined by residues 1, 7 and 9 in the peptide, while the selectivity of the peptide for the MHC was determined by the Asp in position 4. The disease-protective substitution R74Q, leads to reduction in affinity due to changes in local interaction with D4 as well as non-local interaction with other residues. The electrostatic potential on the surface of the DRβ-Arg74 - Tg.2098 complex has a unique signature which may be recognized by T-cell receptors leading to autoimmune thyroiditis. Taken together these findings suggest that Tg.2098, a strong and specific binder to the disease associated HLA-DRβ-Arg74, is a major human T-cell epitope and participant in the pathoetiology of AITD.
Keywords: Thyroglobulin, HLA, gene, thyroiditis
INTRODUCTION
The MHC class II molecules, expressed on the surface of professional antigen presenting cells (APCs, e.g. dendritic cells and B-cells), are composed of α and β chains that form the peptide binding pocket where mostly foreign peptides are packaged and presented to CD4+ T-cells. Recognition of the peptide-MHC II (pMHC II) complex by T-cells, in the presence of co-stimulatory signals, results in T-cell activation leading to an immune response against the host protein from which the presented peptide was derived [1]. When immunogenic self peptides are presented in the context of the MHC II pocket, the result is T-cells activation against self peptides, leading to the onset of autoimmunity [2].
A recurrent theme in the initiation of autoimmunity is the importance of the amino acid signature of the peptide binding cleft of the MHC II molecule. In T1D, it has been shown that the structural features of the MHC class II molecule have a major impact on the presentation of auto-antigenic islet-cell peptides to T-cells [2–6]. Similarly, in rheumatoid arthritis specific HLA-DR pocket sequences have also been found to be associated with disease [7;8], suggesting that this might be a general paradigm in autoimmunity. Our group has identified specific HLA-DR pocket sequences that were strongly associated with autoimmune thyroid disease (AITD), Graves’ disease (GD) [9] and Hashimoto’s thyroiditis (HT) [10]. The key amino acid position in the AITD-associated pocket signature was position DRβ1–74. The presence of arginine at position DRβ1–74 was associated with strong susceptibility to AITD while the glutamine at this position was protective [9;10]. Moreover, we have shown a statistical interaction between amino acid variants in thyroglobulin (Tg), a major tissue specific antigen in autoimmune thyroid disease, and DRβ1-Arg74 resulting in a high odds ratio for GD [11]. This statistical interaction suggested a biological interaction between specific pathogenic Tg peptides and DRβ1-Arg74 HLA-DR. To explore this possibility we have utilized an in vitro system to identify cathepsin generated hTg peptides that bound to DRβ1-Arg74 HLA-DR and only minimally to DRβ1-Gln74, expressed either as recombinant protein or in a cell-based system. Using these novel binding assays we identified four hTg peptides with strong and selective binding to DRβ1-Arg74 HLA-DR: Tg.726, Tg.1571, Tg.1951, and Tg.2098 [12].
Of the four hTg peptides we identified as good binders to DRβ1-Arg74, two (Tg.726 and Tg.2098) have been previously reported [13]. Tg.2098 is especially interesting because it is the only peptide that was bound to MHC II molecules in the thyroids of AITD patients [13], and was able to induce thyroiditis in DR3 transgenic mice when used to immunize the mice [14]. Therefore, the aim of the current study was to extend our in vitro binding studies and to examine, in vivo, both in patients and in a mouse model of EAT, whether Tg.2098 is a major T-cell epitope in thyroid autoimmunity. Our results demonstrated that Tg.2098, which is a strong and specific binder to DRβ1-Arg74, stimulated T-cells from mice and humans that developed autoimmune thyroiditis, while a non-binding peptide (Tg.2766) did not elicit a response.
MATERIALS AND METHODS
Mice
Mice transgenic for DRB1*0301 (DR3) and lacking endogenous murine MHC II were generated as previously described [15;16]. Briefly, the DR3 (DRA1*0101/DRB1*0301) transgenes were inserted into B10.M embryos and the progeny crossed with I-Aβ knockouts (C57BL/6 ×129) to obtain mice lacking murine MHC class II and expressing human DR3. The transgenic mouse line was maintained by intercrossing. The background non-MHC genes in the DR3 transgenic line is 50% C57BL/10 genes and 50% contribution from CBA, C57BL/6, and 129 genes. Mice were bred in a pathogen-free facility (Mount Sinai Medical Center, New York, NY), and expression of HLA-DR3 was tested by PCR and/or by flow cytometry using antibody L243 (American Type Culture Collection [ATCC], Manassas, VA).
Sequencing of exon 2 of the HLA-DR3 gene in DR3 transgenic mice
DNA was extracted from mouse tails using the Puregene kit (Gentra Systems, Minneapolis, MN) according to the manufacturer’s instructions.
In order to identify the HLA-DR sequence at position β-74, we sequenced exon 2 of the HLA-DRB1 gene which encodes the specific amino acids determining the DR specificities. Genomic DNA was amplified and sequenced using the following DR3-specific primers: Forward primer – AACCAGGAGGAGAACGTGCG; reverse primer – GAAGCTCTCCACAACCCCGT. PCRs were performed in a 20 uL reaction mixture containing 50 ng genomic DNA; 5 pmol of each primer; PCR buffer containing 50 mmol/L KCl; 10 mmol/L Tris-HCl (pH 8.3); 2.25 mmol/L MgCl2; 200 mmol/L of each deoxy (d) - dATP, dGTP, dTTP, and dCTP; and 1 U of AmpliTaq DNA polymerase (ABI, Foster City, CA). Reaction mixtures were heated to 94°C for 7 min, and then cycled 30 times as follows: 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C. The product size was 171 bp. The PCR products were sequenced using the same DR3-specific primers used for amplification (see above). Sequencing was performed using the ABI Big Dye DNA sequencing kit (ABI, Foster City, California, USA), and the sequencing products were separated on an ABI-3130 automated sequencer (ABI). Sequence alignment analyses were performed using the Sequencher™ version 3.1 program (Gene Codes Corporation, Ann Arbor, MI). For alignment the known DR3 subtype sequences were obtained from the ImMunoGeneTics project (IMGT)-HLA Informatics Group (HIG) database (http://www.ebi.ac.uk/imgt/hla).
Experimental Autoimmune Thyroiditis
EAT was induced in 10 mice by injecting subcutaneously human thyroglobulin at a concentration of 3 mg/ml (75 ug of hTg per injection), emulsified 1:1 in complete Freund’s adjuvant (CFA) supplemented with Mycobacterium tuberculosis. Two injections of 50 ul, one to the inner thigh and one to the opposite outer hip, were performed at day 0 and again in the opposite thigh and hip at day 7. Mice were sacrificed on day 28.
Lymphocyte isolation
Spleens and draining lymph nodes were collected from mice and kept on ice in Hanks Balanced Salt Solution (HBSS). To harvest lymphocytes, the spleen and lymph nodes were placed in complete RPMI medium [RPMI 1640 medium supplemented with fetal bovine serum (FBS; 10%), L-glutamine (2 mM) and penicillin-streptomycin (100 U/ml, 100 μg/ml)] (Hyclone, Fisher Scientific), and cut in several places. These were then pressed in a circular motion with a plunger from a 6 ml syringe until only fibrous tissue remained. To further disperse clumps in the suspension, it was drawn up and expelled several times through a 6 ml syringe using a 19 gauge needle, and then the suspension was filtered twice through a nylon screen (75um cell strainer) into a 50 ml falcon tube. The cell suspension was then spun for 10 minutes at 200xg and the supernatant was discarded. Cells were again suspended in complete RPMI medium and centrifuged a second time, and the media were discarded. To remove remaining non-lymphocytic cells from spleen, 5 ml of ACK lysis buffer was added to the cells for 5 minutes, after which complete RPMI medium was added to fill the tube, and centrifuged for 10 minutes. The remaining cell pellet was washed and resuspended in 10 ml of complete RPMI medium for counting and plating.
T-cell stimulation assays in DR3 mice immunized with human Tg T-cell proliferation assays
Cells taken from the draining lymph nodes and spleens of EAT mice were pooled and cultured in RPMI 1640 medium supplemented with fetal bovine serum (FBS; 10%), L-glutamine (2 mM) and penicillin-streptomycin (100 U/ml, 100 μg/ml) (Hyclone, Fisher Scientific). Cultures of cells were split into five groups, plated 6 × 105 cells per well in 180ul of medium: Group 1: cells were treated with PBS (negative control); Group 2: cells were stimulated with 2μg/ml Concavalin A (positive control); Group 3: cells were stimulated with human thyroglobulin at a concentration of 40μg/ml; Group 4: cells were stimulated with Tg.2098 (shown to bind to DRβ1-Arg74 HLA-DR) at a concentration of 10μg/ml; and Group 5: cells were stimulated with Tg.2667 (shown not to bind to DRβ1-Arg74 HLA-DR) at a concentration of 10μg/ml. After 48 hrs, cells were pulsed with 1 uCi/well of [3H]thymidine (MP biomedical, Costa Mesa, CA). As negative controls we used non immunized mice of the same strain (DR3 transgenic). Cells were harvested 18 h later, and [3H]thymidine incorporation was measured in a scintillation counter (TopCount·NXT™; PE life sciences, Boston, MA). All assays were performed in quadruplicates. Data are expressed as stimulation index. We calculated the stimulation index by using the following formula: Stimulation index = (CPM of the peptide treated lymphocytes/CPM of PBS treated lymphocytes). The differences between T cell proliferation when cells were incubated with Tg.2098 and Tg.2667 were analyzed using Student’s t-test. P-values of < 0.05 were considered significant.
Interleukin-2 (IL-2) measurements
Lymphocytes isolated from draining lymph nodes and spleens of DR3 mice induced with EAT were plated 2.5 × 106 cells per well in 900 μl complete RPMI 1640 medium in 24 well culture plates. Cells were treated for 48 hours with PBS, 2 μg/ml Concavalin A, 40 ug/ml human thyroglobulin, or 10 ug/ml of peptides (Tg.2098 or Tg.2766). After 48 hours, the supernatant was collected, and used to analyze for IL-2 production. IL-2 levels were assayed using a bead based array (Linco/Millipore, Bellerica, MA). Briefly, in a 96 well plate, 25ul of a mixture of color coded polystyrene beads, was incubated with 25 ul undiluted supernatant, for 1 hour. Beads were washed with wash buffer, and then incubated with biotinylated anti-IL-2 detection antibody solution. Without washing, streptavidin-phycoerythrin was then added to the plate. After washing with wash buffer, sheath fluid was added to the beads, and beads were then read on the Luminex 200 machine (xMAP technology Millipore, Bellerica MA). Standards were run in parallel to the samples.
HLA-DR Typing
Molecular typing of HLA-DR was carried out according to the requirements of The American Society for Histocompatibility [9]. The major alleles of HLA-DR were typed using the technique of group-specific PCR-amplification followed by restriction enzyme digestion as previously described [9].
Direct Sequencing of Exon 2 of the Human HLA-DRB1 Gene
To determine the HLA-DR sequences in AITD patients we sequenced exon 2 of the HLA-DRB1 gene, which encodes the specific amino acids determining the DR specificities, as described above for the DR3 transgenic mice.
T-cell proliferation assays in patients
We tested T-cell proliferation in 4 individuals with AITD, all with high levels of thyroglobulin antibodies. Mononuclear cells were isolated from heparinized whole blood by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Piscataway, NJ). Cells were cultured in RPMI 1640 medium supplemented with FBS; 10%, L-glutamine (2 mM) and penicillin-streptomycin (100 U/ml, 100 μg/ml) (Hyclone, Fisher Scientific). Cultures of cells were plated at 2 × 105 cells per well in 180 ul of media, and split into five groups as described above for mouse lymphocyte proliferation assays,: Group 1: cells were treated with PBS (negative control); Group 2: cells were stimulated with 2 ug/ml Concavalin A (positive control); Group 3: cells were stimulated with 40 ug/ml human thyroglobulin; Group 4: cells were stimulated with 10 ug/ml Tg.2098 (binds to DRβ1-Arg74); and Group 5: cells were stimulated with 10 ug/ml Tg.2667 (does not bind to DRβ1-Arg74). Proliferation was determined by [3H]thymidine incorporation as described above for mouse lymphocytes.
Computer simulations of peptide binding to HLA-DR
The complexes of DRβ1-Arg74 HLA-DR with the Tg.2098 peptide have been constructed with AMBER using the known structure of DR3 with the CLIP peptide (1A6A.pdb). The register of the peptide in the complex was determined by a multiple alignment of the peptides that bind to DR3. The register for Tg.2098 is: ALSSV1VVD4PSIRH9FD. The corresponding 15 residues in the CLIP peptide were substituted with the sequence of the Tg.2098 peptide. The complex of DR3 and Tg.2098 was placed in a periodic box filled with water and neutralized with counterions. The water and ions in the systems were equilibrated by progressively reducing the restraints on the solute [17;18]. Finally the systems were simulated without restraints for 10 ns. Analysis of the trajectory by 2D-RMSD shows that the simulation stabilized after 1 ns and the structures in the remaining time were within a root mean square deviation (rmsd) of 2 Å from each other. Structures from the equilibrated segments were extracted for MM-PBSA analysis, base on reported protocols [19;20]. The binding energy of the peptide to DRβ1-Arg74 was computed with the MM-PBSA approximation. The elements of the calculation consist of the interaction energy including solvation, changes in translational, rotational, and vibrational entropy. The binding energy was decomposed into residue-based contributions as well as into pairwise contributions as described previously [12;19;20]. The decomposition contains the contributions from solvation but not from entropic changes.
RESULTS
HLA class II molecule of humanized DR3 mice is Arg74 positive
Before starting experiments with the humanized DR3 mice, it was important to determine whether the HLA-DR3 of these mice contained the DRβ-Arg74 pocket amino acid which is strongly associated with AITD. Therefore, we sequenced exon 2 of the HLA-DRb1 gene, coding for the position 74 variants. Sequencing results showed that the humanized DR3 mice possess the disease-associated arginine at position 74 of the DRβ1 chain (Figure 1). Therefore, the DR3 mice express the same DR3 pockets that we found to be associated with AITD and to bind specific hTg peptides [9;10;12]. Clearly, the results of testing the presentation of hTg peptides in DR3 mice are translatable to human AITD.
Figure 1.
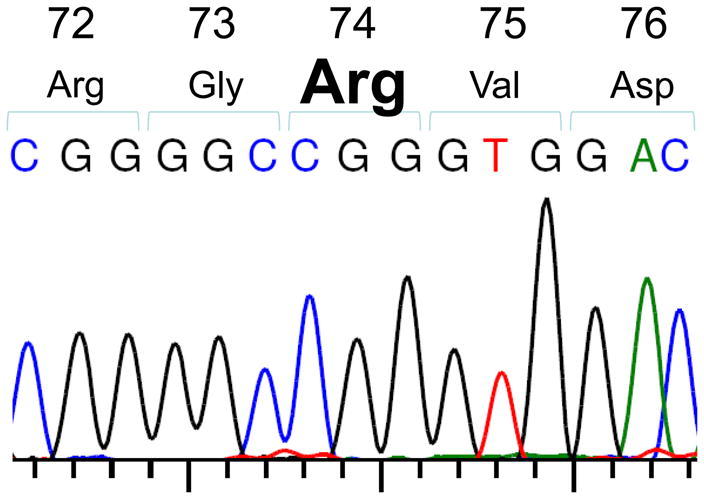
Sequencing of exon 2 of the DRβ1 gene in DR3 transgenic mice demonstrates the presence of arginine (CGG) at position 74 of the DRβ1 chain.
Tg.2098 is a major epitope in the induction of experimental autoimmune thyroiditis (EAT) in DR3 mice
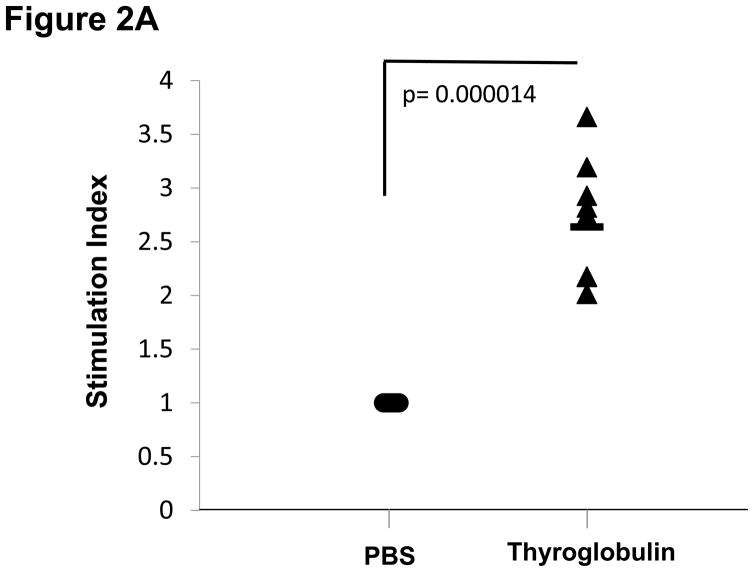
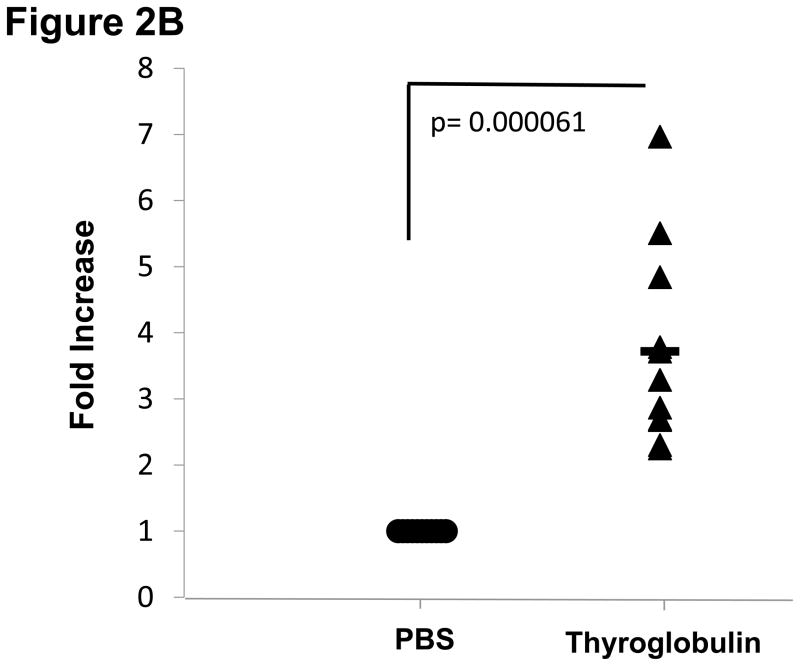
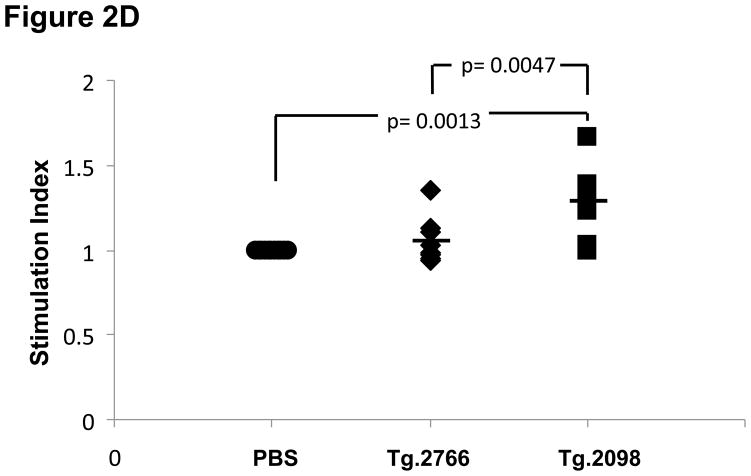
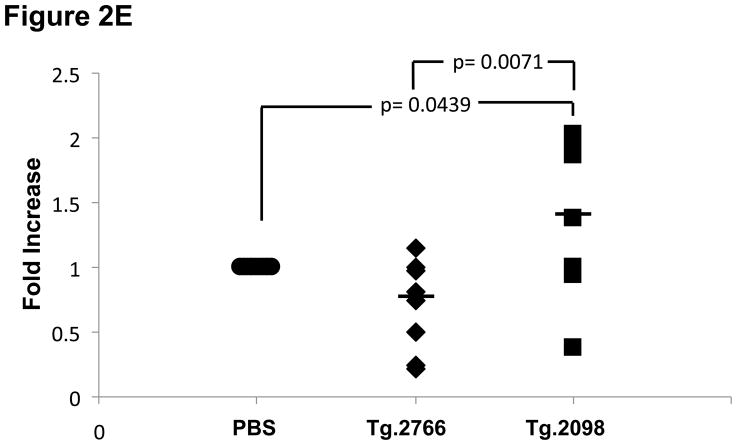
Of the four hTg peptides we identified to bind strongly and specifically to the disease associated HLA-DRβ-Arg74 [12], Tg.2098, was shown to be involved in the etiology of AITD by two other groups [13;14]. Therefore, we tested Tg.2098 for stimulating T-cell proliferation in vivo using the “humanized” DR3 mice. To determine if there was a correlation between peptide binding and immunogenicity, we induced EAT in DR3 mice by injection of full length human thyroglobulin with complete Freund’s adjuvant. After 28 days the mice were sacrificed and T-cells were collected from both the main draining lymph nodes (inguinal and cervical) and spleen. As negative controls we used non-immunized mice. As expected both Tg.2098 and Tg.2766 did not stimulate T-cells from non-immunized mice (stimulation indexes were 1.05 and 1.09, respectively). DR3 mice immunized with hTg developed strong proliferative T-cell responses to hTg (Figure 2A). This T-cell response to hTg was accompanied by IL-2 production (Figure 2B). Next we determined whether Tg.2098, known to bind to DRβ-Arg74 pockets, was a major T-cell epitope in DR3 mice induced with EAT. Lymphocytes from mice immunized with hTg were treated with Tg.2098, and a control non-binding peptide, Tg.2766 (Figure 2C), and proliferation and cytokine production were determined. Treatment with Tg.2098 resulted in significant proliferation of lymphocytes while the non-binding peptide Tg.2766 did not induce cell proliferation (Figure 2D). Similarly, there was significant increase in the production of IL-2 by lymphocytes incubated with Tg.2098 compared to lymphocytes incubated with Tg.2766 (Figure 2E). This suggested that Tg.2098 was presented within HLA-DR on APC’s to T-cells during the immunization of the mice, thereby generating a memory response to it.
Figure 2.
EAT was induced in DR3 mice by injection of full length human thyroglobulin with complete Freund’s adjuvant. After 28 days the mice were sacrificed and T-cells were collected from draining lymph nodes (inguinal and cervical) and spleen. (A) Lymphocytes from mice immunized with hTg were incubated with hTg and proliferation was measured by 3[H]-Thymidine incorporation. Significant proliferation is seen in lymphocytes incubated with hTg compared to PBS. (B) Levels of IL-2 production by lymphocytes incubated with hTG were also significantly elevated. (C) Sequences of the Tg.2098 and Tg.2766 peptide tested in cellular proliferation assays, sequences showed no significant sequence alignment. (D) Lymphocytes from mice immunized with hTg were incubated with hTg peptides Tg.2098 and Tg.2766 and proliferation was measured by 3[H]-Thymidine incorporation. Significant proliferation is seen in lymphocytes incubated with Tg.2098 compared to lymphocytes incubated with PBS or with the non-binding peptide Tg.2766. (E) Levels of IL-2 production by lymphocytes incubated with Tg.2098 were significantly elevated compared to lymphocytes incubated with PBS or with the non-binding peptide Tg.2766.
Tg.2098 is a major epitope in patients
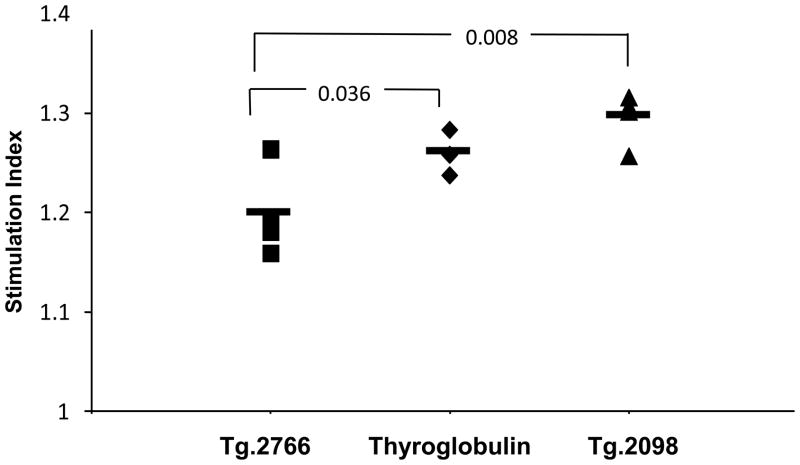
Tg.2098 was also tested for its ability to stimulate T-cell proliferation in vivo. Four individuals positive for Tg antibodies were HLA typed for position 74 amino acids variants: Patient 1 – HLA-DR3 homozygote, Arg/Gln-74; Patient 2 – HLA-DR2/HLA-DR12, Arg/Glu-74; Patient 3 – HLA-DR11/HLA-DR14, Ala/Gln-74; Patient 4 – HLA-DR11/HLA-DR7, Ala/Gln-74. Mononuclear cells from each individual were treated with Tg.2098, and the control non-binding peptide, Tg.2766, and proliferation was determined by [3H]thymidine incorporation. Treatment with Tg.2098 resulted in significant proliferation of lymphocytes compared to incubation with the non-binding peptide Tg.2766 (Figure 3). These data suggested that Tg.2098 is a major thyroglobulin epitope in human AITD in addition to EAT in DR3 mice.
Figure 3.
Lymphocytes from 4 patients who are positive for anti-Tg antibodies were incubated with hTg, and hTg peptides Tg.2098 and Tg.2766. Proliferation was measured by 3[H]-Thymidine incorporation. Significant proliferation is seen in lymphocytes incubated with Tg.2098 compared to lymphocytes incubated with the non-binding peptide Tg.2766.
Tg.2098 peptide fits into the HLA-DRβ-Arg74 peptide binding pocket
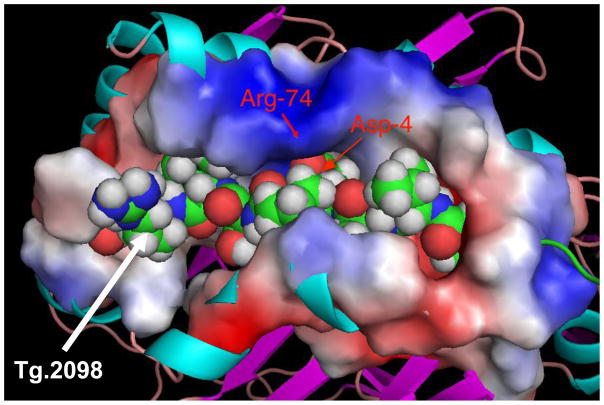
The energetic analysis of binding of Tg.2098 to DRβ-Arg74 pocket is shown in Table 1. A decomposition of the total interaction energy between Tg.2098 and the HLA-DRβ-Arg74 pocket into residue-based contributions shows that the main source of the change lies in the negatively charged residue in the center of the peptide. Thus, the interaction with D4 in Tg.2098 is −3.1 kcal/mole (with respect to the separated molecules), with other important contributions originating from V1 (−7.6 kcal/mol), I7 (−6.2 kcal/mol) and H9 (−8.0 kcal/mol). As in all the peptides studied by us [12] the contributions from the hydrophobic residues are the main source of the stability. However, the interaction with D4 confers the main selectivity of the peptide to DRβ-Arg74 as illustrated by the change in the binding pattern of Tg.2098 to the DRβ-Gln74 variant. The interaction energy between D4 and the DRβ-Gln74 is +0.2 indicating a total contribution to destabilization of +3.3 kcal/mol. Interactions of other residues are also affected by the substitution R74Q: V1 changes to −5.6 kcal/mol, I7 to −3.4 kcal/mol and H9 to −0.6 kcal/mol. These results clearly illustrate that the effect of substitution in the HLA-DRβ-Arg74 pocket has both direct and indirect effects on peptide binding and selectivity. Although other residues gain in interaction energy (not shown) the total binding energy to DRβ-Gln74 is reduced by 1.9 kcal/mol. This change would correspond to a 25-fold reduced affinity of Tg.2098 to DRβ-Gln74. The selectivity of Tg.2098 to DRβ-Arg74 is illustrated in Figure 4, which shows the steric and electrostatic properties of the complex. The peptide is closely packed in the binding groove with strong interaction between the two positive residues K71 and R74 (represented by the positive electrostatic potential on the surface of the complex) and the carboxylate of D4. The hydrophobic residues V1, I7 and H9 are inserted into deep pockets at the bottom of the binding groove and are therefore not visible. The electrostatic potential presented on the surface of the complex is the characteristic and unique signature of the complex of DRβ-Arg74•Tg.2098 not shared with complexes with other peptides.
Table 1.
Interaction energies as a function of the amino acid sequence of the peptide. Final column suggests an ‘optimal’ peptide for binding to HLA-DRβ-Arg74 based on previous [12] and current results.
| P | Tg.2098 | Interaction Energy (kcal/mol) | Optimal a.a. residue |
|---|---|---|---|
| 1 | V | −7.6 | I |
| 2 | V | −5.0 | V |
| 3 | V | −4.3 | F |
| 4 | D | −3.1 | D |
| 5 | P | −3.7 | P |
| 6 | S | −0.1 | V |
| 7 | I | −6.2 | I |
| 8 | R | −1.0 | P |
| 9 | H | −8.0 | H |
Figure 4.
Tg.2098 embedded inside the binding groove, with a characteristic and unique electrostatic potentials displayed on its surface. The ridge opposite the Arg-74 shows a positive potential flanked by negative potential.
DISCUSSION
Thyroglobulin and HLA-DR are two major susceptibility genes for AITD [2;21]. HLA-DR3 has been consistently shown to be associated with both GD and HT [22–25]. Furthermore, our group has identified specific DR pocket amino acid signatures that were associated with AITD [9;10]. When the positively charged amino acid arginine was present at position 74 of the DRβ1 chain there was a strong association with AITD, while glutamine was protective.
Thyroglobulin (Tg) is the major protein in the thyroid gland, and it serves as the precursor and storehouse for thyroid hormones. Linkage and association studies performed in Caucasian and non-Caucasian populations have consistently demonstrated that Tg is a major AITD gene [21;26–31]. Sequencing studies identified amino acid variations in Tg that were strongly associated with AITD [32]. Furthermore, the Tg gene and the DRβ1-Arg74 variant of HLA-DR have shown statistical interaction in predisposing to AITD [11], suggesting that a biological interaction between Tg peptides and DRβ1-Arg74 pockets may play an important role in the etiology of AITD. Therefore, we tested whether DRβ1-Arg74 pockets binds specific hTg peptides that can be presented to T-cells and induce thyroiditis. Indeed, we have identified four hTg peptides with strong and specific binding to the disease-associated DRβ1-Arg74 pocket and not to the disease-protective DRβ1-Gln74 pocket. Of these four peptides one peptide Tg.2098 was found to be important in AITD by two other groups [13;14]. Flynn et al used computer algorithms to predict potential hTg peptides that bind to DR3 and then tested them in DR3 mice. They found that Tg.2098 (designated by them hTg2079) stimulated proliferative responses in hTg immunized mice and was able to induce thyroiditis when mice were immunized with the peptide [14]. These data are consistent with our findings and demonstrate that Tg.2098 is a major hTg epitope. Muixi et al used a different strategy. They sequenced peptides bound to HLA-DR molecules that were isolated from the thyroid glands of GD patients. One of the hTg peptides identified was Tg.2098. Taken together these studies suggested that Tg.2098 is a major T-cell epitope in AITD [13].
Other hTg peptides have also been identified as T-cell epitopes in AITD, including p2340 (a.a. 2340–2359) [33], p179 (a.a. 1790194), p2540 (a.a. 2540–2554), p2529 (a.a. 2529–2545) [34], and others [35]. However, all of these peptides were tested in EAT susceptible mice (e.g. CBA/J, SJL, AKR/J) expressing murine MHC class II and not human DR3. We have previously shown, using sequencing and structural modeling studies, that the murine EAT-associated MHC II pocket has different peptide binding cleft structure than the human AITD-associated HLA-DR pocket signature [10]. Therefore, the hTg pathogenic peptides that were reported to be associated with EAT in mice expressing murine MHC II may not be relevant to human AITD, where hTg peptides are presented within specific HLA-DR pockets (e.g. DRβ1-Arg74). In the current study we have used “humanized” mice, expressing human DR3 (harboring the disease specific DRβ1-Arg74 ) and no murine MHC II, in order to ensure that after immunization with hTg, hTg peptides are being presented to T-cells within the same DR pockets that are associated with human AITD. Using this strategy we have identified Tg.2098 as a major hTg peptide associated with EAT in “humanized” DR3 mice, strongly suggesting that it is a dominant T-cell epitope in human AITD. Moreover, Tg.2098 was also able to stimulate T-cells from patients with antibodies to hTg. These data are consistent with the results of Flynn et all who also used the DR3 “humanized” mice [14].
In this study we have examined the correlation between hTg peptide binding and pathogenicity. Our results showed T-cell responses to the known HLA-DRβ1-Arg74 binder Tg.2098 compared to non-binder Tg.2766 in DR3 mice induced with EAT, and in 4 individuals positive for thyroid antibodies. Furthermore, our computational analysis of the complex of Tg.2098 with the HLA-DRβ1-Arg74 showed a characteristic electrostatic potential on the surface of the complex. We propose that this signature is responsible for selecting the correct T-cell receptor responsible for inducing thyroiditis.
Of the 4 patients, positive for anti-Tg antibodies that were analyzed for their T-cell responses to Tg peptides, only two had DRβ1–74 genotypes. The fact that lymphocytes from two patients without DRβ1-Arg74 stimulated in response to Tg.2098 demonstrated the significant pathogenicity of this peptide, and that it could also bind to pockets that do not contain DRβ1-Arg74, albeit with weaker affinity. This is not unexpected as individuals that are negative for DRβ1-Arg74 still can develop thyroid autoimmunity and anti-Tg T-cell responses.
How can a Tg peptide be important in AITD when anti-TSH receptor (TSHR) and anti-thyroid peroxidase (TPO) antibodies are the clinical hallmarks of GD and HT, respectively? One potential explanation is the concept of epitope spreading. According to the model of epitope spreading it is possible that Tg may be critical to the initiation phase of GD and HT, but after the disease develops and the thyroid is infiltrated with T-cells, TSHR and TPO become the dominant antigens. A similar situation has been shown to occur in celiac disease, where gluten clearly initiates the autoimmune response, but once the disease is initiated tissue transglutaminase (the endomysial antigen) becomes the dominant autoantigen [36;37].
In conclusion, we have shown that Tg.2098 is a major peptide involved in the initiation of AITD. Tg.2098 is the first peptide that has been consistently found to be pathogenic in AITD. These findings have treatment implications since they open the way for possible peptide blocking therapies.
Acknowledgments
This work was supported in part by grants DK61659, DK067555, & DK073681 from NIDDK (to YT), and by a grant from the American Thyroid Association (to EMJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol. 1997;54(2):159–169. doi: 10.1016/s0198-8859(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: From epidemiology to etiology. J Autoimmun. 2008;30(1–2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2(6):501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 4.Wucherpfennig KW. MHC-linked susceptibility to type 1 diabetes: a structural perspective. Ann N Y Acad Sci. 2003;1005:119–127. doi: 10.1196/annals.1288.013. [DOI] [PubMed] [Google Scholar]
- 5.Onengut-Gumuscu S, Concannon P. The genetics of type 1 diabetes: lessons learned and future challenges. J Autoimmun. 2005;25(Suppl):34–39. doi: 10.1016/j.jaut.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Todd JA, Acha-Orbea H, Bell JI, Chao N, Fronek Z, Jacob CO, McDermott M, Sinha AA, Timmerman L, Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen PK. HLA associations with rheumatoid arthritis: a piece of the puzzle. J Rheumatol Suppl. 1992;32:7–11. [PubMed] [Google Scholar]
- 8.Gregersen PK, Silver J, WINCHESTER RJ. Genetic susceptibility to rheumatoid arthritis and human leukocyte antigen class II polymorphism. The role of shared conformational determinants. Am J Med. 1988;85(6A):17–19. doi: 10.1016/0002-9343(88)90374-9. [DOI] [PubMed] [Google Scholar]
- 9.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, Tomer Y. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves’ disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 10.Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, Tomer Y. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U S A. 2008;105(37):14034–14039. doi: 10.1073/pnas.0806584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, Villanueva R, Tomer Y. Possible Interaction Between HLA-DRbeta1 and Thyroglobulin Variants in Graves’ Disease. Thyroid. 2006;16(4):351–355. doi: 10.1089/thy.2006.16.351. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, Li CW, Fadlalla M, Gandhi A, Chaturvedi V, Smith EP, Schwemberger S, Osterburg A, Babcock GF, Tomer Y. Employing a recombinant HLA-DR3 expression system to dissect MHC II- thyroglobulin peptide dynamism: A genetic, biochemical, and reverse immunological perspective. J Biol Chem. 2009 doi: 10.1074/jbc.M109.041574. (ePub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muixi L, Carrascal M, Alvarez I, Daura X, Marti M, Armengol MP, Pinilla C, Abian J, Pujol-Borrell R, Jaraquemada D. Thyroglobulin peptides associate in vivo to HLA-DR in autoimmune thyroid glands. J Immunol. 2008;181(1):795–807. doi: 10.4049/jimmunol.181.1.795. [DOI] [PubMed] [Google Scholar]
- 14.Flynn JC, McCormick DJ, Brusic V, Wan Q, Panos JC, Giraldo AA, David CS, Kong YC. Pathogenic human thyroglobulin peptides in HLA-DR3 transgenic mouse model of autoimmune thyroiditis. Cell Immunol. 2004;229(2):79–85. doi: 10.1016/j.cellimm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Kong YM, David CS, Lomo LC, Fuller BE, Motte RW, Giraldo AA. Role of mouse and human class II transgenes in susceptibility to and protection against mouse autoimmune thyroiditis. IMMUNOGENETICS. 1997;46(4):312–317. doi: 10.1007/s002510050277. [DOI] [PubMed] [Google Scholar]
- 16.Bradley DS, Nabozny GH, Cheng S, Zhou P, Griffiths MM, Luthra HS, David CS. HLA-DQB1 polymorphism determines incidence, onset, and severity of collagen-induced arthritis in transgenic mice. Implications in human rheumatoid arthritis. J Clin Invest. 1997;100(9):2227–2234. doi: 10.1172/JCI119760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixit SB, Beveridge DL, Case DA, Cheatham TE, III, Giudice E, Lankas F, Lavery R, Maddocks JH, Osman R, Sklenar H, Thayer KM, Varnai P. Molecular dynamics simulations of the 136 unique tetranucleotide sequences of DNA oligonucleotides. II: sequence context effects on the dynamical structures of the 10 unique dinucleotide steps. Biophys J. 2005;89(6):3721–3740. doi: 10.1529/biophysj.105.067397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beveridge DL, Barreiro G, Byun KS, Case DA, Cheatham TE, III, Dixit SB, Giudice E, Lankas F, Lavery R, Maddocks JH, Osman R, Seibert E, Sklenar H, Stoll G, Thayer KM, Varnai P, Young MA. Molecular dynamics simulations of the 36 unique tetranucleotide sequences of DNA oligonucleotides. I. Research design and results on d(CpG) steps. Biophys J. 2004;87(6):3799–3813. doi: 10.1529/biophysj.104.045252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gohlke H, Case DA. Converging free energy estimates: MM-PB(GB)SA studies on the protein-protein complex Ras-Raf. J Comput Chem. 2004;25(2):238–250. doi: 10.1002/jcc.10379. [DOI] [PubMed] [Google Scholar]
- 20.Gohlke H, Kiel C, Case DA. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol. 2003;330(4):891–913. doi: 10.1016/s0022-2836(03)00610-7. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farid NR, Bear JC. The human major histocompatibility complex and endocrine disease. Endocrine Reviews. 1981;2:50–86. doi: 10.1210/edrv-2-1-50. [DOI] [PubMed] [Google Scholar]
- 23.Moens H, Farid NR, Sampson L, Noel EP, Barnard JM. Hashimoto’s thyroiditis is associated with HLA-DRw3. N Engl J Med. 1978;299:133–134. doi: 10.1056/NEJM197807202990306. [DOI] [PubMed] [Google Scholar]
- 24.Tandon N, Zhang L, Weetman AP. HLA associations with Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 25.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf) 2002;57(1):81–88. doi: 10.1046/j.1365-2265.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF. Common and unique susceptibility loci in Graves and Hashimoto diseases: Results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73:736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomer Y, Greenberg DA, Concepcion E, Ban Y, Davies TF. Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab. 2002;87(1):404–407. doi: 10.1210/jcem.87.1.8291. [DOI] [PubMed] [Google Scholar]
- 28.Collins JE, Heward JM, Carr-Smith J, Daykin J, Franklyn JA, Gough SCL. Association of a rare thyroglobulin gene microsatellite variant with autoimmune thyroid disease. J Clin Endocrinol Metab. 2003;88:5039–5042. doi: 10.1210/jc.2003-030093. [DOI] [PubMed] [Google Scholar]
- 29.Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. Association of a thyroglobulin gene polymorphism with Hashimoto’s thyroiditis in the Japanese population. Clin Endocrinol (Oxf) 2004;61(2):263–268. doi: 10.1111/j.1365-2265.2004.02096.x. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao JY, Hsieh MC, Tien KJ, Hsu SC, Shin SJ, Lin SR. Association between a C/T polymorphism in exon 33 of the thyroglobulin gene is associated with relapse of Graves’ hyperthyroidism after antithyroid withdrawal in Taiwanese. J Clin Endocrinol Metab. 2007;92(8):3197–3201. doi: 10.1210/jc.2007-0675. [DOI] [PubMed] [Google Scholar]
- 31.Tomer Y, Menconi F, Davies TF, Barbesino G, Rocchi R, Pinchera A, Concepcion E, Greenberg DA. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J Autoimmun. 2007;29(2–3):69–77. doi: 10.1016/j.jaut.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatzioannou A, Liakata E, Karras E, Thrasyvoulides A, Alevizaki M, Lymberi P. Pathogenicity of a human thyroglobulin peptide (2340–2359) in mice with high or low genetic susceptibility to thyroiditis. Immunology. 2007;122(3):343–349. doi: 10.1111/j.1365-2567.2007.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li HS, Jiang HY, Carayanniotis G. Modifying effects of iodine on the immunogenicity of thyroglobulin peptides. J Autoimmun. 2007;28(4):171–176. doi: 10.1016/j.jaut.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Verginis P, Stanford MM, Carayanniotis G. Delineation of five thyroglobulin T cell epitopes with pathogenic potential in experimental autoimmune thyroiditis. J Immunol. 2002;169(9):5332–5337. doi: 10.4049/jimmunol.169.9.5332. [DOI] [PubMed] [Google Scholar]
- 36.Nakachi K, Powell M, Swift G, Amoroso MA, Ananieva-Jordanova R, Arnold C, Sanders J, Furmaniak J, Rees SB. Epitopes recognised by tissue transglutaminase antibodies in coeliac disease. J Autoimmun. 2004;22(1):53–63. doi: 10.1016/j.jaut.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Rief S, Lerner A. Tissue transglutaminase - the key player in celiac disease: a review. Autoimmun Rev. 2004;3:40–45. doi: 10.1016/S1568-9972(03)00065-X. [DOI] [PubMed] [Google Scholar]