Abstract
Sphingolipids and their synthetic enzymes are emerging as important mediators in inflammatory responses and as regulators of immune cell functions. In particular, sphingosine kinase (SK) and its product sphingosine-1-phosphate (S1P) have been extensively implicated in these processes. SK catalyzes the phosphorylation of sphingosine to S1P and exists as two isoforms, SK1 and SK2. SK1 has been shown to be activated by cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin1-β (IL1-β). The activation of SK1 in this pathway has been shown to be, at least in part, required for mediating TNF-α and IL1-β inflammatory responses in cells, including induction of cyclo-oxygenase 2 (COX-2). In addition to their role in inflammatory signaling, SK and S1P have also been implicated in various immune cell functions including, mast cell degranulation, migration of neutrophils, and migration and maturation of lymphocytes. The involvement of sphingolipids and sphingolipid metabolizing enzymes in inflammatory signaling and immune cell functions has implicated these mediators in numerous inflammatory disease states as well. The contribution of these mediators, specifically SK1 and S1P, to inflammation and disease are discussed in this review.
Keywords: sphingosine-1-phosphate, sphingosine kinase, inflammation, ceramide, tumor necrosis factor alpha
INTRODUCTION
Originally, sphingolipids were thought to serve only as structural components of the plasma membrane [1], but current evidence also suggests sphingolipids are pleiotrophic molecules participating in the regulation of numerous cellular functions [2]. Of recent interest is the role that sphingolipids play in inflammation and inflammatory disease. For example, numerous sphingolipid enzymes are activated by inflammatory cytokines and their downstream lipid mediators regulate inflammatory signaling pathways in addition to immune cell functions. The majority of research has focused on the role of sphingosine kinase (SK) and its product sphingosine-1-phosphate (S1P) in these inflammatory processes. Activation of SK has been demonstrated by pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin1-β (IL1-β), leading to production of S1P which has been linked to downstream induction of cyclo-oxygenase (COX2). Additionally, data show that SK and S1P are necessary for migration and maturation of immune cells. This review will discuss the SK/S1P pathway and its involvement in pro-inflammatory signaling and disease.
SPHINGOLIPID METABOLISM
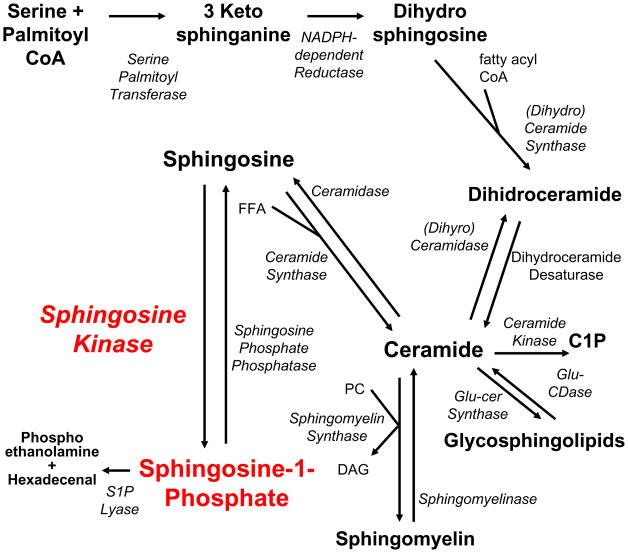
Numerous enzymes are involved in the production and metabolism of sphingolipids, which occur through one of two pathways: de novo synthesis or the salvage pathway (Figure 1). The de novo synthesis of sphingolipids begins in the endoplasmic reticulum with the condensation of palmitoyl Co-A and serine, catalyzed by serine palmitoyl transferase (SPT) to form 3-ketosphinganine. Very recently, evidence suggests that SPT can utilize alanine to produce 3-keto-1-deoxy-sphinganine, a novel sphingoid base lacking the hydroxyl group at the first carbon [3]. Further examination of SPT may lead to the identification of currently unknown species of sphingolipids and important roles for them. The next step of sphingolipid synthesis is reduction of 3-ketosphinganine by a NADH-dependent reductase to produce dihydrosphingosine. Through the addition of differing lengths of acyl chains by ceramide synthase, dihydroceramide is formed [1] and is subsequently desaturated via dihydroceramide desaturase to ceramide. Ceramide can be phosphorylated by ceramide kinase to ceramide-1-phosphate which has recently been identified as a bioactive sphingolipid [4]. After ceramide formation, the remaining reactions to incorporate ceramide into glycolipids and sphingomyelin occur predominantly in the Golgi apparatus. Sphingolipids can also be recycled and ceramide can be produced by the salvage pathway, whereby glucocerebrosidase and sphingomyelinase breakdown various membrane glycolipids and sphingolipids respectively. Through the action of ceramidases, sphingosine is formed by the removal of the acyl chain from a ceramide substrate. Sphingosine can be recycled back to ceramide via ceramide synthases. Alternatively sphingosine is phosphorylated by SK to S1P [5, 6], which can be dephosphorylated by sphingosine-1-phosphate phosphatase (SPP), along with other lipid phosphatases to form sphingosine. Originally viewed as the final step in sphingolipid breakdown, S1P is irreversibly cleaved into ethanolamine phosphate and hexadecenal by S1P lyase.
Figure 1. Pathways of Sphingolipid Metabolism.
Production of bioactive sphingolipids ceramide, sphingosine, and S1P occurs through de novo synthesis or the salvage pathway. De novo synthesis involves the condensation of serine and palmitoyl CoA by serine palmitoyl transferase for form 3-keto-sphinganine, which is rapidly reduced to dihydrosphingosine by an NADPH-dependent reductase. Dihydrosphingosine is converted to dihydroceramide with the addition of fatty acyl CoA by dihydroceramide synthase which is desaturated to form ceramide. Ceramide can be converted to glycosphingolipids by glucosylceramide synthase and back to ceramide by glucosylceramidase. Ceramide can also be formed by the salvage pathway through the action of sphinogmyelinases. Ceramide can be phosphorylated to ceramide-1-phosphate or deacylated by ceramidase to form sphingosine. Sphingosine is quickly phosphorylated by sphingosine kinase to form S1P. S1P can be dephosphorylated by S1P phosphatases, forming sphingosine, which can be converted back to ceramide with the addition of free fatty acid by ceramide synthases. S1P can be terminally degraded by S1P lyase to form hexadecanal and phosphoethanolamine. FFA: free fatty acid, Glu-Cer Synthase: glucosylceramide synthase, Glu-CDase: Glucosylceramidase.
BIOACTIVE SPHINGOLIPIDS
Ceramide, which forms the backbone of all sphingolipids, has an important role in cellular stress responses such as cell cycle arrest, serum and nutrient deprivation, terminal differentiation, apoptosis, and cellular senescence [6]. In addition to a role in cell death pathways, ceramide has also been implicated in inflammation. Specifically, ceramide has been shown to play a role in skin homeostasis—mice treated topically with a ceramide analog had decreased atopic dermatitis [7]. Similarly, C6-ceramide applied topically in a corneal inflammation model decreased inflammation [8].
Phosphorylation of ceramide by ceramide kinase yields ceramide-1-phosphate (C1P), which may have a role in inflammation through its activation of cytosolic phospholipase A2 (cPLA2) [9]. In addition, C1P has been shown to be required for membrane translocation of cPLA2 and downstream production of PGE2 [10]. In macrophages, a C1P analog prevents the production of pro-inflammatory cytokines such as TNF-α; however neither C1P nor S1P prevented cytokine induction [11, 12].
Through the action of ceramidases, ceramides are degraded to sphingosine, which is rapidly phosphorylated to form S1P, which then binds to G-protein coupled receptors; namely, S1P receptors [13]. Upon binding to one of the five known cell surface receptors, S1P initiates signal transduction leading to various cellular responses.
In the last fifteen years S1P has been implicated in many important cell signaling pathways and physiological processes such as, angiogenesis, cell migration and movement, cell survival and proliferation, cellular architecture, cellular contacts and adhesions, heart development, vascular development, atherogenesis, acute lung injury and acute respiratory distress, tumorogenicity and metastasis, and inflammation and immunity [14, 15]. Given the wide variety of cellular and physiological processes in which S1P is involved, elucidating mechanisms behind SK1/S1P pathway regulation is warranted. In summary, sphingolipids possess important structural and signaling duties with ceramide, sphingosine, and S1P having the most established cellular signaling roles.
SPHINGOSINE KINASE
A key step in the sphingolipid pathway is the formation of S1P. Two known isoforms of SK, sphingosine kinase 1 and 2 (SK1 and SK2), are responsible for the production of S1P from sphingosine. SK cloning from yeast has dramatically facilitated the study of the enzyme. In humans, three splice variants of SK1 (SK1a, SK1b, and SK1c) have been identified [16], and a high degree of homology exists between human and mouse SK1 enzyme variants [14]. There is a conserved diacylglycerol kinase (DAG) catalytic domain; also homologous between variants is glycine 181 that is necessary for catalytic activity [17], and aspartate 278 which is required for sphingosine binding [18]. ERK phosphorylation sites [19], phosphatidylserine binding residues [20], ATP binding sites [21], Ca2+/calmodulin [22] and TNF receptor associated factor 2 (TRAF2) binding sites [23] are among other homologous regions of the SK1 enzyme. SK1 resides mostly in the cytosol but can translocate to the plasma membrane upon activation by various stimuli [24]. Cellular localization of SK1 is pivotal in determining the effects of S1P production: when formed near the plasma membrane S1P can be exported before coming into contact with S1P lyase or S1P phosphatase which are ER-localized [25, 26]. There is some evidence that SK1 is secreted from HUVEC cells [16] as well as macrophages [27], allowing for the extracellular formation of S1P; however, further exploration in other cellular systems is necessary.
SK1 is known to be regulated by a multitude of growth factors and cytokines including: platelet derived growth factor (PDGF) [28, 29], vascular endothelial growth factor (VEGF) [30], nerve growth factor (NGF) [31], insulin-like growth factor (IGF) [32], IGF binding protein 3 (IGFBP3) [33], lysophosphatidic acid (LPA) [34], lipopolysaccharide (LPS) [35], compliment 5a (C5a) [36], TNFα [37, 38], and IL-1β [38].
While numerous growth factors and cytokines activate SK, there is only a small body of literature devoted to mechanism(s) by which SK1 is regulated. A 2002 study by Shu et al. probes the mechanism by which VEGF mediates activation of SK1: upon VEGF binding its receptor, PKC becomes activated leading to SK1 activation and S1P generation [30]. IGF was shown to activate SK1 and cause S1P production followed by GFP-S1P1 receptor internalization in HEK-293 cells [32]. As for TNF-α, which was shown to activate SK1 in HUVEC, L929 fibroblasts and monocytes [37, 39, 40], its mechanism of activation has been shown to occur by ERK1/2-mediated phosphorylation at serine residue 225 as well as through association with TRAF2 [19, 23]. Moreover, Chow et al. propose that TNF-α induces the phosphorylation of serine 225 of SK1 and that this phosphorylation causes conformational or electrostatic changes that allow SK1 to remain at the plasma membrane, enhancing the chance of finding its substrate [20]. SK1 has been shown to selectively bind phosphatidylserine at the plasma membrane; moreover, phosphorylation, calmodulin binding, and PKC have also been shown to be required for plasma membrane localization of SK1 [20, 22, 24]. Recently, this SK1 translocation to the plasma membrane has been shown to be facilitated by calcium and integrin binding protein 1 (CIB1) [41 to be inserted]. In yet another study, TNF-α was shown to induce SK1 membrane translocation and activation via a PLD-dependent mechanism in monocytes [40].
A number of SK1-interacting proteins that affect its activity have recently emerged, including Δ-catenin/NRRAP [42], aminocyclase 1 [43], erkaryotic elongation factor 1A [44], filamin A [45], sphingosine kinase 1-interacting protein (SKIP) [46], and platelet endothelial adhesion molecule-1 (PECAM1) [47]. Protein phosphatase 2A (PP2A) has been implicated in the dephosphorylation and deactivation of SK1, whereas cytosolic chaperonin containing TCP-1 (CCT) has been shown to mediate proper folding of the enzyme [48, 49].
Another mechanism of regulation of SK1 is at the transcriptional level whereby the SK1 promoter was shown to be up regulated in response to LPS in RAW macrophages leading to possible protection form apoptosis [27]. In addition the hypoxia-inducible factor 2 α (HIF-2 α) has also been shown to transcriptionally uregulate SK1 but not SK2 expression in glioma cells thus leading to S1P secretion and enhancement of transcellular angiogenesis [50]. It was very recently demonstrated that IL1-β could transcriptionally upregulate SK1 but not SK2 expression in glioblastoma cells, thus also implicating this pathway in the invasivness of these tumors [51].
Another emerging important mechanism of SK1 regulation is its downregulation by proteolysis after prolonged exposure to DNA damaging agents [52] and to TNF-α [53], thus leading to leading to loss of S1P, accumulation of ceramide, and cell death. This SK1 proteolysis was shown to be dependent on p53 activation in Molt-4 leukemia cells [52], and cathepsin-B was implicated in SK1 degradation in MCF-7 breast cancer cells [53]. SK1 activity and protein levels were also shown to decrease in response to reactive oxygen species (ROS) in cardiac cells, again leading to ceramide accumulation and apoptosis [54]. Moreover, knockdown of SK1 using siRNA indeed led to ceramide accumulation, apoptosis, and autophagy [55]. Taken together these studies indicate that perhaps inhibition and or loss of SK1 maybe important for induction of apoptosis not only due to a decrease in the pro-survival lipid S1P but also due to accumulation of the pro-death lipid ceramide. These studies, therefore, underscore an important role for SK1 in ceramide metabolism.
While SK1 has recently been the subject of intense investigation, SK2 has remained the less well-characterized isoform of the enzyme that produces S1P. In addition to being implicated in the induction of apoptosis, SK2 has been shown to have somewhat opposing functions to SK1 [56–58]. Most recently, SK2 has been reported to have a role in the epigenetic regulation of gene expression via modulation of histone acetylation, whereby, nuclear S1P, produced by SK2 activity, was shown to prevent the removal of acetyl groups contained within histone tails via direct interaction with and inhibition of histone deacetylases 1 and 2 (HDAC1 and HDAC2) [59], representing the first direct, intracellular target that has been identified for S1P.
SK1 and SK2 are located on separate genes and are highly homologous, with the exception of an extended N-terminal tail possessed by SK2 [14, 58]. In contrast to SK1, SK2 is thought to be localized to the ER and the nucleus [60]. There is some functional redundancy between SK1 and SK2, evidenced by a lack of phenotype in SK1 single knockout and SK2 single knockout mice [61]; however, SK1/SK2 double knockouts are embryonic lethal due to inadequate blood vessel formation, providing compelling evidence for the necessity of SK in development [62].
SPHINGOSINE-1-PHOSPHATE
SK1 and SK2, along with other sphingolipid metabolizing enzymes, control the fine balance among lipid mediators ceramide, sphingosine, and S1P [15]. S1P can be either dephosphorylated by SPPs or irreversibly degraded into hexadecanal and phosphoethanolamine by S1P lyase [63]. The discovery of five G protein-coupled S1P cell surface receptors (S1PR1-S1PR5) was the catalyst for studies implicating S1P as a multifaceted, bioactive signaling lipid-molecule [4] Understanding the regulation of this lipid-molecule is vital to address the numerous cellular processes and pathologies in which it is involved.
S1P is implicated in both extracellular and intracellular-mediated signaling; however, as yet there is only one, recently identified, direct intracellular S1P target [51, 59]. S1P has been shown to promote growth and survival independent of its G protein-coupled receptors in mouse embryonic fibroblasts devoid of S1P receptors [64]. There is also evidence supporting a role for intracellular S1P in calcium mobilization [65]. To date, most S1P functions have been attributed to receptor-mediated signaling. The five S1P receptors (S1PRs) couple to various α subunits of heterotrimeric G proteins: Gi, Gq and G12/13. S1PR1 signaling is known to be essential for embryonic blood vessel development as the murine knockout is embryonic lethal as a result of hemorrhage [66]. In addition, plasma S1P has been shown to elicit egress of lymphocytes into the blood in an S1PR1-dependent manner [67] and to regulate basal and inflammation-induced vascular leak in vivo [68]. S1PR2 and S1PR3 activate phospholipase-C and Rho, and knockout of both receptors in mice decreases litter size and survival rates [69]. S1P4 and S1P5 are the least studied receptors; although, it is known that S1PR4 is expressed primarily in lymphocytes and is involved in T cell proliferation [70], while S1PR5 is expressed on dendritic and natural killer cells [71]. The five known S1PRs can lead to activation of different downstream targets, such as Rac, ERK, PI3K, adenylyl cyclase, phospholipase C, Rho or JNK, resulting in the aforementioned cellular responses [64]. The pathways mediated by each specific receptor are common; however, because there have been agonists and antagonists that exhibit receptor specificity, it is probable that the S1P receptors are not totally redundant [72]
S1PRs can undergo activation by signaling molecules other than S1P, such as growth factors. Such receptor transactivation is also known as “receptor crosstalk” [73]. For example, PDGF has been shown to activate SK1, the enzyme responsible for catalyzing the formation of S1P from sphingosine, thus activating the S1P receptor [28]. PDGF has been shown to bind its receptor and activate SK1; this specific example results in the transactivation of S1PR1, leading to Rac-mediated cell motility in HEK-293 cells [29]. Given the multiple molecules capable of activating SK1, resulting in S1P production and ultimately S1P receptor transactivation, this could be a common mechanism by which G protein-dependent signals are elicited by non-G protein-coupled receptors.
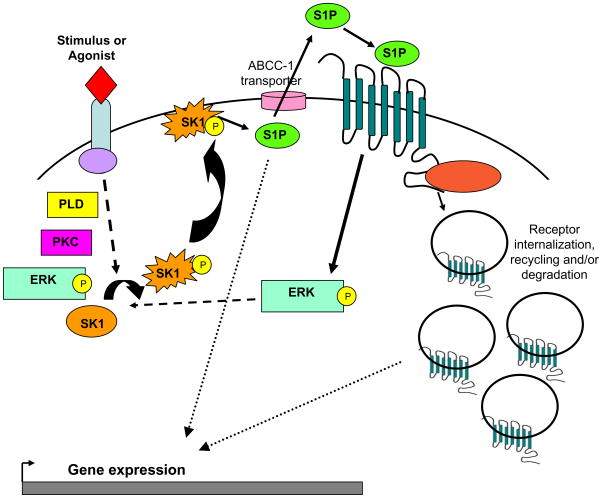
The majority of S1P effects are thought to be attributed to intracellular production, export to extracellular space, and finally activation of the S1P receptors (Figure 2); therefore, it is important to determine the factors which regulate these processes. To further confound S1P signaling, S1P autocrine or paracrine activation of S1PRs are know to activate sphingosine kinase, leading to further production of S1P [74]. The crossing of S1PR pathways with other GPCR and receptor tyrosine kinase (RTK) signaling pathways, along with the activation of SK by numerous agonists or stimuli and “inside-out” signaling capabilities of S1P clearly demonstrate the complexity involved in the SK/S1P signaling pathway. Elucidating various S1P-mediated signals has enormous potential for eliciting possible specific therapeutic targets.
Figure 2. Inside-out S1P Signaling.
Various agonists or stimuli bind and activate their respective receptors, leading to the activation of the SK/S1P pathway by both known and unknown mechanisms. Shown are molecules implicated in the phosphorylation (ERK and PKC) and/or the activation (PLD) of SK. Once activated by phosphorylation or by undefined mechanisms, SK is thought to translocate to the plasma membrane where it comes into contact with its substrate, sphingosine. SK then catalyzes the formation of S1P, which can act as an intracellular signaling molecule, or it can be exported via ABCC transporters to act in an autocrine or paracrine manner. Once activated, S1PRs elicit specific G-protein-mediated signals and this is followed by receptor internalization. Receptors are then recycled or degraded. ERK: extracellular regulated kinase; PKC: protein kinase c; PLD: phospholipase D; SK: sphingosine kinase; S1P: sphingosine 1-phosphate; S1PRs: sphingosine 1-phosphate receptors.
SK1 AND S1P IN INFLAMMATION AND DISEASE
The SK1/S1P pathway has been implicated in inflammation mediated by TNF-α. This TNF-α signaling enhances the expression of adhesion molecules, such as vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) [37]. SK1 was shown to be activated by TNF-α in a dose-dependent manner, an event required for TNF–α-mediated adhesion molecule expression in HUVEC cells [37]. Subsequently, Pettus et al. demonstrated a role for sphingolipid metabolism in the inflammatory response in L929 fibroblasts whereby, it was shown that SK1 and S1P are necessary for TNF-α-induced COX2 and PGE2 production [39]. Billich et al. found increased SK1 mRNA synthesis under inflammatory conditions, again, implicating sphingolipids in inflammation [38]. These studies, along with many others have firmly established that TNF-α signaling leads to activation of the SK1/S1P pathway (Table 1). Other inflammatory signaling molecules such as IL-1β, IFN-γ, IgE and C5a [75], have also been shown to activate SK1, further suggesting the importance of the SK1/S1P pathway in the inflammatory response.
TABLE 1.
TNF-α-Induced Events Mediated by the SK1/S1P Pathway
| Cell Type | Response | Mechanism | Source |
|---|---|---|---|
| HUVEC | Expression of E-selectin and VCAM, ERK and NFkB activation | ND | [37] |
| HUVEC | eNOS activation | S1PR1 and S1PR3 activation | [114] |
| HUVEC | ICAM expression | Akt, ERK and NFkB activation | [115] |
| HAEC | MCP-1 expression and secretion, VCAM expression | p38 | [116] |
| Human neutrophils | Neutrophil priming | ND | [117] |
| RAW 264.7 macrophages | COX-2 expression, PGE2 production | ND | [35] |
| U937 human monocytic cells | ERK and NFkB activation | PLD activation | [40] |
| C6 glioma | Activation of GTP cyclohydrolase I | Intracellular S1P signaling | [118] |
| 1321N1 glioblastoma | Proliferation | Akt and Cyclin D activation | [119] |
| SKNBE human neuroblastoma | eNOS activation | ND | [120] |
| H441 lung epithelial | IL-8 expression | ERK, p38 and AP-1 activation | [121] |
| A549 | Cox-2 expression and PGE2 production, IL-6, RANTES, MCP-1 and VCAM expression | NFkB activation | [38] |
| A549 and L929 | COX-2 and PGE2 production | ND | [39] |
| Fibroblasts | COX-2 expression | ND | [122] |
| Fibroblasts | ERK activation and MMP1 expression | dihydroS1P via Gαi signaling | [122] |
| C2C12 myoblasts | Myogenesis | S1PR2 | [123] |
| MC3T3-E1 osteoblast-like | IL-6 production | PLC and PKC | [124] |
| HEK 293T | NFkB activation | SK:TRAF2 binding | [23] |
Shown are cellular or biological responses induced by TNF in various cell types. These responses elicited by TNF are dependent on the SK1/S1P pathway. The mechanism(s) by which TNF activates the pathway or by which the SK1/S1P pathway is involved in TNF-mediated events is listed when known. ND: not determined
In addition to a role for SK1/S1P in TNF-α-mediated signaling, SK1/S1P and the S1PRs have also been shown to regulate numerous types of immune cells involved in inflammatory diseases. For example, SK1 is necessary for TNF-α-mediated responses in human primary monocytes [76], and it has been suggested, using dimethylsphingosine (DMS), that SK1 is required for catestatin-stimulated migration of monocytes [77]. Macrophages stimulated with LPS increase SK1 message and activity, resulting in generation of S1P and induction of COX2 [35]. In a more recent study, SK1 message and protein have been shown to be increased in LPS-activated microglia [78], perhaps implicating a possible role for the SK1/S1P pathway in neuroinflammation. Also in macrophages, SK1 has been implicated in cytokine production and chemotaxis in response to C5a [36]. Exogenous addition of low concentrations of sphingosine to C5a-primed neturophils stimulated an oxidative burst, which was attributed to activation of SK and generation of S1P [79]. In the same study, neutrophil migration in response to C5a was inhibited by DMS, suggesting a role for SK in neutrophil chemotaxis. S1PR transactivation by FcεRI is necessary for mast cell degranulation and migration [80], whereas mast cell degranulation and cytokine production require SK [81].
In addition to the growing literature implicating SK and S1P in inflammation and immune cell functions, the emergence of FTY720 and other S1PR modulators were instrumental to the discovery that S1PRs are essential for migration of lymphocytes [82, 83]. FTY720 decreases the number of mature circulating lymphocytes and prevents the egress of lymphocytes from thymus and secondary lymphoid tissues through its agonistic effects on and subsequent downregulation of S1PR1 [84–86]. This immunesuppression has prompted the examination of FTY720 treatment in numerous disease states where SK1/S1P has been implicated (Table 2) including colitis [87], arthritis [88], and asthma [89] in addition to Phase III clinical trials for multiple sclerosis.
TABLE 2.
Animal Models of Inflammation and the SK/S1P Pathway
| Disease Model | Animal Model | Inhibitor | Response | Source |
|---|---|---|---|---|
| OVA Albumin Challenge | Mice | DMS/SK-I | Decreased BAL S1P, decreased neutrophil and eosinophil infiltration | [95] |
| OVA Albumin Challenge | Guinea pig | Fumonisin B1 | Decreased CerS activity and ceramide levels in airway epithelium | [96] |
| OVA Albumin Challenge | Mice | FTY720 | Decreased bronchial constriction, eosinophilia, migration of dendritic cells and inhibition of T cell activation | [89] |
| Antigen Challenge | SK1−/− mice | LPA/Thrombin | Increased vascular permeability in lung tissue | [97] |
| Collagen/Adjuvant- Induced Arthritis | Mice | FTY720 | Decreased hindpaw edema, joint destruction and lymphocyte infiltration | [88, 101] |
| Collagen -Induced Arthritis | Mice | DMS | Decreased cytokine production | [99] |
| Collagen -Induced Arthritis | Mice | SK1 and SK2 siRNA | SK2 siRNA resulted in increased disease and cytokine production | [102] |
| Oxazolone-induced colitis | Mice | FTY720 | Decreased cytokine production and macro and microscopic aspects of disease | [104] |
| IL10−/− Mice Colitis | Mice | KRP-203 | Decreased lymphocyte infiltration | [105] |
| CD4+/CD62L+ T cell Transfer Colitis | Mice | FTY720 | Prevented migration of CD4+ T | [87] |
| DSS-Induced Colitis | Mice | ABC294640 and ABC747080 | Decreased cytokine production, tissue destruction and lymphocyte infiltration | [106] |
| DSS-Induced Colitis | SK1−/− mice | Decreased systemic and local inflammatory response | [107] | |
| Intestinal Neoplasia | SK1−/− mice crossed with Apc Min/+ mice | Decreased intestinal adenoma size | [112] | |
| AOM/DSS Colon Carcinogenesis | SK1−/− mice | Decreased polyp size and number | [113] |
Shown are animal models where the SK1/S1P pathway have been shown to influence disease. These responses are elicited by the inhibition or modulation of the SK1/S1P pathway. are dependent on the SK1/S1P pathway.
Further insights into the physiological functions of SK and S1P were anticipated from studies using SK1 and SK2 knockout mice (SK1−/− and SK2−/−). However, this model initially did not provide much information about the in vivo role of these lipid kinases because, unexpectedly, SK1−/− and SK2−/− mice appeared morphologically and functionally normal [90], whereas, the double knockout mice were found to be embryonic lethal due to defects in both neurogenesis and angiogenesis [62]. On closer examination the SK1−/− mice had decreased tissue SK activity and a 50% decrease in serum S1P, but no significant difference in tissue S1P under normal conditions. These mice were rendered lymphopenic in response to the immunosuppressant FTY720 [83], whereas SK2−/− mice were not [91]. In other studies, SK1−/− and SK2 +/− mice were crossed to render SK1−/−SK2+/− mice and these animals had defective lymphocyte egress due to low blood S1P generation [67].
Taken altogether the above studies have begun to establish a key role for the SK1/S1P pathway in inflammatory processes which warrants closer examination of this pathway in specific inflammatory diseases as follows.
ASTHMA
Allergic asthma is characterized by constriction of the smooth muscle cells in the airway and influx of inflammatory cells into the lungs. The onset of this type of asthma is commonly due to an inhaled or ingested allergen. Asthma is typically controlled with inhaled corticosteroids and other medications that prevent or decrease symptom severity. Numerous processes involved in the progression of an asthmatic attack are regulated by or involve SK and S1P. Ingested antigen binding to the FcεR causes mast cell migration and degranluation which is dependent on transactivation of the S1PR2 [80]. S1P induces airway smooth muscle contraction [92] and can influence the migration of inflammatory cells, such as eosinophils [93]. Elevated bronchiolar S1P is found in human ragweed-allergic patients when they are challenged with allergen, compared to normal non-allergic patients [94].
Ovalbumin (OVA) administration is commonly used in rodents to model allergic asthma. Chemical inhibition of SK has been examined in the OVA model using SK-I or DMS. Mice treated with SK-I or DMS had decreased bronchiolar lavage (BAL) levels of S1P and less peroxidase activity and decreased eosinophil migration [95]. Administration of OVA to guinea pigs increased ceramide levels and ceramide synthase activity in the airway epithelium. Inhibition of this pathway with fumonisin B1 decreased ceramide synthase activity, ceramide levels and improved asthma symptoms [96]. In this study, other sphingolipids or sphingolipid metabolizing enzymes were not investigated; however, future experiments to determine whether ceramide was degraded by ceramidases to provide a substrate for SK are needed—most studies have implicated SK/S1P in allergic asthma.
S1PR modulation with FTY720 has also been studied in mouse models of asthma. Mice sensitized to OVA, but pretreated with FYTY720 30 minutes prior to OVA exposure, had less bronchial constriction and eosinophilia than vehicle-pretreated mice. FTY720 prevented migration of dendritic cells, inhibiting T cell activation and improving disease in this model [89].
S1P has also been implicated in vascular leak after antigen challenge. Interestingly, SK1−/− mice have increased inflammation-induced vascular leak in the lungs. SK1−/− mice treated with either LPS or thrombin had increased vascular permeability in lung tissue [97]. Addition of S1P reversed this effect, indicating a crucial role for SK1 and S1P in lung endothelial cell function and barrier integrity. The recent development of conditional knockouts of both SK1 and SK2 in plasma further confirmed that S1P in the plasma mediates vascular integrity [68]. Whereas inhibition of SK1 in asthma may prevent some disease aspects, S1P obviously plays a vital role in preventing vascular leak in the lungs.
RHEUMATOID ARTHRITIS
Rheumatoid arthritis (RA) is a chronic autoimmune disease involving inflammatory cell movement into joint tissue, resulting in tissue destruction and excessive pro-inflammatory cytokine production. Current therapies directed against RA consist of physical therapy, anti-inflammatory medications, and steroids. More recently, therapeutics have been targeted to inhibit increased cytokine production, specifically TNF-α. TNF-α can activate SK1, leading to the production of S1P in synoviocytes from RA patients. The addition of S1P also causes proliferation and cytokine production in these synoviocytes [98]. Furthermore, S1P is elevated in the synovium of patients with RA [99]; also, SK2 protein levels are elevated in synovial fibroblasts from RA patients [100]. These findings suggest that sphingolipid enzyme(s) and their products may play a role in the pathology involved in RA.
In studies utilizing modulation of S1PR function or non-specific inhibition of SK1, RA severity is improved. Modulation of the S1PR1 using FTY720 has been examined in two common animal models of arthritis. Both collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AA) are well established models for studying T-cell dependent RA. In studies conducted using the immunosuppressant FYT720 in rats with both CIA and AA, hindpaw edema and joint destruction was inhibited, and lymphocyte invasion into the joints was decreased [88, 101]. In addition to receptor modulation, non-specific inhibition of SK with DMS has been shown to decrease severity and cytokine production in CIA [99].
Recently, the use of knockout mice and specific knockdown of each SK isoform in RA studies have produced conflicting results. When SK1−/− and SK2−/− mice were utilized in the CIA model, no differences were noted between these strains and wild type (WT) mice [61]. In this study, disease had reached 100% incidence by 40 days post-injection and all mice developed severe joint inflammation [61]. However, in another study, mice were treated intraperitoneally with SK1 siRNA and subjected to CIA, and disease progression was prevented. Mice treated with SK1 siRNA had reduced incidence and severity of disease and decreased cytokine production [102]. In the same study, treatment of mice with SK2 siRNA increased disease incidence and severity suggesting distinct roles for SK1 and SK2 in vivo. Such discrepancies between these two studies could be explained by the collagen dose used to induce disease. Michaud et al. used 100 μg collagen to induce disease, whereas Lai and co-workers used twice that amount. Study outcome differences could also be explained by the mode of enzyme knockdown. Perhaps knockout animals develop compensatory mechanisms to overcome the complete loss of an SK isoform, whereas mice with acute knockdown of either isoform may more closely resemble what would occur with chemical inhibition of the enzyme.
INFLAMMATORY BOWEL DISEASE AND COLON CARCINOGENESIS
Inflammatory bowel disease encompasses ulcerative colitis (UC) and Crohns Disease (CD). Both forms of IBD are characterized by uncontrolled influx of inflammatory cells into the intestinal tract and overproduction of pro-inflammatory cytokines. CD can present in the entire intestinal tract, whereas UC is limited to the colon. IBD is usually treated by immunesuppression with corticosteroids and more recently, like RA, with anti-TNF-α therapies. TNF-α has been shown to activate SK1 in numerous cell types [38, 39] including HT29 colon cancer cells and rat intestinal epithelial cells [103]. This SK1 activation has also been implicated in COX2 induction and PGE2 production [103].
Recent studies with mice deficient in sphingolipid enzymes and experiments with chemical inhibitors have underscored a role for sphingolipids in IBD. Numerous studies depict the efficacy of FTY720 and other S1PR modulators in mouse models of colitis. For example, FTY720 inhibits oxazolone-induced colitis, reducing macro and microscopic aspects of disease, and decreases cytokine production by modulating T-helper type 2 functions [104]. Agonism of S1P1R by KRP-203 also improved colitis in IL-10-deficient mice by decreasing lymphocyte infiltration into the lamina propria [105]. In the CD4+/CD62L+ T cell transfer model of colitis, FTY720 decreased disease by preventing the migration of CD4+ T cells into the colon tissue [87]. These studies, along with others, suggest an important role for S1PR modulation as a potential therapeutic target for IBD.
Chemical inhibition of SK has been shown to improve DSS-induced colitis, both acutely and chronically, improving disease and decreasing cytokine production [106]; however, no iso-enzyme specific inhibitors have been demonstrated in vivo. Similarly, we have demonstrated that both blood and colon tissue S1P increases in WT mice but not in SK1−/− mice with DSS-induced colitis. Systemic inflammation is decreased in SK1−/− mice, when compared to WT mice in this model. In addition, local inflammation, specifically neutrophil infiltration, is decreased in response to DSS in SK1−/− mice. Perhaps most interesting in this study is the absence of COX2 induction in colons of SK1−/− mice, suggesting that SK1 is necessary for induction of COX2 in vivo [107].
Given the increasing evidence linking inflammation and carcinogenesis, it is noteworthy that there is increasing evidence linking SK1 expression and activity to the development and progression of cancer [108–110]. Of particular interest is the role of the SK1/S1P pathway in inflammation-induced cancer. Specifically, SK1 has been shown to be overexpressed in many human cancers including lung and colon [103, 111]. When crossed with SK1−/− mice, intestinal neoplasia model Apc Min/+ mice had decreased intestinal adenoma size [112]. In addition, SK1 has been shown to be overexpressed in human colon cancer samples and SK1−/− mice develop significantly less colon cancer than WT mice in an inflammation-induced colon carcinogenesis model [113].
Thus, with an obvious link between inflammation and cancer, investigation of the role of SK and S1P is needed, especially in view of the fact that many anti-inflammatory medications currently used to treat diseases have deleterious side effects. A better understanding of how SK and S1P facilitate the processes involved in inflammation and disease should lead to improved therapeutics.
CONCLUSION
Inflammation and recruitment of inflammatory cells are important in disease progression and are at least, in part, mediated by the SK1/S1P pathway. This pathway could be manipulated to shift disease processes from pro-inflammatory to anti-inflammatory by decreasing cytokine production and infiltration of inflammatory cells. Future studies involving the modulation and chemical inhibition of SK and S1P levels, in addition to modulation of S1PRs, may lead to improved therapeutics for inflammatory diseases.
Acknowledgments
This work was supported by National Institutes of Health Grants 1F32KD084604-01 (AJS), GM062887 (LMO), and GAANN fellowship P200A070596 and Institutional Training Grant, HL007260 (KAOG). As well as, VA Merit Award (LMO) and by the VAMC Research Excellence Award Program (REAP) (LMO and AJS). We would like to thank Kathy Wiita-Fisk for her administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Mechanisms of ceramide-mediated apoptosis. Adv Exp Med Biol. 1997;407:145–149. doi: 10.1007/978-1-4899-1813-0_22. [DOI] [PubMed] [Google Scholar]
- 3.Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH, Jr, Riley RT. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. The Journal of biological chemistry. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. Journal of cell science. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 5.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. The Journal of biological chemistry. 1998;273:12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 6.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 7.Kang JS, Lee CW, Lee K, Han MH, Lee H, Youm JK, Jeong SK, Park BD, Han SB, Han G, Park SK, Kim HM. Inhibition of skin inflammation and atopic dermatitis by topical application of a novel ceramide derivative, K112PC-5, in mice. Archives of pharmacal research. 2008;31:1004–1009. doi: 10.1007/s12272-001-1260-z. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Fox T, Adhikary G, Kester M, Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. Journal of leukocyte biology. 2008;83:1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura H, Hirabayashi T, Shimizu M, Murayama T. Ceramide-1-phosphate activates cytosolic phospholipase A2alpha directly and by PKC pathway. Biochemical pharmacology. 2006;71:850–857. doi: 10.1016/j.bcp.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Lamour NF, Subramanian P, Wijesinghe DS, Stahelin RV, Bonventre JV, Chalfant CE. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. The Journal of biological chemistry. 2009;284:26897–26907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldsmith M, Avni D, Levy-Rimler G, Mashiach R, Ernst O, Levi M, Webb B, Meijler MM, Gray NS, Rosen H, Zor T. A ceramide-1-phosphate analogue, PCERA-1, simultaneously suppresses tumour necrosis factor-alpha and induces interleukin-10 production in activated macrophages. Immunology. 2009;127:103–115. doi: 10.1111/j.1365-2567.2008.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avni D, Goldsmith M, Ernst O, Mashiach R, Tuntland T, Meijler MM, Gray NS, Rosen H, Zor T. Modulation of TNFalpha, IL-10 and IL-12p40 levels by a ceramide-1-phosphate analog, PCERA-1, in vivo and ex vivo in primary macrophages. Immunology letters. 2009;123:1–8. doi: 10.1016/j.imlet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochimica et biophysica acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 14.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn-Schmiedeberg’s archives of pharmacology. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 15.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochimica et biophysica acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. The Biochemical journal. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, D’Andrea RJ, Wattenberg BW. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. The Journal of biological chemistry. 2000;275:33945–33950. doi: 10.1074/jbc.M006176200. [DOI] [PubMed] [Google Scholar]
- 18.Yokota S, Taniguchi Y, Kihara A, Mitsutake S, Igarashi Y. Asp177 in C4 domain of mouse sphingosine kinase 1a is important for the sphingosine recognition. FEBS Lett. 2004;578:106–110. doi: 10.1016/j.febslet.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 19.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. Embo J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. The Journal of biological chemistry. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 21.Pitson SM, Moretti PA, Zebol JR, Zareie R, Derian CK, Darrow AL, Qi J, D’Andrea RJ, Bagley CJ, Vadas MA, Wattenberg BW. The nucleotide-binding site of human sphingosine kinase 1. The Journal of biological chemistry. 2002;277:49545–49553. doi: 10.1074/jbc.M206687200. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. The Journal of biological chemistry. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- 23.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D’Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. The Journal of biological chemistry. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) The Journal of biological chemistry. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa C, Kihara A, Gokoh M, Igarashi Y. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. The Journal of biological chemistry. 2003;278:1268–1272. doi: 10.1074/jbc.M209514200. [DOI] [PubMed] [Google Scholar]
- 26.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. The Journal of biological chemistry. 2003;278:34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 27.Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79:126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 29.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science (New York, NY) 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 30.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa A, Kita K, Toyomoto M, Fujii S, Inoue S, Hayashi K, Ikeda K. Production of nerve growth factor enhanced in cultured mouse astrocytes by glycerophospholipids, sphingolipids, and their related compounds. Mol Cell Biochem. 2007;305:27–34. doi: 10.1007/s11010-007-9524-4. [DOI] [PubMed] [Google Scholar]
- 32.El-Shewy HM, Johnson KR, Lee MH, Jaffa AA, Obeid LM, Luttrell LM. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. The Journal of biological chemistry. 2006;281:31399–31407. doi: 10.1074/jbc.M605339200. [DOI] [PubMed] [Google Scholar]
- 33.Granata R, Trovato L, Lupia E, Sala G, Settanni F, Camussi G, Ghidoni R, Ghigo E. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost. 2007;5:835–845. doi: 10.1111/j.1538-7836.2007.02431.x. [DOI] [PubMed] [Google Scholar]
- 34.Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. The Journal of biological chemistry. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 35.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2007 doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- 37.Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cellular signalling. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. Faseb J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 40.Sethu S, Mendez-Corao G, Melendez AJ. Phospholipase D1 plays a key role in TNF-alpha signaling. J Immunol. 2008;180:6027–6034. doi: 10.4049/jimmunol.180.9.6027. [DOI] [PubMed] [Google Scholar]
- 41.Jarman KE, Moretti PA, Zebol JR, Pitson SM. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium and integrin binding protein 1. The Journal of biological chemistry. 2009 doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita T, Okada T, Hayashi S, Jahangeer S, Miwa N, Nakamura S. Delta-catenin/NPRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. The Biochemical journal. 2004;382:717–723. doi: 10.1042/BJ20040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maceyka M, Nava VE, Milstien S, Spiegel S. Aminoacylase 1 is a sphingosine kinase 1-interacting protein. FEBS Lett. 2004;568:30–34. doi: 10.1016/j.febslet.2004.04.093. [DOI] [PubMed] [Google Scholar]
- 44.Leclercq TM, Moretti PA, Vadas MA, Pitson SM. Eukaryotic elongation factor 1A interacts with sphingosine kinase and directly enhances its catalytic activity. The Journal of biological chemistry. 2008;283:9606–9614. doi: 10.1074/jbc.M708782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacana E, Maceyka M, Milstien S, Spiegel S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. The Journal of biological chemistry. 2002;277:32947–32953. doi: 10.1074/jbc.M202841200. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda Y, Aoyama Y, Wada A, Igarashi Y. Identification of PECAM-1 association with sphingosine kinase 1 and its regulation by agonist-induced phosphorylation. Biochimica et biophysica acta. 2004;1636:12–21. doi: 10.1016/j.bbalip.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Barr RK, Lynn HE, Moretti PA, Khew-Goodall Y, Pitson SM. Deactivation of sphingosine kinase 1 by protein phosphatase 2A. The Journal of biological chemistry. 2008;283:34994–35002. doi: 10.1074/jbc.M804658200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zebol JR, Hewitt NM, Moretti PA, Lynn HE, Lake JA, Li P, Vadas MA, Wattenberg BW, Pitson SM. The CCT/TRiC chaperonin is required for maturation of sphingosine kinase 1. Int J Biochem Cell Biol. 2009;41:822–827. doi: 10.1016/j.biocel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine Kinase 1 Is Up-regulated during Hypoxia in U87MG Glioma Cells: ROLE OF HYPOXIA-INDUCIBLE FACTORS 1 AND 2. The Journal of biological chemistry. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 51.Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, Milstien S, Spiegel S, Kordula T. Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. The Journal of biological chemistry. 2009;284:3408–3417. doi: 10.1074/jbc.M807170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, Dbaibo GS, Hannun YA, Obeid LM. Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. The Journal of biological chemistry. 2004;279:20546–20554. doi: 10.1074/jbc.M401259200. [DOI] [PubMed] [Google Scholar]
- 53.Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. The Journal of biological chemistry. 2005;280:17196–17202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- 54.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circulation research. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 55.Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. Faseb J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. The Journal of biological chemistry. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 57.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. The Journal of biological chemistry. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 58.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. The Journal of biological chemistry. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 59.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science (New York, NY. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of biological chemistry. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 61.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Veldhoven PP, Gijsbers S, Mannaerts GP, Vermeesch JR, Brys V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1) Biochimica et biophysica acta. 2000;1487:128–134. doi: 10.1016/s1388-1981(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 64.Olivera A, Rosenfeldt HM, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. The Journal of biological chemistry. 2003;278:46452–46460. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- 65.van Koppen CJ, Meyer zu Heringdorf D, Alemany R, Jakobs KH. Sphingosine kinase-mediated calcium signaling by muscarinic acetylcholine receptors. Life Sci. 2001;68:2535–2540. doi: 10.1016/s0024-3205(01)01049-9. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science (New York, NY. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 68.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. The Journal of clinical investigation. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. The Journal of biological chemistry. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 70.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. Faseb J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 71.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 72.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochimica et biophysica acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Baudhuin LM, Jiang Y, Zaslavsky A, Ishii I, Chun J, Xu Y. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR) Faseb J. 2004;18:341–343. doi: 10.1096/fj.03-0302fje. [DOI] [PubMed] [Google Scholar]
- 74.Meyer zu Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rumenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414:145–154. doi: 10.1016/s0014-2999(01)00789-0. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends in endocrinology and metabolism: TEM. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208:109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- 77.Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR, Schratzberger P, Djanani AM, Mahata SK, Kirchmair R. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–111. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, Dheen ST. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 166:132–144. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 79.Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. The Journal of biological chemistry. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]
- 80.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 83.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. The Journal of biological chemistry. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 84.Yanagawa Y, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer’s patches by FTY720-induced lymphocyte homing. Immunology. 1998;95:591–594. doi: 10.1046/j.1365-2567.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yanagawa Y, Sugahara K, Kataoka H, Kawaguchi T, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J Immunol. 1998;160:5493–5499. [PubMed] [Google Scholar]
- 86.Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cellular & molecular immunology. 2006;3:11–19. [PubMed] [Google Scholar]
- 87.Deguchi Y, Andoh A, Yagi Y, Bamba S, Inatomi O, Tsujikawa T, Fujiyama Y. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep. 2006;16:699–703. [PubMed] [Google Scholar]
- 88.Wang F, Tan W, Guo D, He S. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur J Pharmacol. 2007;573:230–240. doi: 10.1016/j.ejphar.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 89.Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. The Journal of clinical investigation. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunology letters. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Kharel Y, Lee S, Snyder AH, Sheasley-O’neill LS, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. The Journal of biological chemistry. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 92.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. Faseb J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 93.Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, De Palma R. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 95.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 96.Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, Comhair SA, Li D, Cuzzocrea S, Matuschak GM, Salvemini D. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. The Journal of pharmacology and experimental therapeutics. 2008;324:548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- 97.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circulation research. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 99.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, McInnes IB, Melendez AJ, Leung BP. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 100.Kamada K, Arita N, Tsubaki T, Takubo N, Fujino T, Soga Y, Miyazaki T, Yamamoto H, Nose M. Expression of sphingosine kinase 2 in synovial fibroblasts of rheumatoid arthritis contributing to apoptosis by a sphingosine analogue, FTY720. Pathol Int. 2009;59:382–389. doi: 10.1111/j.1440-1827.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 101.Matsuura M, Imayoshi T, Okumoto T. Effect of FTY720, a novel immunosuppressant, on adjuvant- and collagen-induced arthritis in rats. Int J Immunopharmacol. 2000;22:323–331. doi: 10.1016/s0192-0561(99)00088-0. [DOI] [PubMed] [Google Scholar]
- 102.Lai WQ, Irwan AW, Goh HH, Melendez AJ, McInnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183:2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 103.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. Faseb J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 104.Daniel C, Sartory NA, Zahn N, Schmidt R, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44:3305–3316. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 105.Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. The Journal of pharmacology and experimental therapeutics. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 106.Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53:997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. Faseb J. 2009;23:143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Safadi-Chamberlain F, Wang LP, Payne SG, Lim CU, Stratford S, Chavez JA, Fox MH, Spiegel S, Summers SA. Effect of a membrane-targeted sphingosine kinase 1 on cell proliferation and survival. The Biochemical journal. 2005;388:827–834. doi: 10.1042/BJ20041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–1816. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 111.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 112.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Molecular and cellular biology. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. Faseb J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 115.Kang JS, Yoon YD, Han MH, Han SB, Lee K, Lee KH, Park SK, Kim HM. Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway: implications of Akt, extracellular signal-regulated kinase, and nuclear factor-kappaB/Rel signaling pathways. Molecular pharmacology. 2006;69:941–949. doi: 10.1124/mol.105.017442. [DOI] [PubMed] [Google Scholar]
- 116.Chen XL, Grey JY, Thomas S, Qiu FH, Medford RM, Wasserman MA, Kunsch C. Sphingosine kinase-1 mediates TNF-alpha-induced MCP-1 gene expression in endothelial cells: upregulation by oscillatory flow. Am J Physiol Heart Circ Physiol. 2004;287:H1452–1458. doi: 10.1152/ajpheart.01101.2003. [DOI] [PubMed] [Google Scholar]
- 117.MacKinnon AC, Buckley A, Chilvers ER, Rossi AG, Haslett C, Sethi T. Sphingosine kinase: a point of convergence in the action of diverse neutrophil priming agents. J Immunol. 2002;169:6394–6400. doi: 10.4049/jimmunol.169.11.6394. [DOI] [PubMed] [Google Scholar]
- 118.Vann LR, Payne SG, Edsall LC, Twitty S, Spiegel S, Milstien S. Involvement of sphingosine kinase in TNF-alpha-stimulated tetrahydrobiopterin biosynthesis in C6 glioma cells. The Journal of biological chemistry. 2002;277:12649–12656. doi: 10.1074/jbc.M109111200. [DOI] [PubMed] [Google Scholar]
- 119.Radeff-Huang J, Seasholtz TM, Chang JW, Smith JM, Walsh CT, Brown JH. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. The Journal of biological chemistry. 2007;282:863–870. doi: 10.1074/jbc.M601698200. [DOI] [PubMed] [Google Scholar]
- 120.De Palma C, Falcone S, Panzeri C, Radice S, Bassi MT, Clementi E. Endothelial nitric oxide synthase overexpression by neuronal cells in neurodegeneration: a link between inflammation and neuroprotection. Journal of neurochemistry. 2008;106:193–204. doi: 10.1111/j.1471-4159.2008.05351.x. [DOI] [PubMed] [Google Scholar]
- 121.Chandru H, Boggaram V. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene. 2007;391:150–160. doi: 10.1016/j.gene.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bu S, Yamanaka M, Pei H, Bielawska A, Bielawski J, Hannun YA, Obeid L, Trojanowska M. Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via activation of ERK1/2-Ets1 pathway in human fibroblasts. Faseb J. 2006;20:184–186. doi: 10.1096/fj.05-4646fje. [DOI] [PubMed] [Google Scholar]
- 123.Donati C, Nincheri P, Cencetti F, Rapizzi E, Farnararo M, Bruni P. Tumor necrosis factor-alpha exerts pro-myogenic action in C2C12 myoblasts via sphingosine kinase/S1P2 signaling. FEBS Lett. 2007;581:4384–4388. doi: 10.1016/j.febslet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Kozawa O, Tokuda H, Matsuno H, Uematsu T. Activation of mitogen-activated protein kinase is involved in sphingosine 1-phosphate-stimulated interleukin-6 synthesis in osteoblasts. FEBS Lett. 1997;418:149–151. doi: 10.1016/s0014-5793(97)01366-5. [DOI] [PubMed] [Google Scholar]