Abstract
Background
Females have a higher risk of experiencing low back pain or injury than males. One possible reason for this might be altered reflexes since longer paraspinal reflex latencies exist in injured patients versus healthy controls. Gender differences have been reported in paraspinal reflex latency, yet findings are inconsistent. The goal here was to investigate gender differences in paraspinal reflex latency, avoiding and accounting for potentially gender-confounding experimental factors.
Methods
Ten males and ten females underwent repeated trunk flexion perturbations. Paraspinal muscle activity and trunk kinematics were recorded to calculate reflex latency and maximum trunk flexion velocity. Two-way mixed model ANOVAs were used to determine the effects of gender on reflex latency and maximum trunk flexion velocity.
Findings
Reflex latency was 18.7% shorter in females than in males (P=0.02) when exposed to identical trunk perturbations, and did not vary by impulse (P=0.38). However, maximum trunk flexion velocity was 35.3% faster in females than males (P=0.01) when exposed to identical trunk perturbations, and increased with impulse (P<0.01). While controlling for differences in maximum trunk flexion velocity, reflex latency was 16.4% shorter in females than males (P=0.04).
Implications
The higher prevalence of low back pain and injury among females does not appear to result from slower paraspinal reflexes.
Keywords: Gender, Paraspinal, Reflex Latency, Spinal Stability Control, Trunk Perturbations, Kinematics, Low Back Pain, Low Back Injury, Female, Male
Introduction
Females have a higher risk of experiencing low back pain (LBP) or injury than males (Feuerstein et al., 1997, Hillman et al., 1996, Jones et al., 1993, Macfarlane et al., 1997) and are more likely to have chronic (Butler et al., 1995, Volinn et al., 1991, Zwerling et al., 1993) or more severe cases (Von Korff et al., 1993). In fact, females experience LBP or injury 240% more frequently than males in occupational settings that involve lifting (Daltroy et al., 1991, Macfarlane et al., 1997). Females are also more likely to experience limited activity capabilities due to LBP one year following an initial incident (Von Korff et al., 1993). The mechanisms behind these gender differences in rate and severity are unclear, yet they are important to discover in order to address this disparity in injury risk.
Paraspinal reflexes are one of many mechanisms that contribute to the control of spinal stability (Panjabi, 1992a), and altered reflexes may contribute to LBP. Longer paraspinal reflex latencies have been reported in LBP patients, compared to healthy controls, after either sudden loading (Wilder et al., 1996) or unloading (Radebold et al., 2000, Radebold et al., 2001) of the trunk that resulted in trunk flexion. Similarly, patients with unilateral low back pain exhibit longer erector spinae reflex latencies on the painful side than on the non-painful side (Wilder et al., 1996).
Longer paraspinal muscle reflex latencies in LBP patients have also been correlated with impaired postural control of the trunk during unstable sitting (Radebold et al., 2001). While it remains unclear whether LBP lead to these increased paraspinal reflex latencies or if altered reflexes contribute directly to the development of LBP, these studies demonstrate an association between LBP and increased paraspinal reflex latency.
Gender differences in paraspinal reflexes may be related to gender differences in LBP. Unfortunately, the few studies investigating gender differences in paraspinal reflexes have not yielded consistent results, perhaps due to confounding experimental factors. Wilder et al. (1996) reported longer paraspinal reflex latencies in females compared to males in response to sudden trunk flexion loading within both LBP patients and healthy controls. However, their subjects participated in a rehabilitation program involving physical conditioning and a cognitive-behavioral approach. Post hoc analyses revealed that males shortened their reflex latencies more than females after the rehabilitation program. Therefore, the gender difference reported could have resulted from the ability of males to shorten their reflex latency more than females following the rehabilitation program, rather than the existence of a true gender difference. Granata et al. (2005) found no gender differences in paraspinal reflex latency following trunk flexion perturbations in individuals without low back symptoms. However, the isotonic trunk extension preloads generated by participants prior to the perturbations could have masked any gender differences in reflex latency by stiffening the spine (Lee et al., 2007) and possibly reduced the need for quicker reflexes. Both Wilder et al. (1996) and Granata et al. (2005) also exposed males and females to the same trunk perturbation magnitudes, which can result in greater trunk flexion velocities in females due to a smaller trunk mass (Granata and Rogers, 2007). Since stretch reflexes are heavily dependent upon the rate of change of muscle length (i.e. trunk flexion velocity) (Kearney and Hunter, 1983, Kearney et al., 1997, Moorhouse and Granata, 2007), comparisons of reflex latency could be confounded when males and females experience different trunk kinematics. Gender differences in reflex latencies have also been reported in other muscles. Women exhibited shorter reflex latencies in the posterior neck musculature following sudden whiplash simulations (Siegmund et al., 2003) and in the quadriceps in response to body weight-scaled perturbations that induced internal and external rotations of a single knee (Shultz et al., 2001).
It is important to recognize any gender differences in paraspinal reflexes to further our understanding of gender differences in LBP. However, existing studies do not provide consistent results and may be confounded by extraneous experimental factors. As a result, the goal of this study was to investigate gender differences in paraspinal reflex latency while avoiding potentially confounding factors, and while controlling for potential gender differences in trunk kinematics resulting from gender differences in trunk mass. Based upon gender differences in reflex latency in the neck musculature (Siegmund et al., 2003) and the quadriceps (Shultz et al., 2001), it was hypothesized that females would exhibit shorter paraspinal reflex latencies than males in response to sudden trunk flexion perturbations.
Methods
Participants included ten males (mass: mean 73.4 (SD 1.5) kg; age: mean 22.6 (SD 2.8) yr) and ten females (mass: mean 65.0 (SD 1.4) kg; age: mean 27.2 (SD 7.8) yr) recruited from the university community. Participants had no history of low back pain or injury. This study was approved by the Virginia Tech Institutional Review Board, and written consent was obtained from all participants prior to participation.
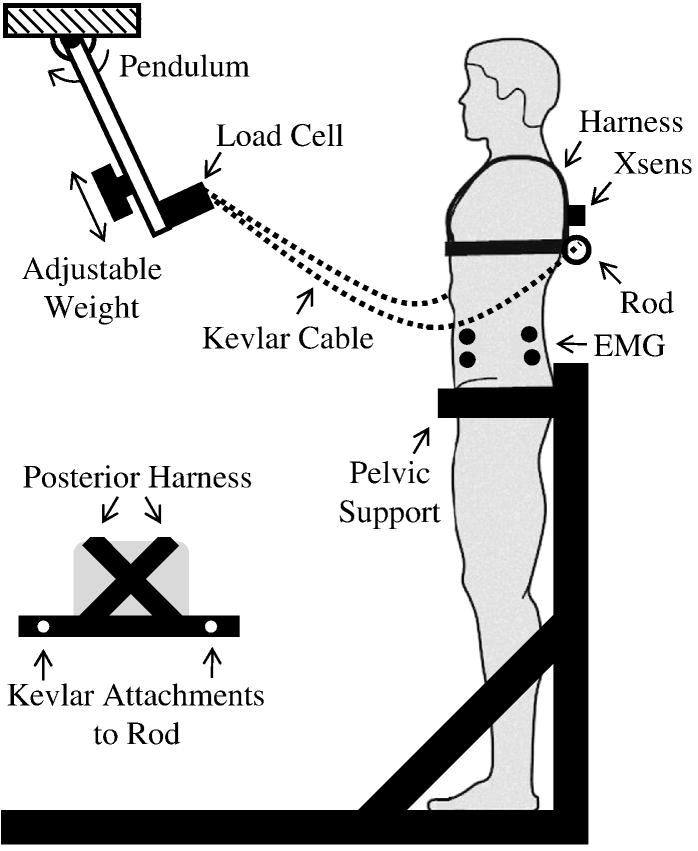
Paraspinal muscle activity and trunk kinematics were recorded following repeated anteriorly-directed trunk perturbations. While in an upright stance, participants were restrained from the pelvis down. Participants were attached to a pendulum (length: 66 cm, mass: 6.6 kg) at the T6/T8 level via Kevlar cable and a harness (Figure 1). Prior to each perturbation, participants were instructed to relax, close their eyes, cross their arms in front of their chest, and calmly return to an upright position following the perturbation. Upon release by an investigator, the pendulum swung down and away from the participants in the mid-sagittal plane, eliciting a brief trunk flexion moment at the instant the pendulum reached vertical and the cable became taut. Five perturbations were applied within about 60 seconds at each of five perturbation levels presented in random order. Perturbation levels were set by adjusting the position of the weight on the pendulum and resulted in torso impulses averaging 3.97, 5.96, 7.67, 9.00, and 10.21 N·s. Participants rested for two minutes between each set of five perturbations. Real-time EMG of the rectus abdominus (RA) and erector spinae (ES) trunk musculature were monitored to ensure no anticipatory stiffening or co-contraction beyond normal activation required to maintain an upright posture.
Figure 1.
Experimental set-up. The pendulum was raised to a mechanical stop and released after a random time delay to elicit trunk flexion perturbations. The final pendulum position was vertical and occurred when the Kevlar cable was taut and horizontal.
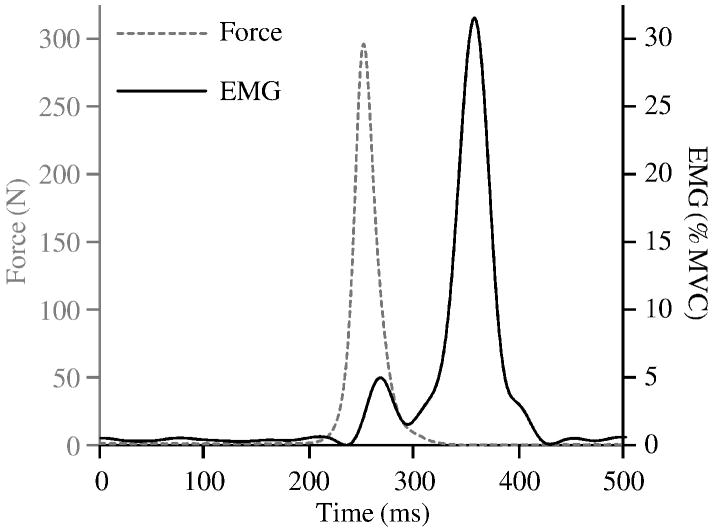
During all perturbations, muscle activity was sampled at 1000 Hz from the right ES, after a 500 Hz anti-aliasing filter, using an EMG system (Measurement Systems, Inc., Ann Arbor, MI, USA) and Ag/AgCl electrodes (VERMED, Bellows Falls, VT, USA) placed at the L3/L4 level of the spine, 3 cm lateral from the midline. These data were then band-pass filtered between 40 and 500 Hz, rectified, smoothed with a 25 Hz low-pass filter (zero-phase-lag 8th order Butterworth), and downsampled to 100 Hz. Trunk angular position was sampled at 60 Hz using a tri-axial Inertial Motion Sensor (Xsens Motion Technologies, Enschede, Netherlands) placed at the T6/T8 level of the spine and subsequently low-pass filtered at 20 Hz (zero-phase-lag 2nd order Butterworth) and upsampled to 100 Hz. Tension force in the pendulum cable was sampled at 1000 Hz using an in-line load cell (Interface SSM-500, Scottsdale, AZ, USA) and subsequently low-pass filtered at 20 Hz (zero-phase-lag 2nd order Butterworth) and downsampled to 100 Hz. Representative perturbation force and muscle activity data are presented in Figure 2.
Figure 2.
Example force impulse with corresponding EMG response activity.
Baseline muscle activity was determined as the mean of the period from 500 to 250 ms prior to each perturbation. Values were normalized to an isometric maximum voluntary contraction (MVC) of the trunk extensors. Two MVCs were performed in the experimental position at the beginning of testing by asking subjects to extend their trunk as hard as possible for 5 seconds while harnessed to a horizontal Kevlar cable fixed to the wall. Reflex latency was quantified as the time between force onset in the pendulum cable and onset of ES muscle activity. Onset times were determined when signals first exceeded a threshold defined as the mean baseline muscle activity level plus two standard deviations, and were confirmed by visual inspection. Latency was restricted to occur within 120 ms of the force onset to exclude voluntary responses (Wilder et al., 1996). Trunk flexion velocity was also quantified due to the velocity-dependent properties of the stretch reflex (Kearney and Hunter, 1983, Kearney et al., 1997, Moorhouse and Granata, 2007). The maximum trunk flexion velocity was found within the time span of force onset in the pendulum cable to the instant of maximum trunk flexion.
A two-way mixed model ANOVA was used to determine the effects of gender, perturbation impulse, and their interaction on reflex latency, maximum trunk flexion velocity, and baseline muscle activity prior to each perturbation. It should be noted that any gender difference in trunk mass would likely result in a gender difference in trunk kinematics when males and females were exposed to the same perturbation impulse. This would confound any gender differences in reflex latency because reflexes are dependent upon muscle velocity. Therefore, a second ANOVA was performed with maximum trunk flexion velocity as an independent variable instead of perturbation impulse to control for potential gender differences in trunk kinematics. This two-way mixed model ANOVA was used to determine the effects of gender, maximum trunk flexion velocity, and their interaction on reflex latency. All statistical analyses were conducted using JMP 7 (SAS Software, Cary, NC, USA) with a significance level of P≤0.05.
Results
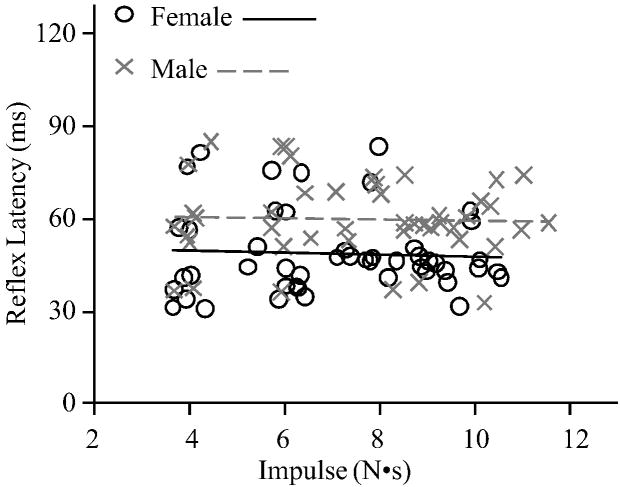
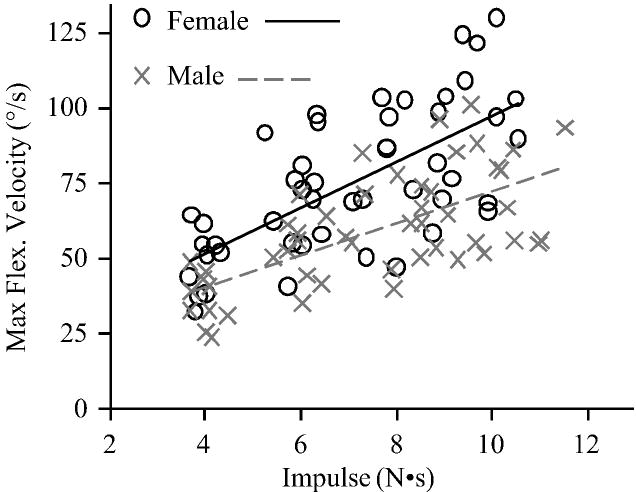
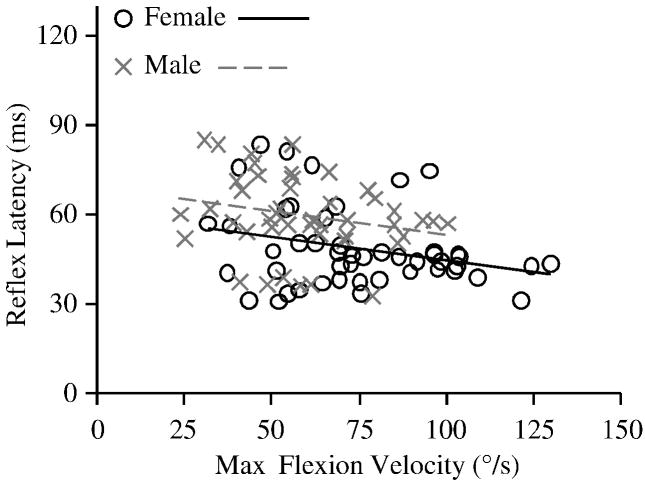
Reflexes were detected after 94% of the perturbations. Prior to each perturbation, baseline muscle activity did not differ between females (mean 1.07 (SD 0.15) %MVC) and males (mean 1.31 (SD 0.15) %MVC; P=0.26) or by impulse (P=0.28). Analyzing reflex latency with respect to impulse (Figure 3), reflex latency was 18.7% shorter in females (mean 48.8 (SD 3.0) ms) than males (mean 60.0 (SD 3.2) ms; P=0.02) and did not vary by impulse (P=0.38). However, maximum trunk flexion velocity (Figure 4) was 35.3% faster in females (mean 76.7 (SD 4.7) deg/s) compared to males (mean 56.7 (SD 4.9) deg/s; P=0.01), and increased with impulse (P<0.01). This was likely due to the 12.9% smaller body mass in females (mean 65.0 (SD 1.4) kg) compared to males (mean 73.4 (SD 1.5) kg; P<0.01), which would result in a similar difference in trunk mass (Moorhouse and Granata, 2005). Analyzing reflex latency with respect to maximum trunk flexion velocity (Figure 5), reflex latency was 16.4% shorter in females (mean 49.4 (SD 2.9) ms) than males (mean 59.1 (SD 3.1) ms; P=0.04) and did not change as maximum trunk flexion velocity increased (P=0.15).
Figure 3.
Reflex latency with respect to applied impulse.
Figure 4.
Maximum trunk flexion velocity with respect to applied impulse.
Figure 5.
Reflex latency with respect to maximum trunk flexion velocity.
There were no interaction effects between gender and impulse for baseline EMG (P=0.12), reflex latency (P=0.87), or maximum trunk flexion velocity (P=0.23). Similarly, there was no interaction effect between gender and maximum trunk flexion velocity for reflex latency (P=0.78).
Discussion
Our goal was to investigate gender differences in paraspinal reflex latency in an effort to improve our understanding of the gender disparity in LBP. Following trunk flexion perturbations, females responded with shorter latencies than males. They also exhibited a faster maximum trunk flexion velocity, which was not unexpected due to a smaller trunk mass in females than males. When controlling for this gender difference in maximum trunk flexion velocity, latency remained shorter in females than in males. Based upon the longer latencies reported in the injured lower back (Radebold et al., 2000, Radebold et al., 2001, Wilder et al., 1996), these results do not appear to support the notion that gender differences in paraspinal reflex latency alone directly contribute to gender differences in LBP.
Our reflex latencies of 48 ms for females and 60 ms for males are comparable to previous studies. Paraspinal reflex latencies have been reported as 68 ms for females and 65 ms for males (Granata et al., 2005) as well as 69 ms (Radebold et al., 2000) and 63 ms (Radebold et al., 2001) for healthy control groups including both males and females. While these values agree in a broad sense, some differences between studies are likely due to methodological differences. Granata et al. (2005), for example, had participants hold an extension preload and applied short stochastic force perturbations to flex the trunk using a motor. Radebold et al. (2000, 2001) suddenly released the resisting forces of isometric contractions, resulting in abrupt trunk flexion. In general, reflex responses are reported as having a monosynaptic stretch reflex ranging in latency from 30-50 ms and a functional polysynaptic reflex ranging from 50-80 ms (Matthews, 1991, Schmidt and Wrisberg, 2008). Although these ranges are generalized, our muscle activity was measured at the spinal level, so it appears that these reflexes are polysynaptic.
Our gender differences are inconsistent with previous investigations of paraspinal reflex latencies, but are consistent with gender differences in other muscles. Wilder et al. (1996) reported longer paraspinal latencies in females, yet this was a main effect of gender and could be confounded by the statistical interaction between gender and rehabilitation. Specifically, males decreased their reflex latency more than females following a rehabilitation program, but gender differences prior to the rehabilitation protocol were not reported. Granata et al. (2005) reported no gender differences in paraspinal reflex latency while participants held various trunk extension preloads. A preload exertion could reduce the need for quicker reflexes by stiffening the spine (Lee et al., 2007) and potentially mask gender differences in reflex latency. In fact, longer paraspinal latencies have been observed within gender for a preload condition versus no preload prior to perturbation (Granata et al., 2004). Despite the inconsistency between these two studies and ours, the 16.4% shorter reflex latency in females reported here is consistent with shorter reflex latencies observed in females in other muscles where experimental procedure did not appear to confound reflex measurements and perturbation kinematics were similar between genders. These include the posterior neck musculature during a whiplash movement where reflex latency was 8% shorter in females than in males (Siegmund et al., 2003), as well as the medial/lateral quadriceps following internal/external knee rotation perturbations where reflex latency (i.e. of the medial quadriceps with external knee rotation) was 12% shorter in females than in males (Shultz et al., 2001).
As described earlier, females are more prone to LBP than males (MacDonald et al., 1997). Since LBP is associated with longer paraspinal reflex latencies (Radebold et al., 2001), it would seem intuitive for females to exhibit longer paraspinal reflex latencies than males if longer latencies contribute to LBP. However, we found that females exhibited shorter paraspinal reflex latencies than males. This suggests that something other than, or in addition to, reflex latency is contributing to LBP. It is possible that the shorter reflexes latencies in females, which would seem to be beneficial from a control perspective, could be compensating for or offset by some other factor within the control of spinal stability. For example, passive trunk stiffness could vary between genders, influencing reflex latency and/or other reflexive parameters. In response to a perturbation, decreased passive stiffness in the spine would cause the active tissues to stretch sooner and thus elicit a reflex earlier. This has been observed in the cervical spine with females exhibiting decreased passive stiffness (McGill et al., 1994) and shorter reflex latencies (Siegmund et al., 2003). Similar gender differences in passive stiffness could exist in the lumbar region. Such gender differences in passive trunk stiffness could involve gender differences in the “neutral zone” of the spine, i.e. the region of intersegmental motion with minimal passive resistance (Panjabi, 1992b). A larger neutral zone would allow for greater trunk movement before passive (and active) tissues stretch. Additional research is necessary to clarify the roles of all factors contributing to spinal stability control, and to understand the causes for gender differences in LBP.
It is also possible that the longer paraspinal reflex latencies associated with LBP (Radebold et al., 2001) may not have contributed to the development of LBP, but instead developed as a consequence to LBP. If so, it would be inappropriate to expect females, or any group predisposed to LBP, to exhibit longer paraspinal reflex latencies. The idea of longer reflex latencies as a result of LBP is supported by additional behaviors and characteristics that have been observed in LBP patients. For example, LBP patients have higher baseline EMG levels than healthy individuals in a normal upright stance (Wilder et al., 1996). Based on these findings, the authors hypothesized this might be a mechanism constantly utilized to help stabilize the back by increasing muscle stiffness, but with the undesirable effect of increasing neuromuscular fatigue in the paraspinal musculature. Therefore, the characteristics seen in LBP patients’ such as decreased reflex magnitudes (Wilder et al., 1996) and poorer performance in trunk balancing tasks (Radebold et al., 2001), as well as delayed or longer reflex latencies (Radebold et al., 2000, Radebold et al., 2001, Wilder et al., 1996), could be influenced by this constant fatiguing stabilization.
Several limitations of this study warrant discussion. First, this study focused on paraspinal reflex latency, which is only one component of multiple biomechanical mechanisms that contribute to spinal stability. Other mechanisms may have larger roles in the gender difference in LBP. Second, reflex magnitude can have a large influence on the contribution of reflexes to the control of spinal stability in addition to reflex latency. However, reflex magnitude was not investigated here due to potential habituation of reflex responses with repeated perturbations (reflex magnitude reduces following repeated perturbations (Siegmund et al., 2003), yet reflex latency is unaffected (Blouin et al., 2003, Siegmund et al., 2003)). Third, maximum trunk flexion velocity was used to quantify the trunk kinematics that elicited paraspinal reflexes due to difficulties in determining the actual trunk flexion velocity (i.e. muscle stretching velocity). While the timing of maximum trunk flexion velocity lags reflex latency by about 80ms, we felt the use of this proxy was reasonable for quantifying trunk kinematics since the time span from perturbation impulse to maximum trunk flexion velocity did not vary between genders (P=0.94). Fourth, trunk angle was measured at the T6/T8 level and used to determine trunk flexion velocity while reflexes were collected at L3/L4. Because of the motion of multiple spinal joints, subtle differences in flexion kinematics could exist between where the angular position was measured and where the reflexes were detected. Fifth, the pelvic angle in the initial testing position was not measured. As such, we cannot rule out slight differences between males and females in initial pelvic tilt during testing. Sixth, only right ES EMG was utilized because the task was sagittally-symmetric. Previous work reported symmetry in paraspinal reflexes using a similar perturbation protocol (Granata et al., 2004). Lastly, our muscle activity threshold of two standard deviations above baseline that was used to detect the onset of the reflex response was relatively arbitrary, but has been used elsewhere (e.g. Granata et al., 2004). While the choice of this threshold may have influenced our specific reflex latency values, it would not have compromised our comparison between males and females.
Conclusion
Females exhibited shorter paraspinal reflex latencies than males after a trunk flexion perturbation, even when controlling for gender differences in kinematics. Based upon the longer latencies reported in the injured lower back (Radebold et al., 2000, Radebold et al., 2001, Wilder et al., 1996), these results do not appear to support the notion that gender differences in paraspinal reflex latency alone directly contribute to gender differences in LBP. Additional research is needed to elucidate the role of paraspinal reflexes in LBP and to understand the gender differences in LBP.
Acknowledgments
This research was funded by grant number 2 RO1 AR046111 from the NIAMS/NIH. The sponsors had no role in the study design, data collection/analysis/interpretation, manuscript writing, or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blouin JS, Descarreaux M, Belanger-Gravel A, Simoneau M, Teasdale N. Attenuation of human neck muscle activity following repeated imposed trunk-forward linear acceleration. Exp Brain Res. 2003;150:458–464. doi: 10.1007/s00221-003-1466-9. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Baldwin ML, Johnson WG. Managing work disability - why 1st return to work is not a measure of success. Ind Labor Relat Rev. 1995;48:452–469. [Google Scholar]
- Daltroy LH, Larson MG, Wright EA, Malspeis S, Fossel AH, Ryan J, et al. A case-control study of risk factors for industrial low back injury: Implications for primary and secondary prevention programs. Am J Ind Med. 1991;20:505–515. doi: 10.1002/ajim.4700200406. [DOI] [PubMed] [Google Scholar]
- Feuerstein M, Berkowitz SM, Peck CA., Jr Musculoskeletal-related disability in us army personnel: Prevalence, gender, and military occupational specialties. J Occup Environ Med. 1997;39:68–78. doi: 10.1097/00043764-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Granata KP, Rogers E. Torso flexion modulates stiffness and reflex response. J Electromyogr Kinesiol. 2007;17:384–392. doi: 10.1016/j.jelekin.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Granata KP, Rogers E, Moorhouse K. Effects of static flexion-relaxation on paraspinal reflex behavior. Clin Biomech (Bristol, Avon) 2005;20:16–24. doi: 10.1016/j.clinbiomech.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, Slota GP, Bennett BC. Paraspinal muscle reflex dynamics. J Biomech. 2004;37:241–247. doi: 10.1016/s0021-9290(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hillman M, Wright A, Rajaratnam G, Tennant A, Chamberlain MA. Prevalence of low back pain in the community: Implications for service provision in bradford, uk. J Epidemiol Community Health. 1996;50:347–352. doi: 10.1136/jech.50.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BH, Bovee MW, Harris JM, 3rd, Cowan DN. Intrinsic risk factors for exercise-related injuries among male and female army trainees. Am J Sports Med. 1993;21:705–710. doi: 10.1177/036354659302100512. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Hunter IW. System identification of human triceps surae stretch reflex dynamics. Exp Brain Res. 1983;51:117–127. doi: 10.1007/BF00236809. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Stein RB, Parameswaran L. Identification of intrinsic and reflex contributions to human ankle stiffness dynamics. IEEE Trans Biomed Eng. 1997;44:493–504. doi: 10.1109/10.581944. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Granata KP, Moorhouse KM. Active trunk stiffness during voluntary isometric flexion and extension exertions. Hum Factors. 2007;49:100–109. doi: 10.1518/001872007779597993. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Sorock GS, Volinn E, Hashemi L, Clancy EA, Webster B. A descriptive study of recurrent low back pain claims. J Occup Environ Med. 1997;39:35–43. doi: 10.1097/00043764-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Macfarlane GJ, Thomas E, Papageorgiou AC, Croft PR, Jayson MI, Silman AJ. Employment and physical work activities as predictors of future low back pain. Spine (Phila Pa 1976) 1997;22:1143–1149. doi: 10.1097/00007632-199705150-00015. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- McGill S, Seguin J, Bennett G. Passive stiffness of the lumbar torso in flexion, extension, lateral bending, and axial rotation. Effect of belt wearing and breath holding. Spine (Phila Pa 1976) 1994;19:696–704. doi: 10.1097/00007632-199403001-00009. [DOI] [PubMed] [Google Scholar]
- Moorhouse KM, Granata KP. Trunk stiffness and dynamics during active extension exertions. J Biomech. 2005;38:2000–2007. doi: 10.1016/j.jbiomech.2004.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhouse KM, Granata KP. Role of reflex dynamics in spinal stability: Intrinsic muscle stiffness alone is insufficient for stability. J Biomech. 2007;40:1058–1065. doi: 10.1016/j.jbiomech.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part i. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992a;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part ii. Neutral zone and instability hypothesis. J Spinal Disord. 1992b;5:390–396. doi: 10.1097/00002517-199212000-00002. discussion 397. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26:724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Wrisberg CA. Motor learning and performance: A situation-based learning approach. 4th Human Kinetics; Champaign, IL: 2008. [Google Scholar]
- Shultz SJ, Perrin DH, Adams MJ, Arnold BL, Gansneder BM, Granata KP. Neuromuscular response characteristics in men and women after knee perturbation in a single-leg, weight-bearing stance. J Athl Train. 2001;36:37–43. [PMC free article] [PubMed] [Google Scholar]
- Siegmund GP, Sanderson DJ, Myers BS, Inglis JT. Rapid neck muscle adaptation alters the head kinematics of aware and unaware subjects undergoing multiple whiplash-like perturbations. J Biomech. 2003;36:473–482. doi: 10.1016/s0021-9290(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Volinn E, Van Koevering D, Loeser JD. Back sprain in industry. The role of socioeconomic factors in chronicity. Spine (Phila Pa 1976) 1991;16:542–548. doi: 10.1097/00007632-199105000-00010. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Deyo RA, Cherkin D, Barlow W. Back pain in primary care. Outcomes at 1 year. Spine (Phila Pa 1976) 1993;18:855–862. doi: 10.1097/00007632-199306000-00008. [DOI] [PubMed] [Google Scholar]
- Wilder DG, Aleksiev AR, Magnusson ML, Pope MH, Spratt KF, Goel VK. Muscular response to sudden load. A tool to evaluate fatigue and rehabilitation. Spine. 1996;21:2628–2639. doi: 10.1097/00007632-199611150-00013. [DOI] [PubMed] [Google Scholar]
- Zwerling C, Ryan J, Schootman M. A case-control study of risk factors for industrial low back injury. The utility of preplacement screening in defining high-risk groups. Spine (Phila Pa 1976) 1993;18:1242–1247. doi: 10.1097/00007632-199307000-00020. [DOI] [PubMed] [Google Scholar]