Abstract
Mutations in collagen II are associated with spondyloepiphyseal dysplasia, a group of heritable diseases whose common features include aberrations of skeletal growth. The mechanisms through which mutations in collagen II affect the cartilaginous tissues are complex and include both intracellular and extracellular processes. One of those mechanisms involves cellular stress caused by excessive accumulation of misfolded collagen II mutants. We investigated whether stabilizing the structure of thermolabile R789C and R992C collagen II mutants would improve their secretion from cells, thereby reducing cellular stress and apoptosis. Employing glycerol and trimethylamine N-oxide (TMAO), chemicals that increase the thermostability of collagen triple helices, we demonstrated that those compounds function as chaperones and stabilize the R789C and R992C mutants, accelerate their secretion, and improve cell survival. Our study provides a scientific basis for considering misfolded triple helices of collagen mutants a target for reducing the deleterious effects caused by their excessive intracellular accumulation.
Introduction
Collagen II is a component of heterotypic fibrils which form scaffolds of mature cartilaginous tissues and provide a template that guides the development of skeletal tissues [1]. Processes leading from the biosynthesis of pro-α1(II) chains in cells to the formation of collagen fibrils in the extracellular space are complex and include: (i) posttranslational modifications of nascent pro-α1(II) chains, (ii) their folding into triple-helical procollagen molecules and secretion from cells, and (iii) self-assembly of collagen molecules into fibrils [2].
Fundamental for the mechanical and biological functions of collagen II is its stable triple-helical conformation. Such a conformation is achieved by the folding of individual pro-α1(II) chains whose main collagenous domain consists of uninterrupted repetitions of the G-X-Y motif in which the “X” and the “Y” positions are frequently occupied by proline and hydroxyproline residues, respectively [2]. The accurate amino acid sequence of collagen II is critical for the correct functions of this protein at the tissue level and mutations in COL2A1 (OMIM#120140) are associated with a broad spectrum of chondrodysplasia phenotypes, generally described as spondyloepiphyseal dysplasia (SED), the main feature of which is abnormal skeletal development [3]
One of the pathological consequences of mutations in collagenous proteins is a loss-of-function effect due to the reduced incorporation of mutants into the extracellular matrix (ECM). It has been suggested that the fundamental cause for the reduced amount of collagen molecules containing mutant α chains is excessive intracellular accumulation and subsequent degradation in a process described as “procollagen suicide” [4].
Due to the possibility of excessive intracellular retention, some diseases that involve collagen mutants are categorized as storage diseases, a group that also includes diseases caused by the excessive accumulation of non-collagenous proteins, lipids, glycoproteins, and other macromolecules [5]. In our studies published elsewhere we demonstrated that the R789C and R992C substitutions in collagen II cause misfolding of mutant molecules, decrease their thermostabilities, promote excessive intracellular retention and aggregation, and increase apoptosis [6; 7; 8]. Thus, in addition to the loss-of-function effect, certain mutations in collagen II may also cause a gain-of-toxicity effect.
Here, we studied the feasibility of reducing cellular stress associated with the R789C and R992C substitutions by stabilizing the mutant molecules and decreasing their intracellular accumulation. Employing glycerol and trimethylamine N-oxide (TMAO), chemicals that stabilize the collagen triple helix, we demonstrated that these compounds increase the thermostability of the R789C and R992C mutants, decrease their intracellular retention, and improve cell survival [9; 10; 11],. Our study provides bases for considering triple helices of thermolabile collagen mutants a new target to reduce the deleterious effects caused by their atypical retention.
Materials and Methods
Mutation nomenclature
The amino acid substitutions are named according to the literature, with amino acid residues numbered from the first glycine of the collagen II triple helix.
Procollagen II mutants
Procollagen II mutants employed in this study are associated with SED [12; 13]. To facilitate various biochemical and microscopic assays, wild type (WT) and mutant procollagen II variants were engineered as chimeras fused with green fluorescent protein (Pro-GFP), as described [6; 8].
Cells expressing collagen II mutants
For the production of Pro-GFP variants in quantities sufficient for assays of their thermostability, these proteins were expressed in HT-1080 cells (ATCC; CCL-121), as described [6; 8; 14]. For analyses of cellular responses to the presence of procollagen II mutants, the chondrocytic cell line SW-1353 (ATCC; HTB-94) was employed, as described [6; 7; 8]. Both cell lines were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 40 μg/ml of L-ascorbic acid phosphate magnesium salt (Wako Chemicals, Inc.), as described [8; 14].
Cell culture in the presence of glycerol and TMAO
Glycerol and TMAO, compounds with chemical chaperone characteristics, were employed to determine the effects of their collagen-stabilizing activities on the structure of the R789C and R992C mutants, and the faith of cells harboring them. For analyses of cell survival, we followed the experimental design described by Hintze et al [7]. In brief, SW-1353 cells were cultured for 12 days post-confluence, and then cell culture media were supplemented with glycerol added to a final concentration of 0.1 M or 0.5 M, or TMAO added at 30 mM or 0.1 M. Subsequently, cell culture was continued for five additional days. After the five-day treatment, cell culture media and cell lysates were collected for further assays. For thermostability analyses, collagen II variants were isolated from HT-1080 cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO, as described [14].
Working concentrations of selected compounds used in this study were determined in pilot experiments. In these experiments cells were cultured in increasing concentrations of glycerol or TMAO. During culture, cells were monitored for proliferation, changes in morphology, and production of procollagen II, as described [6; 8]. The highest concentrations that did not have any apparent negative effects on cultured cells were applied in the presented studies.
Cleaved poly-(ADP-ribose) polymerase (cPARP) and TdT-mediated dUTP Nick-End Labeling (TUNEL) assays
Glycerol and TMAO-treated cells were analyzed for apoptotic markers, as described [7]. In these assays cPARP was detected in cell lysates by Western blot with primary rabbit anti-cPARP antibodies (Cell Signaling Technology, Inc.) and secondary anti-rabbit IgG conjugated to horseradish peroxidase (HRP; Sigma-Aldrich). The same nitrocellulose membranes were also probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The relative amount of cPARP in the analyzed samples was determined by measuring the ratios of the pixel intensities of the cPARP-positive bands and GAPDH-positive bands. In any given group of cells, i.e. expressing GFP-tagged WT, R789C or R992C collagen II, the cPARP/GAPDH ratio derived from non-treated cells was considered 100%. Statistical significances of differences between the means calculated for the relative contents of cPARP in cells cultured in the presence vs. the absence of tested compounds were evaluated by the Student’s t test (GraphPad Software, Inc.).
In another set of experiments the presence of fragmented DNA was analyzed by TUNEL assays that utilize tetramethylrodamine (TMR) as a red fluorophore (Roche). Subsequently the apoptotic index, defined as the percentage of TUNEL-positive nuclei out of the total number of DAPI-positive nuclei, was calculated for each analyzed group, as described [6; 7].
Thermostability assays of collagen II variants
Two experimental approaches were designed; (i) in the first approach we analyzed the thermostabilities of purified collagen II mutants produced by HT-1080 cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO, and (ii) in the second approach these compounds were added to purified collagen II variants produced by cells cultured in the absence of these compounds. The thermostabilities were measured by brief protease digestion, as described [6; 15]. Based on collagen II-melting curves, we determined the temperatures at which the triple helical structure of analyzed collagens was preserved in 75% (Tm75), 50% (Tm50), and 25% (Tm25) of maximum.
Intracellular Pro-GFP variants produced in the presence of glycerol and TMAO
We analyzed whether glycerol and TMAO-mediated stabilization of collagen molecules could also influence their intracellular accumulation. In brief, glycerol or TMAO-treated cells were lysed, and then Pro-GFP variants were detected by Western blot assays with an anti-GFP antibody, as described [6; 8]. The relative content of GFP-positive bands was assayed by densitometry. The results of measurements were normalized to GAPDH and plotted as a percent of the non-treated control (expressed as 100%).
We also studied the effects of glycerol and TMAO on the accumulation of collagen molecules misfolded due to inhibition of critical hydroxylation of proline residues by 2,2′ dipyridyl (Sigma-Aldrich) [16]. The intracellular content of Pro-GFP variants produced in the presence of 2,2′ dipyridyl was assayed as described above.
Results
Apoptotic markers in cells harboring the R789C and R992C mutants cultured in the presence of glycerol and TMAO
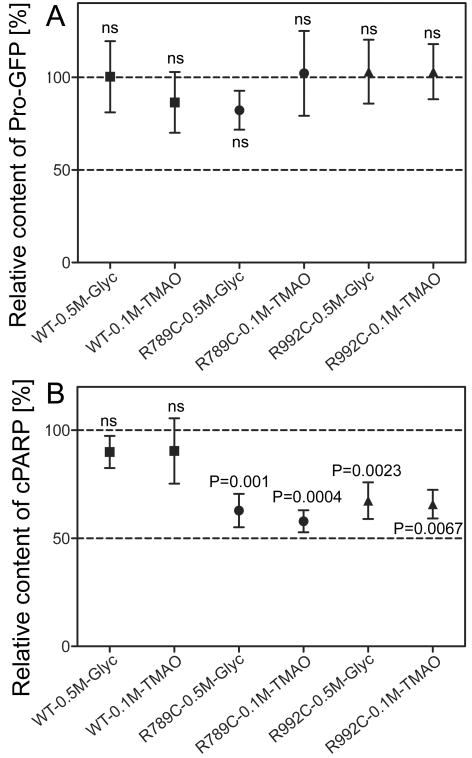
Cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO had a statistically significant decrease in the relative amount of cPARP, indicating an increase of cell survival (Fig. 1 and Supplementary Fig. S1). In the same experimental conditions, the overall amounts of Pro-GFP produced by cells did not change (Fig. 1). The lower concentrations of these compounds used in the pilot studies did not have significant effects on relative amounts of cPARP (data not shown).
Figure 1.
Relative amounts of Pro-GFP variants (A) and cPARP (B) in cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO. The relative amounts of Pro-GFP and cPARP in specific groups of cells treated with glycerol or TMAO are compared corresponding control groups cultured in the absence of these compounds (controls expressed as 100%). Each point represents the mean value from multiple independent assays ± standard error of the mean (SEM). Symbols: WT, samples that include wild type Pro-GFP; R789C and R992C, Pro-GFP mutants; ns, not statistically significant; P values for statistically significant differences between means calculated for analyzed group and non-treated controls are also presented (expressed as 100%).
TUNEL analyses (Supplementary Fig. S2) of cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO support the results of cPARP assays. Specifically, we determined that in comparison to the non-treated cells harboring the WT, R789C or the R992C mutants, the apoptotic indexes for cells cultured in the presence of 0.5 M glycerol decreased from 0.89% (±0.15 SEM), 3.45% (±0.73 SEM), and 0.96% (±0.12 SEM), to 0.82% (±0.14 SEM), 2.34% (±0.3 SEM), and 0.5% (±0.11 SEM), respectively. A similar trend was observed in an analogous experiment where cells were treated with 0.1 M TMAO. Specifically, we determined that in comparison to the non-treated cells harboring the WT, R789C or the R992C mutants (see above) the apoptotic indexes for cells cultured in the presence of 0.1 M TMAO decreased to 0.56% (±0.14 SEM), 2.24% (±0.22 SEM), and 0.67% (±0.08 SEM), respectively.
Thermostabilities of the R789C and R992C mutants produced by cells cultured in the presence of glycerol or TMAO
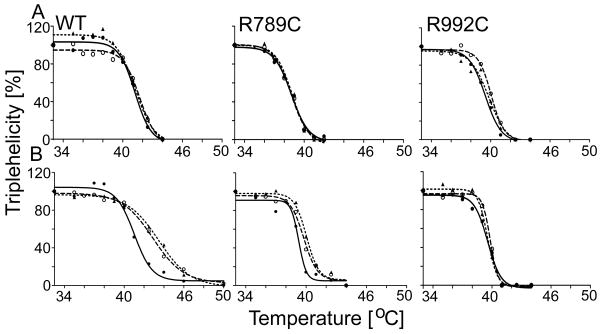
In comparison to the non-treated controls, the thermostabilities of the WT collagen II and the analyzed mutants produced by cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO have increased (Fig. 2A, Supplementary Fig. 3S, and Tab. 1). Based on this observation, however, it has not been clear whether the thermostabilizing effect depended on intracellular interactions of glycerol and TMAO with newly synthesized pro-α1(II) chains or whether these compounds were able exert a stabilizing effect extracellularly. Thus, in another set of experiments, we first prepared collagen II variants isolated from cells cultured in the absence of chaperones. Next, purified collagens were exposed to these compounds for 24-h at 4°C, and then thermostability measurements were performed. Because the thermostabilities of analyzed mutants have increased, these assays suggest that intracellular interaction between the chemical chaperones and procollagen is not a defining factor in stabilizing the collagen II variants (Fig. 2B, Supplementary Fig. 3S, and Tab. 1). When comparing the thermostabilities of the collagen variants purified from cultures of cells grown in the presence of chaperones to those isolated from cells grown in chaperone-free media and treated with chaperones right before the stability assays, it is apparent that the increase in thermostability was greater in the latter group. (Fig. 2 and Tab. 1).
Figure 2.
A, Assays of the thermostabilities of the WT, R789C, and R992C collagen II variants isolated from HT-1080 cells cultured in the presence of 0.5 M glycerol or 0.1 M TMAO. B, Similar assays of the thermostability of the WT, R789C, and R992C collagen II variants isolated from HT-1080 cells cultured in chaperone-free media. Proteins were isolated from the media then incubated with 0.5 M glycerol or 0.1 M TMAO.
Following preincubation at increasing temperatures, collagen II samples were subjected to brief protease digestion. The digested samples were electrophoresed in 7.5% polyacrylamide gels in reducing conditions and then α1(II) chains were visualized by Western blot (Supplementary Figure S3). Subsequently, the relative pixel intensities of bands corresponding to α1(II) chains were measured by densitometry. Graphic representations of changes in the relative amount of triple-helical collagen II in response to increasing temperature are demonstrated (see Table 1). Symbols: WT, wild type collagen II; R789C and R992C, collagen II mutant harboring specific substitution; solid circles (●)and solid line, non-treated control; open circles (○) and dashed line, group treated with 0.5 M glycerol; triangles (▲) and dotted lines, a group treated with 0.1 M TMAO.
Table 1.
Thermostabilities of the R789C and R992C mutant in the presence of glycerol and TMAO.
| Tm75 [°C] | Tm50 [°C] | Tm25 [°C] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Collage n II variant | C | 0.5M Glycerol | 0.1M TMAO | C | 0.5 M Glycerol | 0.1M TMAO | C | 0.5M Glycerol | 0.1M TMAO | |
| Chaperones added during cell culture | WT | 40.4 | 40.6 | 40.5 | 41.3 | 41.5 | 41.3 | 42.2 | 42.3 | 42.2 |
| R789C | 37.7 | 37.8 | 37.9 | 38.6 | 38.6 | 38.7 | 39.6 | 39.5 | 39.6 | |
| R992C | 38.7 | 39.4 | 39.1 | 39.3 | 39.8 | 39.6 | 40.1 | 40.1 | 40.3 | |
| Chaperones added to purified collagen isolated from non-treated cells | WT | 40.4 | 41.0 | 41.5 | 41.5 | 43.0 | 43.4 | 42.7 | 44.9 | 45.2 |
| R789C | 38.6 | 39.0 | 39.4 | 39.1 | 39.7 | 40.1 | 39.6 | 40.6 | 40.9 | |
| R992C | 38.5 | 39.2 | 38.7 | 39.3 | 40.0 | 39.7 | 40.2 | 40.7 | 40.6 | |
Tm75, Tm50, and Tm25 indicate the temperatures at which the triple helical structure of analyzed collagens was preserved in 75%, 50%, and 25% of maximum, respectively; C indicates control samples not treated with chemical chaperones.
Note: As glycerol and TMAO do not change the enzymatic activities of trypsin and chymotrypsin, it is important to emphasize that the observed increase of thermostabilities of collagen II variants was not a result of the decrease of the enzymatic activities of employed proteases [17].
Intracellular accumulation of the R789C and R992C mutants
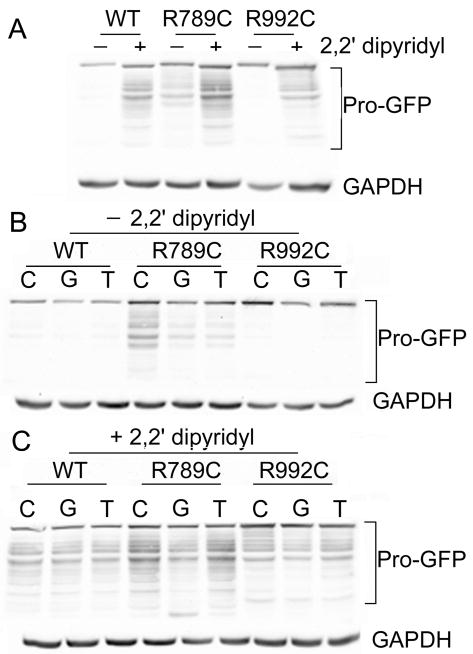
We analyzed the intracellular content of Pro-GFP variants in the presence of glycerol and TMAO. In comparison to the non-treated controls, in cells treated with these compounds the relative amounts of the R789C and R992C variants were reduced (Fig. 3 and Fig. 4). Of note was the observation that in cells expressing the R789C mutant, partial degradation of this protein was observed.
Figure 3.
Intracellular pool of Pro-GFP variants in cells cultured in the presence of glycerol or TMAO with or without addition of 2,2′ dipyridyl. A, Site-by-site comparison of Pro-GFP variants from cells grown in the presence (+) or the absence (−) of 2,2′ dipyridyl but in the absence of glycerol and TMAO. B, Intracellular pool of the Pro-GFP variants in cells grown in the presence of glycerol or TMAO but without 2,2′ dipyridyl. C, Intracellular pool of the Pro-GFP variants in cells grown in the presence of 2,2′ dipyridyl and glycerol or TMAO. Symbols: WT, samples that include wild type Pro-GFP; R789C and R992C, Pro-GFP mutants. Symbols: C, controls grown in the absence of glycerol (G) or TMAO (T).
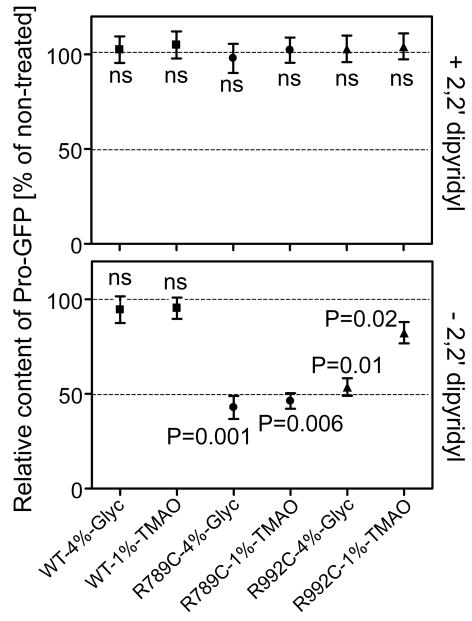
Figure 4.
Graphic representation of intracellular accumulation of Pro-GFP variants in cells cultured in the presence of glycerol or TMAO with or without addition of 2,2′ dipyridyl. The relative amounts of Pro-GFP in specific groups of cells treated with glycerol or TMAO are compared to control groups cultured in the absence of these compounds (controls expressed as 100%). Each point represents the mean value from multiple independent assays ± standard error of the mean (SEM).
As shown in Fig. 3 and Fig. 4 adding 2,2′ dipyridyl into the cell culture increased accumulation of Pro-GFP in all analyzed groups. Moreover, as evident by the presence of GFP-positive fragments, increased intracellular accumulation was associated with degradation of all Pro-GFP variants. In addition, in cells cultured in the presence of this compound, neither glycerol nor TMAO reduced the intracellular accumulation of the Pro-GFP variants (Fig. 3 and Fig. 4).
Discussion
Employing a simple experimental system, we tested whether adverse consequences of intracellular accumulation of the thermolabile R789C and R992C collagen II mutants could be partially reduced by improving their thermostabilities. The strategy proposed here was to employ glycerol and TMAO, compounds that at 1 M concentration increase the thermostability of collagen triple helices by 1°C and 5°C, respectively [9; 10].
We demonstrated that the presence of glycerol and TMAO was associated with reduction of the relative amounts of cPARP in cells harboring the R789C and R992C mutants. At the same time, the presence of these compounds did not affect the overall production of collagen II. In cells expressing WT collagen II, the relative amount of background-level cPARP also decreased, but this change was relatively small compared to that observed for cells harboring collagen II mutants. The decrease in the apoptotic indexes for cells expressing the R789C and R992C variants in the presence of glycerol and TMAO further corroborates the results of quantitative assays of cPARP. Changes in the relative amounts of apoptotic markers in cells cultured in the presence of glycerol or TMAO correlated with the decrease of the intracellular pool of procollagen molecules, suggesting the increase of their secretion. Because the efficient secretion of procollagens depends on the formation of thermostable triple-helical structures, the decrease of intracellular accumulation of inherently thermolabile R789C and R992C procollagen II mutants in the presence of glycerol and TMAO further indicate the chaperone-like collagen-stabilizing effect of these compounds [6; 7; 16; 18; 19]. The collagen-stabilizing activity of selected chemical chaperones, however, is probably limited to mutant molecules with localized structural aberrations. This notion is based on the observation that collagen molecules whose structures are extensively misfolded due to the inhibition of hydroxylation of proline residues by 2,2′ dipyridyl are not stabilized by selected chemical chaperones..
The decrease in the intracellular accumulation of studied mutants indicates that the stabilizing effects of glycerol and TMAO were primarily a result of the intracellular influence of these compounds on the structure of a collagen triple helix. In comparison to the stabilities of collagen II variants purified from cell culture media of cells grown in the presence of glycerol or TMAO, the increase in the thermostability was even greater in another experimental group in which these compounds were added to the purified-collagen samples before thermostability assays and remained in the analyzed samples during these assays. This apparent difference was most likely due to the partial reversal of the stabilizing effects in the first experimental group due to the decrease in the concentration of glycerol and TMAO occurring during the purification of collagen II.
The concept of improving the structure of misfolded mutant proteins by employing chemical chaperones to reduce the harmful effects that such mutants have on cells and tissues has been proposed and tested with a number of proteins associated with various diseases [20; 21; 22; 23; 24]. Specific examples of such an approach include employing TMAO to reduce the effects of mutant keratins that cause various skin disorders and to restore the activity of mutant branched-chain α-ketoacid dehydrogenase associated with maple syrup urine disease [25; 26]. Moreover, chemical chaperones were shown to be effective in stabilizing the structure and accelerating the secretion of mutant proteins involved in cystic fibrosis and Huntington’s disease, as well as liver injury and emphysema caused by an α1-antytrypsin deficiency [27; 28; 29]. Although most studies on chemical chaperones as therapeutic agents were done in cell culture conditions, reduction of ER stress and restoration of glucose homeostasis by administration of 4-phenylbutyric acid, yet another compound with chaperone-like activities, in a mouse model of type 2 diabetes was also demonstrated, thereby indicating a potential utility of chemical chaperones in vivo [30].
The studies presented here provide experimental evidence that the aberrant structure of collagen mutants could be considered a target for approaches aimed at reducing the harmful effects imposed by these mutants. Preliminary tests presented here focused on the thermolabile R789C and R992C collagen II mutants that are associated with apoptosis and whose structural alterations are readily detectable [6; 7; 8; 31]. The structural and biological effects of these mutations, however, do not represent the full spectrum of potential effects that different mutations may impose. For instance, in cells expressing the thermostable R75C, R519C, and G853E collagen II mutants which are also associated with skeletal diseases, an increase in apoptosis was not readily apparent [7]. Thus, the question remains whether targeting the structure of collagens should be considered only for the thermolabile mutants or also for the thermostable mutants whose ER stress-related effects may be long-term and accumulative.
Supplementary Material
Acknowledgments
This work was supported by the National Health Institutes [5R01AR049537-06].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustafsson E, Aszodi A, Ortega N, Hunziker EB, Denker HW, Werb Z, Fassler R. Role of collagen type II and perlecan in skeletal development. Ann N Y Acad Sci. 2003;995:140–50. doi: 10.1111/j.1749-6632.2003.tb03217.x. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 3.Olsen BR. Mutations in collagen genes resulting in metaphyseal and epiphyseal dysplasias. Bone. 1995;17:45S–49S. doi: 10.1016/8756-3282(95)00208-u. [DOI] [PubMed] [Google Scholar]
- 4.Williams CJ, Prockop DJ. Synthesis and processing of a type I procollagen containing shortened pro-alpha 1(I) chains by fibroblasts from a patient with osteogenesis imperfecta. J Biol Chem. 1983;258:5915–21. [PubMed] [Google Scholar]
- 5.Rutishauser J, Spiess M. Endoplasmic reticulum storage diseases. Swiss Med Wkly. 2002;132:211–22. doi: 10.4414/smw.2002.09861. [DOI] [PubMed] [Google Scholar]
- 6.Chung HJ, Jensen DA, Gawron K, Steplewski A, Fertala A. R992C (p.R1192C) Substitution in collagen II alters the structure of mutant molecules and induces the unfolded protein response. J Mol Biol. 2009;390:306–18. doi: 10.1016/j.jmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hintze V, Steplewski A, Ito H, Jensen DA, Rodeck U, Fertala A. Cells expressing partially unfolded R789C/p.R989C type II procollagen mutant associated with spondyloepiphyseal dysplasia undergo apoptosis. Hum Mutat. 2008;29:841–51. doi: 10.1002/humu.20736. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Rucker E, Steplewski A, McAdams E, Brittingham RJ, Alabyeva T, Fertala A. Guilty by association: some collagen II mutants alter the formation of ECM as a result of atypical interaction with fibronectin. J Mol Biol. 2005;352:382–95. doi: 10.1016/j.jmb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Beck K, Chan VC, Shenoy N, Kirkpatrick A, Ramshaw JA, Brodsky B. Destabilization of osteogenesis imperfecta collagen-like model peptides correlates with the identity of the residue replacing glycine. Proc Natl Acad Sci U S A. 2000;97:4273–8. doi: 10.1073/pnas.070050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penkova R, Goshev I, Gorinstein S, Nedkov P. Stability of collagen during denaturation. J Protein Chem. 1999;18:397–401. doi: 10.1023/a:1020632424142. [DOI] [PubMed] [Google Scholar]
- 11.Russell AE. Effect of alcohols and neutral salt on the thermal stability of soluble and precipitated acid-soluble collagen. Biochem J. 1973;131:335–42. doi: 10.1042/bj1310335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan D, Taylor TK, Cole WG. Characterization of an arginine 789 to cysteine substitution in alpha 1 (II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem. 1993;268:15238–45. [PubMed] [Google Scholar]
- 13.Donahue LR, Chang B, Mohan S, Miyakoshi N, Wergedal JE, Baylink DJ, Hawes NL, Rosen CJ, Ward-Bailey P, Zheng QY, Bronson RT, Johnson KR, Davisson MT. A missense mutation in the mouse Col2a1 gene causes spondyloepiphyseal dysplasia congenita, hearing loss, and retinoschisis. J Bone Miner Res. 2003;18:1612–21. doi: 10.1359/jbmr.2003.18.9.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fertala A, Sieron AL, Ganguly A, Li SW, Ala-Kokko L, Anumula KR, Prockop DJ. Synthesis of recombinant human procollagen II in a stably transfected tumour cell line (HT1080) Biochem J. 1994;298:31–7. doi: 10.1042/bj2980031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieron AL, Fertala A, Ala-Kokko L, Prockop DJ. Deletion of a large domain in recombinant human procollagen II does not alter the thermal stability of the triple helix. J Biol Chem. 1993;268:21232–7. [PubMed] [Google Scholar]
- 16.Kao WW, Prockop DJ, Berg RA. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979;254:2234–43. [PubMed] [Google Scholar]
- 17.Kumar R, Serrette JM, Thompson EB. Osmolyte-induced folding enhances tryptic enzyme activity. Arch Biochem Biophys. 2005;436:78–82. doi: 10.1016/j.abb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43:567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- 19.Barsh GS, Byers PH. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981;78:5142–6. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 21.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–9. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 22.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–6. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Baskakov I, Bolen DW. Forcing thermodynamically unfolded proteins to fold. J Biol Chem. 1998;273:4831–4. doi: 10.1074/jbc.273.9.4831. [DOI] [PubMed] [Google Scholar]
- 24.Arakawa T, Ejima D, Kita Y, Tsumoto K. Small molecule pharmacological chaperones: From thermodynamic stabilization to pharmaceutical drugs. Biochim Biophys Acta. 2006;1764:1677–87. doi: 10.1016/j.bbapap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Lee D, Santos D, Al-Rawi H, McNeill AM, Rugg EL. The chemical chaperone trimethylamine N-oxide ameliorates the effects of mutant keratins in cultured cells. Br J Dermatol. 2008;159:252–5. doi: 10.1111/j.1365-2133.2008.08596.x. [DOI] [PubMed] [Google Scholar]
- 26.Song JL, Chuang DT. Natural osmolyte trimethylamine N-oxide corrects assembly defects of mutant branched-chain alpha-ketoacid decarboxylase in maple syrup urine disease. J Biol Chem. 2001;276:40241–6. doi: 10.1074/jbc.M107242200. [DOI] [PubMed] [Google Scholar]
- 27.Brown CR, Hong-Brown LQ, Welch WJ. Strategies for correcting the delta F508 CFTR protein-folding defect. J Bioenerg Biomembr. 1997;29:491–502. doi: 10.1023/a:1022491124939. [DOI] [PubMed] [Google Scholar]
- 28.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reijonen S, Putkonen N, Norremolle A, Lindholm D, Korhonen L. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res. 2008;314:950–60. doi: 10.1016/j.yexcr.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steplewski A, Majsterek I, McAdams E, Rucker E, Brittingham RJ, Ito H, Hirai K, Adachi E, Jimenez SA, Fertala A. Thermostability gradient in the collagen triple helix reveals its multi-domain structure. J Mol Biol. 2004;338:989–98. doi: 10.1016/j.jmb.2004.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.