Abstract
Chronic stress may have different effects on hippocampal CA3 and CA1 neuronal morphology and function depending upon hormonal status, but rarely are manipulations of stress and gonadal steroids combined. Experiment 1 investigated the effects of chronic restraint and 17β-estradiol replacement on CA3 and CA1 dendritic morphology and spatial learning in ovariectomized female Sprague-Dawley rats. Ovariectomized rats were implanted with 25% 17β-estradiol, 100% cholesterol or blank silastic capsules, and then chronically restrained (6h/d/21d) or kept in home cages. 17β-estradiol or cholesterol prevented stress-induced CA3 dendritic retraction, increased CA1 apical spine density, and altered CA1 spine shape. The combination of chronic stress and 17β-estradiol facilitated water maze acquisition compared to chronic stress + blank implants and nonstressed controls + 17β-estradiol. To further investigate the interaction between 17β-estradiol and stress on hippocampal morphology, Experiment 2 was conducted on gonadally intact, cycling female rats that were chronically restrained (6h/d/21d) and then euthanized at proestrus (high ovarian hormones) or estrus (low ovarian hormones). Cycling female rats failed to show chronic stress-induced CA3 dendritic retraction at either estrous phase. Chronic stress enhanced the ratio of CA1 basal spine heads to headless spines as found in Experiment 1. In addition, proestrous rats displayed increased CA1 spine density regardless of stress history. These results show that 17β-estradiol or cholesterol protect against chronic stress-induced CA3 dendritic retraction in females. These stress- and 17β-estradiol-induced morphological changes may provide insight into how dendritic complexity and spine properties contribute to spatial ability.
Keywords: Morris water maze, dendritic retraction, neuroprotection, rat, locomotion, estrous cycle
INTRODUCTION
Numerous studies using a variety of species including humans show that chronic stress and prolonged elevations in corticosterone impair hippocampal function. Chronic stress impairs hippocampal-dependent spatial learning and memory on a variety of tasks including the Y-maze (Bellani et al., 2006; Conrad et al., 1996; Wright and Conrad, 2005), T-maze (Sunanda et al., 2000), hole-board task (Ohl and Fuchs, 1999), radial arm maze (Luine et al., 1994; Luine et al., 1993), water maze (Ma et al., 2007; Park et al., 2001; Song et al., 2006; Walesiuk et al., 2005; Wright and Conrad, 2008), and radial arm water maze (Gerges et al., 2004; Srivareerat et al., 2009). Humans with a history of elevated corticosterone show impaired cognitive performance on tasks requiring hippocampal function (Lupien et al., 1996; Starkman et al., 1992). Thus, chronic stress in general influences cognition, with hippocampal-dependent memory being particularly sensitive to its impairing effects.
The impairment in hippocampal-dependent spatial memory by chronic stress can be mediated through a variety of mechanisms. As examples, in the hippocampus, chronic stress decreases neurogenesis, alters spine density, changes inhibitory tone, and restructures dendritic arbors (for review, see McEwen, 2001; McEwen et al., 1997). Stress-induced restructuring of hippocampal dendritic arbors has received much attention because spatial learning and memory is often impaired under conditions that produce CA3 dendritic retraction (Kleen et al., 2006), but not under conditions in which stress fails to produce CA3 dendritic retraction (Luine et al., 1996; McLaughlin et al., 2007). As examples, daily restraint for fewer than 21days (6hr for 7 to 14 days, Luine et al., 1996; McLaughlin et al., 2007), does not alter CA3 dendritic arbors, and fails to impair spatial learning or memory (Luine et al., 1996; McLaughlin et al., 2007). When rats are allowed to recover from stress-induced CA3 dendritic retraction (Conrad et al., 1999; Vyas et al., 2004), they show functional spatial memory (Luine et al., 1994). Pharmacological interventions also provide compelling evidence that stress-induced CA3 dendritic retraction underlies spatial memory deficits. Chronically stressed rats treated daily with antidepressants (i.e., tianeptine, fluoxetine) to block CA3 dendritic retraction (Luo and Tan, 2001; Magariños et al., 1999; Watanabe et al., 1992a), show functional spatial memory (Conrad et al., 1996; Luine et al., 1994). These studies support the hypothesis that chronic stress remodels CA3 dendrites, which in turn, contributes to impaired hippocampal-dependent spatial memory (Conrad, 2006).
Of particular interest is that chronic stress causes robust CA3 dendritic retraction in the hippocampus of males, a more robust effect than that observed in cycling females. One report showed that chronic stress caused basal CA3 dendritic retraction in gonadally intact females, which differed from the apical CA3 dendritic retraction in gonadally-intact males (Galea et al., 1997). In a subsequent study, chronic stress caused robust CA3 dendritic retraction in ovariectomized (OVX) female rats, with reduced dendritic arborization in both apical and basal CA3 regions (McLaughlin et al., 2005). This latter finding supports the hypothesis that ovarian hormones may offer neuroprotection against stress-induced CA3 dendritic retraction. However, females are capable of performing well in spatial tasks following chronic stress (Bisagno et al., 2003; Bowman et al., 2003; Bowman et al., 2001; Conrad et al., 2003; Kitraki et al., 2004), even in conditions that produce CA3 dendritic retraction (McLaughlin et al., 2005). Ovarian hormones may mediate female performance since chronically stressed (ovariectomized) females implanted with 17β-estradiol exhibit better spatial ability than chronically stressed females implanted with cholesterol or non-stressed females given either type of implant (Bowman et al., 2002). Moreover, enhanced CA1 dendritic spine shape correlated with spatial ability in chronically stressed female rats (McLaughlin et al., 2005). Therefore, chronic stress exerts sex-specific effects on hippocampal morphology and function and ovarian hormones likely modulate chronic stress effects in females. However, research has yet to evaluate both hippocampal morphology and function in the same female subjects following chronic stress and 17β-estradiol treatment.
The following study investigated whether chronic stress and 17β-estradiol influence hippocampal CA3 and CA1 morphology and function within the same OVX female rats. We wanted to optimize the procedures that alter CA1 dendritic spines because CA1 spine properties correlate with spatial and nonspatial performance (McLaughlin et al., 2005; Wallace et al., 2006). Given that hippocampal CA1 dendritic spines are highly sensitive to hormonal, neurochemical, and behavioral experiences (Eyre et al., 2003; Frick et al., 2004; Garza-Meilandt et al., 2006; Kirov et al., 2004; Leggio et al., 2005), silastic capsules were implanted during ovariectomy surgery and great care was taken to equalize handling, training and testing experiences across groups during the experiment. In addition, 17β-estradiol silastic implants increase antioxidant reactivity in the hippocampus (Prediger et al., 2004), reverse toxin-induced dendritic retraction in the basal forebrain (Saenz et al., 2006), and increase CA1 dendritic spine density (Garza-Meilandt et al., 2006) similarly as two injections of estradiol benzoate or 17β-estradiol (McLaughlin et al., 2008; Woolley, 1998; Woolley and McEwen, 1992). Pilot data from our lab has confirmed that with our implant methodology, cholesterol and estradiol both yield circulating levels of estradiol in serum, although the actions of cholesterol on hippocampal morphology remain unknown. However, based upon previous research from our lab using chronically stressed ovariectomized female rats, we hypothesized that continuous, chronic 17β-estradiol replacement would attenuate stress-induced CA3 dendritic retraction. In addition, we hypothesized that chronic stress and 17β-estradiol would have interacting effects on hippocampal-dependent behavior, which was assessed using both the Y-maze and Morris water maze. Based upon the findings in Experiment 1, we also investigated the influence of the phase of the estrous cycle on chronic stress-induced CA3 dendritic retraction in rats euthanized at proestrus or estrus, when ovarian hormone levels would be at their highest and lowest, respectively, as dendritic remodeling can change within hours in mammals (Magariños et al., 2006; Popov et al., 1992; von der Ohe et al., 2006).
MATERIALS AND METHODS
Subjects
All subjects and procedures were approved by the Arizona State University Institutional Animal Care and Use Committee in Experiment 1, the University of Illinois Institutional Animal Care and Use Committee in Experiment 2, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996).
In Experiment 1, 90 female Sprague-Dawley rats were purchased from Charles River Labs (Hartford, CT) and housed at Arizona State University housing facilities. In Experiment 2, 26 female Sprague-Dawley rats were purchased from Harlan (Oregon, WI) and housed at the University of Illinois housing facilities. Rats were about three months of age and the control and stressed rats were housed in separate, but identical chambers (21–22° C) under a 12/12 light-dark cycle (lights off at 6:00AM), with access to food and water ad libitum.
Experiment 1: Effect of Chronic stress and 17β-Estradiol on CA3 Dendritic Complexity, and Maze Performance
Surgery and Hormone Administration
Rats were divided into six experimental groups according to stress treatment and hormone administration: non-stress control-blank (NX), non-stress control-cholesterol (NC), non-stress control-17β-estradiol (NE), stress-blank (SX), stress-cholesterol (SC), stress-17β-estradiol (SE). One week following arrival, all rats underwent OVX surgery as previously described (McLaughlin et al., 2005; McLaughlin et al., 2008) and were implanted with silastic capsules at the time of surgery. Silastic implants were made from silastic tubing (Dow Corning medical grade tubing, 5 mm long; 1.96 mm outer diameter, 1.47 mm inner diameter) and sealed on both ends with glue (Dow Corning Silastic medical adhesive silicone, type A). There were three implant groups: glue-filled implants (blank), 100% cholesterol, and 25% 17β-estradiol diluted in cholesterol. Cholesterol is commonly used as a control condition in rodent hormone replacement studies because estrogen administration is diluted in cholesterol (Bowman et al., 2002; Daniel et al., 1997; Daniel et al., 2006; Garza-Meilandt et al., 2006). However, previous pilot data from our lab comparing circulating estradiol levels obtained from silastic implants (given to OVX female Sprague-Dawley rats) to estradiol levels found in intact, cycling female Sprague-Dawley rats showed that 100% cholesterol implants provided serum estradiol levels similar to those obtained during diestrus. Moreover, 25% 17β-estradiol silastic implants yielded similar estradiol serum levels as female rats in proestrus (unpublished data). Therefore, the glue subset (blank condition) served as a model of the female system free of hormonal treatment, while cholesterol and 17β-estradiol served as treatment conditions. Implants were inserted just below the nape of the neck with a 10-gauge Precision trochar (Innovative Research of America, Sarasota, FL) during OVX surgery, at a location where the implant would not be disrupted during decapitation.
Chronic Stress and Handling Procedure
Two weeks after ovariectomy, wound clips were removed and half of the rats began 6h/d/21d restraint stress using wire mesh restraints, a well documented stress paradigm used to induce CA3 hippocampal dendritic retraction in both male (Conrad et al., 1999; Kleen et al., 2006; Magariños and McEwen, 1995a; Magariños and McEwen, 1995b; McLaughlin et al., 2007; Vyas et al., 2002; Watanabe et al., 1992b) and female rats (Galea et al., 1997; McLaughlin et al., 2005). Both non-stressed controls and stressed rats were briefly handled beginning on day 7 of restraint to reduce any stress associated with the experimenter and behavioral testing. Separate investigators participated in handling duties and restraint procedures.
Rats were weighed on days 1, 7, 14, and 21 of restraint to verify that restraint was perceived as stressful (Conrad et al., 2001; McKittrick et al., 2000; McLaughlin et al., 2005; Wright and Conrad, 2005). Both chronic restraint stress (McLaughlin et al., 2005; McLaughlin et al., 2007) and estradiol treatment (Daniel and Lee, 2004; Geary et al., 1994; Gentry and Wade, 1976) were expected to decrease weight gain in females. Five rats were removed due to surgical complications and another five were removed due to inconsistencies with 17β-estradiol levels detected in the blood. Thus, eighty rats contributed to body weight data on Day 0 (implant and OVX surgery) and then on days 1, 7, 14, and 21 of restraint: NX (n = 13), NC (n = 16), NE (n = 12), SX (n = 13), SC (n = 14), SE (n = 12).
Behavioral Tasks
Following the end of the chronic stress procedure, rats were tested on two tasks that have been used successfully to measure spatial ability in male rats: the Y-maze and water maze (Conrad, 2006; Wright and Conrad, 2008). These tasks were chosen because spatial ability can be assessed relatively quickly, an important consideration as stress-induced CA3 dendritic retraction is dynamic and is present four days after chronic stress has ended, but by ten days post-stress, CA3 dendritic remodeling is no longer observed (Conrad et al., 1999). Moreover, we did not want to continue the chronic stress procedure during behavioral testing, which would confound the acute stress effects of restraint with the history of chronic stress.
Y-maze
The day after chronic restraint stress ended (Day 22), rats were tested on the Y-maze, a hippocampal-dependent task used to assess spatial memory (Bellani et al., 2006; Conrad et al., 1996; Kleen et al., 2006; McLaughlin et al., 2005). The Y-maze consisted of three unilaterally intersecting Plexiglas arms (58 cm long by 19 cm wide by 38 cm high). Spatial cues included vertical metal poles position around the outside perimeter of the maze and high enough to be viewed the black Plexiglas sides, as well as geometric shapes painted on the wall. The floor of the maze was covered with corncob bedding and mixed between trials to prevent utilization of odor cues.
Rats were tested in random order and place in an arm of the Y-maze (start arm) with one of the arms blocked off (novel arm) and allowed to explore the start and remaining arm (other arm) for 15 minutes. Rats were then removed from the maze, the novel arm unblocked, and the bedding mixed. After a 4 hour delay, rats were placed back in the start arm and allowed to explore all arms freely for 5 minutes. The start, novel and other arm were held constant for a given rat, but randomized across rats and treatments. Because rats tend to explore novel environments, functional memory was measured as the number of entries into the novel arm compared to the other arm. Poor memory would lead to relatively equal entries into the novel and other arms, indicating that the animals had forgotten the experience in the other arm, and would view it as an unexplored arm.
Behavior was videotaped and quantified off-line. Intact spatial memory was defined as a preference for the novel arm, which was determined by comparing the percent of entries into the novel arm to those made in the other, previously explored arm. Difference scores (entries into the novel - other arms) were also computed, with positive scores reflecting preference for the novel arm and negative scores reflecting preference for the other arm. Total entries were calculated to assess potential differences in motivational factors and changes in locomotor activity. Two rats were excluded from Y-maze data (one rat entered the blocked arm during training and one rat’s behavior could not be clearly scored from the video), leaving 78 rats for statistical analyses: NX (n = 13), NC (n = 16), NE (n = 12), SX (n = 13), SC (n = 12), SE (n = 12).
Morris Water Maze
On day 23, rats were trained and tested on the water maze, a spatial task that depends upon the hippocampus (Morris et al., 1982; Moser and Moser, 1998) and successfully implemented in our lab (Conrad and Roy, 1993; Wright and Conrad, 2008). The water maze consisted of a white, circular metal pool (diameter = 152.4 cm, height = 61 cm) filled with water maintained at approximately 21–23° C. Water maze testing occurred in a separate room from Y-maze testing and with different extra maze cues. During the training sessions, a white circular escape platform (diameter = 14 cm, height 43.2 cm) was placed in a fixed location of one quadrant, approximately 2 cm under the water surface. Pool water was mixed with non-toxic white tempera paint (Rich Art Color Co., Northale, NJ) to create an opaque environment that concealed the escape platform and any other visual cues within the pool. The water maze procedure (training and testing) occurred within a 24-hr timeframe (8 training trial protocol, similar to Berry et al., 1997). This protocol allowed rats to complete both behavioral tasks (Y-maze and water maze) within the time that stress-induced dendritic retraction is present in male rats (Conrad et al., 1999).
Water maze training included 8 acquisition trials where rats swam for a maximum of 60s or until they located the escape platform. If a rat failed to locate the platform within 60s, it was guided to the platform. Rats remained on the platform for 20s, and then began the next trial. Rats started each acquisition trial from one of four different points equidistant around the pool. Four hours after the last training trial, the escape platform was removed and rats completed a 60s probe trial. The probe trial was then followed by a cued trial where latency to reach a visible platform within the maze was recorded. Rats were placed in the same start location used for the probe trial; however the visible cue was located at the center of the opposite quadrant within the water maze.
Behavior analysis in the water maze included latency to the platform, distance swam, and total speed across the eight training trials. Pairs of trials were grouped together to represent four separate blocks for both latency and distance measures. For the probe trial, behavior was assessed by time and distance covered in four virtual quadrants. The quadrant that previously contained the escape platform was defined as the target quadrant. The total amount of time spent and distance swam in the target was compared to time spent and distance swam in the opposite quadrant. Rats with intact spatial memory were expected to spend more time and swim a greater percentage of their total distance in the target quadrant compared to the opposite quadrant. Total distance traveled and speed were also measured during the probe trial to elucidate potential differences in locomotor activity and/or strategy. Following the probe trial, the latency to reach a visible cue in the maze was recorded. Data from six rats were removed from analyses (one rat jumped out of the maze; five rats did not have recorded data due to equipment failure/experimental error). Thus, 74 rats were included in the water maze analyses: NX (n = 13), NC (n = 14), NE (n = 12), SX (n = 11), SC (n = 12), SE (n = 12).
Euthanasia
Immediately after completing the behavioral tasks, the rats were euthanized by rapid decapitation and the brains and trunk blood were collected for Golgi processing and 17β-estradiol measurements, respectively. Blood was centrifuged for 30 minutes at 4000 rpm at 4°C using a Heraeus centrifuge (model Megafuge 1.0R, VWR). The serum was collected and then frozen at − 70° C.
Golgi & Histology
Golgi staining was conducted according to FD Rapid Golgistain™ Kits (FD NeuroTechnologies, Baltimore, MD) on unperfused brain tissue. Sections were cut using a Microm Cryostat (−23°C, 100 μm) and staining procedures were similar to that previously described (McLaughlin et al., 2005; McLaughlin et al., 2008; McLaughlin et al., 2007). Approximately 20 to 25 sections were obtained for each rat that spanned the rostro-caudal extent of the dorsal hippocampus. Neurons were selected from either hemisphere, provided that they met the following criteria: 1) location within the CA3 and CA1 dorsal hippocampus, 2) dark and consistent staining throughout the full extent of the neuron, 3) relatively isolated from nearby neurons to be able to accurately reconstruct the neuronal dendrites, 4) cell body in the middle of the section to avoid truncated dendrites from tissue sectioning. Neurons that met these criteria in the CA3 region yielded an average of 4–8 neurons per neuronal type (short shaft = SS, long shaft = LS). CA3 neurons were labeled as SS or LS, depending on their relative location in the stratum pyramidale and proximal apical shaft length (Fitch et al., 1989). Each rat had one value calculated for SS and LS dendritic properties, which represented an average value of dendritic complexity for each neuronal type. Separate measures for apical and basal dendritic properties as well as CA3 neuronal type were computed. Due to the sensitivity of Golgi staining, some rats did not have appropriately stained or isolated hippocampal cells. Therefore, the final analyses for CA3 dendritic morphology included a total of 44 rats: NX (n = 9), NC (n = 7), NE (n = 7), SX (n = 6), SC (n = 9), SE (n = 6).
CA1 cell types were analyzed together since dendritic complexity is similar among neuronal types and spine densities were determined using previously established protocols (Gould et al., 1990; McLaughlin et al., 2005; McLaughlin et al., 2008; Woolley et al., 1997). For the CA1 region, 3–5 neurons were averaged to obtain one value for each rat, and separate analyses were conducted for apical and basal dendritic properties. CA1 arborization analyses included the following number of rats/group: NX (n = 9), NC (n = 10), NE (n = 8), SX (n = 8), SC (n = 10), SE (n = 7).
CA1 spines were counted and reported as the number of spines/10 μm of dendrite. Spines were also categorized as having a head, or being headless (Kasai et al., 2003; McLaughlin et al., 2005; McLaughlin et al., 2008). Counting the number of spine heads and then computing a ratio of heads to headless spines determined spine shape ratio. All spine data are represented per 10μm segment. Due to the high level of magnification required for data collection (1250×), we were unable to clearly view spine segments from one rat in the NX group, resulting in the following final number of 51 subjects: NX (n = 8), NC (n = 10), NE (n = 8), SX (n = 8), SC (n = 10), SE (n = 7).
17β-Estradiol Assay
Serum from trunk blood was shipped over night on dry ice to the Core Endocrine Laboratory, Pennsylvania State University, headed by Laurence M. Demers. 17β-estradiol levels were determined by a solid phase radioimmunoassay kit utilizing 125I-labeled estradiol (Kit manufactured by Siemens Medical Solutions Diagnostics, Los Angeles, CA). The interassay precision at a concentration of 43pg/ml was 11%, with functional sensitivity at 10pg/ml. The assay was performed in duplicate and rats with 17β-estradiol levels greater than two standard deviations between duplicate assays were removed from morphological and behavioral analyses. In addition, a Grubbs outlier test detected several outliers for blood levels within each group. The final number of subjects per group were: NX (n = 15), NC (n = 17), NE (n = 14), SX (n = 16), SC (n = 16), SE (n = 15).
Experiment 2: Effect of Chronic Stress and Phase of the Estrous Cycle on CA3 Dendritic Morphology
Experiment 2 General Procedures
The restraint procedures were similar to those described for Experiment 1. In addition, vaginal smears were taken daily between 9 AM and 11AM throughout restraint to determine estrous cycle stage as done previously (Conrad et al., 2004a; Korol et al., 2004). Vaginal cells were collected by gently inserting a small sterile swab (calcium alginate tips, Fisher Scientific, Houston, TX) soaked in saline into the vagina of the rat. The collected cells were placed on slides fixed with 95% ethanol, air dried, and stained with hematoxylin and eosin solutions (Sigma Diagnostics, St. Louis, MO). Smears were classified by Long and Evans (1922) methodology: estrous smears had cornified epithelial cells without nuclei; diestrous smears had leukocytes with few epithelial cells; and proestrous smears had a predominance of nucleated epithelial cells. All rats maintained regular cycling throughout the experiment. Body weight was also taken on days 1, 7, 14, and 21–24. The last day depended upon the stage of the estrous cycle: when a rat was determined to be in an estrous stage other than proestrus or estrus, the rat was placed back in restraint (or returned to the animal colony if a nonstressed control) for another day. This procedure was repeated until the vaginal smear indicated that the rat was in estrus or proestrus during days 21 – 24 of restraint.
At the chosen estrous phase, rats were euthanized by rapid decapitation, the brains removed and shipped overnight on dry ice to Arizona State University for processing using the Golgi technique as described in Experiment 1. In addition, adrenal glands were removed, cleaned of any excess fat and weighed. Chronic stress is likely to increase adrenal gland weight due to increased metabolic demands and dysregulation of stress hormones (Armario et al., 1990; Ulrich-Lai et al., 2006). Because uterine tissue is sensitive to changing circulating estrogen levels across the estrous cycle (Kuiper et al., 1996; Saiduddin and Zassenhaus, 1977), uterine horns were removed and immediately weighed to verify estrous stage.
For the CA3 region, SS and LS cells were averaged separately before being averaged together to obtain one measurement for each subject (total of 6–9 neurons analyzed per rat). This procedure was performed because five rats had just two LS or SS neurons. In order to maintain statistical power, each cell type was averaged separately and then LS and SS values were averaged to obtain one measurement to represent CA3 dendritic complexity. Moreover, Experiment 1 revealed that CA3 dendritic retraction occurred in the apical regions of both SS and LS neurons and so combining the values in Experiment 2 maximized statistical power. One rat died during the study and one was excluded from CA3 analyses due to insufficient staining, resulting in the following subjects for final analyses: non-stress control estrus (NE) n=5, non-stress control proestrus (NP) n=6, stress estrus (SE) n=6 and stress proestrus (SP) n=7.
For the CA1 region, 4–6 neurons were averaged to obtain one value for each subject. All rats had successful CA1 staining and final analyses for dendritic complexity and spine properties included the following: non-stress control estrus (NE) n=5, non-stress control proestrus (NP) n=6, stress estrus (SE) n=7 and stress proestrus (SP) n=7.
Statistical Analyses
The statistical software package, STATISTICA (Statsoft Inc., Tulsa, OK) was used for all analyses. ANOVAs analyzed parametric data and were followed by Newman-Keuls post hoc tests, when P≤0.05. Wilcoxon tests were computed for nonparametric data.
RESULTS
Experiment 1: Effect of Chronic stress and 17β-Estradiol on CA3 Dendritic Complexity and Maze Performance
CA3 Dendritic Morphology
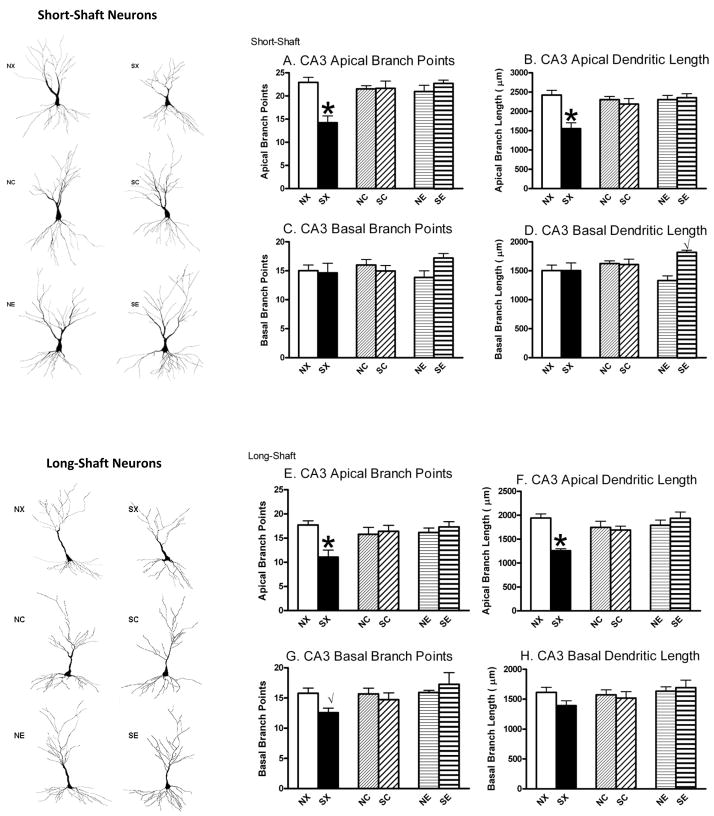
The overall finding was that chronic stress decreased CA3 apical dendritic complexity in both SS and LS neurons as measured by the total number of branch points and total dendritic length in OVX female rats with blank implants, and these effects were prevented by 17β-estradiol or cholesterol implants (Figure 1). A 2 × 3 ANOVA for stress (non-stress, stress) and hormone (blank, cholesterol, 17β-estradiol) on CA3 apical branch number in SS neurons revealed significant effects of chronic stress, F(1,38)=4.74, P<0.05, hormone, F(2,38)=4.06, P<0.05, and interaction, F(2,38)=9.73, P<0.01. Post hoc analyses showed that the SX group had significantly fewer apical branch points compared to all other groups (NX, NC, SC, NE, SE, Figure 1A). This outcome is replicated for CA3 apical dendritic length for SS neurons: A 2 × 3 ANOVA performed on SS apical branch length showed significant effects of chronic stress, F(1,38)=9.00, P<0.01, hormone, F(2,38)=3.87, P<0.05, and interaction, F(2,38)=7.45, P<0.01. Post hoc analyses showed that SX rats had shorter total branch length compared to all other groups (NX, NC, SC, NE, SE, Figure 1B). The LS neurons within CA3 showed similar patterns as the SS neurons. A 2 × 3 ANOVA on LS apical branches revealed a significant interaction between chronic stress and hormone, F(2,38)=6.57, P<0.01. Post hoc analyses showed that the SX group had significantly fewer apical branch points compared to all other groups (NX, NC, SC, NE, SE, Figure 1E). A 2 × 3 ANOVA on CA3 LS apical branch length also showed significant effects of chronic stress, F(1,38)=5.75, P<0.05, hormone, F(2,38)=3.30, P<0.05, and interaction, F(2,38)=8.98, P<0.01. Post-hoc analyses showed SX rats had shorter total branch length compared to all other groups (NX, NC, SC, NE, SE, Figure 1F).
Figure 1.
Effects of Chronic Stress and 17β-Estradiol Implants on Hippocampal CA3 Dendritic Arborization. Camera Lucida tracings (320X) represent both SS and LS hippocampal neurons to the left. Chronically stressed rats with blank implants (SX) showed CA3 dendritic retraction in SS apical branch points (A) and length (B) and in LS apical and basal branch points (E, G) and apical length (F). In addition, rats in the SE group had significantly greater branch length compared to rats in the NE group (P<0.01, D). Non-stress control-blank (NX), non-stress control-cholesterol (NC), non-stress control-17β-estradiol (NE), stress-blank (SX), stress-cholesterol (SC), stress-17β-estradiol (SE). *P<0.05 compared to NX, NC, SC, NE, SE; √P<0.05 compared to the nonstressed control given the same hormone implant.
The CA3 basal region of SS or LS neurons showed mixed responses to chronic stress and hormonal treatment. A 2 × 3 ANOVA on CA3 basal branch points for SS neurons failed to detect significant effects (Figure 1C). However, a 2 × 3 ANOVA on CA3 SS basal branch length found a significant effect of chronic stress, F(1,38)=4.58, P<0.05, and interaction between chronic stress and hormone F(2,38)=4.78, P<0.05. Post hoc analyses showed that rats in the SE group had significantly greater branch length compared to rats in the NE group (P<0.01, Figure 1D). No other effects were significant. For LS neurons in the CA3 region, a 2 × 3 ANOVA failed to find significant effects on CA3 LS neuronal basal branch points or branch length. However, a prior study showed that LS neurons display dendritic retraction in response to chronic stress so we conducted planned comparisons between the SX and NX groups, which would be similar to the OVX rats used in that study (McLaughlin et al., 2005). These results showed that rats in the SX group had significantly fewer basal branches compared to rats in the NX group (P<0.05, Figure 1G). A similar pattern was observed for basal branch length (Figure 1H), although it did not reach statistical significance (P=0.09).
CA1 Dendritic Arborization
17β-estradiol enhanced CA1 apical dendritic arborization compared to cholesterol or blank treatments, and these effects were unaltered by stress history (Figure 2). Separate 2 × 3 ANOVAs showed significant effects of hormone on CA1 apical branch points, F(1,46)= 4.39, P<0.05, and on CA1 apical branch length, F(1,46)= 4.09, P<0.05 (Figure 2A, B). Post-hoc results showed that rats treated with 17β-estradiol (NE, SE) had significantly greater CA1 apical arborization (branch points and length) compared to cholesterol (NC, SC; P<0.01) and blank-treated rats (NX, SX; P=0.05). No significant effects were found for CA1 basal arborization (Figure 2C, D).
Figure 2.
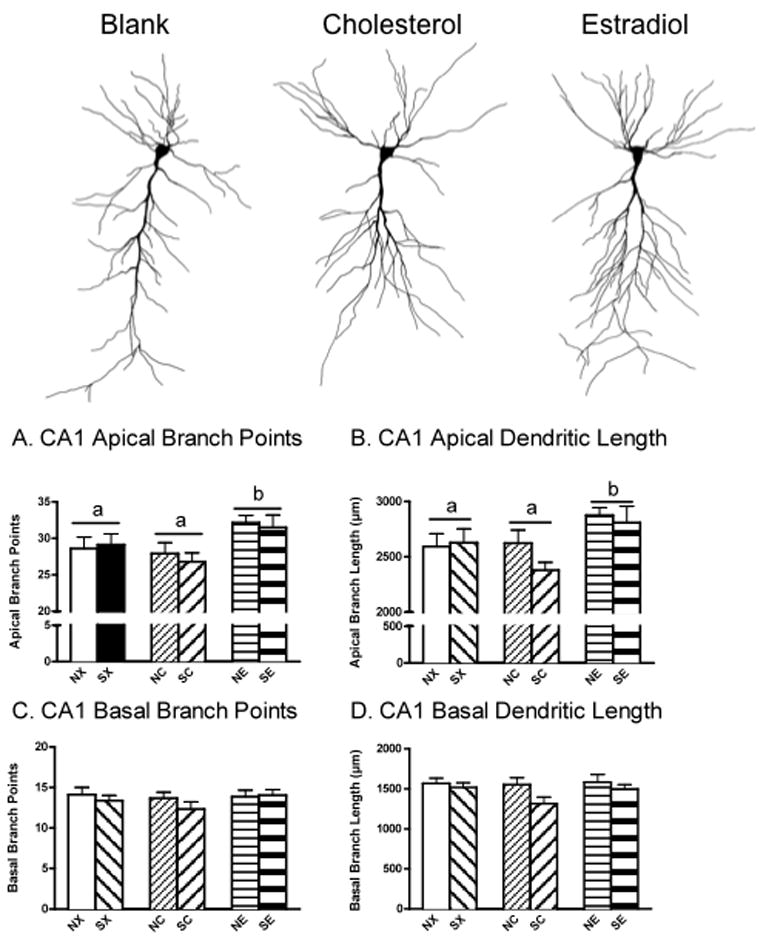
Effects of Chronic Stress and 17β-estradiol Implants on Hippocampal CA1 Dendritic Arborization. Camera Lucida tracings (320×) represent CA1 neurons at the top. 17β-estradiol (NE, SE) increased CA1 apical branch points (A) and apical branch length (B) compared to cholesterol (NC, SC) and blank-treated rats (NX, SX) and these effects were unaltered by stress history. There were no significant differences in CA1 basal arborization (C, D). Non-stress control-blank (NX), non-stress control-cholesterol (NC), non-stress control-17β-estradiol (NE), stress-blank (SX), stress-cholesterol (SC), stress-17β-estradiol (SE). Means with different letters indicate values that are statistically different (P<0.05, i.e., compare “a” vs. “b”), whereas means with similar letters represent values that are statistically similar (i.e., compare “a” vs. “a”).
CA1 Dendritic Spines
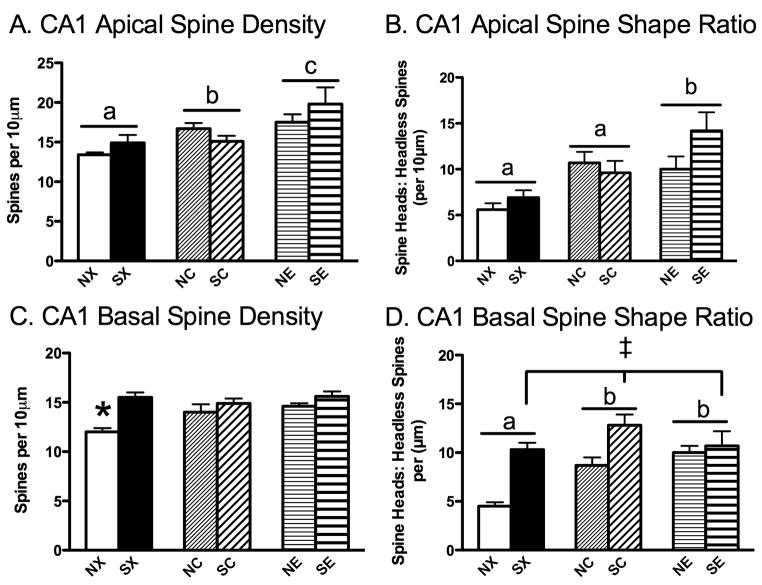
In the apical region, 17β-estradiol and cholesterol implants increased CA1 apical spine density and ratio of spine shape, and these effects were unaltered by stress history (Figure 3A, B). A 2 × 3 ANOVA on CA1 apical spine density revealed a significant main effect of hormone, F(2,45)=9.22, P<0.001. Post hoc analyses showed that rats treated with 17β-estradiol, regardless of stress history (NE, SE) had more apical spines than rats treated with cholesterol (NC, SC, P<0.01) or blank implants (NX, SX, P<0.001, Figure 3A). Moreover, planned comparisons showed that rats with cholesterol implants (NC, SC) had more spines compared to rats treated with blank implants (NX, SX, P<0.05), collapsed across stress history. Additionally, a 2 × 3 ANOVA on the ratio of spine heads to headless spines demonstrated a significant effect of hormone, F(2,45)=9.40, P<0.001. Post hoc tests showed that both 17β-estradiol (NE, SE) and cholesterol-treated (NC, SC) rats had a greater proportion of spine heads to headless spines compared to blank-treated rats (NX, SX, P<0.01, Figure 3B).
Figure 3.
Effects of Chronic Stress and 17β-Estradiol Implants on Hippocampal CA1 Spine Density and Shape Ratio. (A) 17β-estradiol (NE, SE) increased CA1 apical spine density compared to cholesterol (NC, SC), which were increased compared to blank silastic implants (NX, SX). (B) 17β-estradiol (NE, SE) increased CA1 apical spine shape ratio compared to cholesterol and blank silastic implants (NC, SC, NX, SX), and the latter two groups were statistically similar. (C) Nonstressed controls with blank implants (NX) had fewer CA1 basal dendritic spines compared to all remaining groups (SX, NC, SC, NE, SE). (D) In general, chronic stress (SX, SC, SE) increased the ratio of spine heads to headless spines, while blank implants (NX, SX) reduced the ratio compared to cholesterol (NC, SC) and 17β-estradiol (NE, SE). Non-stress control-blank (NX), non-stress control-cholesterol (NC), non-stress control-estradiol (NE), stress-blank (SX), stress-cholesterol (SC), stress-estradiol (SE). Means with different letters indicate values that are statistically different (P<0.05, i.e., compare “a” vs. “b”), whereas means with similar letters represent values that are statistically similar (i.e., compare “a” vs. “a”), *P<0.05 compared to SX, NC, SC, NE, SE; ‡ P<0.01 for a significant main effect for stress (SX, SC, SE) compared to nonstressed controls (NX, NC, NE), collapsed across hormone treatment.
In the basal region, chronic stress, cholesterol, or estrogen treatment prevented an OVX-induced decrease in CA1 basal spine density and ratio of spine shape (Figure 3C, D). A 2 × 3 ANOVA on CA1 basal spine density showed a significant effect of chronic stress, F(1,45)=15.52, P<0.01, and interaction between chronic stress and hormone, F(2,45)=3.35, P<0.05. Post hoc tests revealed that all groups (SX, NC, SC, NE, SE) had significantly more CA1 basal spines compared to the NX group. The ratio of CA1 basal spines with heads to headless spines showed a slightly different pattern than observed with CA1 basal spine density. In general, chronic stress increased the ratio of CA1 basal spines for heads to headless spines, as revealed by a significant main effect of chronic stress on basal spine shape ratio, F(1,45)=26.88, P<0.001. Moreover, 17β-estradiol or cholesterol also influenced CA1 basal spine ratio, as determined by a significant main effect of hormone, F(2 45)=5.96, P<0.01. Post hoc testing revealed that cholesterol (NC, SC) and 17β-estradiol (NE, SE) significantly increased the ratio of CA1 basal spine heads to headless spines compared to the blank condition (NX, SX, P=0.05).
Y-Maze
Based upon past data showing that rats habituate quickly to the Y-maze (Conrad et al., 2003; McLaughlin et al., 2005; Wright et al., 2006), the first minute of testing was analyzed. The number of arms visited in the first minute statistically differed among treatment conditions (Table 1). Rats treated with 17β-estradiol (NE, SE) entered significantly more arms than did rats treated with blank implants (NX, SX). A 2 × 3 ANOVA on total entries showed a significant effect of hormone, F(2,72)=6.01, P<0.01, and chronic stress and hormone interaction, F(2,72)=3.17, P<0.05. Post hoc tests revealed that 17β-estradiol-treated rats (NE, SE) entered more arms compared to blank-treated rats (NX, SX, P<0.01), and rats in the NX group entered significantly fewer arms compared to rats in the NC, NE, and SE groups (P<0.05).
Table 1.
Data from Experiment 1, representing means ± standard error of the mean.
| Stress History: Hormone: Group: |
Non-Stress Control + Blank (NX) | Non-Stress Control + Cholesterol (NC) | Non-Stress Control + 17β-Estradiol (NE) | Stress + Blank (SX) | Stress + Cholesterol (SC) | Stress + 17β-Estradiol (SE) |
|---|---|---|---|---|---|---|
| Behavioral Measures from the Y-Maze | ||||||
| %Novel Arm Entries | 46.9 ± 4.2* | 42.7 ± 2.5 | 52.0 ± 2.4* | 41.2 ± 6.4 | 51.4 ± 5.3* | 48.7 ± 2.4* |
| %Other Arm Entries | 31.0 ± 3.0 | 31.0 ± 3.0 | 28.3 ± 2.2 | 37.7 ± 4.2 | 27.8 ± 4.6 | 26.3 ± 2.2 |
| Difference Scores | 15.9 ± 5.9 | 9.8 ± 5.0 | 23.7 ± 3.9 | 3.5 ± 10.2 | 23.6 ± 9.0 | 22.4 ± 4.3 |
| Total Entries | 3.9 ± 0.3a | 5.4 ± 0.3a | 5.3 ± 0.4b | 4.5 ± 0.5a | 4.3 ± 0.4a | 5.6 ± 0.3b |
| Physiological Measures | ||||||
| Serum Estradiol (pg/ml) | 9.5 ± 2.3a | 20.0 ± 1.0b | 53.2 ± 3.0c | 12.0 ± 2.5a | 21.9 ± 1.4b | 58.1 ± 4.0c |
| Body Wt Day 0 (g) | 240.2 ± 4.6a | 240.8 ± 3.9a | 240.0 ± 2.2a | 239.5 ± 2.6a | 237.9 ± 2.8a | 240.2 ± 4.1a |
| Body Wt Day 21 (g) | 329.6 ± 5.8b | 329.0 ± 5.3b | 254.6 ± 4.5d | 287.5 ± 3.4c | 290.3 ± 4.0c | 245.4 ± 3.9a |
Indicates preference for the “Novel” arm over the previously explored “Other” arm within a given column. Within a given row for Total Entries or Serum Estradiol Levels, means with different letters indicate values that are statistically different (P<0.05, i.e., compare “a” vs. “b”), whereas means with similar letters represent values that are statistically similar (i.e., compare “a” vs. “a”). For Body Weight, means with different letters indicate values that are statistically different (P<0.05, i.e., compare “a” vs. “b”) across two rows: Day 0 and Day 21.
Four of the six groups (NX, NE, SC, SE) entered the novel arm significantly more than the other arm during the first minute of testing according to a Wilcoxon matched-pairs test (P<0.05, Table 1). Rats in the NC and SX groups made a statistically similar number of entries in the novel arm compared to the other arm during min 1. Difference scores were calculated by subtracting the percentage of entries made into the novel and other arms (novel – other) during minute 1 to assess group comparisons. A 2 × 3 ANOVA failed to detect any significant differences among groups for difference scores.
Water Maze
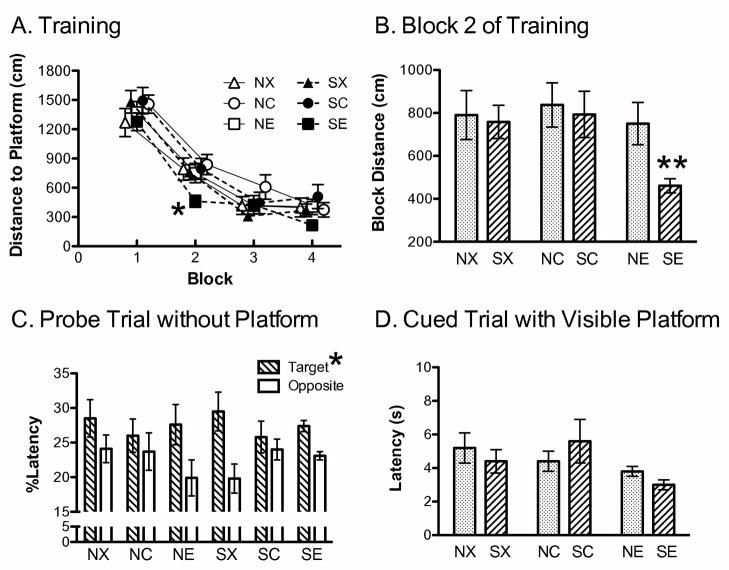
Rats in all groups learned the platform location as displayed by decreased swim distance to the platform as the trials progressed. A 2 × 3 × 4 mixed factor ANOVA was performed with block as a repeated measure, and showed a significant repeated effect of block on distance to locate the platform, F(3,204)=154.1, P<0.0001, and significant effect for hormone, F(2,68)=3.25, P<0.05 (Figure 4A). Post hoc analyses on hormone revealed that across all four blocks of training, 17β-estradiol-treated rats (NE, SE) swam shorter distances to find the platform compared to cholesterol-treated rats (NC, SC), although NE and SE covered statistically similar distances as the blank-treated rats (NX, SX). By the end of the last training block, all groups learned the platform location and covered less distance than at the beginning.
Figure 4.
Effects of Chronic Stress and 17β-Estradiol Implants on the Water Maze. (A) A significant main effect of hormone treatment was found for distance to locate the platform, with SE and NE covering significantly less distance before finding the platform compared to SC and NC. Note: the means were shifted on the x-axis in to facilitate visualization of the data. Each block represents the average of two training trials. *P<0.05 for SE and NE vs. NC, SC. (B) A second analysis was performed on the first two blocks and revealed that SE covered significantly less distance to locate the platform compared to NE and SX. ** P≤0.01 for SE vs. SX or NE. (C) The percentage of time spent searching during the probe trial. Rats spent more time searching the quadrant that previously contained the platform (Target) compared to the opposite quadrant (Opposite, *P=0.01). (D) Latency to find the visible platform. No significant differences were observed across groups and all rats quickly located the visible platform. Non-stress control-blank (NX), non-stress control-cholesterol (NC), non-stress control-estradiol (NE), stress-blank (SX), stress-cholesterol (SC), stress-estradiol (SE).
Previous findings reported group differences in water maze acquisition tended to emerge shortly after the initial training trial (Bimonte-Nelson et al., 2006; Frye, 1995; Rubinow et al., 2004), most likely because rats lack any prior experience on the maze during the first trial. Consequently, we focused the next analysis comparing the initial learning between blocks 1 and 2 of training (Rubinow et al., 2004) for the 17β-estradiol-treated rats compared to the blank-treated rats, as those groups were predicted to reveal the most robust effects (McLaughlin et al., 2008). A 2 × 2 × 2 mixed factor ANOVA for chronic stress (nonstress, stress) and hormone (blank, 17β-estradiol) across blocks 1 and 2 of distance swam during training revealed a significant interaction between chronic stress and hormone, F(1,44)=6.701, P=0.01, and a significant effect of block, F(1,44)=64.95, P<0.0001, with no other significant effects. The 17β-estradiol-treated rats that were chronically stressed, covered less distance than did the blank-implanted stressed rats (P<0.01) and the 17β-estradiol-treated non-stress control rats (P<0.05), but were statistically similar to the blank-implanted non-stress controls (Figure 4B). Moreover, all groups covered less distance to find the platform by block 2, indicating that they were learning the platform location soon after block 1 of training.
Swim speed fluctuated across training with rats swimming faster during the latter trials. A 2 × 3 × 4 mixed factor ANOVA was performed with block as a repeated measure, and revealed a significant effect for block, F(3,204)=4.04, P<0.01. Post hoc tests showed that rats swam slower when searching for the platform in blocks 1 and 2 compared to blocks 3 and 4 (data not shown), indicating that the rats were learning the platform location as trials progressed. No other effects were significant.
During the probe trial, the swim pattern of the rats indicated that they learned the platform location. A 2 × 3 × 2 mixed factor ANOVA was performed on time spent in the quadrants (target, opposite) as a repeated measure, revealed a significant effect for quadrant, F(1,68)=6.637, P=0.01, with no other significant effects. Rats swam more in the target quadrant that previously contained the hidden platform compared to the quadrant that was opposite of the target (Figure 4C). Moreover, a 2 × 3 × 2 mixed factor ANOVA performed on velocity within the two quadrants (target, opposite) showed a similar outcome as latency, with a significant effect for quadrant, F(1,68)=12.675, P<0.001. Rats swam slower in the target quadrant than they did in the opposite quadrant. There were no other significant effects. An ANOVA failed to detect significant effects for swim distance between the target and opposite quadrant. Following the probe trial, a 2 × 3 ANOVA did not detect any group differences in swim latency to a visible cue located within the maze (Figure 4D).
Verification Measures: Chronic Stress and Serum Estradiol Levels
Both chronic stress and 17β-estradiol decreased body weight gain (Table 1). A 2 × 3× 4 mixed-factor ANOVA, with day as a repeated measure, for body weight gain during the 21 days of restraint showed significant effects of chronic stress F(1,74)=42.66, P<0.01), hormone, F(2,74)=85.10, P<0.01), day, F(3,222)=398.24, P<0.01, and a significant three way interaction, F(6,222)=5.12, P<0.01. While all groups showed similar weights at the beginning of the study, both chronic stress and 17β-estradiol reduced body weight gain by day 21, with rats in the SE and NE groups gaining the least amount of weight compared to the other groups.
Both 25% 17β-estradiol and 100% cholesterol-filled silastic implants yielded greater serum estradiol levels than those found in rats given blank implants. A 2 × 3 ANOVA for serum blood levels showed a significant effect of hormone F(2,87)=173.95, P<0.01. Post hoc analyses showed that each implant group provided estradiol levels significantly different from all other groups, with 17β-estradiol implants providing the greatest levels of circulating estradiol (NE, SE), followed by 100% cholesterol (NC, SC), and then blank-filled implants (NX, SX, Table 1).
Experiment 2: Effect of Chronic Stress and Phase of the Estrous Cycle on CA3 Dendritic Morphology
Given the finding in Experiment 1 that cholesterol-filled silastic implants yielded low, physiological 17β-estradiol levels and protected against chronic stress-induced CA3 dendritic retraction, Experiment 2 was conducted to investigate chronic stress effects on CA3 dendritic retraction in gonadally-intact, cycling female rats. To our knowledge, just one study investigated the effects of chronic restraint stress on CA3 dendritic complexity in gonadally-intact females (Galea et al., 1997). While CA3 dendritic retraction takes three weeks to develop following restraint stress (McLaughlin et al., 2007), there is evidence that CA3 dendrites can rapidly remodel within hours under some circumstances (for review, see Conrad, 2006; Magariños et al., 2006; Popov et al., 1992; von der Ohe et al., 2006) or days (Sandi et al., 2003). This opens the possibility that rapid CA3 dendritic remodeling may occur when ovarian hormones are at their highest in proestrus and lowest at estrus. The phase of the estrous cycle was not determined in previous work (Galea et al., 1997), thus, Experiment 2 investigated the effects of chronic restraint stress and the phase of the estrous cycle on CA3 dendritic complexity.
CA3 Dendritic Arborization
Chronic stress and the phase of the estrous cycle did not alter CA3 dendritic complexity (Figure 5). A 2 × 2 ANOVA for stress history (non-stress, stress) and estrous phase (estrus, proestrus) did not reveal any statistically significant effects for CA3 apical dendritic branch points (P≥0.28), apical dendritic branch length (P≥0.3), basal dendritic branch points (P≥0.1) and basal dendritic branch length (P>0.18).
Figure 5.
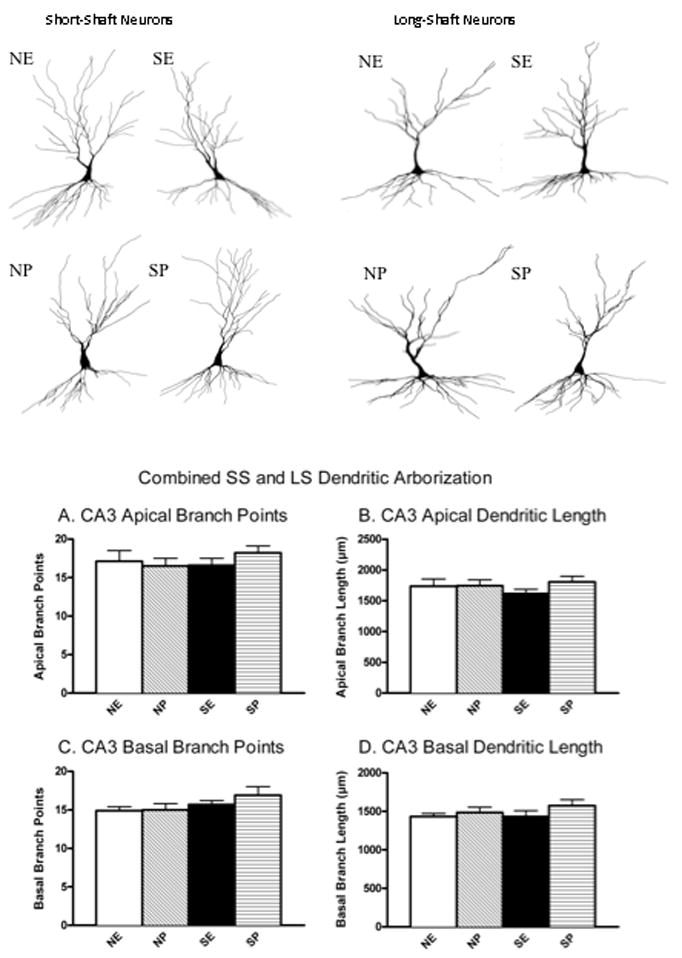
Effects of Chronic Stress and the Phase of the Estrous Cycle on CA3 Dendritic Arborization. Chronic stress, proestrus and estrus did not alter CA3 dendritic properties as measured by apical dendritic branch points (A), apical dendritic length (B), basal branch points (C), basal dendritic length (D). Non-stress control-estrus (NE), Non-stress control-proestrus (NP), stress-estrus (SE), stress-proestrus (SP). Short-shaft (SS) and long-shafted (LS) neurons were averaged for each subtype separately and then these two values were averaged to obtain one measure, resulting in a total of 4 to 6 neurons per rat. The number of rats per group ranged from 5 to 7.
CA1 Dendritic Arborization
Chronic stress and estrous cycle stage did not influence CA1 apical or basal dendritic arborization. A 2 × 2 ANOVA for stress history (non-stress, stress) and estrous phase (estrus, proestrus) failed to detect any statistically significant effect for CA1 apical branch points (P≥0.15), apical branch length (P≥0.10), basal branch points (P≥0.40) or basal branch length (P≥0.33, data not shown).
CA1 Dendritic Spines
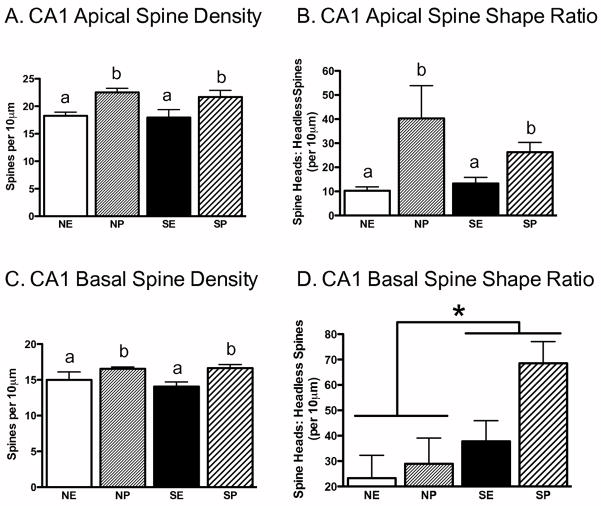
Rats in proestrus demonstrated increased apical and basal CA1 spine density compared to rats in estrus; these effects were unaltered by stress history. 2 × 2 ANOVAs revealed a significant main effect of estrous cycle on CA1 apical spine density, F(1,20)=12.53, P<0.01, and CA1 basal spine density, F(1,20)=10.19, P<0.01, without significant interactions for the apical (Figure 6A) and basal regions (Figure 6C). In addition, for apical dendrites, a 2 × 2 ANOVA performed on the ratio of spines to headless spines showed a significant main effect of estrous cycle, F(1,20)=8.45, P<0.01. Proestrus (CP, SP) elevated apical spine ratios compared to estrus (CE, SE, Figure 6B), and these effects were unaltered by stress exposure. In contrast, analyses of CA1 basal spine shape ratio (heads: headless spines) showed a significant main effect of stress, F(1,20)=4.10, P=0.05. Chronic stress (SE, SP) enhanced the ratio of basal spine heads to headless spines (CE, CP; Figure 6D). No other effects were significant.
Figure 6.
Effects of Chronic Stress and the Phase of the Estrous Cycle on CA1 Spine Density and Shape Ratio. Proestrus (NP, SP) increased CA1 apical spine density (A), CA1 apical spine head to headless spine ratio (B) and CA1 basal spine density (C), and these effects were not influenced by chronic stress history. (D) In contrast, stress history (SE, SP) increased the ratio of heads to headless spines. Non-stress control-estrus (NE), Non-stress control-proestrus (NP), stress-estrus (SE), stress-proestrus (SP). Means with different letters indicate values that are statistically different (P<0.05, i.e., compare “a” vs. “b”), whereas means with similar letters represent values that are statistically similar (i.e., compare “a” vs. “a”). *P<0.05.
Verification Measures: Chronic stress and Stage of Estrous
Chronically stressed rats (SE, SP) gained weight more slowly than did non-stress control rats (CE, CP) throughout restraint: non-stress control day 1 = 264.4 ± 2.2 g, non-stress control last day = 275.9 ± 2.8 g, stress day 1 = 266.7 ± 3.3 g, stress last day = 264.2 ± 3.9 g. This observation was supported by a significant interaction for stress across days (1, 7, 14, 21–24) on body weight, F(3,72)=19.85, P<0.001, followed by post hoc tests.
Chronic stress increased adrenal weights: stress (SE, SP) = 73.5 ± 2.4 mg, non-stress control (CE, CP) = 64.4 ± 2.1 mg. A 2 × 2 ANOVA confirmed these observations with a significant main effect of stress on adrenal weight, F(1,23)=5.63, P<0.05.
Proestrus increased uterine horn weights compared to estrus, as demonstrated by a 2 × 2 ANOVA showing a significant main effect of estrous cycle on uterine weight, F(1,23)= .20, P<0.05: Proestrus (CP, SP) = 1.015 ± 0.121 g, Estrus (CE, SE) = 0.704 ± 0.060 g.
DISCUSSION
These findings are the first to show that 17β-estradiol prevents chronic stress-induced CA3 hippocampal dendritic retraction in OVX female rats. Moreover, we extend these findings to show that cholesterol implants also provide neuroprotection from chronic stress-induced CA3 dendritic remodeling. The 17β-estradiol and cholesterol silastic implants supplied dose-dependent physiological levels of serum 17β-estradiol that corresponded to levels found within rats at proestrus (approximately 40 – 100 pg/ml, Dayas et al., 2000; Holmes et al., 2002; Prediger et al., 2004) and diestrus (approximately 15–30 pg/ml, Foster et al., 2003; Holmes et al., 2002; Lerner et al., 1990; Prediger et al., 2004) and were significantly greater than serum 17β-estradiol levels associated with OVX females (approximately 0–15 pg/ml, Dayas et al., 2000; Foster et al., 2003; Prediger et al., 2004). Why cholesterol-filled implants reliably yielded low circulating levels of estradiol in our study is unclear, especially given that others have used cholesterol-filled implants as a control condition for hormone replacement (Bowman et al., 2002; Daniel et al., 1997; Garza-Meilandt et al., 2006). In some cases, serum estradiol levels were not measured (Bowman et al., 2002; Garza-Meilandt et al., 2006), as body weight gain produced a reliable measure of 17β-estradiol treatment effectiveness (Hamosh and Hamosh, 1975; Ramirez, 1981). In another case, serum estradiol levels were near the low range, but the 17β-estradiol implant increased CA1 spine density compared to the cholesterol implant (Daniel et al., 1997). However, we observed a similar outcome with 17β-estradiol increasing CA1 spine density compared to cholesterol. Furthermore, cholesterol enhanced CA1 spines density compared to the blank groups. Consequently, the effects produced by cholesterol would not have been detected unless a blank implant was used. Our study may be the first to observe this novel outcome because we incorporated all three conditions in our paradigm.
Given the morphological findings from Experiment 1, Experiment 2 was performed on gonadally intact, cycling female rats and brains were taken at the end of the chronic restraint period that corresponded to endogenously high (proestrus) and low (estrus) levels of ovarian hormones. Chronic stress did not alter CA3 dendritic complexity in gonadally intact, cycling females, which complements the findings from Experiment 1 and supports the hypothesis that estrogen prevents chronic stress-induced CA3 dendritic retraction. Moreover, Experiment 1 included behavioral data using two spatial mazes to determine whether spatial ability corresponded to changes in the hippocampus. The results from the Y-maze may have been influenced by motor confounds; however, all groups successfully learned the water maze task, with the combination of chronic stress and 17β-estradiol facilitating acquisition during block 2 of training. This outcome was unlikely attributed to improved motor function and/or motivation, because the former was not sensitive to distance measures and the latter was not detected by the cued trials. By the end of training, all rats learned the task, as revealed by the significant effect of latency and swim speed in the probe trial, showing that rats favored the quadrant that previously contained the platform compared to the quadrant located on the opposite side of the maze. Consequently, rats navigated in the water maze using spatial strategies. Importantly, the group exposed to both chronic stress and 17β-estradiol showed amplified CA1 spine density simultaneously with increased proportion of spines with heads, and also displayed functional benefits during spatial learning. This finding supports our hypothesis that chronic stress and 17β-estradiol have interacting effects on hippocampal-dependent behavior. Together, these data demonstrate that 17β-estradiol and cholesterol may offer neuroprotection against chronic stress-induced CA3 dendritic retraction, and that spatial learning may occur despite the presence of CA3 dendritic retraction, perhaps through mechanisms involving CA1 spine density and shape.
Neuroprotection by 17β-Estradiol and Cholesterol on Stress-Induced CA3 Dendritic Retraction
An important and novel finding was that 17β-estradiol and cholesterol prevented chronic stress from producing CA3 dendritic retraction in OVX female rats. The neuroprotection arising from 17β-estradiol is consistent with numerous findings that estrogens benefit brain and hippocampal health (for reviews, see Garcia-Segura et al., 2001; Manthey and Behl, 2006; McCullough and Hurn, 2003; Roof and Hall, 2000). A surprising finding was that cholesterol-filled silastic implants protected against stress-induced CA3 dendritic retraction. While the neuroprotective effects of cholesterol are currently unknown, cholesterol is a key regulator of neuronal health affecting cell structure and function (Pfrieger, 2003; Suzuki et al., 2007; Suzuki et al., 2004) and cholesterol dysregulation is associated with memory impairments (Kadish et al., 2009). Additionally, cholesterol can promote synaptogenesis (Fester et al., 2009; Goritz et al., 2005; Pfrieger, 2003). Therefore, cholesterol may exert similar actions as 17β-estradiol on hippocampal morphology.
Another interpretation of cholesterol’s actions may be that cholesterol indirectly affects hippocampal neurons because it is a precursor to all steroid hormones, including 17β-estradiol, which can be aromatized in many tissues, such as the hippocampus (Holloway and Clayton, 2001; Kawato, 2004; Prange-Kiel and Rune, 2006; Rune and Frotscher, 2005; Rune et al., 2006; Wehrenberg et al., 2001). In particular, steroidogenic factors needed to convert cholesterol to estrogen are highly expressed within the CA1, CA3 and dentate regions (Hojo et al., 2004; Hojo et al., 2008; Mukai et al., 2006a; Mukai et al., 2006b; Wehrenberg et al., 2001). The conversion of cholesterol into 17β-estradiol supports emerging evidence that the hippocampus can produce estrogen from cholesterol de novo. Alternatively, cholesterol could be converted into progesterone or testosterone, either of which can exert neuroprotective actions or alter CA1 spines (Fargo and Sengelaub, 2004; Fester et al., 2009; Leranth et al., 2004; Wright et al., 2007). Another explanation may be that cholesterol exerts it actions in the periphery, which would be consistent with the finding that low levels of estradiol were found in the serum of rats implanted with cholesterol, and were similar to serum levels of gonadally-intact female rats in diestrus (Holmes et al., 2002; Lerner et al., 1990; Prediger et al., 2004). Similarly, brain and hippocampal levels of glucocorticoids do not always parallel the changes in plasma, and this supports brain-specific mechanisms for retaining and/or synthesizing steroids (Croft et al., 2008). While the exact action of cholesterol remains unknown, cholesterol clearly has the potential to modulate hippocampal morphology through several mechanisms.
The finding that chronic stress caused CA3 dendritic retraction in OVX females given blank implants supports an earlier study also using OVX females (McLaughlin et al., 2005); however the failure to find CA3 dendritic retraction in steroid-replaced females may contradict a previous report that gonadally-intact females express CA3 dendritic retraction in the basal CA3 dendritic region (Galea et al., 1997). Consequently, we designed Experiment 2 using gonadally intact female rats and sacrificed them at the end of chronic restraint when the rats were in proestrus or estrus, representing the peak and dip of ovarian hormone secretion, respectively. Experiment 2 failed to find CA3 dendritic retraction following chronic stress, despite confirmation that proestrus increased uterine horn weight and CA1 dendritic spine density. Determining estrous cycle stage was important because evidence of rapid CA3 dendritic remodeling can occur within hours (Conrad, 2006; Magariños et al., 2006; Popov et al., 1992; von der Ohe et al., 2006) or days (Sandi et al., 2003). Consequently, controlling for the stage of estrus at the time of brain removal was important in the event that such rapid remodeling occurs in female rats. Another point to consider was that the prior study found CA3 dendritic retraction in the basal branch points without an effect in the basal length (Galea et al., 1997). While the outcome was interpreted to reflect stress-induced CA3 dendritic retraction, a conservative interpretation may be warranted since branch points and length were inconsistent. Taken together, these new data support the interpretation raised previously, that females are protected against stress-induced CA3 dendritic retraction because of ovarian hormones, such as 17β-estradiol (McLaughlin et al., 2005).
Stress and Hormone-Mediated Morphological Effects in the Hippocampus
These findings show that chronic stress, 17β-estradiol, and cholesterol influence hippocampal CA3 and CA1 dendritic morphology. Chronic stress has robust effects on CA3 apical dendritic retraction because it is revealed in both SS and LS neuronal types and both measures of branch points and dendritic lengths. We previously found CA3 LS neurons to be more sensitive to dendritic retraction than CA3 SS neurons (McLaughlin et al., 2005) and other studies reveal differences in neuronal type sensitivity to environmental manipulations (Bartesaghi and Severi, 2002). In the current study, CA3 dendritic retraction was found in the LS basal branch points, but was not significant for LS basal length. We previously speculated that incorporating more LS than SS neurons could have provided some of the findings by others (Galea et al., 1997), although the proportion of neuronal type was not reported in earlier studies. The current study also found that chronic stress and 17β-estradiol increased CA3 apical dendritic length of SS neurons; however, this finding was not evident in the other measure using branch points, or in Experiment 2. While it may be possible that the prolonged and continual elevation of 17β-estradiol is needed to enhance CA3 dendritic arborization, we are reluctant to speculate further about this outcome without additional support. Importantly, a consistent finding is that chronic stress increased the proportion of spines with heads in the basal CA1 region in all hormone conditions. We observed this outcome previously (McLaughlin et al., 2005) and another study found chronic stress increased the CA1 post-synaptic density surface area/spine surface area ratio (Donohue et al., 2006). Increasing the proportion of spines with heads may allow stronger synaptic contacts that could facilitate hippocampal function (Diamond et al., 2006; Kasai et al., 2003; Li et al., 2004; McLaughlin et al., 2005; McLaughlin et al., 2008). Similar arguments have been made about 17β-estradiol-mediated increases in CA1 spine density and hippocampal function (Garza-Meilandt et al., 2006; Luine et al., 2006; McLaughlin et al., 2008), and so chronically stressed female rats in proestrus may benefit from both increased proportion of CA1 spine heads and density. The current study also found increased CA1 dendritic complexity in both branch points and length with 17β-estradiol treatment, but this outcome was not found in gonadally-cycling female rats in proestrus. Whether this morphological change requires prolonged exposure to high and tonic levels of 17β-estradiol will need to be studied further, but clearly this enhanced CA1 dendritic arborization is not a characteristic in naturally cycling female rats. Consequently, both chronic stress and 17β-estradiol influence CA3 and CA1 dendritic morphology.
Possible Correspondence Between CA1 Spine Density and Shape with Spatial Acquisition
We previously speculated that CA1 spine shape contributes to optimal female spatial navigation and the data from the current study support a modified form of this hypothesis. In a previous study, we observed that chronically stressed, OVX female rats expressed robust CA3 dendritic retraction, but performed well on a spatial memory Y-maze task (McLaughlin et al., 2005). In that study, we reported that both non-stress control and chronically stressed rats showed functional spatial memory, but the chronically stressed rats performance corresponded to an increase in the shape of CA1 basal spine heads (McLaughlin et al., 2005), a finding also reported in males with synthetic glucocorticoid treatment (Komatsuzaki et al., 2005). Consequently, it is possible for rats with CA3 dendritic retraction to exhibit similar spatial learning as rats without CA3 dendritic retraction, a finding that is consistent with rats being able to learn in spatial tasks despite dentate gyrus granule cell loss (Conrad and Roy, 1993; Conrad and Roy, 1995). We propose that changes in CA1 spine shape or heads may provide alternative mechanisms for learning in chronically stressed and nonstressed control animals.
Changes in hippocampal CA1 spine shape and/or density may impart functional benefits, with some qualifications. The current study found that the SE group showed statistically significant rapid spatial learning and was the only condition to experience alterations in both CA1 spine density and shape. If increases in either CA1 spine density or shape by 17β-estradiol or chronic stress, respectively, was a key mechanism to facilitate spatial learning, then improved spatial learning should have been observed with the 17β-estradiol-treated nonstressed controls (NE) and the chronically stressed groups with blank (SX) or cholesterol implants (SC). However, none of these groups exhibited facilitated spatial learning as was found for the chronically stressed rats implanted with 17β-estradiol (SE). One difference between the current study and our previous report (McLaughlin et al., 2005) is that the present data revealed effects in spatial learning on the water maze, whereas our previous paradigm found effects with spatial recognition memory, an outcome that was confounded by motor issues when using the Y-maze in the current study. Another difference was that we altered both CA1 spine density and shape in the current study, whereas we influenced just CA1 spine heads previously (McLaughlin et al., 2005). This latter issue may be relevant because testing conditions could mask the effects of chronic stress or 17β-estradiol on spatial learning and memory (Bohacek and Daniel, 2007; Wright and Conrad, 2008), especially as the aversive nature of the testing paradigm is elevated (Conrad, 2005; Sandi and Pinelo-Nava, 2007). Given that the current study capitalized on motivation to escape an aversive experience, then detecting differences among groups may have been difficult or masked unless rats expressed changes in both CA1 spine density and morphology. Significantly, the chronic stress + 17β-estradiol-induced facilitated learning corroborates findings using the radial arm maze (Bowman et al., 2002; Bowman et al., 2001) and a report that optimal spatial learning occurs in rats exhibiting enhanced CA1 spinogenesis (Diamond et al., 2006). Therefore, we speculate that stress history in corroboration with increased CA1 spine properties, such as enhanced proportion of spines with heads and increased CA1 spine density, provide functional benefits on spatial learning, and perhaps other hippocampal functions.
Estrogens are well known to influence spatial learning and memory. In general, increases in circulating levels of estradiol facilitate the use of hippocampal-dependent spatial strategies (Davis et al., 2005; Korol, 2004; Korol and Kolo, 2002; Zurkovsky et al., 2007; Zurkovsky et al., 2006) and alters how cues are used (Daniel and Lee, 2004). Tonic systemic estradiol treatment tends to facilitate spatial ability (Garza-Meilandt et al., 2006; Iivonen et al., 2006); however, not when administered orally. We were unable to detect an effect of a 17β-estradiol on the performance of non-stress controls despite using silastic implants, a delivery route and type of estrogen used by others. In the Y-maze, confounds may have arisen from locomotor issues because 17β-estradiol-treated rats (control and stress) entered more arms than did rats in the other conditions. While the Y-maze is highly useful for measuring spatial memory in male rats (Bellani et al., 2006; Conrad et al., 1996; Kleen et al., 2006; Wright and Conrad, 2005; Wright et al., 2006), it is less so in female rats because of potential confounds from motor ability (Conrad et al., 2003; McLaughlin et al., 2008) and handling (Bohacek and Daniel, 2007; Frick et al., 2004). For the water maze, 17β-estradiol-treated non-stressed rats performed similarly to the blank-treated non-stressed rats. We have recently observed that water temperature could mask chronic stress effects on spatial memory (Wright and Conrad, 2008) and another study found that water temperature influences female navigation (Rubinow et al., 2004). Moreover, aversive components of the task may influence navigation strategy (Conrad, 2005; Kim et al., 2001), with the water maze eliciting greater stress responses than other mazes (Harrison et al., 2009). Therefore, the type of task and aversive component could have influenced female navigation to make it difficult to detect differences in spatial ability.
Significance
These findings show that chronic stress influences female hippocampal morphology in substantially different ways than previously reported for males. Chronic stress produces robust CA3 dendritic retraction in males (Conrad et al., 1999; Kleen et al., 2006; Magariños and McEwen, 1995a; Magariños et al., 1996), but the current study shows that females may be protected against CA3 dendritic retraction because of circulating estradiol levels. While the consequences of CA3 dendritic retraction on female spatial ability were not readily apparent from the Y-maze and water maze, testing of females in other types of tasks, such as object placement (McLaughlin et al., 2008), place and response learning tasks (Korol, 2004; Park et al., 2008), and contextual conditioning (Baran et al., 2009) may be useful. Moreover, CA3 dendritic retraction may have other significant consequences on hippocampal connectivity and health (McLaughln et al., In Press). In particular, conditions producing CA3 dendritic retraction may make the hippocampus vulnerable to metabolic or neurotoxic challenges (for review, Conrad, 2008). Specifically, challenging the hippocampus with ibotenic acid under conditions when dendritic retraction is present causes more cell loss compared to controls (Conrad et al., 2007). Moreover, chronically stressed, gonadally-intact males are more susceptible to exacerbated hippocampal damage than are chronically stressed, gonadally-intact females (Conrad et al., 2004b). The current findings shed light on these studies because chronically stressed females may be able to withstand a metabolic challenge, in part, because they fail to express CA3 dendritic retraction under conditions that produce CA3 dendritic retraction in males. Taken together, these data show that females are resistant to the detrimental effects of chronic stress on CA3 dendritic arborization, in part through estrogen neuroprotection, and that normal spatial acquisition can occur even when CA3 dendritic retraction is present, perhaps through other neuroplastic changes that may involve CA1 spines.
Acknowledgments
The authors acknowledge Sarah Baran, Rudy Bellani, and Jonathan Kleen for their contributions to this research. The authors also thank Pierre Deviche for assisting with the silastic implant procedure, Clark Presson and David Diamond for reviewing the honor’s thesis, and the Barrett Honors College at Arizona State University. This work was part of a doctoral dissertation (KJM, 2007) and honor’s thesis for Wilson (2007) and Wieczorek (2004).
Support: This work was funded by NIH MH64727 (Conrad), Institute for Mental Health Research (Conrad), Arizona Biomedical Research Commission (Conrad), NSF IBN-0081061 (Korol), funds from ASU School of Life Sciences and the Howard Hughes Medical Institute through the Undergraduate Science Education Program (Harman, Wilson, Wieczorek), ASU Minority Access to Research Careers Program (Gomez) and the ASU Barrett Honors College (Wilson, Wieczorek).
References
- Armario A, Marti J, Gil M. The serum glucose response to acute stress is sensitive to the intensity of the stressor and to habituation. Psychoneuroendocrinology. 1990;15(5–6):341–347. doi: 10.1016/0306-4530(90)90059-i. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. The Neurobiology of Learning and Memory. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi R, Severi S. Effects of early environment on field CA3a pyramidal neuron morphology in the guinea-pig. Neuroscience. 2002;110(3):475–488. doi: 10.1016/s0306-4522(01)00469-9. [DOI] [PubMed] [Google Scholar]
- Bellani R, Luecken L, Conrad CD. Peripubertal anxiety profile can predict spatial memory impairments following chronic stress. Behavioural Brain Research. 2006;166(2):263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a hippocampal-dependent task. Behavioral Neuroscience. 1997;111(2):267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. European Journal of Neuroscience. 2006;24(1):229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Bowman R, Luine V. Functional aspects of estrogen neuroprotection. Endocrine. 2003;21(1):33–41. doi: 10.1385/endo:21:1:33. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Hormones & Behavior. 2007;52(2):237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Hormones and Behavior. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity in Biology, Toxicology and Medicine. 2005;3(1):57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral and Cognitive Neuroscience Reviews. 2006;5(1):41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Reviews in the Neurosciences. 2008;19(6):395–412. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze, and this effect is blocked by tianeptine pretreatment. Behavioral Neuroscience. 1996;110(6):1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. The Neurobiology of Learning and Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute restraint stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacology Biochemistry and Behavior. 2004a;78(3):569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise L. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004b;125(3):759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4(4):305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. The Journal of Neuroscience. 2007;27(31):8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Roy EJ. Selective loss of hippocampal granule cells following adrenalectomy: Implications for spatial memory. The Journal of Neuroscience. 1993;13(6):2582–2590. doi: 10.1523/JNEUROSCI.13-06-02582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Roy EJ. Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus. 1995;5:1–15. doi: 10.1002/hipo.450050103. [DOI] [PubMed] [Google Scholar]
- Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Research. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiology of Learning and Memory. 2004;82(2):142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Sulzer JK, Hulst JL. Estrogen increases the sensitivity of ovariectomized rats to the disruptive effects produced by antagonism of D2 but not D1 dopamine receptors during performance of a response learning task. Hormones & Behavior. 2006;49(1):38–44. doi: 10.1016/j.yhbeh.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84(2):132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. Journal of Neuroendocrinology. 2000;12(8):784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis R. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PLA, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: A stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140(2):597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Eyre MD, Richter-Levin G, Avital A, Stewart MG. Morphological changes in hippocampal dentate gyrus synapses following spatial learning in rats are transient. The European Journal of Neuroscience. 2003;17(9):1973–1980. doi: 10.1046/j.1460-9568.2003.02624.x. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. Journal of Neurobiology. 2004;60(3):348–359. doi: 10.1002/neu.20027. [DOI] [PubMed] [Google Scholar]
- Fester L, Zhou L, Butow A, Huber C, von Lossow R, Prange-Kiel J, Jarry H, Rune GM. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus. 2009 doi: 10.1002/hipo.20548. In Press. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Research. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24(6):839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19(11):3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology and Behavior. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behavioral Neuroscience. 2006;120(4):905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiology & Behavior. 1994;56(2):281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. Journal of Comparative Physiological Psychology. 1976;90:18–25. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behavioural Brain Research. 2004;155(1):77–84. doi: 10.1016/j.bbr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Molecular and Cellular Neuroscience. 2005;29(2):190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of Neuroscience. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh M, Hamosh P. The effect of estrogen on the lipoprotein lipase activity of rat adipose tissue. Journal of Clinical Investigation. 1975;55(5):1132–1135. doi: 10.1172/JCI108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioral Brain Research. 2009;198(1):247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(3):865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Molecular and Cellular Endocrinology. 2008;290(1–2):31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nature Neuroscience. 2001;4(2):170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puoliväli J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137:1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. The Journal of Neuroscience. 2009;29(6):1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends in the Neurosciences. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kawato S. Endocrine disrupters as disrupters of brain function: a neurosteroid viewpoint. Environmental Sciences. 2004;11(1):1–14. [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han J-S, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. The Journal of Neuroscience. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behavioral Neuroscience. 2006;120(4):842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki Y, Murakami G, Tsurugizawa T, Mukai H, Tanabe N, Mitsuhashi K, Kawata M, Kimoto T, Ooishi Y, Kawato S. Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochemical & Biophysical Research Communications. 2005;335(4):1002–1007. doi: 10.1016/j.bbrc.2005.07.173. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning and Memory. 2004;82(3):309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behavioural Brain Research. 2005;163(1):78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. The Journal of Neuroscience. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner SP, Meredith S, Thayne WV, Butcher RL. Age-related alterations in follicular development and hormonal profiles in rats with 4-day estrous cycles. Biology of Reproduction. 1990;42(4):633–638. doi: 10.1095/biolreprod42.4.633. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magariños AM, Allen PB, Greengard P, Luine V, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Memoir of University of California. 1922;6:1–148. [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Research. 2006;1126(1):183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology & Behavior. 1996;59(1):27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Research. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Luo L, Tan RX. Fluoxetine inhibits dendrite atrophy of hippocampal neurons by decreasing nitric oxide synthase expression in rat depression model. Acta Pharmacologica Sinica. 2001;22(10):865–870. [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NPV. Longitudinal study of basal cortisol levels in healthy elderly subjects: evidence for subgroups. Neurobiology of Aging. 1996;17(1):95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Ma WP, Cao J, Tian M, Cui MH, Han HL, Yang YX, Xu L. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neuroscience Research. 2007;59(2):224–230. doi: 10.1016/j.neures.2007.06.1474. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European Journal of Pharmacology. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995a;69(1):83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. The Journal of Neuroscience. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(49):18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey D, Behl C. From structural biochemistry to expression profiling: Neuroprotective activities of estrogen. Neuroscience. 2006;138:845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: An integrated view. Trends in Endocrinology and Metabolism. 2003;14(5):228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Annals New York Academy of Sciences. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magariños AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. European Neuropsychopharmacology. 1997;7:S323–S328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135(4):1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson HA, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Hormones & Behavior. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughln KJ, Baran SE, Conrad CD. Sex-specific neuromorphological changes in limbic structures and their functional outcomes. Molecular Neurobiology. doi: 10.1007/s12035-009-8079-7. In Press. (accepted pending revisions) [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser M-B, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. The Journal of Neuroscience. 1998;18(18):7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii H-t, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006a;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology. 2006b;84(4):255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Ohl F, Fuchs E. Differential effects of chronic stress on memory processes in the tree shrew. Cognitive Brain Research. 1999;7:379–387. doi: 10.1016/s0926-6410(98)00042-1. [DOI] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in rats. Biological Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learning & Memory. 2008;15(4):271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Role of cholesterol in synapse formation and function. Biochimica et Biophysica Acta - Biomembranes. 2003;1610(2):271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48(1):45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on the rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Prediger ME, Gamaro GD, Crema LM, Fontella FU, Dalmaz C. Estradiol protects against oxidative stress induced by chronic variate stress. Neurochemical Research. 2004;29(10):1923–1930. doi: 10.1023/b:nere.0000042219.98446.e7. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Estradiol-induced changes in lipoprotein lipase, eating, and body weight in rats. American Journal of Physiology. 1981;240(5):E533–E538. doi: 10.1152/ajpendo.1981.240.5.E533. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. Journal of Neurotrauma. 2000;17(5):367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behavioral Neuroscience. 2004;118(4):863–868. doi: 10.1037/0735-7044.118.4.863. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: Role in synaptic plasticity. Neuroscience. 2005;136(3):833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Rune GM, Lohse C, Prange-Kiel J, Fester L, Frotscher M. Synaptic plasticity in the hippocampus: effects of estrogen from the gonads or hippocampus? Neurochemical Research. 2006;31(2):145–155. doi: 10.1007/s11064-005-9004-8. [DOI] [PubMed] [Google Scholar]
- Saenz C, Dominguez R, de Lacalle S. Estrogen contributes to structural recovery after a lesion. Neuroscience Letters. 2006;392(3):198–201. doi: 10.1016/j.neulet.2005.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiduddin S, Zassenhaus HP. Estradiol-17beta receptors in the immature rat ovary. Steroids. 1977;29(2):197–213. doi: 10.1016/0039-128x(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Sandi C, Davies HA, Cordero MI, Rodriquez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. European Journal of Neuroscience. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007;2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Biochemistry and Behavior. 2006;83(2):186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biological Psychiatry. 2009;65(11):918–926. doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Sunanda, Shankaranarayana Rao BS, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Current Science. 2000;79:14581–1584. [Google Scholar]
- Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. The Journal of Neuroscience. 2007;27(24):6417–6427. doi: 10.1523/JNEUROSCI.0690-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. Journal of Cell Biology. 2004;167(6):1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. American Journal of Physiology - Endocrinology & Metabolism. 2006;291(5):E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. The Journal of Neuroscience. 2006;26(41):10590–10598. doi: 10.1523/JNEUROSCI.2874-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Walesiuk A, Trofimiuk E, Braszko JJ. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacological Reports. 2005;57(2):176–187. [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Research. 2006;1126(1):176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. European Journal of Pharmacology. 1992a;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992b;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. Journal of Neurochemistry. 2001;76(6):1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. The Journal of Neuroscience. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic output: Correlation with dendritic spine density. Journal of Neuroscience. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Annals of Emergency Medicine. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8(2):151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behavioural Brain Research. 2008;187(1):41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. European Journal of Neuroscience. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144(1):26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiology of Learning and Memory. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]