Abstract
The selective cyclooxygenase (COX)-2 inhibitor, celecoxib, and the vitamin E isoform, γ-tocotrienol, both display potent anticancer activity. However, high dose clinical use of selective COX-2 inhibitors has been limited by gastrointestinal and cardiovascular toxicity, whereas limited absorption and transport of γ-tocotrienol by the body has made it difficult to obtain and sustain therapeutic levels in the blood and target tissues. Studies were conducted to characterize the synergistic anticancer antiproliferative effects of combined low dose celecoxib and γ-tocotrienol treatment on mammary tumor cells in culture. The highly malignant mouse +SA mammary epithelial cells were maintained in culture on serum-free defined control or treatment media. Treatment effects on COX-1, COX-2, Akt, NFκB and prostaglandin E2 (PGE2) synthesis was assessed following a 3- or 4-day culture period. Treatment with 3–4 μM γ-tocotrienol or 7.5–10 μM celecoxib alone significantly inhibited +SA cell growth in a dose-responsive manner. However, combined treatment with subeffective doses of γ-tocotrienol (0.25 μM) and celecoxib (2.5 μM) resulted in a synergistic antiproliferative effect, as determined by isobologram analysis, and this growth inhibitor effect was associated with a reduction in PGE2 synthesis, and decrease in COX-2, phospho-Akt (active), and phospho-NFκB (active) levels. These results demonstrate that the synergistic anticancer effects of combined celecoxib and γ-tocotrienol therapy are mediated by COX-2 dependent and independent mechanisms. These findings also suggest that combination therapy with these agents may provide enhanced therapeutic response in breast cancer patients, while avoiding the toxicity associated with high-dose COX-2 inhibitor monotherapy.
Keywords: γ-tocotrienol, celecoxib, breast cancer
1. Introduction
Over the past thirty years there has been increasing interest in the role of the cyclooxygenase (COX) family of prostaglandin synthases in the growth and progression of various types of cancer [1, 2]. Cyclooxygenase-2 (COX-2) is an inducible form of COX that catalyzes the conversion of arachidonic acid to prostaglandins and plays a major role in the inflammatory response [3, 4]. It is now clearly established that overexpression of COX-2 plays a major role in nearly all stages of tumor development, growth and progression [4, 5]. As such, a great deal of effort has been focused on the development of agents that target and inhibit COX-2 activity for use in cancer chemotherapy. Unfortunately, use of high therapeutic doses of selective COX-2 inhibitors is associated with a variety of gastrointestinal and cardiovascular toxicities and these adverse effects have greatly limited their clinical use in cancer chemoprevention and treatment [6, 7].
Vitamin E is a general term representing a family of compounds that is further divided into two subgroups called tocopherols and tocotrienols. Tocopherols are commonly found in high concentrations in a wide variety of foods, whereas tocotrienols are relatively rare and found in appreciable levels only in a few specific vegetable fats, such as palm oil [8]. Although chemically very similar, tocopherols have a saturated, whereas tocotrienols have an unsaturated phytyl chain attached to a chromane ring structure. Previous investigations have clearly established that tocotrienols, but not tocopherols, display potent antiproliferative and apoptotic activity against neoplastic mammary epithelial cells at treatment doses that have little or no effect on normal cell growth and function [9, 10]. However, studies have also established that it is very difficult to obtain and/or sustain therapeutic levels of γ-tocotrienol in the blood and target tissues by simple oral administration because absorption and transport mechanisms within the body display significant preference for α-tocopherol [11].
The intracellular mechanism mediating the anticancer effects of γ-tocotrienol have been shown to be associated with the attenuation of EGF receptor dependent mitogenic signaling, particularly the phosphatidylinositol 3-kinase (PI3K)/PI3K-dependent kinase (PDK)/Akt, and NFκB signaling pathways [11]. PI3K is a lipid signaling kinase that subsequently activates PDK-1, which then phosphorylates and activates Akt. Activated Akt phosphorylates various target proteins associated with cell proliferation and survival [12]. NFκB signaling is a complex multi-step process, and activation of NFκB can be initiated by several different kinases, such as Akt and NFκB-inducing kinase. Activated NFκB translocates to the nucleus, binds to DNA, and initiates gene transcription associated with enhanced cell proliferation and survival [13].
Recent studies have shown that combined treatment of γ-tocotrienol with other anticancer agents, such as statins, resulted in synergistic antiproliferative effects [14]. Therefore, it was hypothesized that combined treatment with low doses of γ-tocotrienol and a COX-2 inhibitor might also result in an enhanced therapeutic response. Studies were conducted to characterize the effects of combined low dose treatment of celecoxib, a selective COX-2 inhibitor, with low doses of γ-tocotrienol on the growth of the highly malignant +SA mouse mammary epithelial cells in vitro. Additional studies were conducted to determine the intracellular signaling mechanisms involved in mediating the inhibitory effects of combined low dose γ-tocotrienol and celecoxib treatment on EGF-dependent mitogenesis in these cells.
2. Materials and Methods
2.1. Reagents and chemicals
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated. Isolated γ-tocotrienol (>98% pure) was provided by Carotech Bhd. (Ipoh, Malaysia). Celecoxib was a gift from Pfizer Inc. (New York). Antibodies for COX-2, Akt, phospho-Akt (Ser473), phospho-NFκB were purchased from Cell Signaling Technology (Beverly, MA). COX-1 primary antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Goat anti-rabbit secondary antibody was purchased from PerkinElmer Biosciences (Boston, MA). Mouse anti-actin and peroxidase goat anti-mouse antibody were purchased from Calbiochem (San Diego, CA). The PGE2 EIA-Monoclonal assay kit was purchased from Cayman Chemical Co. (Ann Arbor, MI).
2.2. Cell line and culture conditions
Experiments conducted in the present study represent a logical continuation of previous studies that have extensively characterized the antiproliferative and apoptotic effects of γ-tocotrienol in the highly malignant +SA mammary epithelial cell line [15]. The highly malignant +SA mammary epithelial cell line was derived from an adenocarcinoma that developed spontaneously in a BALB/c female mouse [16]. The +SA cell line is characterized as being highly malignant, estrogen-independent, and displays anchorage-independent growth when cultured in soft agarose gels. When +SA cells are injected back into the mammary gland fat pad of syngeneic female mice, they form rapidly growing anaplastic adenocarcinomas that are highly invasive and metastasize to the lung [16]. Cell culture and the experimental procedures used in this present study have been previously described in detail [9]. Briefly, cells were grown and maintained in serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 control media containing 5 mg/ml bovine serum albumin (BSA), 10 μg/ml transferrin, 100 μg/ml soybean trypsin inhibitor, and 100 U/ml penicillin and 100 μg/ml streptomycin, 10 μg/ml insulin, and 10 ng/ml EGF as a mitogen. Cells were maintained at 37.0 °C in a humidified atmosphere of 95.0% air and 5.0% CO2.
2.3. Measurement of viable cell number
Viable cell number was determined using the 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) colorimetric assay as previously described in detail [9]. Briefly, at the end of the treatment period, media in all treatment groups was removed and replaced with fresh control media containing 0.42 mg/mL MTT, and the cells were returned to the incubator for a period of 4 h. At the end of the incubation period, media was removed, the MTT crystals were dissolved in 1 ml of isopropanol, and the optical density of each sample was read at 570 nm on a microplate reader (SpectraCount, Packard BioScience Company, Meriden, CT). Cell number was calculated against a standard curve prepared by plating known concentrations of cells, as determined by the hemocytometer, at the start of each experiment.
2.4. Experimental treatments
For all experiments, an aqueous stock solution of highly lipophilic γ-tocotrienol was prepared as previously described [9]. Briefly, an appropriate amount of γ-tocotrienol was first dissolved in 100 μL of 100% ethanol, then added to a small volume of sterile 10% BSA in water and incubated overnight at 37°C with continuous shaking. This stock solution was then used to prepare various concentrations of 0–7 μM γ-tocotrienol-supplemented treatment media. A stock solution of celecoxib was prepared by dissolving a known amount in sterile dimethyl sulfoxide (DMSO) at the start of each experiment. This stock solution was then used to prepare various concentrations of 0–20 μM celecoxib-supplemented treatment media. Final concentration of DMSO and/or ethanol was maintained as the same in all treatments groups within a given experiment and never exceeded 0.1%.
2.5. Growth studies
+SA cells were initially plated at a density of 5 × 104 cells/well (6 wells/group) in serum-free defined control media in 24-well culture plates and allowed to adhere overnight. The following day, cells were divided into different treatment groups and media was removed and replaced with fresh control or treatment media, and then returned to the incubator. Cells were treated with celecoxib (0–20 μM) or γ-tocotrienol (0–7 μM) alone and in combination. Cells in their respective treatment groups were fed fresh media every day throughout experimentation.
2.6. Measurement of prostaglandin E2 (PGE2) levels
+SA cells were initially plated at a density of 5 × 104 cells/well (6 wells/group) in serum-free defined control media in 24-well culture plates and allowed to adhere overnight. The following day, cells were divided into different treatment groups and media was removed and replaced with fresh control or treatment media, and then returned to the incubator for a 72 h culture period. Cells were treated alone or in combination with vehicle, celecoxib (2.5 μM or 20 μM) or γ-tocotrienol (0.25 μM or 3 μM). In these particular experiments, media was not replaced at any time after the start of treatment exposure. At the end of the 72 h treatment period, media was collected and assayed for PGE2 according to the methods described in the EIA kit provided by the manufacturer (Cayman Chemical Co. Ann Arbor, MI). Optical density was measured at 420 nm on a Synergy-2 Multi Mode Microplate Reader (BioTek Instruments Inc., Winooski, VT).
2.7. Electrophoresis and western blot analysis
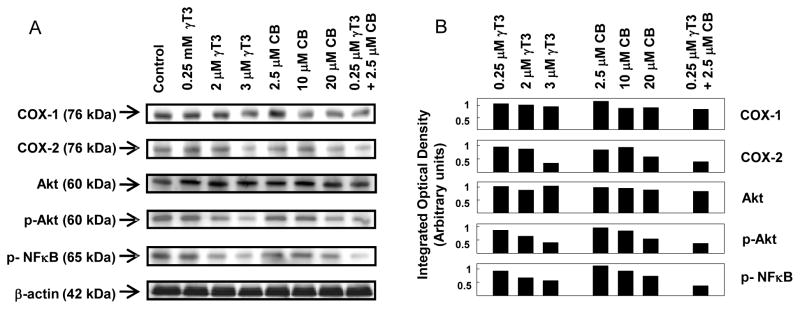
+SA cells were plated at a density of 1 × 106 cells/100 mm culture plates and grown in serum-free defined control or treatment media. At the end of 96 h treatment period, cells were isolated with trypsin, washed, and then whole cell lysates were prepared for subsequent electrophoresis, as previously described in detail [17]. Briefly, protein concentration in each sample was determined using the BioRad protein assay kit (BioRad, Hercules, CA). Equal amounts of protein (30 μg) from each sample were loaded on 10% SDS-polyacrylamide minigels and electrophoresed. Proteins were then transblotted (30 V for 12–16 h at 4 °C) to polyvinylidene difluoride (PVDF) membranes (Dupont, Boston, MA) and then blocked with 2% bovine serum albumin (BSA) in 0.1% Tween Tris buffered saline (TBST) for 2 h. The PVDF membranes were probed with specific primary antibodies against COX-1, COX-2, Akt, phospho-Akt, and phospho-NFκB diluted 1:2000 in 2% BSA/TBST for 2 h at room temperature. Membranes were washed five times with TBST and then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody diluted 1:4000 in 2% BSA/TBST for 1 h followed by washing. Antibody bound proteins were visualized with the SuperSignal enhanced chemiluminescence kit (Pierce, Rockford, IL). The Kodak Gel Logic-1500 imaging system (Carestream Molecular Imaging, New Haven, CT) was used to visualize the luminescent proteins. All experiments were repeated at least three times. A representative Western blot image from each experiment is shown in Fig. 4. The visualization of β-actin was used to ensure equal sample loading in each lane. Densitometric analysis was performed using Kodak Molecular Imaging Software 4.5 (Carestream Health Inc, New Haven, CT). For quantification, the values obtained from densitometry of Western blot images for the various treatment groups were normalized to their respective β-actin and control densitometric values to clearly visualize the differences between treatment groups.
Fig. 4.
(A) Western blot analysis of γ-tocotrienol and celecoxib treatment alone and in combination on COX-1, COX-2, total Akt, phospho-Akt (Ser473) and phospho-NFκB in neoplastic +SA mammary epithelial cells. Cells were initially plated at a density of 1×106 cells/100 mm culture dishes, divided into different treatment groups, and then maintained on their respective control or treatment media for a 4-day treatment period. Afterwards, cells were isolated and prepared for Western blot analysis. (B) Scanning densitometric analysis was performed for each blot to visualize the relative levels of COX-1, COX-2, total Akt, phospho-Akt (Ser 473), and phospho-NFκB. Integrated optical density of each band was normalized with their corresponding β-actin and control treatment bands and then shown in bar graphs. Vertical bars indicate the fold-change in protein levels in various treatment groups as compared with their respective controls.
2.8. Statistical analysis
The level of interaction between celecoxib and γ-tocotrienol was evaluated by isobologram method [18]. A straight line was formed by plotting IC50 doses of γ-tocotrienol and individual statins on the x-and y-axes, respectively as determined by non-linear regression curve fit analysis using GraphPad Prism 4 (GraphPad Software Inc. La Jolla, CA). The data point in the isobologram corresponds to the actual IC50 dose of combined γ-tocotrienol and celecoxib treatment. If a data point is on or near the line, this represents an additive treatment effect, whereas a data point that lies below or above the line indicates synergism or antagonism, respectively.
Differences among the various treatment groups in cell growth and viability studies were determined by analysis of variance (ANOVA) followed by Dunnett’s t-test using the SAS software package (SAS Institute Inc., NC). A difference of P<0.05 was considered to be significant as compared to vehicle-treated controls.
3. Results
3.1. Antiproliferative effects of γ-tocotrienol and celecoxib
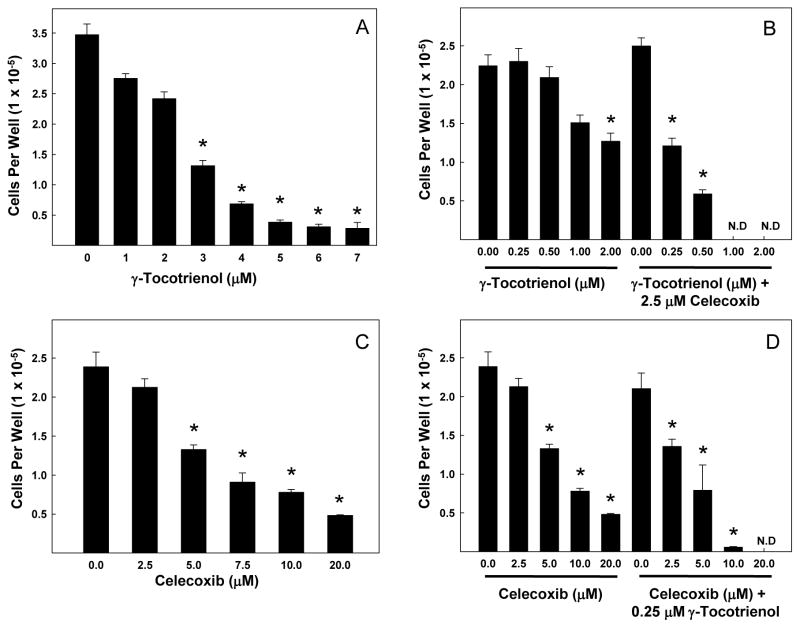
The effects of various doses of γ-tocotrienol and celecoxib on neoplastic +SA mammary epithelial cell growth are shown in Fig. 1. Treatment with 0–7 μM γ-tocotrienols for 4 days significantly inhibited +SA cell growth in a dose-responsive manner (Fig. 1A) with an IC50 dose calculated to be 3.3μM. However, combined treatment of 0–2 μM γ-tocotrienol with 2.5 μM celecoxib (subeffective dose) resulted in a greater inhibition in +SA cell growth as compared to cells treated with similar doses of γ-tocotrienol alone (Fig. 1B). Similarly, treatment with 0–20 μM celecoxib for 4 days caused a significant dose-responsive decrease in +SA cell growth (Fig. 1C) with an IC50 dose calculated to be 8.6 μM. Combined treatment of similar doses of celecoxib with 0.25 μM γ-tocotrienol (subeffective dose) resulted in a greater inhibition in +SA cell growth as compared to cells treated with similar doses of celecoxib alone (Fig. 1D).
Fig. 1.
Antiproliferative effects of γ-tocotrienol and celecoxib treatment alone and in combination on neoplastic +SA mouse mammary epithelial cells. Cells were initially plated at a density of 5×104 cells/well (6 wells/group) in 24-well plates and exposed to (A) 0–7 μM γ-tocotrienol, (B) 0–20 μM celecoxib, (C) 0–0.25 μM γ-tocotrienol in combination with 0–20 μM celecoxib, or (D) 0–2.5 μM celecoxib in combination with 0–7 μM γ-tocotrienol for a 4-day treatment period. Afterwards, viable cell number was determined using the MTT colorimetric assay. Vertical bars indicate the mean cell count ± SEM in each treatment group. *P<0.05 as compared to the vehicle-treated control group.
3.2. Synergistic antiproliferative effects of combined γ-tocotrienol and celecoxib treatment
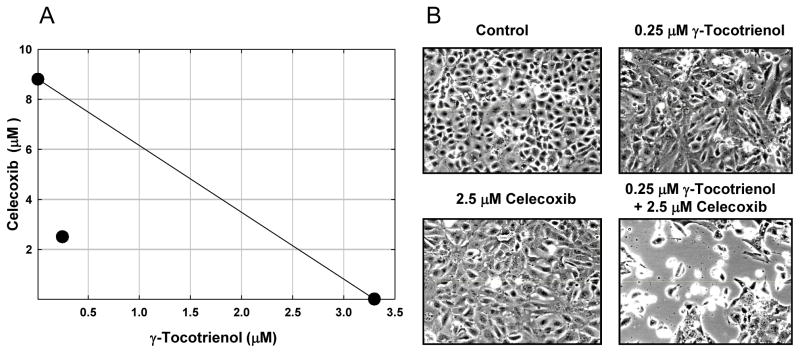
Isobologram analysis of combined treatment effects of γ-tocotrienol and celecoxib is shown in Fig. 2A. The growth inhibitory effect of combined γ-tocotrienol and celecoxib treatment was found to be statistically synergistic, as evidenced by the location of the data point in the isobologram being well below the line defining an additive effect Fig. 2A. Photomicrographs show that treatment with 0.25 μM γ-tocotrienol (subeffective dose) or 2.5 μM celecoxib (subeffective dose) alone had only slight effects on +SA cell number and morphology, whereas combined treatment caused a large suppression in cell proliferation following the 4-day treatment period (Fig 2B).
Fig. 2.
(A) Isobologram analysis of γ-tocotrienol and celecoxib antiproliferative effects on +SA mouse mammary epithelial cells. Individual IC50 doses for γ-tocotrienol and celecoxib were calculated and then plotted on the x and y axes, respectively. The line connecting these points represents the drug doses of each compound that would induced the same growth inhibition when used in combination if the interaction between these compounds were additive. The data point on the isobologram represents the actual doses of combined γ-tocotrienol and celecoxib treatment that results in 50% growth inhibition. Since the data point is positioned well below the line, a strong synergistic antiproliferative effect is indicated. (B) Photomicrographs of neoplastic +SA mammary epithelial cells in different groups after a 4-day treatment period. Magnification in each micrograph is 100X.
3.3. Effects of γ-tocotrienol and celecoxib on PGE2 synthesis
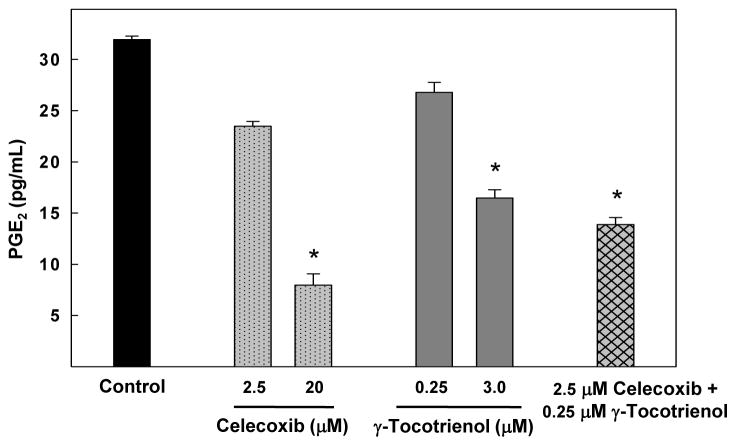
Treatment with 2.5 or 20 μM celecoxib or 0.25 or 3 μM γ-tocotrienol alone resulted in dose-responsive decrease in PGE2 synthesis, but these decreases were found to be significant only at the higher doses of celecoxib and γ-tocotrienol tested. However, combined treatment with 0.25 μM γ-tocotrienol and 2.5 μM celecoxib resulted in a significant decrease in PGE2 synthesis as compared to the vehicle-treated control group (Fig. 3).
Fig. 3.
Effects of celecoxib and γ-tocotrienol treatment alone and in combination on PGE2 synthesis in neoplastic +SA mammary epithelial cells. Cells were initially plated at a density of 5×104 cells/well in 24 well culture plates, divided into the different treatment groups, and then maintained on their respective control or treatment media for a 72 hr treatment period. Afterward, media was collected from the different treatment groups and prepared for use in the EIA assay for PGE2. Vertical bars indicate the mean cell count ± SEM in each treatment group. *P<0.05 as compared with the vehicle-treated control group.
3.4. Effects of γ-tocotrienol and celecoxib treatment on COX-1 and COX-2 levels, and Akt and NFκB activation
Western blot (Fig. 4A) and scanning densitometric analysis (Fig. 4B) showed that treatment with 0.25 μM γ-tocotrienol or 2.5 μM celecoxib alone had no effect on the relative levels of COX-1, COX-2, total Akt, phospho-Akt (activated) or phospho-NFκB (activated) levels in +SA cells. However, combined treatment with subeffective doses of γ-tocotrienol (0.25 μM) and celecoxib (2.5 μM) caused a large relative decrease in COX-2, phospho-Akt (activated) and phospho-NFκB (activated) levels as compared to +SA cells in the vehicle-treated control group (Fig. 4A and 4B). In contrast, treatment with higher doses of γ-tocotrienol (2–3 μM) or celecoxib (10–20 μM) alone caused only a relatively modest decrease in COX-2, phospho-Akt (activated) and phospho-NFκB (activated) as compared to vehicle-treated controls (Fig. 4A and 4B).
4. Discussion
These results demonstrate that combined treatment with low doses of γ-tocotrienol and celecoxib synergistically inhibited the growth of highly malignant +SA mammary epithelial cells in culture. Combined treatment with 0.25 μM γ-tocotrienol (subeffective dose) and 2.5–20 μM celecoxib, or combined treatment with 2.5 μM celecoxib (subeffective dose) and 0.25–2.0 μM γ-tocotrienol caused a greater reduction in +SA cell growth as compared to cells treated with corresponding doses of γ-tocotrienol or celecoxib alone. Additional studies showed that the synergistic antiproliferative effects of these combined treatments were associated with reduction in COX-2, but not COX-1 levels, and a corresponding suppression in PGE2 synthesis and decrease in Akt and NFκB activation.
Previous studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) can enhance the anticancer effects of various chemotherapies both in vitro and in vivo [19, 20], whereas treatment with a mixture of various tocopherols and tocotrienols was shown to reduce COX-2 expression in monocytes [21]. The present study extends these previous findings by demonstrating that the synergistic antiproliferative effect of combined celecoxib and γ-tocotrienol treatment is mediated by both COX-2-dependent and -independent mechanisms. Combined treatment with low doses of celecoxib (2.5 μM) and γ-tocotrienol (0.25 μM) resulted in a relatively large decrease in COX-2 expression as compared to treatment with either agent alone. Furthermore, treatment with subeffective doses of celecoxib and γ-tocotrienol was found to cause a corresponding decrease in phosphorylated Akt (activated) and phosphorylated NFκB (activated) levels, an effect that was not observed when cells were treated with the same dose of either agent alone. Other investigations have shown that treatment with COX-2 inhibitors can significantly inhibit cell proliferation in COX-2 deficient cell lines, providing further evidence that COX-2 independent mechanisms are involved in mediating the antiproliferative effects of COX-2 inhibitors [22].
Studies have shown that COX-2-derived PGE2 is the major prostaglandin produced by breast cancer cells[23]. The present study shows that moderate dose of celecoxib (20 μM) or γ-tocotrienol (3 μM) alone caused a significant decrease, whereas treatment with low doses of these agents had no effect on PGE2 synthesis in +SA mammary tumor cells. However, combined treatment with these same low doses of celecoxib (2.0 μM) and γ-tocotrienol (0.25 μM) caused a significant reduction in PGE2 levels. None of the treatment groups showed alterations in COX-1 expression as compared to the vehicle-treated control group. These data provide evidence that the synergistic anticancer effect of combined low dose celecoxib and γ-tocotrienol treatment is associated with an inhibition of COX-2 dependent PGE2 synthesis and is independent of COX-1 expression.
Overexpression of COX-2 is a characteristic that is displayed in a wide range of cancer cell types and is associated with enhanced angiogenesis, metastatic phenotype appearance and behavior, and resistance to apoptosis [24–27]. Therefore, much effort has been directed towards the development and use of COX-2 inhibitors for the prevention and treatment of metastatic cancers. Celecoxib is such a selective COX-2 inhibitor that has been approved for treatments of familial adenomatous polyposis (FAP) [28]. However, severe gastrointestinal and cardiovascular toxicities associated with moderate to high dose use of selective COX-2 inhibitors have limited their clinical usefulness [6, 7].
γ-Tocotrienol displays potent anticancer activity at treatment doses that have little or no effect on normal cell growth and viability [9, 10]. Studies have shown that treatment with 4 μM γ-tocotrienol inhibited PI3K/PDK/Akt mitogenic signaling and these effects were not found to be associated with increases in PTEN and PP2A phosphatase activity in +SA mammary tumor cells [11]. In addition, γ-tocotrienol treatment caused a large decrease in NFκB transcriptional activity, apparently by suppressing IκB-kinase (IKK)-α/β activation, an enzyme associated with inducing NFκB activation [11]. Since Akt and NFκB are intimately involved in mammary tumor cell proliferation and survival, these findings strongly suggest that the antiproliferative effects of γ-tocotrienol are mediated by a reduction in Akt and NFκB activation and mitogenic signaling in +SA mammary tumor cells. Nevertheless, although tocotrienols display significantly anticancer activity in a variety of in vitro experimental models, it has been difficult to reproduce these findings in vivo because of low bioavailability. It is now clearly evident that absorption and transport mechanisms within the body display selective preference for α-tocopherol [11]. As a result, it is extremely difficult to obtain and/or sustained therapeutic levels of γ-tocotrienol in the blood and target tissues following oral administration [11]. However, treatment doses of γ-tocotrienol used in the present study are physiologically relevant based on serum concentrations of tocotrienols that were found to ranged between 2–4 μM in individuals given a single oral dose of mixed tocotrienols (300 mg) under fed or fasted conditions [29].
Celecoxib has also been shown to suppress Akt and NFκB activation and mitogenesis in various tumor cell types [22, 30]. Results in the present study showed that treatment with moderate doses of either celecoxib or γ-tocotrienol alone inhibited, whereas treatment with low doses of celecoxib or γ-tocotrienol alone had no effect on Akt and NFκB phosphorylation (activation). However, combined treatment with these same low doses of celecoxib and γ-tocotrienol resulted in a large decrease in Akt and NFκB phosphorylation. Although studies have shown that moderate doses of γ-tocotrienol attenuate EGF-dependent EGF-receptor family activation [31], additional studies are required to determine if the antiproliferative effects of combined low dose celecoxib and γ-tocotrienol are mediated through a similar suppression in EGF-receptor activation or through an EGF-receptor independent mechanism.
5. Conclusion
These studies demonstrate the potent synergistic antiproliferative effects of combined low dose treatment of γ-tocotrienol and celecoxib against +SA mammary tumor cells in culture. Furthermore, these findings suggest that combined low dose γ-tocotrienol and celecoxib treatment may greatly improve therapeutic response in the treatment of breast cancer, while at the same time greatly reduce or eliminate the severe gastrointestinal and myocardial toxicities that are associated with the use of high therapeutic doses of COX-2 inhibitor monotherapy.
Acknowledgments
This work was supported in part by grants from NIH (grant # CA86833) and First Tech International Ltd. The authors would like to thank Carotech Bhd. for generously providing isolated γ-tocotrienol and Pfizer Inc. for generously providing celecoxib for use in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal A, Fentiman IS. NSAIDs and breast cancer: a possible prevention and treatment strategy. Int J Clin Pract. 2008;62:444–9. doi: 10.1111/j.1742-1241.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 2.Antonakopoulos N, Karamanolis DG. The role of NSAIDs in colon cancer prevention. Hepatogastroenterology. 2007;54:1694–700. [PubMed] [Google Scholar]
- 3.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4:17–23. [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Laible B. COX-2 inhibitors and cardiovascular toxicity: a class effect? S D J Med. 2005;58:93–4. [PubMed] [Google Scholar]
- 7.Shafiq N, Malhotra S, Pandhi P, Nada R. Comparative gastrointestinal toxicity of selective cyclooxygenase (COX-2) inhibitors. Indian J Exp Biol. 2005;43:614–9. [PubMed] [Google Scholar]
- 8.Sylvester PW, Shah S. Intracellular mechanisms mediating tocotrienol-induced apoptosis in neoplastic mammary epithelial cells. Asia Pac J Clin Nutr. 2005;14:366–73. [PubMed] [Google Scholar]
- 9.McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224:292–301. doi: 10.1046/j.1525-1373.2000.22434.x. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids. 2000;35:171–80. doi: 10.1007/BF02664767. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ, Sylvester PW. Gamma-tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp Biol Med (Maywood) 2005;230:235–41. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 12.Toker A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol Pharmacol. 2000;57:652–8. [PubMed] [Google Scholar]
- 13.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 14.Wali VB, Sylvester PW. Synergistic antiproliferative effects of gamma-tocotrienol and statin treatment on mammary tumor cells. Lipids. 2007;42:1113–23. doi: 10.1007/s11745-007-3102-0. [DOI] [PubMed] [Google Scholar]
- 15.Sylvester PW. Vitamin E and apoptosis. Vitam Horm. 2007;76:329–56. doi: 10.1016/S0083-6729(07)76012-0. [DOI] [PubMed] [Google Scholar]
- 16.Danielson KG, Anderson LW, Hosick HL. Selection and characterization in culture of mammary tumor cells with distinctive growth properties in vivo. Cancer Res. 1980;40:1812–9. [PubMed] [Google Scholar]
- 17.Shah S, Gapor A, Sylvester PW. Role of caspase-8 activation in mediating vitamin E-induced apoptosis in murine mammary cancer cells. Nutr Cancer. 2003;45:236–46. doi: 10.1207/S15327914NC4502_14. [DOI] [PubMed] [Google Scholar]
- 18.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–72. [PubMed] [Google Scholar]
- 19.Gianni L. The future of targeted therapy: combining novel agents. Oncology. 2002;63 (Suppl 1):47–56. doi: 10.1159/000066197. [DOI] [PubMed] [Google Scholar]
- 20.Lev-Ari S, Zinger H, Kazanov D, Yona D, Ben-Yosef R, Starr A, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed Pharmacother. 2005;59 (Suppl 2):S276–80. doi: 10.1016/s0753-3322(05)80045-9. [DOI] [PubMed] [Google Scholar]
- 21.Wu SJ, Liu PL, Ng LT. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol Nutr Food Res. 2008;52:921–9. doi: 10.1002/mnfr.200700418. [DOI] [PubMed] [Google Scholar]
- 22.Pang RP, Zhou JG, Zeng ZR, Li XY, Chen W, Chen MH, et al. Celecoxib induces apoptosis in COX-2 deficient human gastric cancer cells through Akt/GSK3beta/NAG-1 pathway. Cancer Lett. 2007;251:268–77. doi: 10.1016/j.canlet.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429–34. [PubMed] [Google Scholar]
- 25.Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–12. [PubMed] [Google Scholar]
- 26.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–90. [PubMed] [Google Scholar]
- 27.Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26–30. doi: 10.1002/1096-9098(200101)76:1<26::aid-jso1005>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Davies NM, Jamali F. COX-2 selective inhibitors cardiac toxicity: getting to the heart of the matter. J Pharm Pharm Sci. 2004;7:332–6. [PubMed] [Google Scholar]
- 29.Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol. 2001;53:67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]
- 30.Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004;173:2011–22. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 31.Samant GV, Sylvester PW. gamma-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. 2006;39:563–74. doi: 10.1111/j.1365-2184.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]