Abstract
Bipolar Disorder (BD) is a neuropsychiatric disorder characterized by symptoms ranging from a hyperactive manic state to depression, with periods of relative stability, known as euthymia, in between. Although prognosis for BD sufferers remains poor, treatment development has been restricted due to a paucity of validated animal models. Moreover, most models focus on the manic state of BD with little done to characterize the longitudinal behavior of these models. We recently presented two dopamine transporter (DAT) mouse models of BD mania: genetic (DAT knockdown; KD, mice) and pharmacological (the selective DAT inhibitor GBR 12909). These models exhibit an exploratory profile consistent with the quantified exploratory profile of manic BD patients observed in the cross-species translational test, the Behavioral Pattern Monitor (BPM).
To further explore the suitability of these models, we examined the effects of reduced DAT function on the behavior of mice testing after familiarization to the BPM environment. Testing with 16 mg/kg GBR 12909 in familiarized mice resulted in a consistent mania-like profile. In contrast, the mania-like profile of DAT KD mice disappears in a familiar environment, with partial reinstatement elicited by the introduction of novelty. In addition, we found that a sub-threshold dose of GBR 12909 (9 mg/kg) reinstated the mania-like profile in DAT KD mice without affecting wildtype behavior.
Thus, the mania-like exploratory profile of DAT KD mice is reduced in a familiar environment, partially reinstated with novelty, but is fully restored when administered a stimulant that is ineffective in wildtype mice. These mice may provide a model of BD from mania to euthymia and back again with stimulant treatment. Acute blockade of the DAT by GBR 12909 however, may provide a consistent model for BD mania.
Introduction
Bipolar Disorder (BD) is a major and debilitating psychiatric disorder, affecting approximately 1-2% of the population. The heritability of BD has been estimated between 50-80%, suggesting a strong genetic component to the disorder. Despite treatment availability for BD, prognosis remains poor with 15% committing suicide. While some treatments were discovered serendipitously through chance observations, such as lithium (Gould and Einat, 2007), others were originally designed for other psychiatric disorders, such as antipsychotic treatment for schizophrenia. Thus, individuals with BD have not benefited from a treatment strategy that was designed specifically to treat the pathology of the disorder, possibly contributing to poor prognosis (Einat, 2006).
One rate-limiting step in developing treatments for BD has been the lack of suitable animal models (Gould and Einat, 2007). While some models exist, they are not necessarily derived from the limited understanding we possess about the neuropathology of BD. For example, amphetamine administration was first used as a model of mania based upon the observed behavior of rats after administration, not as an a priori hypothesis on the neurobiological underpinnings of the disorder (Davies et al., 1974, Randrup and Munkvad, 1974, Rushton and Steinberg, 1963). This lack of etiological validity may limit the development of novel therapeutics for treating BD, thus explaining why current treatments were only serendipitously discovered or adopted from other psychiatric conditions (Gould and Einat, 2007).
A model of BD mania we have proposed is based on the putative reduced functioning of the dopamine transporter (DAT) in BD patients. Genetic linkage studies have linked the DAT and BD (Greenwood et al., 2001, Greenwood et al., 2006, Kelsoe et al., 1996), with lower levels of the DAT being reported in BD (Amsterdam and Newberg, 2007). Moreover, reduced expression of DAT has been observed in BD patients (Horschitz et al., 2005). We observed that mice with reduced functioning DAT levels (via genetic or pharmacological manipulation), exhibit a phenotype in the mouse Behavioral Pattern Monitor (BPM) that is consistent with that of acutely manic BD patients in a human BPM, specifically increased activity, increased specific exploration, and reduced spatial d (Perry et al., 2009, Young et al., 2010, Young et al., 2007).
Mania is the cardinal feature of BD, as exemplified by the fact that it is a core symptom in the diagnosis of both Type I and Type II BD according to the DSM IV. Other behavioral abnormalities exist in BD however, with patients classically alternating between episodes of mania and depression. Between these episodes patients are typically in a euthymic state characterized by relative behavioral stability with little to no hyperactive or manic behaviors. Very few animal models of BD attempt to mimic the full spectrum of the disorder. The kindling and intermittent cocaine (Post, 2007) models attempt to model cycling effects in rodents, but fall short of modeling specific behavioral phenotypes exhibited by BD patients (Young and Geyer, in press).
The genetic and pharmacological DAT models we presented previously appear to mimic the symptoms of the manic phase of BD (Perry et al., 2009, Young et al., 2010). These models include DAT knockdown (KD) mice, which exhibit chronic low level expression (10%) of the DAT (Zhuang et al., 2001) and selective inhibition of the DAT with GBR 12909 (Heikkila and Manzino, 1984). Mice from each model exhibited hyperactivity and increased specific exploration, as well as greater straight line movements represented by the fractal geometry measure spatial d. This behavioral profile was consistent with the abnormal exploratory behavior of manic BD patients (Perry et al., 2009). These models may therefore prove useful in the development of treatments specifically targeted for the treatment of BD mania. To prove useful as a drug discovery model in which animal use is maximized however, it would be beneficial to know whether the mania-like exploratory profile of these models are observed following subsequent exposures to the testing environment. Thus, in the present studies, we investigated the exploratory behavioral profile of these models following initial familiarization to the BPM testing environment. Specifically, we examined whether the mania-like behavioral profiles observed in these BD models would still be observed with repeated testing.
Methods
Animals
DAT KD mice were generated by inserting altered embryonic stem cells of the 129Sv/J mouse strain in C57BL/6J blastocysts. These stem cells were altered as detailed by Zhuang et al (2001). In brief, a 7.5-kb HindIII fragment containing the first two exons of the DAT gene was excised from a phage DNA isolated from a mouse 129Sv/J genomic library. A Not1 and Asc1 cassette was inserted to generate the targeting construct. This cassette contained the tetracycline-dependent transactivator tTA, the neomycin resistance gene, the tetoperators, and the human cytomegalovirus minimal promoter. An extra 4-kb DNA sequence was then inserted, which resulted in a region and temporal unspecific reduction in DAT expression. The generated C57BL/6JX129Sv/J chimera was mated with 129Sv/J females to generate heterozygous mutants on a 129Sv/J genetic background (Zhuang et al., 2001). The DAT KD cohort was sent to our laboratory from Columbia University and all subsequent mice were derived from a breeding colony in the vivarium at the University of California, San Diego. The DAT KD and wildtype (WT) mice assessed in this study were from the 11th generation and were generated by matings of heterozygous parents. The mice were between 5-6 months old, weighed approximately 20-40 g, and had previously been exposed to the mouse BPM two-weeks prior to the current studies (Perry et al., 2009). To assess the effects of repeated dosing of GBR 12909 C57BL/6J mice (Jackson labs, Harbor, Maine), previously exposed to GBR 12909 and the mouse BPM (Young et al., 2010), were assessed at 4 months old and weighed between 20-40 g.
All mice were maintained on a reversed day-night cycle (lights on at 8.00 PM, off at 8.00 AM) and housed in groups of maximum 4 per cage at the University of California San Diego (UCSD) vivarium. The animals had unlimited access to water and food (Harlan, Madison, WI) except during testing. Prior to testing, all mice were acclimatized to the testing room for a period of at least 60 minutes. All testing occurred between 9.00 AM and 6.00 PM. All behavioral testing procedures were approved by the UCSD Institutional Animal Care and Use Committee before the start of the experiments. All mice were maintained in an animal facility that meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Drugs
GBR 12909 dihydrochloride (Sigma, St Louis, MO) was dissolved in saline after sonicating for 2-4 hours at 40°C. Due to the poor solubility of GBR 12909 in saline, the injection volume was increased to 10 ml/kg. Free-base drug weights were used in all drug calculations. All drugs were administered by intraperitoneal injection.
Mouse Behavioral Pattern Monitor
Spontaneous locomotor and exploratory behavior was examined in 10 mouse BPM chambers as described previously (Halberstadt et al., 2009, Risbrough et al., 2006). Each chamber consists of a 30.5 × 61 × 38 cm area with a Plexiglas hole board floor equipped with 3 floor holes and 8 wall holes (3 along each side of the long walls, and two holes in the front and back walls; Fig. 1). Each chamber is illuminated from a single light source above the arena (producing 350 lux in the center, and 92 lux in the 4 corners). Each hole is equipped with an infrared photobeam to detect nose poking behavior. A grid of 12 × 24 infrared photobeams (Fig. 1A) 1 cm above the floor recorded the location of the mouse every 0.1 s. The position of the mouse was defined across nine unequal regions (four corners; 9.375 × 16.875 cm, four wall regions; long, 9.375 × 26.25 cm, short, 11.25 × 16.875 cm, and a center; 11.25, 26.25 cm, Fig. 1B (Geyer et al., 1986, Risbrough et al., 2006). An array of 16 infrared photobeams 2.5 cm above the floor and aligned with the long axis of the chamber was used to register rearing behavior. At the start of each test session, mice were placed in the bottom left hand corner of the chamber, facing the corner and the test session started immediately.
Figure 1. Schematic of the mouse Behavioral Pattern Monitor.
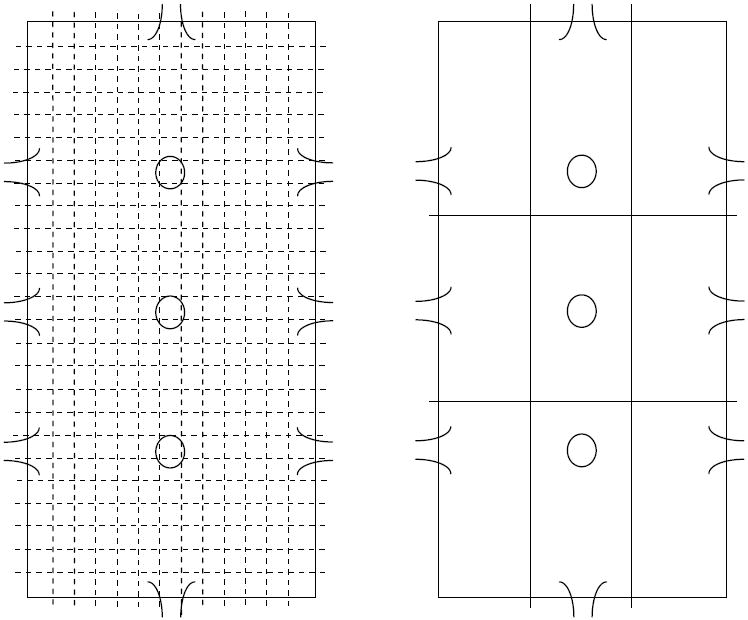
The mouse BPM is an open activity chamber (30.5 × 61 cm) which records the spatial location of the mouse using a grid of 12 × 24 photobeams located 1 cm above the floor (A). The chamber contains eleven 1.4 cm diameter holes (3 in the floor, 3 on each long wall, 1 on each short wall), each provided with an infrared photobeam to detect investigatory holepokes (B). The chamber is divided into 9 unequal regions (B), with transitions between regions utilized as a measure of investigatory activity.
The primary dependent variables of interest were; locomotor activity as measured by transitions (calculated as a movement across a defined region) and center entries (cumulative entries into the center region); specific exploration as measured by holepoking, rearing, and center duration (cumulative time spent in the center); locomotor exploratory pattern as measured by spatial d. Spatial d uses analyses based on fractal geometry to quantify the geometrical structure of the locomotor path, where a value of 2 represents highly circumscribed localized movement, 1 represents straight line distance covering movements (Paulus and Geyer, 1991).
Experiment 1: Exploratory profile of DAT mutant mice in a familiar BPM environment (previous exposure to the BPM: 2 weeks prior to testing)
Previously we reported that DAT KD mice appear to represent a viable model of BD mania, insofar as they exhibit a phenotype in the mouse BPM consistent with that of patients with BD mania in the human BPM (Perry et al., 2009). We assessed the consistency of the DAT KD phenotype upon repeated testing. DAT KD mice that had previously been exposed to the mouse BPM were retested two weeks later, consisting of male DAT KD (n=11), male DAT WT (n=8), female DAT KD (n=10) and female DAT WT (n=7).
Experiment 2: Novelty-induced modification of the exploratory profile of DAT mutant mice (previous exposures to the BPM: 7 and 5 weeks prior to testing)
Upon repeated testing of DAT KD mice, the hyperactive, exploratory, and perseverative profile previously observed in these mice (Perry et al., 2009), was diminished. This change may have been due to the loss of novelty of the testing procedure or environment. We therefore assessed the activity of the same DAT KD and WT mice five weeks later in the same, or a novel, environment. We hypothesized that creating a novel environment for these mice may lead to a re-emergence of the behavioral profile observed in Experiment 1. To create a novel environment for this study, the floors of the mBPM chambers were covered with sandpaper and small objects were placed into three of the wall holes. Each object was consistent between chambers but different within the chamber, with differing olfactory cues in an attempt to stimulate exploratory behavior. Exploration was assessed in male DAT KD (n=10), male DAT WT (n=8), female DAT KD (n=10) and female DAT WT (n=7) mice in this novel arena. Exploratory behavior of male DAT KD (n=11), male DAT WT (n=8), female DAT KD (n=10) and female DAT WT (n=7) mice was assessed in the arena consistent with previous testing (above) as a control.
Experiment 3: GBR 12909-induced alterations in exploration in mice familiar with the BPM environment (previous exposures to the BPM: 2 and 7 weeks prior to testing)
Previously, we demonstrated that pharmacological inhibition of the selective DAT inhibitor GBR 12909 induces a behavioral profile consistent with that of BD patients (Perry et al., 2009, Young et al., 2010). We investigated whether GBR 12909 administration would produce consistent effects following repeated exposure. Seven weeks after initial exposure, and two weeks after the first GBR 12909 study in the BPM, male C57BL/6J mice were administered the following doses of GBR 12909: 0 mg/kg (n=13), 9 mg/kg (n=13), 10.9 mg/kg (n=13), 13.21 mg/kg (n=13), 16 mg/kg (n=13), and 28.5 mg/kg (n=13). While the doses of 9, 16, and 28.5 mg/kg were duplicated from the previous study to establish whether this pharmacological model was subject to habituation confounds, we also assessed 10.9 and 13.21 mg/kg to gain greater information on the dose response effect of GBR 12909 and for dose consistency with previous studies in 129 mice (Young et al., 2010). To minimize carry-over effects from previous studies, we counter-balanced drug groups here from previous treatment groups.
Experiment 4: Effects of a subthreshold dose of GBR 12909 (9 mg/kg) on exploratory behavior of DAT mutant mice familiar with the BPM (previous exposure to the BPM: 2, 7, and 5 weeks prior to testing)
Previously, we determined that 9 mg/kg GBR 12909 did not affect exploratory behavior in 129 (Young et al., 2010), or C57BL/6J mice (above). We therefore assessed whether this dose would affect exploratory behavior in DAT mutant mice habituated to the test chambers. Given the apparent increased sensitivity of BD patients to stimulants, which may trigger episodes of mania, we hypothesized that this dose, while ineffective in DAT WT mice, would reinstate the mania-like profile of DAT KD mice. This study was conducted in the same cohort of mice tested in Experiment 2 five weeks later. The exploration of DAT KD (n=17) and WT (n=16) mice was assessed in the mBPM after acute administration of GBR 12909 or vehicle. GBR 12909 was administered in a dose of 9 mg/kg to 8 DAT KD and 8 DAT WT mice, while saline was administered to the control groups which consisted of 9 DAT KD and 8 DAT WT mice.
Statistics
The measures of each experiment were analyzed using two- or three- way ANOVAs, with sex, genotype, or treatment as between subject variables and time (six 10 min periods within a test session) as a within subject variable. When no main effect of sex or interaction with sex was observed, the data were collapsed across sex and reanalyzed. Significant main effects were analyzed using Tukey post hoc analyses. The data were analyzed \using the Biomedical Data Programs (BMDP) statistical software (Statistical Solutions Inc,. Saugus, MA). The alpha level was set to 0.05.
Results
Experiment 1: Exploratory profile of DAT mutant mice in the a familiar BPM environment (previously exposed to the BPM two weeks prior to testing)
DAT KD and WT mice were re-assessed in the mBPM two weeks after initial exposure to determine whether the mania-like phenotype of these mice was consistent with repeated testing.
Locomotor activity
A significant gene by time interaction (F(5,170)=3.83, p<0.005) confirmed the increased number of transitions in DAT KD mice (Fig. 2A). No main effect of sex or sex interaction with time was observed. Center entries were also increased in DAT KD mice, as reflected in a significant gene by time interaction (F(5,170)=2.29, p<0.05). No main effects of sex were observed, nor was there a sex by time interaction for center entries.
Figure 2. Activity levels of DAT KD and WT mice on repeated testing.
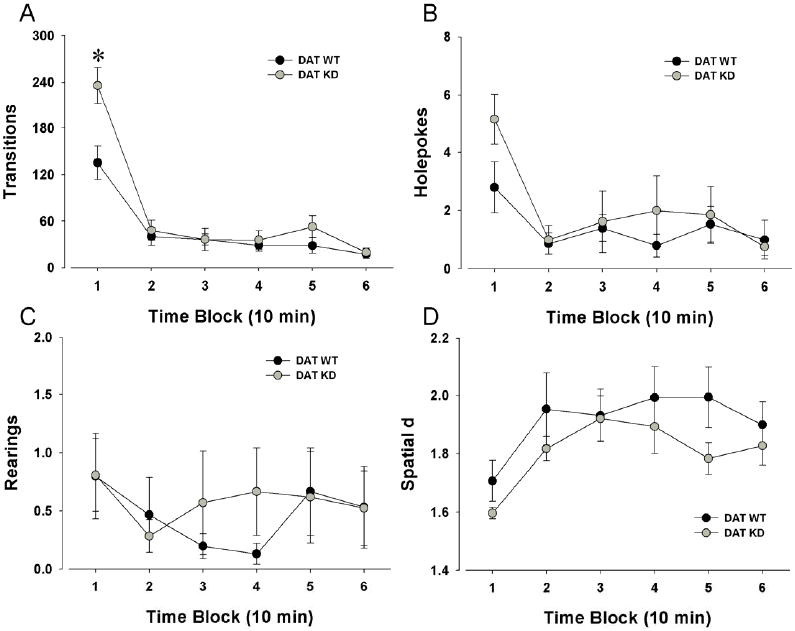
Previous reports indicate a mania-like phenotype in BPM-naïve DAT KD mice. The present data reassessed the consistency of this phenotype by retesting the same animals in the BPM. The mania-like phenotype was almost entirely absent upon repeated testing, with an increase in transitions observed only during the first 10 min (A). In contrast to behavior when BPM naïve, DAT KD mice did not exhibit an increase in holepoking (B). No difference in rearing was observed, consistent with previous reports, but in contrast, no change in spatial d was evident (D). Data collapsed across sex and presented as mean ± s.e.m., * denotes p < 0.05 when compared to vehicle control.
Exploratory behavior
No significant main effect of gene was observed for hole-poking (Fig. 2B), or rearing (Fig. 2C), nor were there any interactions of these measures with time (F<1, ns). A significant main effect of time was observed for holepoking (F(5,170) = 6.52, p<0.0001), but no effect of time was observed for rearing. No main effect of sex was observed nor were there any interactions with between subject factors for holepoking or rearing.
Locomotor patterns
For spatial d, no main effect of gene was observed, nor was there an interaction with time (F<1, ns; Fig. 2D). A main effect of time was observed however (F(5,170) = 7.86, p<0.0001). No main effect of sex, nor interaction with time or gene was observed (p>0.1).
Experiment 2: Novelty-induced modification of the exploratory profile of DAT mutant mice (previous exposures to the BPM: 7 and 5 weeks prior to testing)
DAT mutant mice were re-examined in the BPM five weeks later, with the behavior of half examined in an unaltered BPM (normal environment), and the other half in an altered BPM environment (novel environment).
Locomotor activity
For transitions, significant time by gene (F(5,290)=2.70, p<0.05; Fig. 3A) and time by environment (F(5,290)=3.82, p<0.005) interactions were observed demonstrating a novelty induced-increase in activity in KD mice only. No significant main effect of sex was detected, nor were there significant interactions with sex and other factors. For center entries, a significant interaction between time and environment (F(5,290)=3.58, p<0.005) was observed for center entries. Again, no significant main effect of sex or genotype was observed, nor was there a significant time by sex or time by genotype interaction.
Figure 3. Exploratory behavior of DAT WT and KD mice in a modified BPM environment.
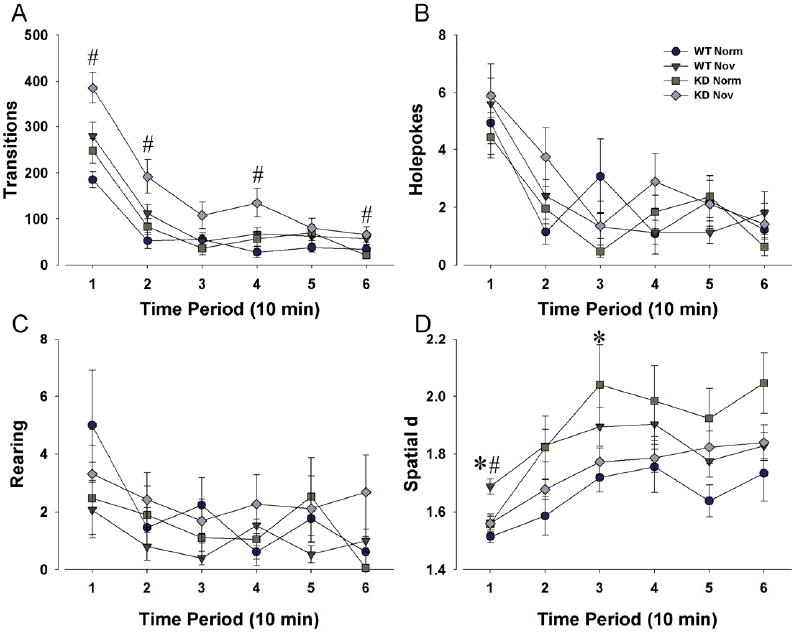
The same DAT WT and KD mice were reassessed in the BPM but with a modified environment in half of the BPM chambers. The BPM was altered by placing sandpaper on the floor, as well as inserting novel objects in four of the holes in the chambers. This novel environment was created to ascertain whether it would reinstate the mania-like phenotype of DAT KD mice observed when first placed in the BPM. The novel environment did elicit a significant increase in transitions in DAT KD, but not WT mice (A). Although the novel environment increased holepoking in DAT KD mice consistent with the increase in transitions, this effect was not significant (B). No effect of environment on holepoking was observed in WT mice either (B). Environment had no effect on rearing (C) in DAT KD or WT mice, with varying effects on spatial d in these mice (D). Data collapsed across sex and presented as mean ± s.e.m., * denotes p < 0.05 when compared to WT control mice, # denotes 0.05 when compared to KD mice in the normal (control) environment.
Exploratory behavior
No main effects of sex, genotype, or environment were observed for holepoking (Fig. 3B), nor were there any significant interactions between any factors (p>0.05). For rearing, a significant interaction between time, sex, genotype, and environment was observed (F(5,290)=2.68, p<0.05; Fig. 3C), although discernible group differences were observed.
Locomotor patterns
A gene by environment interaction for spatial d suggested that WT mice in the novel environment initially exhibited higher spatial d compared to KD mice and WT mice in the normal environment (F(1,58)=7.06, p <0.05; Fig. 3D). No main effect of sex was observed, nor was there a time by sex by environment interaction.
Experiment 3: GBR 12909-induced alterations in exploration in mice familiar with the BPM environment (previous exposures to the BPM: 2 and 7 weeks prior to testing)
Here we investigated whether mice administered GBR 12909 previously would exhibit a consistent phenotype upon re-testing two-weeks later. We chose doses based on previous findings to examine both the reproducibility of our previous main findings (9, 16, and 28.5 mg/kg), as well as to identify doses between effective and ineffective doses (10.9 and 13.21 mg/kg) to acquire greater information on the dose-response effect of GBR 12909 on exploratory behavior.
Locomotor activity
Drug by time interactions demonstrated a GBR 12909-induced increase in activity as measured by transitions (F(25,335)=5.01, p<0.0001; Fig. 4A). GBR 12909 increased center entries compared to saline (F(5,67)=3.44, p<0.05), although there was no drug by time interaction.
Figure 4. Effects of GBR 12909 on exploratory behavior in BPM non-naïve C57BL/6J mice.
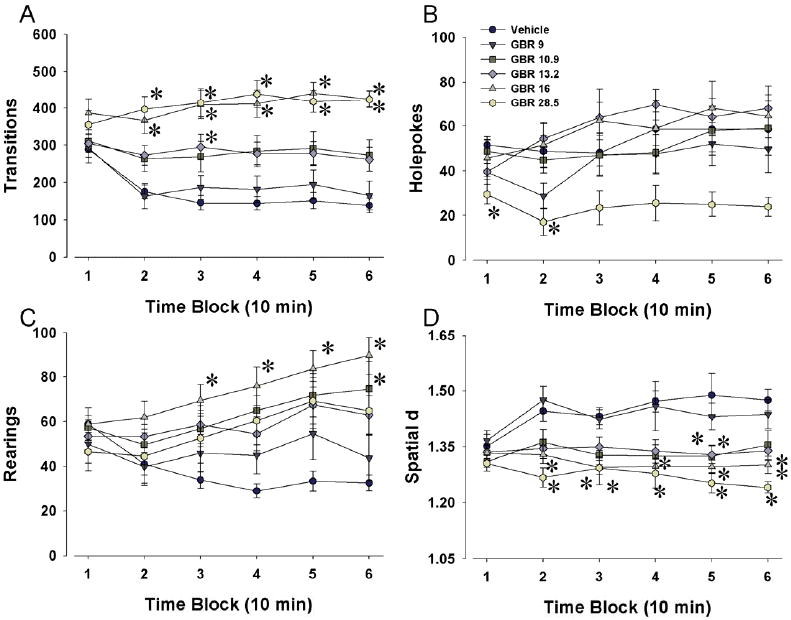
Mice that had previously exposed to the BPM were administered various doses of the selective DAT inhibitor GBR 12909 prior to their assessment in the mouse BPM. The doses used in the present study induced a consistent effect on exploratory behavior to that of the previous study. Both 16 and 28 mg/kg GBR 12909 increased transitions, with doses 10 and 13 mg/kg exhibiting a modest increase in transitions, while 9 mg/kg did not affect activity in mice (A). Again, consistent with previous reports, 16 mg/kg induced a modest increase in holepokes while 28 mg/kg reduced holepoking (B). GBR 12909 at 16 mg/kg also consistently increased rearing (C). Numerous doses of GBR 12909 including 10, 13, and 16 mg/kg, significantly reduced spatial d (D). Data presented as mean ± s.e.m. where * denotes p < 0.05 when compared to vehicle administered control mice.
Exploratory behavior
GBR 12909 increased rearing compared to saline at later time points as observed by a drug by time interaction (F(25,335)=3.99, p<0.0001; Fig. 4C). GBR 12909 reduced holepoking at 28 mg/kg (F(5,67)=3.17, p<0.05), with a trend toward a drug by time interaction (F(25,335)=1.44, p=0.0815; Fig. 4B) indicative of increased holepoking at 16 mg/kg toward the end of the session.
Locomotor patterns
For spatial d, a significant drug by time interaction was observed (F(25,335)=2.68, p<0.0001; Fig. 4D) resulting in a dose-dependent reduction in spatial d.
Experiment 4: Effects of a subthreshold dose of GBR 12909 (9 mg/kg) on exploratory behavior of DAT mutant mice familiar with the BPM (previous exposure to the BPM: 2, 7, and 5 weeks prior to testing)
It was apparent that GBR 12909 administration resulted in a mania-like phenotype irrespective of the repeated testing, unlike DAT KD mice. We tested the hypothesis that DAT KD mice may be more sensitive to stimulation compared to WT mice. We administered GBR 12909 to DAT KD and WT mice at 9 mg/kg, a dose that was ineffective in altering exploration of C57BL/6J mice.
Locomotor activity
For transitions, both time by drug (F(5,145)=8.93, p<0.0001) and genotype by drug (F(1,29)=5.03, p<0.05) interactions were observed demonstrating a selective effect of GBR 12909 on increasing activity in KD mice only (Fig. 5A). For center entries, a significant main effect of drug (F(1,29)=8.99, p<0.01) was observed. No main effects of genotype or time were observed, although there was a trend toward a drug by genotype by time interaction (F(5,145) = 2.20, p = 0.0573).
Figure 5. Sensitivity of DAT KD mice to a sub-threshold dose of GBR 12909.
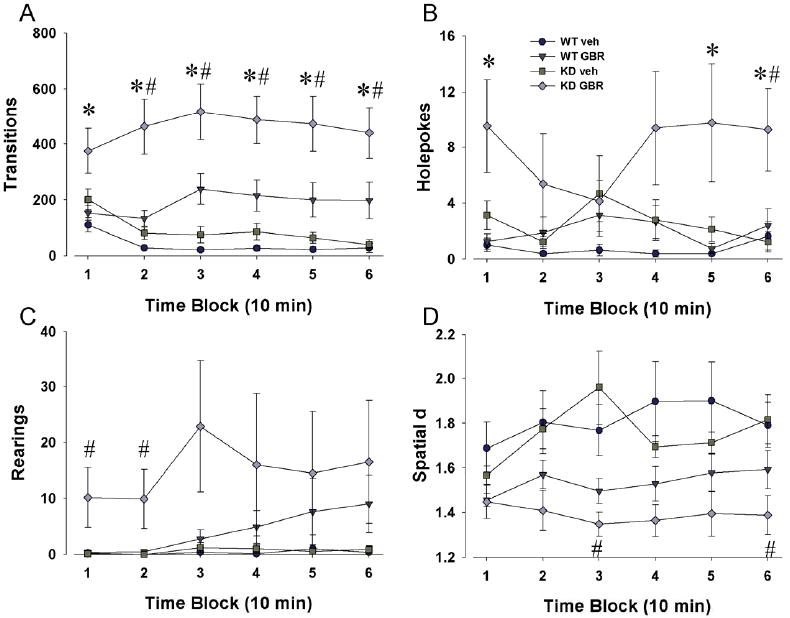
DAT WT and KD mice were reassessed in the BPM, with half administered 9 mg/kg GBR 12909, a dose determined to be ineffective to altering exploratory behavior in C57BL/6J mice. GBR 12909 significantly increased transitions (A), holepoking (B), and rearing (C) in DAT KD mice but not WT mice. DAT KD mice also exhibited reduced spatial d when administered GBR 12909, again in contrast with WT mice (D). Thus DAT KD mice exhibited a sensitivity to GBR 12909 at a dose that was ineffective in altering behavior in WT mice. Data collapsed across sex and presented as mean ± s.e.m. where * denotes p < 0.05 when compared to WT control mice at the same dose. # denotes p < 0.05 compared to KD mice administered vehicle.
Exploratory behavior
DAT KD mice administered GBR 12909 exhibited increased holepokes compared to WT mice as observed by a significant main effect of genotype (F(1,29)=7.47, p<0.05) and drug (F(1,29)=5.59, p<0.05; Fig. 5B). A trend toward a genotype by drug by time interaction was observed (F(5,145)=1.94, p=0.0916). A trend towards a main effect of drug was observed for rearing (F(1,29)=3.59, p=0.0681; Fig. 5C), with no main effect of genotype observed, nor were there any significant interactions although only KD mice exhibited GBR 12909-induced increased rearing.
Locomotor patterns
For spatial d, a main effect of drug (F(1,29)=15.96, p<0.0005; Fig. 5D) was observed with GBR 12909 treatment lowering spatial d mainly in KD mice. No main effects of genotype or time were observed, nor were there any significant interactions between these factors. No main effects of sex or sex by between subject factor interactions were observed for any measure.
Discussion
In the present studies, we performed a longitudinal assessment of the exploratory behavior of our pharmacological and genetic mouse models of BD mania. We previously reported that DAT KD mice and mice administered GBR 12909 exhibit an exploratory profile in the mouse BPM consistent with that of patients with bipolar mania assessed in the human BPM, specifically increased activity and specific exploration, as well as reduced spatial d (Perry et al., 2009, Young et al., 2010). The present studies on repeated testing suggest that the mania-like profile of DAT KD mice is dependent upon the novelty of the BPM. Although increasing the environmental novelty in the BPM did not fully reinstate the mania-like profile of DAT KD mice, this profile re-emerged after the administration of a low, subthreshold dose of GBR 12909. Importantly these latter data are consistent with stimulant-induced mania observed in BD (Wingo and Ghaemi, 2008). In contrast, the mania-like profile of mice administered GBR 12909 acutely is consistent even after repeated testing and familiarization with the BPM chamber. Hence, further studies using more systematic within-subjects designs are warranted to clarify the respective contributions of the subjects’ experimental history and prior drug exposures to the behavioral profiles of these genetic and pharmacological models of reduced DAT function.
Upon initial testing, DAT KD mice exhibit increased activity, increased specific exploration, and reduced spatial d in the mouse BPM, consistent with the exploratory profile of manic BD patients (Perry et al., 2009). The present studies suggest however that, after familiarization to the BPM chamber, the same cohort of DAT KD mice exhibited a less dramatic phenotype. Activity levels were increased in DAT KD compared to WT mice only in the first 10 min of their second exposure to the BPM chambers (Fig. 2A), while no increase in specific exploration or altered spatial d of DAT KD mice was observed (Fig. 2C,D). Thus the mania-like phenotype of DAT KD mice might be a result of their introduction to a novel environment, which could alternatively be viewed as a disruption of their environmental rhythm. Exposure to novelty induces increased exploration (File and Wardill, 1975, Flicker and Geyer, 1982) as well as increased dopamine release in the nucleus accumbens (Legault and Wise, 2001) in normal rodents. Moreover, it has been suggested that reduced DAT expression is linked to increased novelty preference (Zhu et al., 2007). We therefore introduced novel features to the BPM chambers in an attempt to stimulate greater exploration in DAT KD mice. The novel features we introduced included inserting objects into 3 of the 11 wall holes (Flicker and Geyer, 1982), and by changing the texture and color of the chamber floor. This altered environment resulted in DAT KD mice exhibiting significantly higher levels of transitions compared to DAT KD mice in the normal environment, an effect not observed in the WT mice (Fig. 3A). In contrast to previous reports in rats however (Flicker and Geyer, 1982, Legault and Wise, 2001), access to novel objects in the holes did not induce an increase in specific exploration in DAT KD or WT mice (Fig. 3A,B). Previous reports of novelty-induced increase in specific exploration utilized methodologies both consistent (Flicker and Geyer, 1982) and different (Legault and Wise, 2001) to those reported here. It may therefore be that such novelty does not induce increases in specific exploration in mice. The introduction of the novel environment differentially affected spatial d over time in WT or KD mice. WT mice exhibited an initial (0-10 min) novelty-induced increase in spatial d, while KD mice were unaffected by the novel environment, although KD mice in the normal environment exhibited an increase in spatial d between 20-30 min (Fig. 3D). The cause of these minor alterations in spatial d in two separate 10 min time-bins remain unknown and would require further investigation. Thus introducing novelty in the BPM only partially reinstated the mania-like phenotype of DAT KD mice, as measured by hyperlocomotion. In support of these findings, environmental novelty resulted in hyperlocomotion in Lewis compared to Fischer 344 rat strains, which correlated to lower DAT function in Lewis rats (Gulley et al., 2007).
Novelty and environment may have an important role to play in the behaviors observed in BD. The social zeitgeber theory of mood disorders suggests that novel environmental factors may disrupt a subjects’ circadian rhythm, resulting in manic episodes in subjects predisposed to the disorder (Ehlers et al., 1988, Malkoff-Schwartz et al., 1998, Malkoff-Schwartz et al., 2000). In fact, Interpersonal and Social Rhythm Therapy designed to maintain environmental consistencies and manage rhythm dysregulation may buffer against future episodes (Frank et al., 2005). Further support for this theory is observed where novel environmental events which disrupted social rhythm predicted episodes of (hypo)mania (Sylvia et al., 2009). Thus, while repeated testing-induced loss of phenotype in DAT KD mice could reflect simple habituation to the testing chambers, these data could also be interpreted as consistent with BD where environmental stability can buffer against provoking mania-like behavior.
One striking feature of BD is that a manic episode can be triggered by stimulant administration. Hence during periods of euthymia, where BD patient behaviors are no longer ‘manic’ and less clearly distinguishable from controls, stimulant-induced mania has been observed (Wingo and Ghaemi, 2008). Moreover, stimulant abuse can also lead to manic episodes (Lake et al., 1983). The present studies demonstrate that consistent with BD, DAT KD mice are sensitive to the stimulant effects of GBR 12909, resulting in the mice once again exhibiting a mania-like profile in the BPM. Given that these effects were observed in DAT KD mice at a dose that did not affect WT, C57BL/6J, or 129/SJ mice (present studies; Young et al., 2010), suggests that KD may in fact be hypersensitive the effects of GBR 12909. These data are consistent with previous reports of increased sensitivity of DAT KD mice to cocaine (Tilley et al., 2007). The effects of psychostimulants in these mice are in contrast with mice that have no DAT (knockout mice), where psychostimulants such as amphetamine and cocaine actually reverse their hyperactive behavior (Gainetdinov et al., 1999). Since psychostimulants are used to treat attention deficit hyperactivity disorder (ADHD) patients, it has been proposed that DAT knockout mice may prove to be a useful model of ADHD (Gainetdinov et al., 1999). Despite apparent predictive validity for treatment, the construct validity of such a DAT knockout model has been questioned (Hewitt et al., 2009), given that increased striatal DAT are observed in ADHD patients (Krause et al., 2003, Madras et al., 2002). Thus the hyersensitivity to stimulants in DAT KD mice and observations of increased motivation for food rewards - which may be interpreted as hedonistic behavior consistent with BD (Cagniard et al., 2006) - provide support for these mice as a more appropriate model for investigating BD manic behaviors.
To date, the exploratory profile of patients with BD during periods of euthymia has yet to be determined. Given that environmental stability may buffer against episode reoccurrence (Sylvia et al., 2009), repeated testing of patients with BD in the hBPM may provide further information for the interpretation of the present studies (loss of phenotype observed in DAT KD mice with repeated exposure, partial reinstatement with novelty, full reinstatement with a subthreshold dose of GBR 12909; Fig. 6). Moreover, assessing patients with BD in the human BPM that may represent differing subgroups of the disorder (MacQueen et al., 2005) might also prove useful when interpreting the data presented here. Furthermore, behaviors which vary dependent upon BD state, such as risk taking behavior as measured by the Iowa Gambling Task (Adida et al., 2008, Clark et al., 2001, Frangou et al., 2008), would also prove useful to examine in mouse models of BD mania such as DAT KD mice. Such studies may provide evidence further behavioral consistency of DAT KD mice with varying stages of BD.
Figure 6. Summary of repeated testing of DAT KD mice in the mBPM.
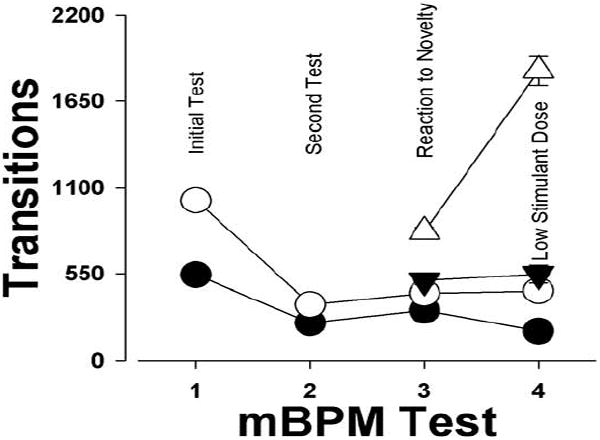
DAT KD mice in a normal mBPM environment (○) initially exhibited hyperactivity compared to WT mice (●) (Perry et al., 2009) that disappeared by the second stage of testing (experiment 1). The introduction of novelty stimulated activity in the DAT KD mice (△) but not WT mice (▼) in the third stage of testing (experiment 2), while a sub-threshold dose of GBR 12909 vastly increases activity in DAT KD mice (△) compared to WT mice (▼), and KD mice administered vehicle (○) (experiment 3). Data collapsed across sex and presented as mean.
Acute pharmacological challenge in mice with the selective DAT inhibitor GBR 12909 resulted in a behavioral profile that is consistent with that of BD mania (Perry et al., 2009). We observed increased specific exploration in mice administered 16 mg/kg GBR 12909, which was reduced at the 28 mg/kg dose, in both C57BL/6J and 129/SJ mice (Young et al., 2010). While earlier studies reported reduced rearing in mice administered GBR 12909 (Kliethermes and Crabbe, 2006), this was at 20 mg/kg, suggesting a narrow dose response window of the effects of this selective DAT inhibitor. In the present studies, we measured the exploratory behavior of mice previously exposed to GBR 12909 in the BPM: a) to assess the effect of a narrower dose response window of GRB 12909, between 9 and 16 mg/kg in C57BL/6J mice; and b) to examine whether 16 and 28 mg/kg would produce consistent effects on exploratory activity of mice after repeated testing. Consistent with previous reports, GBR 12909 administration at 16 mg/kg increased activity (Fig. 4A), increased specific exploration (Fig. 4C), and lowered spatial d (Fig. 4D). Administration of 28 mg/kg GBR 12909 mice also produced consistent effects to those previously reported, namely increased activity, reduced specific exploration, and reduced spatial d (Young et al., 2010). Interestingly, doses of GBR 12909 between the ineffective and effective activity-altering doses (9 and 16 mg/kg respectively), produced similar effects to 16 mg/kg, but at a reduced expression (Fig. 4A, C and D). Again consistent with previous data, 9 mg/kg GBR 12909 did not affect exploratory activity in any measure assessed in the present study. Thus it appears that GBR 12909 administration consistently increased activity in C57BL/6J mice irrespective of their habituation to the test chambers, consistent with previous reports (Zalcman, 2001).
While the DAT-manipulated mouse models presented here may provide a model of BD mania with some etiological validity, other models with putative etiological validity also exist. Polymorphisms of the Clock gene have been identified in BD patients (Benedetti et al., 2003). Clock gene mutant mice exhibit BD mania-like phenotypes including increased responsiveness to novelty as well as hyperactivity (Coyle, 2007, Le-Niculescu et al., 2009, Roybal et al., 2007). The anti-manic agents valproate and lithium may act via the glycogen synthase kinase-3 (GSK-3; (Kozikowski et al., 2007), and mice overexpressing GSK-3 beta also exhibit hyperactivity (Prickaerts et al., 2006). The consistency of the Clock mutant or GSK-3 phenotype upon repeated testing have not been established however, nor has their exploratory behavior been examined in the BPM. Thus it would be interesting to examine the acute and chronic behavior of these BD model mice in the BPM.
In conclusion, we have presented evidence for two mouse models of BD mania,, both of which are mediated by reducing DAT function. These two models differ however, in the mechanism by which DAT function is reduced. The pharmacological model represents an acute challenge, while the genetic model represents a chronic reduction in DAT function. The present studies suggest that repeated assessment of DAT KD mice in the BPM resulted in a loss of their mania-like phenotype as compared to those historically observed in the same mice as reported in Perry et al (2009). Further studies of exporatory behavior in euthymic BD patients and more systematic experiments in the genetic and pharmacological mouse models will be needed to better understand the implications of this apparent lack of the hyperactivity phenotype in DAT KD mice in a familiar environment. The sensitivity of DAT KD mice to GBR 12909 at doses that does not elicit alterations in behavior of WT mice suggests consistency with BD sensitivity to stimulant effects. While the consistency of expression of each model may differ, each may represent a viable model of BD, perhaps reflecting differing stages of the disorder, or differing subgroups within BD. Future studies examining the validity of these models could be performed by assaying the effects of chronic treatment with antimanic agents, such as valproate, on their exploratory behavior.
Acknowledgments
We thank Richard Sharp, Virginia Masten, and Mahálah Buell for their support. This study was supported by NIH grants R01-DA02925, R21-MH085221, and R01-MH071916, as well as by a NARSAD Young Investigators Award (JWY) and by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin JM, et al. Lack of insight may predict impaired decision making in manic patients. Bipolar disorders. 2008;10:829–37. doi: 10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Newberg AB. A preliminary study of dopamine transporter binding in bipolar and unipolar depressed patients and healthy controls. Neuropsychobiology. 2007;55:167–70. doi: 10.1159/000106476. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–6. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–70. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. The American journal of psychiatry. 2001;158:1605–11. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- Coyle JT. What can a clock mutation in mice tell us about bipolar disorder? Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6097–8. doi: 10.1073/pnas.0701491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Sanger DJ, Steinberg H, Tomkiewicz M, U’Prichard DC. Lithium and alpha-methyl-p-tyrosine prevent “manic” activity in rodents. Psychopharmacologia. 1974;36:263–74. doi: 10.1007/BF00421808. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Archives of general psychiatry. 1988;45:948–52. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Einat H. Modelling facets of mania--new directions related to the notion of endophenotypes. Journal of psychopharmacology (Oxford, England) 2006;20:714–22. doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44:53–9. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Flicker C, Geyer MA. Behavior during hippocampal microinfusions. I. Norepinephrine and diversive exploration. Brain Res. 1982;257:79–103. doi: 10.1016/0165-0173(82)90006-6. [DOI] [PubMed] [Google Scholar]
- Frangou S, Kington J, Raymont V, Shergill SS. Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry. 2008;23:300–8. doi: 10.1016/j.eurpsy.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of general psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biological psychiatry. 1999;46:303–11. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, biochemistry, and behavior. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neuroscience and biobehavioral reviews. 2007;31:825–31. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, et al. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. American journal of medical genetics. 2001;105:145–51. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Molecular psychiatry. 2006;11:125–33. 15. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Everett CV, Zahniser NR. Inbred Lewis and Fischer 344 rat strains differ not only in novelty- and amphetamine-induced behaviors, but also in dopamine transporter activity in vivo. Brain Res. 2007;1151:32–45. doi: 10.1016/j.brainres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR 12909, GBR 13069 and GBR 13098: specific inhibitors of dopamine uptake. Eur J Pharmacol. 1984;103:241–8. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Marsden CA, Hollis CP, Fone KC. Behavioural characterisation of the effects of acute and repeated administration of GBR 12909 in rats: further evaluation of a potential model of ADHD. Neuropharmacology. 2009;57:678–86. doi: 10.1016/j.neuropharm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Molecular psychiatry. 2005;10:1104–9. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Sadovnick AD, Kristbjarnarson H, Bergesch P, Mroczkowski-Parker Z, Drennan M, et al. Possible locus for bipolar disorder near the dopamine transporter on chromosome 5. American journal of medical genetics. 1996;67:533–40. doi: 10.1002/(SICI)1096-8628(19961122)67:6<533::AID-AJMG4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Crabbe JC. Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacology, biochemistry, and behavior. 2006;85:57–65. doi: 10.1016/j.pbb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, et al. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007;129:8328–32. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, la Fougere C, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neuroscience and biobehavioral reviews. 2003;27:605–13. doi: 10.1016/j.neubiorev.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Lake CR, Tenglin R, Chernow B, Holloway HC. Psychomotor stimulant-induced mania in a genetically predisposed patient: a review of the literature and report of a case. Journal of clinical psychopharmacology. 1983;3:97–100. [PubMed] [Google Scholar]
- Le-Niculescu H, Patel SD, Bhat M, Kuczenski R, Faraone SV, Tsuang MT, et al. Convergent functional genomics of genome-wide association data for bipolar disorder: comprehensive identification of candidate genes, pathways and mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:155–81. doi: 10.1002/ajmg.b.30887. [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. The European journal of neuroscience. 2001;13:819–28. doi: 10.1046/j.0953-816x.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Molecular psychiatry. 2005;10:811–26. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter: relevance to attention deficit hyperactivity disorder (ADHD) Behav Brain Res. 2002;130:57–63. doi: 10.1016/s0166-4328(01)00439-9. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Archives of general psychiatry. 1998;55:702–7. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychological medicine. 2000;30:1005–16. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Archives of general psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neuroscience and biobehavioral reviews. 2007;31:858–73. doi: 10.1016/j.neubiorev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Pharmacology and physiology of stereotyped behavior. Journal of psychiatric research. 1974;11:1–10. doi: 10.1016/0022-3956(74)90062-4. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D(1), D(2), and D(3) receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton R, Steinberg H. Dose-response relations of amphetamine-barbiturate mixtures. Nature. 1963;197:1017–8. doi: 10.1038/1971017a0. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Alloy LB, Hafner JA, Gauger MC, Verdon K, Abramson LY. Life events and social rhythms in bipolar spectrum disorders: a prospective study. Behav Ther. 2009;40:131–41. doi: 10.1016/j.beth.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC neuroscience. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Ghaemi SN. Frequency of stimulant treatment and of stimulant-associated mania/hypomania in bipolar disorder patients. Psychopharmacology bulletin. 2008;41:37–47. [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010 doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neuroscience and biobehavioral reviews. 2007;31:882–96. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalcman SS. Interleukin-2 potentiates novelty- and GBR 12909-induced exploratory activity. Brain Res. 2001;899:1–9. doi: 10.1016/s0006-8993(01)02090-x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. The European journal of neuroscience. 2007;26:717–28. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


