Abstract
Phagocytes engulf foreign cells but not ‘self’ in part because self cells express CD47 as a ligand for signal regulatory protein SIRPα, which inhibits phagocytosis. Motivated by reports of upregulation of CD47 on both normal and cancerous stem cells [1] and also by polymorphisms in SIRPα [2], we show here that inhibition of engulfment correlates with affinity of CD47 for SIRPα – but only at low levels of CD47. One common human polymorph of SIRPα is studied and binds more strongly to human-CD47 than to mouse-CD47 (Kd ≈ 0.12 μM and 6.9 μM, respectively) and does not bind sheep red blood cells (RBC) – which are well-established targets of human macrophages; in comparison, a common mouse polymorph of SIRPα binds with similar affinity to human and mouse CD47 (Kd ≈ 0.22 μM). Using immunoglobulin (IgG)-opsonized particles with varying levels of either human- or mouse-CD47, the effective inhibition constants Ki for blocking phagocytosis are then determined with both human- and mouse-derived macrophages. Only human phagocytes show significant differences in man versus mouse Ki's and only at CD47 levels below normal densities for RBCs. While phospo-signaling through human-SIRPα shows similar trends, consistent again with the affinity differences, saturating levels of CD47 (> Ki) can signal and inhibit phagocytosis regardless of man versus mouse. Quantitative analyses here prompt more complete characterizations of both CD47 levels and SIRPα polymorphisms when attempting to study in vivo effects of these key proteins in innate immunity.
Introduction
Critical to innate immunity, macrophages engulf a foreign cell or particle in a coordinated process of adhesion, activation, and internalization, but macrophages come into contact with ‘self’ cells more frequently than foreign. Opsonization of a target by immunoglobulin-G (IgG) facilitates binding to the macrophage's Fcγ receptors (FcγRs), which signal assembly of a focal adhesion-like synaptic structure for firm attachment to the target Error! Reference source not found.Error! Reference source not found.], but self cells are also opsonized by Ig [5]. While an analogy to integrin-based focal adhesions rightly suggests the importance of ligand densities and various phospho-Tyrosine (pTyr) signaling proteins as well as myosin-II to pull on a nascent adhesion to promote adhesion maturation [6], the processes by which macrophages distinguish foreign objects from autologous cells of self remain largely unclear. Studies of CD47-knockout mice have shown that red blood cells (RBC) that lack the membrane receptor CD47 are readily taken up by normal mouse macrophages – though not by CD47-null macrophages [6] A.E. Brown and D.E. Discher. Conformational changes and signaling in cell and matrix physics, Current Biology 19 (2009) pp.R781–R789.
[3]. Human RBCs with considerably reduced levels of CD47 (10-20% of normal in some Rh variants) show no evidence of enhanced interaction with phagocytes [4[5[6], but hematopoietic stem cells (HSC) in the circulation as well as leukemic cancer stem cells have been reported to overexpress CD47 by 2- to 4-fold (for human and mouse HSC, respectively) relative to marrow resident normal cells [1]. Extra CD47 on cells in both mouse and man is speculated to protect from phagocytic clearance, but quantitative relations to underlying protein-protein interactions and downstream signals have remained obscure.
CD47 binds with high specificity to the inhibitory membrane receptor SIRPα [7], which is expressed at particularly high densities on macrophages [8[9[10[11]. SIRPα belongs to a ‘paired receptors’ class of membrane proteins analogous to Natural Killer (NK) cell receptors involved in MHC recognition [12]. SIRPs have three extracellular immunoglobulin domains, a variable region at the N-terminal and distinct transmembrane and cytoplasmic sequences that function as activating or – as with SIRPα – inhibitory signal motifs [13[14[15]. Macrophages distinguish self from foreign through binding of SIRPα's N-terminal to the lone Ig domain of ‘marker of self’ CD47 on target cells. The interaction leads to pTyr modifications of SIRPα's immune-receptor Tyrosine-based inhibitory motif (ITIM) with downstream activation of SHP-1 phosphatase and subsequent deactivation of myosin-II and the contractile cytoskeletal activity involved in pulling a target into a macrophage [16]. Differences in CD47-SIRPα binding presumably affect signaling and inhibitory function, but linearity or not of signal transmission is unclear and has implications for up- or down-regulation of CD47. In fact, saturation in binding and signaling should be anticipated.
If the hypothesis of CD47 as a marker of self is valid, then macrophages should be able to discriminate between cells of different species and perhaps between different ‘strains’ or polymorphisms. Earlier studies have indicated limited cross-reactivity across species between SIRPα and CD47 [17]. Initial studies here demonstrate that sheep-RBCs as well as mouse-RBCs bind less soluble human SIRPα when compared to human-RBCs (Figure 1A). Based on this divergence, human macrophages might be expected to engulf opsonized sheep RBCs – an expectation borne out by many prior studies using sheep-RBCs as targets for human macrophages in phagocytosis (e.g. [18[19[20]). In addition to the recognition of SIRPα and CD47 between species, there exists considerable polymorphism in SIRPα between mouse strains [21] and between humans [2]; indeed, from a very limited but worldwide study of 37 human haplotypes, 10 variants of SIRPα's ~100 amino acid N-terminal domain were reported. Polymorphisms in SIRPα in humans have been suggested, based on xenografts of human-HSC into mice [2], to explain stem cell compatibility differences in humans [22]. SIRPα polymorphisms that alter interactions thus have major implications for human health, contributing perhaps to anemias and autoimmune diseases [23]. Here we exploit man versus mouse differences in quantitation of CD47-SIRPα binding, phagocytosis, and signaling as we seek to define mechanisms by which macrophages discern foreign from self.
Figure 1. Species-specific binding of soluble human-SIRPα to various species RBCs and CD47-coated beads.

(A) Fresh human, sheep, and mouse RBC binding to soluble hSIRPα (4μM of GST conjugate), as detected by FITC-anti-GST. ‘Bkgd’ is obtained with RBC plus anti-GST. Species-specific affinities of hCD47- and mCD47-coated beads binding to soluble (B) hSIRPα or (C) mSIRPα based on mean fluorescence in flow cytometry. Saturation binding fits gave the indicated dissociation constants, Kd.
RESULTS and DISCUSSION
SIRPα Binding to CD47 is Species-specific for human but not mouse
In order to clarify some of the limits of CD47 as a “self” marker, we assessed the extent to which soluble human SIRPα (hSIRPα) binds to red blood cells (RBC) and purified CD47 from different species. Soluble human SIRPα used in our studies and expressed in the human-derived THP-1 macrophages below express hSIRPα variant 2 – ‘V2’ in the nomenclature of [2] – as assessed by reverse transcriptase PCR (RT-PCR) and sequencing. RBC from mice as well as sheep respectively show weak to no binding to this recombinant hSIRPα when compared to human RBCs (Figure 1A). Based on the lack of sheep RBC interactions with soluble hSIRPα, human macrophages would be expected to phagocytose IgG-opsonized sheep RBC – which is indeed commonly reported in studies of Fcγ receptor mediated phagocytosis [18[19[20]. RBC membranes are nonetheless complex, with many additional differences between species: for example, CD47 on human RBCs is less mobile than on mouse RBCs in part due to human CD47's association with the species-specific Rh complex [24]. To eliminate such differences in measurements of binding, the immunoglobulin-like domain of human CD47 (hCD47) and also of mouse CD47 (mCD47) were recombinantly expressed with a spacer domain plus a C-terminal biotinylation site [25[16]. The attachment of the hCD47 and mCD47 to avidin-coated beads was confirmed using antibodies against the specific species by flow cytometry (Figure S1), and the density of CD47 was adjusted to that of normal RBC [26] with binding affinity then determined by varying the concentration of soluble hSIRPα. Flow cytometry data was fit to saturation binding, giving dissociation constants (Kd) of 0.12 and 6.93 μM that indicate a ~60-fold weaker binding of mCD47 to hSIRPα (Figure 1B).
The SIRPα-V2 used here together with past results for SIRPα-V1 [27] suggest similar binding affinities for human CD47 despite 9 residue differences in the N-terminal domain that binds CD47. With the same types of beads, interactions of soluble mSIRPα were also examined; the N-terminal domain of this mSIRPα – referred to as 129-IgV [2] – differs in only minor ways from mSIRPα on J774A.1 macrophages (used below), which has the sequence of that reported in BalbC mice (see Methods). In contrast, the latter mSIRPα differs from human at 43 residues, including key amino acids at or near hCD47's binding site for hSIRPα [28]. No difference in binding was detected in the moderately high affinity interactions of mSIRPα with both mCD47- coated particles and hCD47-coated particles (Kd = 0.22 μM; Figure 1C). The lack of species specificity with mouse SIRPα needs to be understood better at a detailed structural level, but phagocytosis results below with macrophages will be seen to be consistent with these binding results.
Species-specific phagocytosis is weakly inhibited by soluble CD47
In a first study of phagocytosis, sheep and human RBC were IgG-opsonized and fed to macrophages. The opsonization level is linear with anti-serum dilution (Figure 2A) while phagocytosis of sheep-RBC by human-derived THP-1 macrophagesas well as by mouse-derived J774A.1 macrophages is non-linear, exhibiting a saturable type of response (Figure 2B,C). Addition of soluble human-CD47 at μM concentrations (> Kd) showed at most a slight inhibition of phagocytosis of sh-RBC by THP-1 macrophages (Figure 2C, inset) and by J774A.1 macrophages (not shown). Because phagocytosis is a highly localized process on the macrophage, signaling at the phagocytic synapse would likely be tested better, we thought, if the various recombinant CD47's were attached to otherwise identical target particles.
Figure 2. Phagocytosis of Ig-opsonized sheep-RBC and human-RBC by human-THP-1 macrophages.
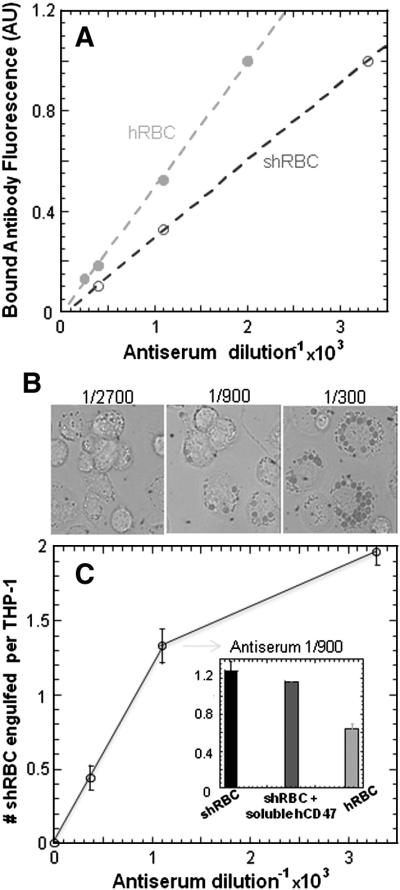
(A) Fresh human and sheep RBC were incubated with antiserum at different dilution ratios and detected by fluorescent secondary antibodies, exhibiting a linear opsonization based on mean fluorescence in flow cytometry. (B) Phagocytosis of shRBC by human-derived THP-1 macrophages at different opsonization level observed by DIC microscopy, which showed a saturable type of response (C). Addition of soluble human-CD47 at μM concentrations showed at most a slight inhibition of phagocytosis of sh-RBC by THP-1 macrophages but not enough to reach hRBC phagocytosis levels at 1/900 antiserum dilution ratio.
Inhibition of phagocytosis by CD47 is species-specific for human but not mouse
The same type of avidin-coated particles as used for binding in Figure 1B,C (but made red fluorescent in Figure 3A) were IgG-opsonized with anti-streptavidin for FcγR-mediated phagocytosis and then fed to human or mouse macrophages. These particles of course lack membrane proteins (eg. Rh proteins) and standardize the opsonization as well as the CD47 presentation. After the phagocytosis assay, non-engulfed particles were detected with an anti-Fc (green) against the IgG-opsonin. Phagocytosed particles were protected from the anti-Fc because cells were not permeablized, thus providing a method to quantify the internalized beads per phagocyte for both human THP-1 and mouse J774A.1 macrophages. With increasing opsonization human macrophages phagocytosed the uncoated “CD47-null” beads significantly more than beads displaying hCD47 (Figure 3B). The two ligands, CD47 and IgG-opsonin, do not compete and do not interfere with SIRPα binding [16], and the phagocytosis results for mCD47 coated beads proved consistent with the hCD47 coated beads (Figure 3C). The dependence on the level of IgG-opsonization fits a saturation binding process, indicative of the specificity of Fcγ-R mediated phagocytosis as seen with RBC (Figure 2C).
Although seminal studies of CD47-knockout mice implicated CD47 as a “marker of self” on mouse RBCs [6] A.E. Brown and D.E. Discher. Conformational changes and signaling in cell and matrix physics, Current Biology 19 (2009) pp.R781–R789.
Figure 3. CD47 is sufficient to inhibit phagocytosis.
(A) Microbeads with streptavidin were coated with anti-streptavidin IgG as the opsonin ± biotinylated human-CD47 or mouse-CD47. Phagocytosis of beads (red) by THP-1 or J774A.1 cells was assessed in DIC and fluorescence with non-ingested beads (green) visible with rabbit anti-streptavidin plus a second, fluorescent goat anti-rabbit antibody. White arrow indicates non-phagocytosed beads. Scale bar, 10μm. Streptavidin beads coated with both anti-streptavidin IgG as the opsonin and ± biotinylated (B) human CD47 particle targets with THP-1 macrophages or (C) mouse CD47 particle targets with J774A.1 in a phagocytosis assay.
[3], human Rhnull genotypes with major co-deficiencies of CD47 on RBCs show no evidence of increased macrophage interactions [5[6]. One could also hypothesize that overexpression of CD47, such as reported for circulating hematopoietic and leukemic stem cells in man or mouse [1], could mediate strong adhesion and thereby promote phagocytosis rather than inhibit it (i.e. adhesion dominates signaling).
Based on results from a 40-fold range of CD47 densities, human-CD47 proved more potent than mouse-CD47 in inhibiting phagocytosis by THP-1 macrophages (Figure 4). The species-specific inhibition constant, Ki differed by 5-fold at high IgG-opsonin and 2.2-fold at low opsonin (Table 1 and Figure S2A). However, maximum inhibition proved to be the same for human- and mouse-CD47 and is achieved in vitro here with particles at CD47 densities at or below those estimated for CD47 on human RBC (~250 molecules/μm2). Even at several fold higher densities, the same maximum inhibition was found, indicating that CD47-mediated signaling dominates CD47-mediated adhesion. Primary human peripheral blood monocytes incubated with IgG-opsonized particles at high densities of hCD47 or mCD47 (~400 molecules/μm2) also showed statistically similar reduction in phagocytosis (Figure 4A, inset).
Figure 4. Inhibition of phagocytosis by CD47 is density dependent.
IgG-opsonized particles with human (solid line) and mouse CD47 (dashed line) were used as targets for (A) human THP-1 macrophages or (B) mouse J744A.1 macrophages. Grey shaded vertical bar indicates normal RBC density (~250 CD47/μm2). Species-specific differences are quantitated by fits to y = a – b xm / (Kim + xm) with effective Ki [molecules/mm2] that differ by 5-fold at high opsonin, although the maximum inhibition is similar (see Table 1). A Hill coefficient of m = 2 suggests cooperativity.
Table 1. Human THP-1 phagocytosis of beads.
Summary of conditions used to fit the data points for both hCD47 and mCD47 coated beads at high and low opsonization. The Ki for each of conditions are summarized with low Ki having a higher inhibitory effect.
|
a |
b |
m |
Ki |
|
|---|---|---|---|---|
| hCD47 (High Opson.) |
1.07 |
0.53 |
2 |
21 |
| mCD47 (High Opson.) |
1.07 |
0.53 |
2 |
110 |
| hCD47 (Low Opson.) |
0.46 |
0.25 |
2 |
87 |
| mCD47 (Low Opson.) | 0.46 | 0.25 | 2 | 190 |
Mouse J774A.1 macrophages, in contrast to human cells, showed levels of inhibition of phagocytosis IgG-opsonized particles that were similar for both human-CD47 and mouse-CD47 at all CD47 densities (Figure 4B). The lack of difference in phagocytosis by mouse macrophages is fully consistent with the lack of difference in binding affinities of soluble mSIRPα for mCD47 and hCD47 (Figure 1C). More importantly, the results for human and mouse showed that even 10-20% of normal CD47 densities are sufficient to inhibit phagocytosis for CD47-SIRPα within the same species whereas weaker binding can be compensated by increasing CD47 density.
The surface concentrations of CD47 above are not small. Assuming a phagocytic synapse with a gap between macrophage and target of ~10 nm [28], the Ki range of of 20-200 molecules/μm2 corresponds to a gap concentration Ki3D ≈ 3-30 μM. Because Ki3D >> Kd, there seem to be impediments to the effectiveness of inhibitory signaling by CD47 – such as overcoming Fcγ receptor activation within the same signaling synapse – which probably explains the relative ineffectiveness of soluble CD47 at ~1 μM (Figure 2C, inset). CD47 on the macrophage [26] might also interact in cis with SIRPα and thereby compete with CD47 in trans (on the target's surface), which would effectively reduce the inhibitory interaction. The fits of phagocytosis inhibition also suggest a Hill coefficient of m = 2 which implies cooperative interactions that are characteristic of dimers and might further explain the limited inhibition by soluble CD47. Nano-resolution imaging of the molecular rearrangements within the phagocytic synapse might help to clarify such structure-function issues.
CD47 signaling through SIRPα is species-specific
Based upon the binding and phagocytosis studies above (Figure 1 and 4A), SIRPα binding to CD47 sends species-specific signals primarily through hSIRPα. Recent results have also documented hSIRPα localization to the phagocytic synapse with targets presenting CD47 [16], which is consistent with ligand-receptor interactions that are expected to phospho-activate SIRPα's immune-tyrosine based inhibitory motif (ITIM) which then activates SHP-1 phosphatase [29[30]. We therefore hypothesized for a last set of experiments that hCD47 would also prove more effective than mCD47 at inducing SIRPα phosphorylation during phagocytosis. Immunoprecipitation of SIRPα followed by Western blot analysis of phospho-Tyrosine (pTyr) showed a clear but saturable difference in signaling by hCD47 versus mCD47 (Figure 5). Normalization of CD47 densities to the phagocytosis inhibition constant for human-CD47 (Figure 4A), denoted as Ki-h, and normalization of pTyr levels to SIRPα intensities showed that the effective signaling constant Ks not only approximated the Ki for each species but also differed by ~10-fold between human and mouse.
Figure 5. Species-specific signaling through SIRPα.
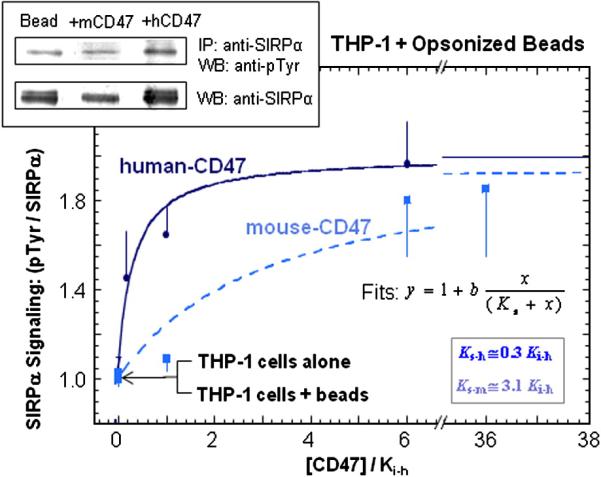
hCD47 or mCD47 were bound at varying densities to opsonized beads and phagocytosed by THP-1 macrophages. From macrophage lysates, SIRPα was immunoprecipitated and immunoblotted (inset) for quantitation of phospho-Tyr and total SIRPα for normalization. Fits of the data gave effective signaling constant Ks for each species that depends on the CD47 density; all densities are scaled by hCD47's inhibitory constant (Ki-h) as determined in Figure 4.3A at the same opsonization.
CONCLUSIONS
Normal macrophages are efficient at removing typical targets such as foreign cells or particles and apoptotic cells [31], but cells or particles that express a recognizable CD47 are engulfed in vitro at a lower frequency. The decision of a macrophage to ‘eat’ a target is in part made by the extent of target opsonization, and Ig concentrations being very high in bodily fluids seems to lead to absorption or perhaps weak binding at some level to all cells [], especially aged blood cells [36]. Figure 6 summarizes our quantitative studies of IgG-opsonized beads decorated with CD47 and phagocytosed by human or mouse macrophages. A higher CD47-SIRPα binding strength (set by species) results in higher potency inhibition and this increases with increasing opsonin activation of the cell. Such signal amplification through antagonistic interactions might seem counter-intuitive, but enhanced CD47 signaling with increased IgG-opsonization might occur because IgG binding to FcγR [33] promotes intimate adhesion between target and macrophage, narrowing the gap between interfaces and thereby promoting CD47 interactions with SIRPα within the phagocytic synapse.
Figure 6. Potency and binding strength depend on species-specific CD47-SIRPα interactions.

IgG-opsonization modulates the density-dependent activity of hCD47 or mCD47 with either human THP-1 macrophages (hSIRPα) or mouse J774A.1 macrophages (mSIRPα). Each point on the plot represents a Kd obtained from binding studies per Figure 4.1B and a Ki obtained per Figure 4.3A as fitted by a Hill-type model. Larger symbols indicate results with higher opsonin, which promotes synapse formation and thereby more potent inhibition than at lower opsonin.
Based on the various man versus mouse results here, a 60-fold higher binding affinity of CD47 for SIRPα (Figure 1B) produces a 10-fold more specific pTyr signal in SIRPα (Figure 5), which leads to a 5-fold more specific inhibition of phagocytosis at high opsonin (Table 1). Biochemical differences propagate but seem blunted rather than amplified in this process of signaling self. Specificity is also lost altogether at high CD47 density, consistent with a single saturable process of binding → signaling → local inhibition. While the interactions might be crucial to some species-specific recognition, polymorphisms in SIRPα within a given species have been reported for human [34[35[2] and mouse [21] and raise important questions about altered interactions. Structural analysis of the human SIRPα-CD47 complex has suggested that SIRPα polymorphisms occur outside the binding site and therefore seem unlikely to directly affect CD47 binding [28]; polymorphisms have been speculated to frustrate pathogens that seek to exploit the SIRPα inhibitory signal [28]. Other paired receptors seem to use this strategy including Ly49 which interacts with viral particles [36] and the paired immunoglobulin-like receptor (PIR) that binds bacteria [37]. However, human hematopoietic stem cells (HSCs) have been shown to engraft efficiently only into the NOD strain of mouse because human-CD47 binds sufficiently strongly to SIRPαNOD but not other mouse SIRPα's [2]. Our binding, signaling, and phagocytosis results motivate additional characterization of CD47 expression levels on all cells involved, especially in light of changes in CD47 expression levels on HSCs [1]. The results here nonetheless help to make it clear that in man, and probably in mouse, innate immune cells distinguish cells or other taregts for clearance through specific CD47-initated “self” signals that ultimately inhibit engulfment.
Materials and Methods
Chemicals
Dulbecco's phosphate-buffered saline (DPBS) without Ca2+ or Mg2+ (Invitrogen) was supplemented with either 1% BSA or 1% BSA + 0.05% Tween 20 (Sigma-Aldrich). PKH26 (Sigma-Aldrich) hydrophobic dye was used for red cell labeling. Tris-buffered saline (TBS) and TBS with Tween 20 (TTBS) were used in Western blotting.
Antibodies
The fluorescein-labeled antibody B6H12-FITC was used against human CD47 (BD Biosciences). Opsonizing antibodies against human and sheep RBCs included rabbit anti-human RBC, and rabbit anti-sheep RBC (Sigma-Aldrich); these were used as IgG opsonin in phagocytosis assays. Antibodies against 1.1μm radius streptavidin-coated polystyrene beads (Spherotech) included rabbit anti-streptavidin (Sigma-Aldrich); and rabbit anti-streptavidin conjugated with FITC (Rockland Immunochemicals) used also as IgG opsonin in phagocytosis assays. Secondary antibodies used for detecting opsonin levels and uningested beads included goat anti-rabbit FITC or goat anti-rabbit F(ab’)2 R-PE (Sigma-Aldrich). Secondary antibodies used for in conjuction with soluble SIRPα or CD47 binding included anti-GST Alexa 488 (Invitrogen) and anti-biotin Cy5 (Sigma-Aldrich) respectively.
SIRPα reverse transcriptase PCR and sequence
The SIRPα variant expressed in THP-1 macrophages was confirmed by RNA extraction (Qiagen) using a one step reverse transcriptase PCR (RT-PCR) amplification (Invitrogen). Samples of the PCR product were run on 1% agarose gel and gel purified for sequencing. Primers used for both RT-PCR sequencing include the following 5′-GGGTGAGGAGGAGCTGCAGGTGATT-3′ and 5′-GCGCTCGAGCCGTTCATTAGATCC-3′. In tha case of mSIRPα expressed in J774A.1 macrophages the primers used were 5′-CGTCCTGTTTCTGTACAGGA-3′ and 5′-AGTTCACTTTCTGGTCAGGT-3′. J774A.1 cells are derived from Balb/C mice, and compared to our soluble mSIRPα, the mSIRPα in the J774A.1 cells differs in the N-terminal domain by 8 residues, with 6 conservative changes and the other 2 being far from the site of CD47 interaction [28].
Cell culture
COS-1, CHO-K1, J774A.1, and THP-1 cells (American Type Culture Collection) were respectively maintained in DMEM, MEMα, and RPMI 1640 media (Invitrogen) supplemented with 10% FBS (Sigma-Aldrich). Differentiation of THP-1 cells was achieved in 100 ng/mL phorbol myristate acetate (PMA) (Sigma-Aldrich) for 2 days and confirmed by attachment of these cells to tissue-culture plastic. Peripheral blood monocytes from human donors were obtained through the Human Immunology Core at Penn (University of Pennsylvania). Human blood was obtained from finger pricks of healthy donors. Blood from other species was obtained from Covance and washed 3x in PBS plus 0.4% BSA.
Soluble human and mouse SIRPα production
COS-1 cells were transfected with pcDNA3-based vector [10] encoding a human SIRPα extracellular domain (variant: NA18949_V2) fused to GST using Lipofectamine 2000 (Invitrogen). Secreted SIRPα1-GST (referred as hSIRPα) was affinity-purified using Glutathione Sepharose 4B (Amersham Biosciences) and dialyzed against PBS (Invitrogen). The protein was stored at -20°C with or without addition of 10% v/v glycerol (Fisher Scientific). The extracellular domain of mouse SIRPα (variant: 129_IgV) was also prepared as a GST fusion and will be referred as mSIRPα.
Production of recombinant human and mouse CD47
Plasmid encoding the extracellular domain of human and mouse CD47 were PCR amplified, digested with XbaI and SalI (New England Biolabs, Inc.), and ligated to similarly digested vector, pEF-BOS-XB [15], which results in an in-frame fusion of CD4d3+4-biotin at the C-terminus of the extracellular domain of CD47. The above vector containing the extracellular domain of CD47 was transfected into CHO(-K1) cells using Lipofectamine 2000 (Invitrogen). Secreted CD47-CD4d3+4 was concentrated using a 10K MWCO Amicon filter (Millipore) and biotinylated at the C-terminus using a biotin-protein ligase (Avidity, LLC) and dialyzed against PBS (Invitrogen). The protein was affinity-purified using monomeric avidin (Promega) and dialyzed against PBS (Invitrogen).
Preparation and quantification of CD47 density on polystyrene beads
Streptavidin-coated polystyrene beads of 2.1μm diameter (Spherotech) were washed and blocked 3x in PBS plus 0.4% BSA. Biotinylated human CD47 was attached to streptavidin-coated beads at room temperature for 30 min, washed 3x and resuspended in PBS plus 0.4% BSA. The attachment of mouse CD47 to streptavidin coated beads was performed in the same way.
The density of human and mouse CD47 present on the beads was assessed by labeling with saturating levels of B6H12-FITC and mIAP301-FITC (BD Biosciences) respectively for 30 min at room temperature. Beads were washed and resuspended in PBS/0.4% BSA and flow cytometry measurements were performed using BD Calibur (BD Immunocytometry Systems). Mean fluorescence intensities were calibrated against uncoated streptavidin beads labeled with saturating B6H12-FITC/ mIAP301-FITC levels (Figure S1). The fluorescent intensities were standardized using Quantum FITC Molecule of Equivalent Soluble Fluorochrome (MESF) units (Bangs Laboratories). The MESF value for the human and mouse CD47 beads were then divided by the number of fluorophore molecules per antibody to obtain the number of molecules per bead. The density of CD47 molecules on the streptavidin beads was determined by dividing the surface area (SA) of the 2.1 μm bead (SA = 13.9 μm2). The density of CD47 molecules on human RBC (SA = 128 μm2) and mouse RBC (SA = 85 μm2) was also determined as described for the CD47 beads.
Binding isotherm for soluble hSIRPαex and mSIRPαex for CD47 coated beads
Human and mouse CD47 were attached at the identical density as described above. The binding isotherm of soluble hSIRPαex and mSIRPαex were performed for the different species over a range of concentration using flow cytometry. Forward scatter, side scatter, and fluorescence (FL1, FL2, FL3, FL4 channels in logarithmic mode) were acquired for at least 104 events using a FACScan or FACSCalibur (BD Immunocytometry Systems). Data points from flow cytometry was plotted and fitted.
Phagocytosis assay
For phagocytosis assays, macrophages were plated in 4-cm2 Lab-Tek II chambered coverglasses (Nalge Nunc International) at 105/cm2. Streptavidin polystyrene beads or RBCs were added to macrophages at a ratio of 20:1 and allowed to incubate at 37°C for 45 min. Non-phagocytosed beads was washed with PBS. Cells were fixed with 5% formaldehyde (Fischer Scientific) for 5 min, followed by immediate replacement with PBS. For differentiation of non-internalized beads, beads were labeled with a primary antibody, rabbit anti-streptavidin (Sigma-Aldrich) at 1:1,000, 1:2,000, or 1:8,000 in PBS for 20 min at 25°C. A second antibody, anti-rabbit R-PE (Sigma-Aldrich) was added at 1:1,000 in PBS to the cells and incubated for an additional 20 min at 25°C. Cells were then washed with PBS/ 0.4% BSA and then quantified by light and fluorescent microscopy. At least 200 cells were scored per well and experiments were repeated at least three times.
For stimulated phagocytosis assays, beads with or without CD47 were incubated with rabbit anti-streptavidin serum (Sigma-Aldrich) as the opsonin. Beads were opsoninized at the respective concentrations for 30 min at RT. In some experiments, human CD47 was blocked with a F(ab′)2 monoclonal antibody against CD47. The Fc fragment of mAb B6H12 were removed by enzymatic digestion with immobilized Ficin (Pierce) and separation by protein A chromatography after 10 min of the addition of opsonin. Opsoninized beads were washed 2x and resuspended in 50μl of PBS/0.4% BSA.
Immunofluorescence microscopy
Immunostaining was performed after cells were fixed and blocked for 1 h with 5% BSA in PBS. Staining with primary antibody anti-rabbit PE-conjugated (1:200) was used for detection of non-phagocytosed beads for 1 h at room temperature in PBS. After washing, samples were fixed with 5% formaldehyde and imaged. In order to ensure that the cells were not permeable to labeling an antibody against myosin IIA was used to confirm not labeling occurred (Sigma-Aldrich).
Images were acquired on an inverted microscope (IX71; Olympus) with a 40x or a 60x (oil, 1.4 NA) objective using a Cascade CCD camera (Photometrics). Image acquisition was performed with Image Pro software (Media Cybernetics, Inc.). All subsequent image analysis was done using ImageJ.
Immunoprecipiation and Western blotting
Human phagocytes, THP-1 cells (2×106) were cultured and differentiated in 6-well plates for 48 hours after PMA differentiation. Human and mouse CD47 was attached to 2.1μm beads at specific densities as described in the text and added at a bead to cell ratio of 20:1 for 2, 5, 10, or 30 minutes. Following the incubation time, the cells were washed with ice-cold PBS and then lysed on ice in 400 μl of lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1mM EDTA, 1% NP-40, 1% protease inhibitor cocktail [Sigma-aldrich] and 2mM activated sodium orthovanadate). For immunoprecipitation, whole lysate was mixed with 2μg of anti-SIRPα antibody (SE7C2 clone)(Santa Cruz Biotechnology, Inc.) and protein G (Fischer Scientific) at 4°C overnight. Precipitated proteins were washed with lysis buffer 3x and resuspended in 2x LDS buffer (Invitrogen) and boiled at 90°C. The supernatant was collected and run on a 10% SDS-PAGE gel (Invitrogen), transferred to PVDF membrane for Western blotting, where anti-phosphotyrosine HRP-conjugated antibodies and anti-SIRPα (clone c-20) followed by anti-goat HRP-conjugated (Santa Cruz Biotechnology, Inc.) as a primary antibody.
Supplementary Material
Supplemental Figures
Figure S1: Functional interaction of recombinant CD47 on beads
Flow cytometry data shows binding of (A) anti-HuCD47 antibody, B6H12 or (B) anti-mouseCD47 antibody, MIAP301 to recombinant human or mouse CD47 attached to particles respectively.
Figure S2: Low density IgG-opsoninized particles show CD47 inhibition
IgG opsonized beads with human (solid line) and mouse (dashed line) CD47 was used as target for (A) human THP-1 or (B) mouse J774A.1 macrophages. Inhibition of phagocytosis depends on density of human-CD47 or mouse-CD47 on beads at low IgG opsonin based on Figure 4.2. Species-specific differences are evident from fits with effective Ki that differ by 5-fold at low opsonin with human THP-1 macrophages.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell. 2009;138(2):271–85. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–23. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg S, Burridge K, Silverstein SC. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1990;172(6):1853–6. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177(2):529–34. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turrini F, Mannu F, Arese P, Yuan J, Low PS. Characterization of the autologous antibodies that opsonize erythrocytes with clustered integral membrane proteins. Blood. 1993;81(11):3146–52. [PubMed] [Google Scholar]
- 6.Brown AE, Discher DE. Conformational changes and signaling in cell and matrix physics. Current Biology. 2009;19:R781–R789. doi: 10.1016/j.cub.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101(10):4180–8. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 5.Mouro-Chanteloup I, Delaunay J, Gane P, Nicolas V, Johansen M, Brown EJ, Peters LL, Van Kim CL, Cartron JP, Colin Y. Evidence that the red cell skeleton protein 4.2 interacts with the Rh membrane complex member CD47. Blood. 2003;101(1):338–344. doi: 10.1182/blood-2002-04-1285. [DOI] [PubMed] [Google Scholar]
- 6.Arndt PA, Garratty G. Rh(null) red blood cells with reduced CD47 do not show increased interactions with peripheral blood monocytes. Br J Haematol. 2004;125(3):412–4. doi: 10.1111/j.1365-2141.2004.04911.x. [DOI] [PubMed] [Google Scholar]
- 7.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457–64. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 8.Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273(35):22719–28. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 9.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274(2):559–62. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 10.Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, Brown EJ, Ullrich A, Buhring HJ. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94(11):3633–43. [PubMed] [Google Scholar]
- 11.Vernon-Wilson EF, Kee WJ, Willis AC, Barclay AN, Simmons DL, Brown MH. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol. 2000;30(8):2130–7. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 13.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16(12):6887–99. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13(3):345–53. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 15.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005;35(6):1670–7. doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 16.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian S, Boder ET, Discher DE. Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity. Implications for the binding site. J Biol Chem. 2007;282(3):1805–18. doi: 10.1074/jbc.M603923200. [DOI] [PubMed] [Google Scholar]
- 18.Lowry MB, Duchemin AM, Robinson JM, Anderson CL. Functional separation of pseudopod extension and particle internalization during Fc gamma receptor-mediated phagocytosis. J Exp Med. 1998;187(2):161–76. doi: 10.1084/jem.187.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151(7):1353–68. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooney DS, Phee H, Jacob A, Coggeshall KM. Signal transduction by human-restricted Fc gamma RIIa involves three distinct cytoplasmic kinase families leading to phagocytosis. J Immunol. 2001;167(2):844–54. doi: 10.4049/jimmunol.167.2.844. [DOI] [PubMed] [Google Scholar]
- 21.Sano S, Ohnishi H, Kubota M. Gene structure of mouse BIT/SHPS-1. Biochem J. 1999;344(Pt 3):667–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Berg TK, van der Schoot CE. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29(5):203–6. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Shi FD, Ljunggren HG, Sarvetnick N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends Immunol. 2001;22(2):97–101. doi: 10.1016/s1471-4906(00)01821-4. [DOI] [PubMed] [Google Scholar]
- 24.Dahl KN, Parthasarathy R, Westhoff CM, Layton DM, Discher DE. Protein 4.2 is critical to CD47-membrane skeleton attachment in human red cells. Blood. 2004;103(3):1131–6. doi: 10.1182/blood-2003-04-1331. [DOI] [PubMed] [Google Scholar]
- 25.Brown MH, Barclay AN. Expression of immunoglobulin and scavenger receptor superfamily domains as chimeric proteins with domains 3 and 4 of CD4 for ligand analysis. Protein Eng. 1994;7(4):515–21. doi: 10.1093/protein/7.4.515. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRP{alpha} Blood. 2006;107(6):2548–2556. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Tong Q, Zhou Y, Lee HW, Yang JJ, Buhring HJ, Chen YT, Ha B, Chen CX, Yang Y, Zen K. Functional elements on SIRPalpha IgV domain mediate cell surface binding to CD47. J Mol Biol. 2007;365(3):680–93. doi: 10.1016/j.jmb.2006.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatherley D, Graham SC, Turner J, Harlos K, Stuart DI, Barclay AN. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31(2):266–77. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 30.Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich A, Delespesse G, Sarfati M. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167(5):2547–54. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- 31.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg P-A, Michalak M, Henson PM. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through trans-Activation of LRP on the Phagocyte. Cell. 2005;123(2):321. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Fossati-Jimack L, Azeredo da Silveira S, Moll T, Kina T, Kuypers FA, Oldenborg PA, Reininger L, Izui S. Selective increase of autoimmune epitope expression on aged erythrocytes in mice: implications in anti-erythrocyte autoimmune responses. J Autoimmun. 2002;18(1):17–25. doi: 10.1006/jaut.2001.0563. [DOI] [PubMed] [Google Scholar]
- 33.Duchemin AM, Ernst LK, Anderson CL. Clustering of the high affinity Fc receptor for immunoglobulin G (Fc gamma RI) results in phosphorylation of its associated gamma-chain. J Biol Chem. 1994;269(16):12111–7. [PubMed] [Google Scholar]
- 34.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386(6621):181–6. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 35.Van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175(12):7781–7. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 36.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–6. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama M, Underhill DM, Petersen TW, Li B, Kitamura T, Takai T, Aderem A. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol. 2007;178(7):4250–9. doi: 10.4049/jimmunol.178.7.4250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures
Figure S1: Functional interaction of recombinant CD47 on beads
Flow cytometry data shows binding of (A) anti-HuCD47 antibody, B6H12 or (B) anti-mouseCD47 antibody, MIAP301 to recombinant human or mouse CD47 attached to particles respectively.
Figure S2: Low density IgG-opsoninized particles show CD47 inhibition
IgG opsonized beads with human (solid line) and mouse (dashed line) CD47 was used as target for (A) human THP-1 or (B) mouse J774A.1 macrophages. Inhibition of phagocytosis depends on density of human-CD47 or mouse-CD47 on beads at low IgG opsonin based on Figure 4.2. Species-specific differences are evident from fits with effective Ki that differ by 5-fold at low opsonin with human THP-1 macrophages.




