Abstract
A combined cohort of 8,884 North American, 2,893 British and 1,574 Nordic subjects with Wilms tumor (WT) diagnosed before 15 years of age during 1960–2004 was established to determine the risk of secondary malignant neoplasms (SMN). After 169,641 person-years (PY) of observation through 2005, 174 solid tumors (exclusive of basal cell carcinomas) and 28 leukemias were ascertained in 195 subjects. Median survival time following a solid SMN diagnosis 5 years or more from WT was 11 years; it was 10 months for all leukemia. Age-specific incidence of secondary solid tumors increased from approximately 1 case per 1,000 PY at age 15 to 5 cases per 1,000 PY at age 40. The cumulative incidence of solid tumors at age 40 for subjects who survived free of SMNs to age 15 was 6.7%. Leukemia risk, by contrast, was highest during the first 5 years following WT diagnosis. Standardized incidence ratios (SIRs) for solid tumors and leukemias were 5.1 and 5.0, respectively. Results for solid tumors for the 3 geographic areas were remarkably consistent; statistical tests for differences in incidence rates and SIRs were all negative. Age-specific incidence rates and SIRs for solid tumors were lower for patients whose WT was diagnosed after 1980, though the trends with decade of diagnosis were not statistically significant. Incidence rates and SIRs for leukemia were highest among those diagnosed after 1990 (p-trend =0.003). These trends may reflect the decreasing use of radiation therapy and increasing intensity of chemotherapy in modern protocols for treatment of WT.
Keywords: Wilms tumor, childhood cancer, secondary malignant neoplasm
Introduction
Wilms tumor (WT) is an embryonal tumor of the kidney that affects approximately one child in every 10,000 before the age of 15 years in Europe and North America.(1) During the 20th century, with the development first of curative radiation therapy techniques and then of effective chemotherapy regimens, WT case fatality rates declined from 90% to 10%.(2) The increasing numbers of survivors of WT and other childhood cancers, however, have substantial risk of developing adverse medical conditions related to treatment of their disease.(3) One of the most serious late consequences of treatment for cancer is development of a secondary malignant neoplasm (SMN). Recent studies in North America (4;5), Britain (6) and Scandinavia (7) have demonstrated a heightened occurrence of SMNs among WT survivors in comparison with the general population.
The goal of the present study was to combine the resources used in these prior investigations, extending patient populations and follow-up periods where possible, to obtain more precise estimates of second cancer risks in WT survivors. We sought to characterize the patterns of second cancer in terms of tumor type, time since WT diagnosis and age at SMN diagnosis; to compare incidence rates of SMN among the different geographic areas; and to investigate changes in the nature of SMN risk over calendar time. Two of the earlier studies (5;6) involved survivor cohorts and thus excluded SMNs diagnosed within 5 years of the WT. Our goal was to provide a more complete description of SMN risk by inclusion of all WT patients. Since uniform treatment data were not available from all sources, no attempt was made in this descriptive study to characterize SMN risk according to actual treatments received.
Material and methods
The study comprised 3 cohorts of patients under the age of 15 years who were diagnosed with WT in 1960 or later.
North American cohort
The National Wilms Tumor Study (NWTS) conducted 5 clinical trials of children with renal tumors diagnosed between October, 1969 and April, 2002 in the United States and Canada. (8–12) The registering institution submitted clinical records for at least 5 years after diagnosis and follow-up was maintained indefinitely thereafter through attempted annual contact with the institution, the family or the adult patient as part of the NWTS Late Effects Study.(13) SMNs ascertained through this process were confirmed by pathology report. Loss to follow-up was estimated at approximately 1% per year, depending on ethnicity.(14) Consent from a parent or guardian for participation in the NWTS clinical trials was obtained by the patient’s institution on enrollment in the study; continuing consent for participation in the Late Effects Study was obtained directly from adult survivors at age 18. The Late Effects protocol was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
The Childhood Cancer Survivor Study (CCSS) was a collaborative project among 26 institutions in the United States and Canada, some of which also contributed to the NWTS.(15) The CCSS cohort comprised 5-year survivors of childhood cancer diagnosed between 1970 and 1986. SMNs were ascertained initially by patient or family self-report in a baseline or follow-up questionnaire. They were confirmed by pathology or, when such reports could not be obtained, by CCSS investigators using other data sources.(5) Of 1,182 CCSS patients who met eligibility criteria for the present study, 756 were also NWTS participants. The Institutional Review Boards of all participating CCSS institutions reviewed and approved the CCSS protocol. The North American cohort consisted of participants in either study who met eligibility criteria, with the 426 participants from CCSS but not NWTS entered into SMN analyses 5 years after WT diagnosis.
British cohort
The British cohort comprised children ascertained by the population-based National Registry of Childhood Tumours (NRCT) as having been diagnosed in England, Scotland or Wales with childhood cancer between 1962 and 2002 inclusive.(16) Most subjects who were diagnosed before 1992 and who survived 5 or more years from diagnosis were also included in the British Childhood Cancer Survivor Study (BCCSS).(17) The BCCSS also included patients diagnosed during 1940–1961, before the NRCT was considered to be population-based. Information on their experience of SMNs has been reported recently, and these patients are not considered here.(6) SMNs were initially identified by linkage to reports of cancer diagnoses in the population-based National Health Service Central Registers, by BCCSS questionnaire, by linkage to routine notifications to the NRCT of cancers diagnosed in childhood, or by direct notification to the BCCSS, and were confirmed by pathology report. The NRCT and BCCSS each obtained permission to conduct the study from the UK Patient Information Advisory Group and the Office of National Statistics.
Nordic cohort
The Nordic cohort consisted of children ascertained by one of 5 national, population based cancer registries as having been diagnosed with a childhood renal tumor before the end of 2002 (Norway), 2003 (Denmark, Iceland, Sweden) or 2004 (Finland). Although these registries started operation at various times between 1943 and 1958, only children diagnosed in 1960 or later were considered here in view of the very substantial mortality experienced before then. SMNs were ascertained by record linkage to the national cancer registries using personal identification numbers. The study was approved by the Data Protection Agency, a governmental institution, of each Nordic country.
Histologic subtype
During the 1970’s two rare histologic variants, clear cell sarcoma and rhabdoid tumor of kidney, were discovered to have distinct clinical features and therapeutic response profiles and are no longer considered to be WT per se.(18) Patients known to have either variant were excluded. Excluded patients in Britain comprised 2% of the total for those diagnosed prior to 1982, during which period diagnosis of histologic subtype was incomplete, and 6% thereafter. The corresponding percentages for North America, where histologic diagnoses were applied restrospectively, were 5% for both periods. No patients were excluded from the Nordic cohort since histologic subtype was not recorded.
Analysis of SMN occurrence
SMNs identified by various coding schemes in different countries were re-classified using ICD-O site and morphology codes.(19) After exclusion of non-melanotic skin cancers, all basal cell carcinomas, they were divided into two major types: leukemias and solid tumors. Benign brain tumors and myelodysplastic syndrome (MDS) were included with the malignant solid tumors and leukemias, respectively, depending on local practice as reflected in official statistics for each region. Secondary malignancies of each type were also classified according to whether they were the first such tumor diagnosed following WT, the second or the third. Cancers diagnosed within 1 month of the WT diagnosis were reported but were not treated as SMNs in the statistical analyses.
Participants entered follow-up 1 month after WT diagnosis (5 years for CCSS) and were withdrawn at the earliest of death, loss-to-follow-up or study closing at the end of 2004 (Britain) or 2005 (NWTS/CCSS, Nordic). Leukemias and solid second tumors were evaluated separately using statistical methods appropriate for the analysis of recurrent events.(20) The risk of a secondary malignant tumor was expressed in three ways: (i) incidence rate as a function both of time since WT diagnosis and of attained age; (21) (ii) cumulative incidence to age 40 for those who survived to age 15, treating death as a competing risk; (22) and (iii) standardized incidence ratio (SIR), the ratio of the number of cases observed to the number expected based on population rates specific for age and calendar period.(23) Subjects were considered at risk from time of study entry until death or withdrawal from observation. Consequently, although SMNs occurring prior to 5 years in the CCSS cohort and after loss-to-follow-up in the NWTS and CCSS cohorts were not ascertained, incidence rates and ratios would be unbiased so long as patients who developed an SMN were neither more nor less likely to be lost than others. All secondary occurrences of tumors of each type were counted in incidence rates and SIRs, but only the first such occurrence was used in calculation of cumulative incidence. Incidence rates (hazards) were estimated as (Epachenikov kernel) weighted averages of jumps in the cumulative hazard within 5 or 10 year intervals (bandwidths) of each time point.(24;25) Differences in incidence among subgroups were evaluated using log rank, likelihood ratio and non-proportionality tests based on the Cox model.(26;27) Mortality risks were based on the Kaplan-Meier estimate and mortality risk ratios following SMN diagnoses on the Cox model with a time-dependent covariate.(28) Calculations were performed using the survival and cmprsk packages in R (http://www.r-project.org/).
Population based cancer incidence rates were supplied by the Nordic registries and BCCSS based on data from their national statistics offices. SEER incidence data were used as the standard for North American.(29) The standard rates were extrapolated backwards and forwards in time to cover calendar years of follow-up not available from official statistics. SIRs were estimated and differences in SIRs were evaluated by Poisson regression using the Epi package in R.(23)
Results
The 3 cohorts comprised 13,351 subjects followed for a median of 11.6 years (Table 1). Ages at WT diagnosis and median follow-up times were comparable among the 3, but larger fractions of the British and Nordic cohorts were followed for longer periods in view of their earlier start dates. All but 2 of the 45 SMNs in Britain were ascertained through the routine record systems. Three malignant neoplasms were identified within 1 month of WT diagnosis: a ganglioneuroblastoma and a hepatoblastoma, each in patients with the Beckwith-Wiedemann syndrome (BWS), and an osteogenic sarcoma. These were not considered in the statistical analyses.
Table 1.
Description of the Cohorts
| Cohort | N. America | Britain | Nordic | Combined |
|---|---|---|---|---|
| Number of WT patients | 8,884 | 2,893 | 1,574 | 13,351 |
| Years of WT diagnoses | 1969–2002 | 1962–2002 | 1960–2004 | 1960–2004 |
| Mean age at WT diagnosis ± standard deviation (yrs) | 3.7 +/− 2.6 | 3.5 +/− 2.6 | 3.6 +/− 2.7 | 3.6 +/− 2.6 |
| Median follow-up (yrs) (25th – 75th percentile) | 12.1 (5.3, 19.1) | 9.2 (1.5, 22.0) | 10.7 (1.6, 23.2) | 11.6 (4.2, 19.9) |
| No. of second (third) [fourth] solid tumors | 99 (4) [1] | 41 (0) [0] | 29 (0) [0] | 169 (4) [1] |
| No. of second (third) leukemias | 23 (1) | 4 (0) | 0 (0) | 27 (1)* |
The patient with 2 leukemias also had 1 solid tumor, so the total number of patients with a SMN of either type was 195.
Solid tumors
A total of 174 solid tumors was identified in 169 patients (Tables 1 and 2). Three patients developed two secondary solid tumors: histiocytoma (age 19) followed by malignant melanoma (29); histiocytoma (22) followed shortly by hepatocellular carcinoma (22); and osteosarcoma (11) followed by thyroid cancer (15). The single patient with 3 secondary solid tumors developed a malignant peripheral nerve sheath tumor, a renal cell carcinoma and a papillary bladder carcinoma, all at ages 20–21.
Table 2.
Secondary solid tumors and leukemias, by time from WT to SMN diagnosis
| Time from WT to SMN (yr) | 0–9 | 10–19 | 20–29 | 30+ | Total |
|---|---|---|---|---|---|
| Solid tumors (by 2-digit primary site code): | 37 | 69 | 49 | 19 | 174 |
| Lip, oral cavity, pharynx (C07,09) | 2 | 3 | 1 | 0 | 6 |
| Digestive organs (C16-8,20,22,25-6) | 4 | 12 | 12 | 3 | 31 |
| Respiratory, intrathoracic (C34,37-8) | 2 | 5 | 2 | 0 | 9 |
| Bones, joints, cartilage (C40-1) | 5 | 11 | 4 | 0 | 20 |
| Skin (malignant melanoma) (C44) | 2 | 1 | 1 | 2 | 6 |
| Peripheral, autonomic nervous system (C47) | 0 | 3 | 2 | 0 | 5 |
| Retroperitoneum, peritoneum (C48) | 1 | 1 | 1 | 0 | 3 |
| Connective, subcutaneous, soft tissue (C49) | 3 | 4 | 4 | 0 | 11 |
| Breast (C50) | 0 | 7 | 8 | 8 | 23 |
| Female and male genital organs (C53-5, 62-3) | 1 | 1 | 5 | 1 | 8 |
| Urinary tract (C64,67) | 3 | 4 | 3 | 0 | 10 |
| Eye, brain other CNS (C69-72) | 9 | 3 | 4 | 1 | 17 |
| Thyroid and other endocrine (C73-5) | 2 | 11 | 2 | 3 | 18 |
| Lymph nodes (C77) | 3 | 3 | 0 | 0 | 6 |
| Other and ill defined (C76) | 0 | 0 | 0 | 1 | 1 |
| Leukemias (by morphology): | 24 | 2 | 2 | 0 | 28 |
| Acute lymphoblastic leukemia (M9821) | 2 | 0 | 1 | 0 | 3 |
| Myeloid and monocytic leukemia (M9861,63,66,91) | 17 | 2 | 1 | 0 | 20 |
| Myelodysplastic syndrome (M9987) | 5 | 0 | 0 | 0 | 5 |
Nine of the 174 solid tumors were lymphomas, 2 of Hodgkin and 1 of Burkitt type, of which 6 were assigned to lymph nodes as primary site and the others to liver, tonsils and mediastinum. The most common primary sites were digestive organs (including 8 hepatocellular carcinomas), breast, thyroid (15 of the 18 listed as “thyroid and other endocrine”), bone (11 osteosarcomas, 4 chondrosarcomas and 3 Ewing sarcomas) and CNS. Five of the 6 oral tumors were in the parotid gland. Four of the 10 urinary tract tumors were renal cell carcinomas that occurred in the remaining kidney at ages 7, 20, 22 and 26 years. There were clear differences in the times of occurrence of SMNs of different types, with CNS tumors tending to occur soon after WT diagnosis, for example, while breast cancers developed many years later. Three benign brain tumors reported by the Nordic registries to their official statistics bureaus were included, but those found among North American survivors were excluded in accordance with official practice.(29)
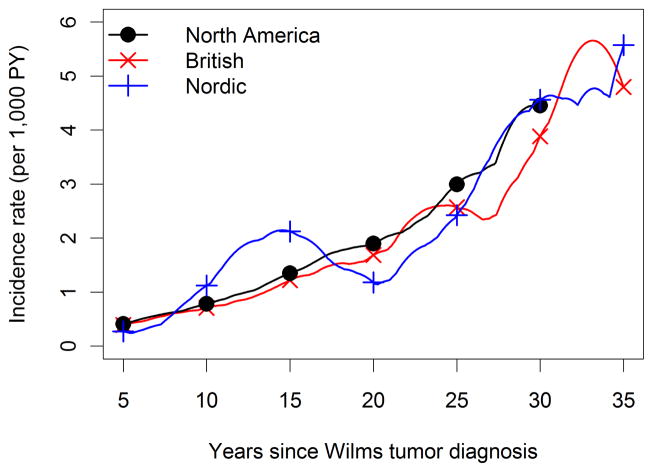
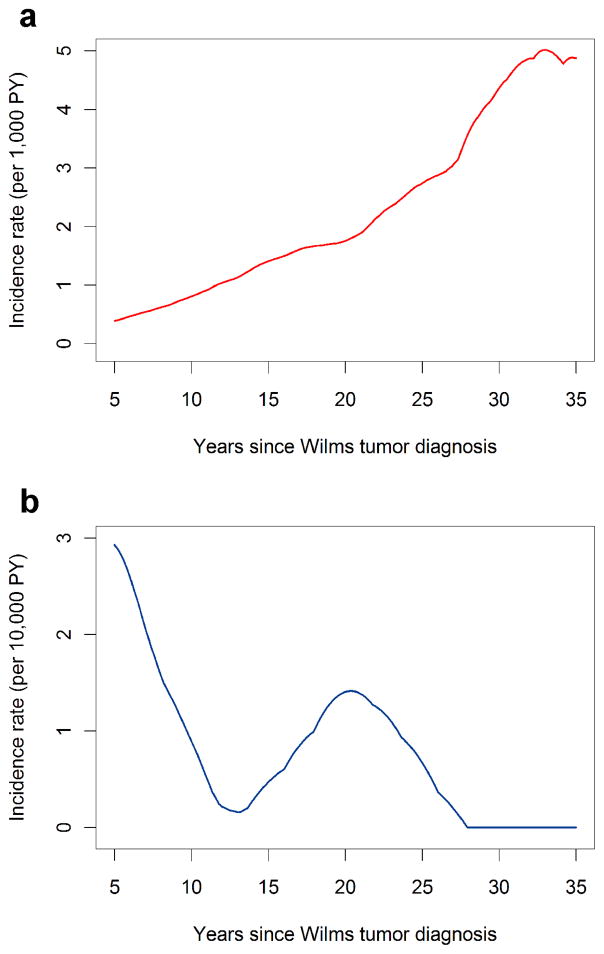
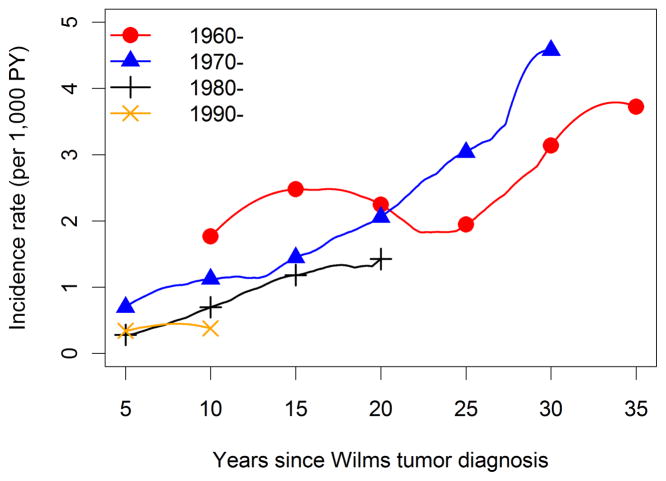
The overall average incidence of solid tumors was 1 SMN per thousand survivors per year (174 tumors observed during 169,641 person-years of follow-up). Time (Figure 1, p=0.67) and age (not shown, p=0.70) specific incidence patterns were consistent among the 3 cohorts. Incidence increased rapidly with time since WT diagnosis (Figure 2) and attained age (not shown), from approximately 1 solid tumor per 1,000 survivors per year at 10 years from diagnosis or age 15 to 5–6 solid tumors per 1,000 person-years at 35 years past diagnosis or age 40. Incidence rates for more recent calendar periods of WT diagnosis declined, but the trend was not statistically significant when evaluated using either time since diagnosis (Figure 3, p-trend=.10) or age (not shown, p-trend=0.29). Age-specific SMN rates were higher (rate ratio = 1.47, 95% confidence interval (CI) 1.07–2.02, p = 0.03) for survivors whose WT was diagnosed after age 5 compared to those diagnosed earlier. Among WT patients who survived to age 15 with no SMN, the cumulative incidence of a solid SMN by age 40 was 6.7%, and this varied little with cohort (Table 3).
Figure 1.
Incidence of solid tumors by time since WT diagnosis and cohort, calculated as weighted averages of jumps in cumulative hazards using 5 year bandwidths around each time point. Results for North America were truncated at 30 years due to limited follow-up thereafter.
Figure 2.
Incidence of solid tumors (panel a, per 1,000 person-years) and leukemia (panel b, per 10,000 person-years) by time since WT diagnosis, calculated using 5 year bandwidths.
Figure 3.
Incidence of solid tumors by time since WT diagnosis and decade of WT diagnosis, calculated using 5 and 10 year (for 1960–69) bandwidths and truncated at 10 year intervals depending on decade of diagnosis.
Table 3.
Cumulative incidence of solid SMN by attained age for WT patients who survived to age 15 years without SMN, by cohort (in percent ± standard error)
| Attained age (yrs.) | N. America | Britain | Nordic | Combined |
|---|---|---|---|---|
| 20 | 0.46 +/− 0.11 | 0.28 +/− 0.16 | 0.63 +/− 0.32 | 0.44 +/− 0.09 |
| 25 | 1.24 +/− 0.21 | 1.24 +/− 0.36 | 1.41 +/− 0.50 | 1.26 +/− 0.17 |
| 30 | 2.34 +/− 0.35 | 2.43 +/− 0.55 | 2.49 +/− 0.73 | 2.39 +/− 0.27 |
| 35 | 4.81 +/− 0.74 | 3.99 +/− 0.84 | 3.68 +/− 1.00 | 4.31 +/− 0.48 |
| 40 | 5.78 +/− 1.01 | 6.57 +/− 1.34 | 7.53 +/− 1.96 | 6.74 +/− 0.83 |
The SIR for solid tumors, equal to 174/33.9=5.1 overall, was quite consistent when examined by gender and cohort (Table 4). There was some suggestion that the SIRs first increased and then decreased with decade of WT diagnosis, attained age and time since diagnosis; however, none of the univariate tests for trend or heterogeneity was statistically significant. The SIR for children age 5 or older at diagnosis was 49% higher than for those diagnosed earlier (p=0.01). In a multiple regression analysis with grouped linear terms for the ordered factors and indicator terms for the other factors shown in Table 4, there was an average decline of 18% (95% CI -4%–35%) in the adjusted SIR for solid tumors with each decade of WT diagnosis after 1960–69, but this too lacked statistical significance (p=0.11).
Table 4.
Person-years (PY) of follow-up and standardized incidence ratios (SIR) of solid tumor and leukemia SMNs according to various study factors (observed/expected = SIR)
| Factor | Level | PY | Solid tumors | p* | Leukemias | p |
|---|---|---|---|---|---|---|
| Gender | Male | 81,282 | 81/14.8=5.5 | 0.435 | 15/3=4.9 | 0.970 |
| Female | 88,359 | 93/19.1=4.9 | 13/2.6=5.0 | |||
| Decade | 1960–1969 | 12,173 | 25/5.1=4.9 | 0.187 | 0/0.3=0.0 | 0.026 |
| of WT | 1970–1979 | 53,817 | 88/14.6=6.0 | (0.142) | 5/1.5=3.2 | (0.003) |
| 1980–1989 | 63,808 | 47/10.2=4.6 | 6/2.1=2.9 | |||
| 1990–2003 | 39,843 | 14/4=3.5 | 17/1.7=9.9 | |||
| Age at | 0–4 | 132,016 | 109/24.2=4.5 | 0.013 | 20/4.7=4.3 | 0.126 |
| WT (yrs) | 5–14 | 37,624 | 65/9.8=6.7 | 8/1=8.1 | ||
| Attained | 0–9 | 64,538 | 17/5.5=3.1 | 0.108 | 16/3.1=5.1 | 0.823 |
| age (yrs) | 10–19 | 66,260 | 52/8.5=6.1 | (0.208) | 10/1.6=6.2 | (0.349) |
| 20–29 | 30,483 | 64/12.3=5.2 | 2/0.7=3.0 | |||
| 30+ | 8,360 | 41/7.6=5.4 | 0/0.2=0.0 | |||
| Time | 0–9 | 92,923 | 38/8.5=4.5 | 0.264 | 24/3.8=6.3 | 0.308 |
| since | 10–19 | 52,802 | 68/10.9=6.2 | (0.957) | 2/1.2=1.6 | (0.100) |
| WT (yrs) | 20–29 | 20,224 | 49/10.7=4.6 | 2/0.5=4.2 | ||
| 30+ | 3,692 | 19/3.7=5.1 | 0/0.1=0.0 | |||
| Cohort | N. America | 112,975 | 104/20.8=5.0 | 0.747 | 24/3.9=6.2 | 0.586 |
| Britain | 36,417 | 41/8.2=5.0 | 4/1.1=3.5 | |||
| Nordic | 20,249 | 29/5=5.8 | 0/0.7=0.0 |
p-values in parentheses are for trend with ordered category
From the total observed and expected numbers of cases and PY of follow-up, one may calculate the absolute excess risk of a solid tumor as (174-33.9)/169,641=8.3 additional tumors per 10,000 survivors per year. The excess risk may be similarly calculated for each of the patient/time subgroups shown in Table 4 using the observed, expected and PY data reported therein.
The occurrence of a solid SMN dramatically affected survival prospects. Age-specific mortality rates increased 15-fold (95% CI 11–21) following such an occurrence. Considering the 9,486 subjects who survived 5 years beyond their WT diagnosis without an SMN, 62 deaths were recorded among the 155 who developed a solid tumor thereafter. Median time to death from the (first) solid SMN diagnosis was 11 years.
Leukemias
Twenty-seven patients developed a total of 28 leukemias (Tables 1 and 2). One child diagnosed with WT during the first year of life was found to have neurofibromatosis at age 17 months, developed acute lymphoblastic leukemia at age 6 and had both acute myeloid leukemia and a primitive neuroectodermal (PNET) brain tumor at age 8.
No leukemia was ascertained in the Nordic cohort. There was some evidence for a difference in incidence between the three cohorts using the likelihood ratio test (p=0.02), with fewer leukemias diagnosed later in time since WT diagnosis in Britain than in North America (not shown). Pooling data for all cohorts, the highest leukemia incidence was observed shortly after WT diagnosis (Figure 2) and, consequently, at younger ages (not shown). Figure 2 demonstrates the sharp contrast between leukemias and solid tumors both in terms of overall incidence (the leukemia rates are shown per 10,000 person-years, the solid tumor rates per 1,000) and the time/age incidence patterns. By 5 years from diagnosis leukemia incidence was already sharply declining, with a few cases subsequently observed around 20 years from diagnosis. Leukemia incidence was roughly four fold higher for WT patients diagnosed in 1990–2003 compared with those diagnosed in previous decades (p-trend=0.003).
Results of the SIR analyses were similar (Table 4) though the differences between cohorts now were not statistically significant. Relative leukemia risk increased during later decades of WT diagnoses, reaching 9.9 times background for patients diagnosed after 1990 (p=0.003 for trend). Leukemia risk decreased to levels closer to background (SIR close to 1) with time since WT diagnosis and with attained age, but both observed and expected numbers were small and there was substantial statistical uncertainty. Gender, age at WT diagnosis and cohort had no detectable effect on the SIR. Eighteen deaths were recorded among patients who developed leukemia; the median time to death was approximately 10 months from diagnosis of the (first) leukemia.
The higher incidence of leukemia observed during the most recent decade and in North America was partly due to inclusion of MDS among the leukemias (Table 2). This inclusion was based on re-classification of MDS as malignant in ICD-O-3, a change adopted officially by SEER in 2001. MDS cases diagnosed as early as 1994 appeared in the SEER database and were thus used to determine expected numbers. MDS did not occur in British and was not reported for Nordic WT survivors. When the five cases of MDS were removed from the leukemia diagnoses, the SIR for WT patients diagnosed during 1990–2003 declined to 7.0 and the SIR for North America to 4.9. The tests for an increase in incidence with decade of diagnosis, however, remained statistically significant (p=.03 when based on the Cox model and p=0.04 when based on the SIR).
Discussion
This is the largest study to date of SMNs following a diagnosis of WT, with a combined cohort of 13,351 subjects in whom 174 solid tumors and 28 leukemias were observed. Results for solid tumors were remarkably consistent among the 3 cohorts, with age/time-specific and cumulative incidence curves nearly super-imposable. Such consistency increases confidence in the validity of the results and in the comparisons made for other factors such as gender and age that use pooled data. Results for leukemia were less consistent, with no cases observed for the Nordic cohort and differences apparent between the North American and British cohorts in timing of the diagnoses. There were many fewer leukemias, however, and statistical tests were largely inconclusive. Consequently, factors such as differences in WT treatment protocols must compete with chance as a possible explanation for the findings
There was a marked difference between solid tumors and leukemias in the age-time incidence patterns. For solid tumors the risk sharply increased with age and time since WT diagnosis, but decreased with calendar period of WT diagnosis. For leukemias the risk was highest during the few years immediately following the WT diagnosis and in patients whose WT was diagnosed during the most recent calendar period. This latter finding was largely based on results from North America and is subject to substantial statistical uncertainty. Nonetheless, the contrasting incidence patterns suggest that different mechanisms are likely at work in causing the excess secondary tumors of each type.
Besides having received intensive radiation and chemotherapy, WT survivors have heightened risk for secondary tumors by virtue of the same genetic factors that predisposed them to WT. Such factors are multiple and complex, however, and not easily amenable to quantitative study.(30;31) In the absence of routinely collected tissue samples for genotyping large numbers of patients, evidence for a specific genetic role may be largely anecdotal.(32) The 2 NWTS patients in whom another neoplasm was diagnosed at the same time as WT both had BWS. The NWTS patient with neurofibromatosis who developed WT, then two leukemias and a PNET brain tumor was reported previously, though the brain tumor was earlier stated to be a medulloblastoma.(33) One British patient with AML had the WT-aniridia syndrome and harbored a separate WT1 mutation in the leukemia.(34) Many more of the patients with SMN included in this series would be found to have interesting clinico-pathologic and genetic features were detailed histories and biological material available for them.
This study identified four cases of renal cell carcinoma diagnosed at ages 7–26 years in WT survivors, of which 2 were previously reported by BCCSS (6) and 2 were newly identified by NWTS, 1 in a patient with 2 other solid SMNs. Based on 3 cases in 30,483 PY of observation (Table 4), the observed incidence for the age decade 20–29 was approximately 1 case per 10,000 PY. Since population rates of all kidney cancer for both the US and UK are approximately 0.4 cases per 100,000 PY during this age interval (http://seer.cancer.gov/canques/incidence.html, http://www.statistics.gov.uk/downloads/theme_health/Mb1_31/Mb1_31.pdf), WT survivors indeed seem at increased risk of developing renal cell carcinoma in the remaining kidney. Recent case reports of such occurrences in adult survivors have led to calls for nephron sparing surgery in primary treatment protocols.(35;36) While one can anticipate that additional cases will occur as the study cohorts age, it is also important to recognize that the absolute risk observed so far is not great.
Earlier studies have demonstrated a specific role for treatment factors, particularly radiation. The recent report from the BCCSS cohort, which overlaps to a large extent the British cohort included here, noted that 35 of 39 solid tumors of the thorax, abdomen or pelvis developed within irradiated fields.(6) Previous analyses of the NWTS cohort demonstrated a clear dose-response gradient in the SIR for SMNs, both solid tumors and leukemia, with abdominal irradiation.(4) Many other investigators have similarly demonstrated a relationship between irradiation of childhood cancer patients and secondary cancers of bone, bone marrow, breast, thyroid and other sites.(37–40) Standard treatment protocols for WT during the 1960s involved large (40 Gy) doses of radiation to the renal fossa for all patients. By the 1990s, due in part to increasing concern about SMNs, radiation therapy (RT) was limited to “high risk” patients and starting doses were typically only 10 Gy. Thus the observed decrease in time-specific (Figure 3) and age-specific (not shown) incidence rates and SIRs (Table 4) for solid tumors in the later decades, albeit not statistically significant, may well reflect changes in treatment protocols. Similarly, the fact that WT patients diagnosed at older ages tend to have higher stage disease and receive RT may explain the higher SIRs observed for them (Table 4).(41, 42)
By contrast with solid tumors, incidence rates and SIRs for secondary leukemias were substantially higher for WT patients diagnosed during 1990–2003 compared with earlier decades. This finding persists even if one eliminates MDS from consideration as an SMN. It most likely reflects intensification of chemotherapy over time, particularly the greater use of doxorubicin in primary treatment protocols and of epipodophyllotoxins for treatment of relapse. Doxorubicin may act as a potentiator of RT in causing secondary AML among WT patients.(43;44) Epipodophyllotoxins have been linked to secondary AML among childhood cancer patients more generally.(5;45;46) As observed here, the occurrence of such secondary leukemia often proves rapidly fatal.
No attempt was made to directly evaluate treatment factors in this study because uniform treatment data were not available for the various cohorts. In Europe, most children with WT are treated using the protocols of the International Society of Paediatric Oncology (SIOP) whereas in North America most are treated using the protocols of the NWTS Group, which has been superseded by the Renal Tumor Committee of the Children’s Oncology Group (COG). Prior to 1991 these protocols were quite similar. The primary difference today in treatment strategy has to do with the timing of nephrectomy. The SIOP protocols recommend administration of a short period of pre-nephrectomy combination chemotherapy. The NWTS/COG protocols are based on surgical and pathological staging determined at the time of nephrectomy and prior to administration of any chemotherapy. Current treatment regimens for patients treated by SIOP include cumulative anthracycline doses of 250 mg/m2 for stage II and III patients with low or intermediate risk histology and 300 mg/m2 for all patients with high risk histology. Patients with low or intermediate risk histology and stage II N1 or stage III disease receive 15 Gy of abdominal irradiation. Those with high risk histology receive 30 Gy of abdominal irradiation.(47) The treatment regimens recommended by COG include a cumulative dose of 150 mg/m2 of anthracycline and 10.8 Gy abdominal irradiation for all patients with stage III/favorable histology disease unless they have loss of heterozygosity for both 16q and 1p. This subset of patients receives treatment with 195 mg/m2 of anthracycline and 10.8 Gy of abdominal irradiation (J. Dome, personal communication, October, 2009)
A comparison of exposures to radiation therapy and anthracyclines, using the treatment regimens of NWTS – 4 (11) and SIOP – 9 (48) and based on the stage distributions in NWTS – 3 (10) and SIOP – 6 (49), demonstrates that approximately 50% more European than North American patients with non-mestastatic WT of non-anaplastic histology will be treated with an anthracycline (SIOP-6: 45.5%, 201/442; NWTS-3: 29.3%, 449/1528), whereas approximately 50% more North American than European patients will be treated with abdominal irradiation (SIOP: 18.0%, 80/442; NWTS: 29.3%, 449/1528).(50) Consequently, although no such differences were evident in the present study, patients with unilateral Wilms tumor treated using NWTS regimens eventually may have a greater risk of radiation related second malignant tumors whereas those treated using SIOP regimens may have a greater risk of topoisomerase II inhibitor related leukemias.
In conclusion, this study has confirmed the serious risk of SMN in patients treated for WT in Europe and North America. While indications are that the risk of secondary solid tumors may have started to moderate somewhat with decreasing use of RT, the even more devastating risk of secondary leukemia seems to be on the rise. The leukemia risk is largely concentrated during the first 5 years or so following the WT diagnosis, however, and is not nearly as great in absolute terms. While SIRs for solid SMNs decline slightly following a peak at 10–19 years from WT diagnosis (Table 4), they nonetheless remain near 5 and are not trending sharply downwards. This means that as much as 80% of the total incidence of solid tumors, shown in Figure 2 by time since WT diagnosis, represents excess incidence associated with WT or its treatment. As the cohorts are followed into middle age and beyond, therefore, the excess cancer burden borne by WT survivors may become even more serious as the risk multipliers act on increasing background rates. Designing better studies to identify those at highest risk by virtue of genetic susceptibility is a high priority for future research efforts. (32)
Acknowledgments
The authors gratefully acknowledge the Association of Nordic Cancer Registries for compilation of data from the five Nordic countries. The CCSS is supported by Grant No. U24 CA55727 from the US National Cancer Institute, with additional support provided to St. Jude Children’s Research Hospital, Memphis, TN, by the American Lebanese Syrian Associated Charities. The NWTS Late Effects Study is supported by Grant No. R01 CA054498 from the US National Cancer Institute. The Childhood Cancer Research Group receives core funding from the Department of Health and the Scottish Ministers. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health and the Scottish Ministers.
Abbreviations
- AML
acute myeloid leukemia
- BCCSS
British Childhood Cancer Survivor Study
- BWS
Beckwith-Wiedemann syndrome
- CNS
central nervous system
- CCSS
Childhood Cancer Survivor Study
- COG
Children’s Oncology Group
- MDS
myelodysplastic syndrome
- NWTS
National Wilms Tumor Study
- NRCT
National Registry of Childhood Tumours
- PNET
primitive neuroectodermal
- PY
person-years
- RT
radiation therapy
- SEER
Surveillance, epidemiology and end results
- SIR
standardized incidence ratio
- SMN
secondary malignant neoplasm
- WT
Wilms tumor
References
- 1.Stiller CA, Parkin DM. International variations in the incidence of childhood renal tumours. Br J Cancer. 1990;62:1026–30. doi: 10.1038/bjc.1990.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Angio GJ. Oncology seen through the prism of Wilms’ tumor. Med Pediatr Oncol. 1985;13:53–8. doi: 10.1002/mpo.2950130202. [DOI] [PubMed] [Google Scholar]
- 3.Rosoff PM. Focus on research: The two-edged sword of curing childhood cancer. N Engl J Med. 2006;355:1522–3. doi: 10.1056/NEJMp068168. [DOI] [PubMed] [Google Scholar]
- 4.Breslow NE, Takashima JR, Whitton JA, Moksness J, D’Angio GJ, Green DM. Second malignant neoplasms following treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 1995;13:1851–9. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 5.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Winter DL, Pritchard-Jones K, Stiller CA, Frobisher C, Lancashire ER, Reulen RC, Hawkins MM. Second primary neoplasms in survivors of Wilms’ tumour - a population-based cohort study from the British Childhood Cancer Survivor Study. Int J Cancer. 2008;122:2085–93. doi: 10.1002/ijc.23333. [DOI] [PubMed] [Google Scholar]
- 7.Olsen JH, Moller T, Anderson H, Langmark F, Sankila R, Tryggvadottir L, Winther JF, Rechnitzer C, Jonmundsson G, Christensen J, Garwicz S. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009 Jun 2;101:806–13. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 8.D’Angio GJ, Evans AE, Breslow N, Beckwith B, Bishop H, Feigl P, Goodwin W, Leape LL, Sinks LF, Sutow W, Tefft M, Wolff J. The treatment of Wilms’ tumor: Results of the National Wilms’ Tumor Study. Cancer. 1976;38:633–46. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.D’Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, Goodwin W, Leape L, Palmer N, Sinks L, Sutow W, Tefft M, et al. The treatment of Wilms’ Tumor - Results of the Second National Wilms’ Tumor Study. Cancer. 1981;47:2302–11. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.D’Angio GJ, Breslow N, Beckwith B, Evans A, Baum E, deLorimier A, Fernbach D, Hrabovsky E, Jones B, Kelalis P, Othersen B, Tefft M, et al. Treatment of Wilms’ tumor: Results of the Third National Wilms’ Tumor Study. Cancer. 1989;64:349–60. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Green DM, Breslow NE, Beckwith JB, Finklestein JZ, Grundy PE, Thomas PRM, Kim T, Shochat SJ, Haase GM, Ritchey ML, Kelalis PP, D’Angio GJ. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:237–45. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 12.Grundy PE, Breslow NE, Li S, Perlman E, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D’Angio GJ, Donaldson M, Coppes MJ, Malogolowkin M, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–21. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 13.Evans AE, Norkool P, Evans I, Breslow N, D’Angio GJ. Late effects of treatment for Wilms’ tumor. Cancer. 1991;67:331–6. doi: 10.1002/1097-0142(19910115)67:2<331::aid-cncr2820670202>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Breslow N, Olshan A, Beckwith B, Moksness J, Feigl P, Green D. Ethnic variation in the incidence, diagnosis, prognosis, and follow-up of children with Wilms’ tumor. J Natl Cancer Inst. 1994;86:49–51. doi: 10.1093/jnci/86.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 16.Stiller C. Childhood cancer in Britain: incidence, survival, mortality. Oxford: Oxford University Press; 2007. p. 270. [Google Scholar]
- 17.Hawkins MM, Lancashire ER, Winter DL, Frobisher C, Reulen RC, Taylor AJ, Stevens MCG, Jenney M. The British Childhood Cancer Survivor Study: Objectives, methods, population structure response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50:1018–25. doi: 10.1002/pbc.21335. [DOI] [PubMed] [Google Scholar]
- 18.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms’ tumor: results from the First National Wilms’ Tumor Study. Cancer. 1978;41:1937–48. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. International classification of diseases for oncology (ICD-O) 1. Geneva: World Health Organization; 1976. p. 131. [Google Scholar]
- 20.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J Roy Stat Soc B. 2000;62:711–30. [Google Scholar]
- 21.Yasui Y, Liu Y, Neglia JP, Friedman DL, Bhatia S, Meadows AT, Diller LR, Mertens AC, Whitton J, Robison LL. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–13. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Breslow NE, Day NE. The design and analysis of cohort studies. II. Lyon: International Agency for Research on Cancer; 1987. Statistical methods in cancer research; p. 406. [PubMed] [Google Scholar]
- 24.Aalen O. Nonparametric inference for a family of counting processes. Ann Statist. 1978;6:701–26. [Google Scholar]
- 25.Ramlau-Hansen H. Smoothing counting process intensities by means of kernel functions. Ann Statist. 1983;11:453–66. [Google Scholar]
- 26.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Roy Stat Soc A. 1972;135:185–206. [Google Scholar]
- 27.Cox DR. Regression models and life-tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations - Total U.S. (1969-2005) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released February 2008.
- 30.Pritchard-Jones K, Breslow NE. Wilms tumour and other childhood renal tumours. In: Eeles RA, Easton DF, Eng C, Ponder B, editors. Genetic predisposition to cancer. 2. London: Arnold; 2004. pp. 119–31. [Google Scholar]
- 31.Huff V. Wilms tumor genetics: A new, unX-pected twist to the story. Cancer Cell. 2007;11:105–7. doi: 10.1016/j.ccr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele A, Figg WD, Gospodarowicz MK, et al. Cancer survivorship - genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 33.Perilongo G, Felix CA, Meadows AT, Nowell P, Biegel J, Lange BJ. Sequential development of Wilms-tumor, T-cell acute lymphoblastic-leukemia, medulloblastoma and myeloid-leukemia in a child with type 1 neurofibromatosis: a clinical and cytogenetic case-report. Leukemia. 1993;7:912–5. [PubMed] [Google Scholar]
- 34.Pritchard-Jones K, Renshaw J, King-Underwood L. The Wilms tumour (WT1) gene is mutated in a secondary leukemia in a WAGR patient. Hum Mol Genet. 1994;3:1633–7. doi: 10.1093/hmg/3.9.1633. [DOI] [PubMed] [Google Scholar]
- 35.Cherullo EE, Ross JH, Kay R, Novick AC. Renal neoplasms in adult survivors of childhood Wilms tumor. J Urol. 2001;165:2013–6. doi: 10.1097/00005392-200106000-00059. [DOI] [PubMed] [Google Scholar]
- 36.Kraushaar G, Wiebe S. Renal cell carcinoma as a second malignant neoplasm in a patient with non-syndromic hemihypertrophy and previous Wilms tumor. Pediatr Radiol. 2005;35:1208–11. doi: 10.1007/s00247-005-1540-5. [DOI] [PubMed] [Google Scholar]
- 37.Tucker MA, D’Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, Stone BJ, Green DM, Lombardi F, Newton W, Hoover RN, Fraumeni JF., Jr Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–93. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 38.Tucker MA, Jones PH, Boice JD, Jr, Robison LL, Stone BJ, Stovall M, Jenkin RD, Lubin JH, Baum ES, Siegel SE, Meadows AT, Hoover RN, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. Cancer Res. 1991;51:2885–8. [PubMed] [Google Scholar]
- 39.Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745–51. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 40.Haddy N, Le Deley MC, Samand A, Diallo I, Guerin S, Guibout C, Oberlin O, Hawkins M, Zucker JM, de Vathaire F. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer. 2006;42:2757–64. doi: 10.1016/j.ejca.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Breslow N, Churchill G, Beckwith JB, Fernbach DJ, Otherson HB, Tefft M, D’Angio GJ. Prognosis for Wilms’ tumor patients with nonmetastatic disease at diagnosis – results of the second National Wilms’ Tumor Study. J Clin Oncol. 1985;3:521–31. doi: 10.1200/JCO.1985.3.4.521. [DOI] [PubMed] [Google Scholar]
- 42.Breslow NE, Churchill G, Nesmith BS, Thomas PRM, Beckwith JB, Othersen HB, D’Angio GJ. Clinicopathologic features and prognosis for Wilms’ tumor patients with metastases at diagnosis. Cancer. 1986;58:2501–11. doi: 10.1002/1097-0142(19861201)58:11<2501::aid-cncr2820581125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 43.Meadows AT. Curing cancer in children: minimizing price, maximizing value. J Clin Oncol. 1995;13:1837–9. doi: 10.1200/JCO.1995.13.8.1837. [DOI] [PubMed] [Google Scholar]
- 44.Shearer P, Kapoor G, Beckwith JB, Takashima J, Breslow N, Green DM. Secondary acute myelogenous leukemia in patients previously treated for childhood renal tumors: A report from the National Wilms Tumor Study Group. J Pediatr Hematol Oncol. 2001;23:109–11. doi: 10.1097/00043426-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Sandler ES, Friedman DJ, Mustafa MM, Winick NJ, Bowman WP, Buchanan GR. Treatment of children with epipodophyllotoxin-induced secondary acute myeloid leukemia. Cancer. 1997;79:1049–54. doi: 10.1002/(sici)1097-0142(19970301)79:5<1049::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins MM, Wilson LMK, Stovall MA, Marsden HB, Potok MHN, Kingston JE, Chessells JM. Epipodophyllotoxins, alkylating-agents, and radiation and risk of secondary leukemia after childhood-cancer. Br Med J. 1992;304:951–8. doi: 10.1136/bmj.304.6832.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhard H, Semler O, Burger D, Bode U, Flentje M, Gobel U, Gutjahr P, Leuschner I, Maass E, Niggli F, Scheel-Walter HG, Stockle M, et al. Results of the SIOP 93-01/GPOH trial and study for the treatment of patients with unilateral nonmetastatic Wilms Tumor. Klin Padiat. 2004;216:132–40. doi: 10.1055/s-2004-822625. [DOI] [PubMed] [Google Scholar]
- 48.Tournade MF, Com-Nougue C, de Kraker J, Ludwig R, Rey A, Burgers JM, Sandstedt B, Godzinski J, Carli M, Potter R, Zucker JM International Society of Pediatric Oncology Nephroblastoma Trial and Study Committee. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms’ tumor in children older than 6 months: results of the Ninth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study. J Clin Oncol. 2001;19:488–500. doi: 10.1200/JCO.2001.19.2.488. [DOI] [PubMed] [Google Scholar]
- 49.Tournade MF, Com-Nougue C, Voute P, Lemerle J, de Kraker J, Delemarre JF, Burgers M, Habrand JL, Moorman CGM, Burger D, Rey A, Zucker JM, et al. Results of the Sixth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study: a risk-adapted therapeutic approach in Wilms’ tumor. J Clin Oncol. 1993;11:1014–23. doi: 10.1200/JCO.1993.11.6.1014. [DOI] [PubMed] [Google Scholar]
- 50.Green DM. Controversies in the management of Wilms tumour – immediate nephrectomy or delayed nephrectomy? Eur J Cancer. 2007;43:2453–6. doi: 10.1016/j.ejca.2007.07.022. [DOI] [PubMed] [Google Scholar]