Abstract
The central transcriptional response to hypoxia is mediated by the Prolyl Hydroxylase Domain protein (PHD):Hypoxia Inducible Factor (HIF) pathway. In this pathway, PHD prolyl hydroxylates and thereby negatively regulates the α-subunit of the transcription factor HIF (HIF-α). An important HIF target gene is that for Erythropoietin (EPO), which controls red cell mass. Recent studies have identified PHD2 as the critical PHD isoform regulating the EPO gene. Other studies have shown that the inducibility of the HIF pathway diminishes as a function of age. Thus, an important question is whether the PHD2:EPO pathway is altered in the aging. Here, we employed a mouse line with a globally-inducible Phd2 conditional knockout allele to examine the integrity of the Phd2:Epo axis in young (six to eight month old) and aging (sixteen to twenty month old) mice. We find that acute global deletion of Phd2 results in a robust erythrocytosis in both young and aging mice, with both age groups showing marked extramedullary hematopoiesis in the spleen. Epo mRNA is dramatically upregulated in the kidney, but not in the liver, in both age groups. Conversely, other Hif targets, including Vegf, Pgk1, and Phd3 are upregulated in the liver but not in the kidney in both age groups. These findings have implications for targeting this pathway in the aging.
Keywords: Erythropoietin, Prolyl Hydroxylase Domain protein, Hypoxia Inducible Factor, Prolyl hydroxylation, Gene regulation
Introduction
The HIF pathway plays a central role in coordinating cellular, local, and systemic responses to hypoxia [1; 2]. In this pathway, HIF-α (of which there are two main isoforms) is site-specifically prolyl hydroxylated by a family of three PHDs (also known as HIF Prolyl Hydroxylases and Egg Laying Defective Nine proteins) [3]. The three PHD isoforms are PHD1, PHD2, and PHD3. This modification provides a recognition motif for the von Hippel Lindau tumor suppressor protein (VHL), a component of an E3 ubiquitin ligase complex that specifically targets hydroxylated HIF for degradation by the ubiquitin-proteasome pathway [4]. Under normoxic conditions, this modification occurs constitutively and hence, HIF-α is maintained at very low steady state levels. This modification is inherently oxygen dependent [5] and is inhibited by hypoxia-generated reactive oxygen species [6; 7; 8]. Therefore, under hypoxic conditions the modification is inhibited, allowing the stabilization of HIF-α. HIF-α then heterodimerizes with a distinct protein, HIF-β, and binds to hypoxia response elements (HREs) in the promoters and enhancers of HIF target genes, allowing the transactivation of these genes.
The prototypical HIF target gene is that encoding for EPO [9; 10; 11; 12]. EPO is a glycoprotein that is the central growth factor controlling red cell mass. In situations such as anemia, EPO gene transcription is induced, leading to increased circulating levels of EPO. EPO then binds to the EPO receptor on red cell progenitors in the bone marrow, initiating signaling through the JAK:STAT pathway, and leading to expansion of red cell mass. The expanded red cell mass increases delivery of oxygen to the tissues of the body, and this in turn downregulates HIF and hence EPO gene transcription. This negative feedback loop thus ensures that HIF maintains EPO at a level that is appropriate for a given tissue oxygenation. EPO gene transcription is also regulated in a developmental and tissue specific manner. In the mammalian fetus, the liver is the predominant source of EPO [13]; postpartum, the site of EPO production shifts from the liver to the kidney.
The multiplicity of PHD isoforms raises the question of which, if any, are involved in EPO regulation. Studies of patients with idiopathic erythrocytosis, an uncommon condition characterized by elevated red cell mass, have revealed that a subset of these patients harbor heterozygous mutations in the PHD2 gene [14; 15; 16; 17]. These mutations comprise missense, nonsense, and frameshift mutations. PHD2 consists of an N-terminal non-catalytic domain, and a C-terminal catalytic domain that is homologous to a larger family of 2-oxoglutarate dependent prolyl hydroxylases that include the collagen 4-prolyl hydroxylases [3]. All reported missense mutations studied to date affect the catalytic domain and severely impair enzymatic activity [16; 17; 18]. The frameshift mutations occur within the N-terminal domain and thereby delete the catalytic domain, and the single nonsense mutation reported is predicted to delete the C-terminal 50 amino acids [14].
Studies of genetically engineered mice have demonstrated a critical role for Phd2 in red blood cell control. Conventional knockout of Phd2 leads to embryonic lethality between day E12.5 to E14.5 due to placental and cardiac defects [19], necessitating a conditional knockout (CKO) approach to studying its role in adult red cell control. Employing a global, tamoxifen-inducible system, we and others have found that acute deletion of Phd2 in the mouse results in marked erythrocytosis [20; 21]. In contrast, neither Phd1 −/− nor Phd3 −/− mice display erythrocytosis, but interestingly, Phd1 −/− ; Phd3 −/− double knockout mice exhibit a mild erythrocytosis [20]. Collectively, the human and mouse studies point to PHD2 as being the central PHD isoform regulating red cell mass.
These and other findings have stimulated great interest in targeting this pathway for therapeutic benefit [22]. Based on available evidence, inhibition of PHD2 would be predicted to increase EPO and thereby stimulate erythropoiesis, which could be of benefit in situations such as anemia. However, an important caveat to the mouse studies mentioned above is that they were performed on young mice (two to three months of age). Therapeutic targeting of this pathway, in contrast, would likely have its greatest impact in the aging population, which is the population that is preferentially affected by anemia due to causes that include end stage renal disease and chemotherapy. It should also be noted that a substantial segment of the aging population has anemia of unknown cause [23; 24]. Anemia in the aging is associated with increased mortality risk, with the degree of risk correlated with the degree of anemia [25].
This issue is all the more relevant because there is substantial evidence that there are aging-associated changes in the HIF pathway. Thus, there is impaired hypoxia-induced HRE binding activity in senescent mice [26], a defect in hypoxia-induced HIF-1 α activation in aging aortic smooth muscle cells from rabbits [27], decreased hypoxia-induced activation of HIF-1 α in rat carotid bodies as a function of age [28], decreased hypoxia-induced HRE-binding activity in aging rat lungs [29], impaired induction of HIF-1 α protein by ischemia in hindlimb skeletal muscle of aging mice [30], decreased hypoxia-induced HIF-1 α expression in the aging mouse brain [31], and diminished ischemia-induced HIF-1 α activation in tissue flaps of aging mice [32]. Collectively, these studies suggest an impairment of the HIF activation pathway as a function of aging.
This then raises the important question of whether there is a fundamental defect in the HIF pathway in aging mammals. The PHD2:EPO pathway, with its high level of inducibility and its ability to be assessed quantitatively by indices that include hematocrit (Hct) and hemoglobin (Hb), provides an ideal and clinically relevant system for examining this. To pursue this, we employed a genetic model in which Phd2 can be temporally knocked out in order to examine young and aging mice. Contrary to what might have been expected based on previous published reports on aging and the HIF pathway, we find that loss of Phd2 in both age groups induces dramatic induction of erythrocytosis. The kidney continues to be the source of Epo in these aging mice. Furthermore, other Hif target genes are inducibly upregulated in the liver in both age groups. These findings indicate that the PHD2:EPO pathway is robustly inducible in aging mammals.
Materials and Methods
Mice
The generation of mice in which exon 2 of the Phd2 gene has been flanked by loxP sites (floxed) has been described previously [19]. These mice were maintained in a mixed CD1/129/BL6 background. Mice expressing either a constitutively active Cre recombinase or a tamoxifen-inducible CreERT2 fusion protein from the Rosa26 locus were obtained from Taconic [the strain designations are C57BL/6-Gt(ROSA)26Sortm16(cre)Arte (which we hereafter refer to as Rosa26-Cre) and C57BL/6-Gt(ROSA)26Sortm9(cre/Esr1)Arte (which we hereafter refer to as Rosa26-CreERT2), respectively]. Phd2 +/− mice were generated by crossing Phd2 f/f (where f = flox) mice with the constitutive active Rosa26-Cre deleter mouse. Phd2 f/−; Rosa26-CreERT2 and Phd2 f/+ mice were generated by crossing Phd2 f/f mice with Phd2 +/−; Rosa26-CreERT2 mice. For genotyping or determination of Phd2 exon 2 deletion efficiency, DNA was isolated from tails or various organs using a DNeasy tissue kit (Qiagen). For genotyping the Phd2 allele, we employed the following three primers: PHD2rec55: 5’-AGG GCT TCT GGC ATT AGT TGA CC-3’; PHD2mouseR: 5-TCA ACT CGA GCT GGA AAC C-3’; and Pint 1–4 5’: 5’-ATG AAT CAG AGT TCC CCG TG-3’. The wild type Phd2 allele produces a 1.09 kb band with the PHD2rec55 and PHD2mouseR primers, the floxed Phd2 allele produces a 0.95 kb band with the Pint1–4 5’ and PHD2mouseR primers, and the knockout Phd2 allele produces a 0.8 kb band with the PHD2rec55 and PHD2mouseR primers. For genotyping the Cre allele, we employed the following three primers: Rosa26-1: 5’-TGG AGG CAG GAA GCA CTT GCT CTC-3’; Rosa26-2: 5’-CAT ACT GTA GTA AGG ATC TCA AGC-3’; Cre-1(R): 5’-GCA TGT TTA GCT GGC CCA AAT G-3’. The wild type Rosa26 locus produces a 0.55 kb band with the Rosa26-1 and Rosa26-2 primers. The Rosa26 locus containing either the Cre or CreERT2 transgene produces a 0.69 kb band with the Rosa26-1 and Cre-1(R) primers. All animal procedures were approved by the Institutional Animal Care and Use Committees at the University of Pennsylvania in compliance with Animal Welfare Assurance.
Tamoxifen administration
A 10 mg/ml stock solution of tamoxifen free base (MP Biomedicals) was prepared in corn oil with shaking at 37 °C, and then aliquoted and stored at −20 °C. Tamoxifen was administered by oral gavage for five consecutive days (2 mg doses for young mice; 1 mg doses for aging mice).
Hematologic analysis
Peripheral blood samples were obtained from the retroorbital cavity and collected in Microvette 100 lithium heparin tubes (Sarstedt). Hematocrit was measured using a Critspin hematocrit reader (Iris). Hemoglobin measurements and complete blood counts were determined using a Hemavet FS950 hematology analyzer (Drew Scientific).
Epo assay
Plasma was obtained by centrifuging the retroorbital blood samples at 20 min at 2,000 × g for 20 min at room temperature. Plasma Epo levels were then determined using a rodent Quantikine Epo Immunoassay kit (MEP00, R&D Systems). Enzyme linked immunoabsorbent assay (ELISA) readings were made on a Tecan Sunrise microplate reader.
Erythroid Burst Forming Unit (BFU-E) assay
Cells were obtained from either bone marrow or spleen, and red blood cells were lysed in a solution containing 150 mM NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA for 5 min at room temperature. The remaining cells were suspended in Iscove’s modified Dulbecco’s medium. Cells (1 × 105) were plated in 35 mm dishes containing methylcellulose media and varying concentrations of EPO (prepared by mixing appropriate amounts of Methocult M3436, which contains 3 U/ml EPO, and Methocult M3236, which is the EPO-deficient base media for M3436). After fourteen days of culture at 37 °C in a humidified chamber with 5% CO2, BFU-E colonies were counted using an Olympus CK2 phase contrast microscope.
Real Time PCR analysis (RT-PCR)
Total RNA was isolated from kidney, liver, and bone marrow using TRIzol reagent (Invitrogen). Reverse transcription of 0.8 µg of total RNA was then performed using an ABI High Capacity cDNA Synthesis Kit (Applied Biosciences). RT-PCR was then performed on 20 ng cDNA equivalents using a SYBR green PCR master mix (Applied Biosciences) and an ABI 7300 Real Time PCR System. Relative quantification was performed employing the ΔΔCt method and β-actin as the endogenous control.
The primers employed for RT-PCR were as follows. Phd2, 5’-AGG CAA CGG AAC AGG CTA TG-3’ and 5’-CGC ATC TTC CAT CTC CAT TTG-3’; Epo, 5’-CAT CTG CGA CAG TCG AGT TCT G-3’ and 5’-CAC AAC CCA TCG TGA CAT TTT C-3’; Vegf (A isoform), 5’-TGT CAC CAC CAT GCC ATC AT-3’ and 5’-GAC CCA AAG TGC TCC TCG AA-3’; Pgk1, 5’-GGA AGC GGG TCG TGA TGA-3’ and 5’-GCC TTG ATC CTT TGG TTG TTT G-3’; Tfr1, 5’-TGG AGA CAG ATG CTC CCT CC-3’ and 5’-TTT GTG CTC TGT GTA TGT GGT AAG G-3’; Phd3, 5’-CTC TGC CCA CGT CGT TCA G-3’ and 5’-CCC GGC AAG AAA ACA TGA AG-3’; Hepcidin, 5’-TGT CTC CTG CTT CTC CTC CT-3’ and 5’-CTC TGT AGT CTG TCT CAT CTG TTG-3’; Alas2, 5’-TCC AAG GCA TTC GCA ACA-3’ and 5’-CCT GGG TCA TTG TGT CTG AAG A-3’; Dmt1, 5’-GGC TTT CTT ATG AGC ATT GCC TA-3’ and 5’-GGA GCA CCC AGA GCA GCT TA-3’; EpoR, 5’-CGG CTC CGT GCG TTT CT-3’ and 5’-CGG CAC AAA ACT CGA TGT GT-3’; Tf, 5’-CCT GGC CCA AGC TCC AA-3’ and 5’-CCG GGC TGC CTT CTC TTT-3’; β-actin, 5’-GGC CAA CCG TGA AAA GAT GA-3’ and 5’-CAC AGC CTG GAT GGC TAC GT-3’.
Histologic analysis
Mouse liver and spleen were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut and stained with hematoxylin and eosin. Photomicrographs were obtained with a Leica DM2500 upright light microscope fitted with a DFC420 digital camera. The eyepiece lens was 10×, and the objective lens was 10× (total magnification = 100×).
Statistical analysis
Data are presented as the mean ± standard deviation. Groups were compared by unpaired Student’s t test. p values below 0.05 were considered to be significant.
Results
We and others have previously shown that acute global deletion of Phd2 in young mice results in marked erythrocytosis [20; 21]. In the studies that we conducted, the mice were two to three months of age. This leaves open a number of important questions, namely (1) whether acute global deletion in aging mice also results in erythrocytosis, (2) whether the kidney continues to be the source of Epo, and (3) whether other Hif target genes are inducible to a comparable degree in the aging mice.
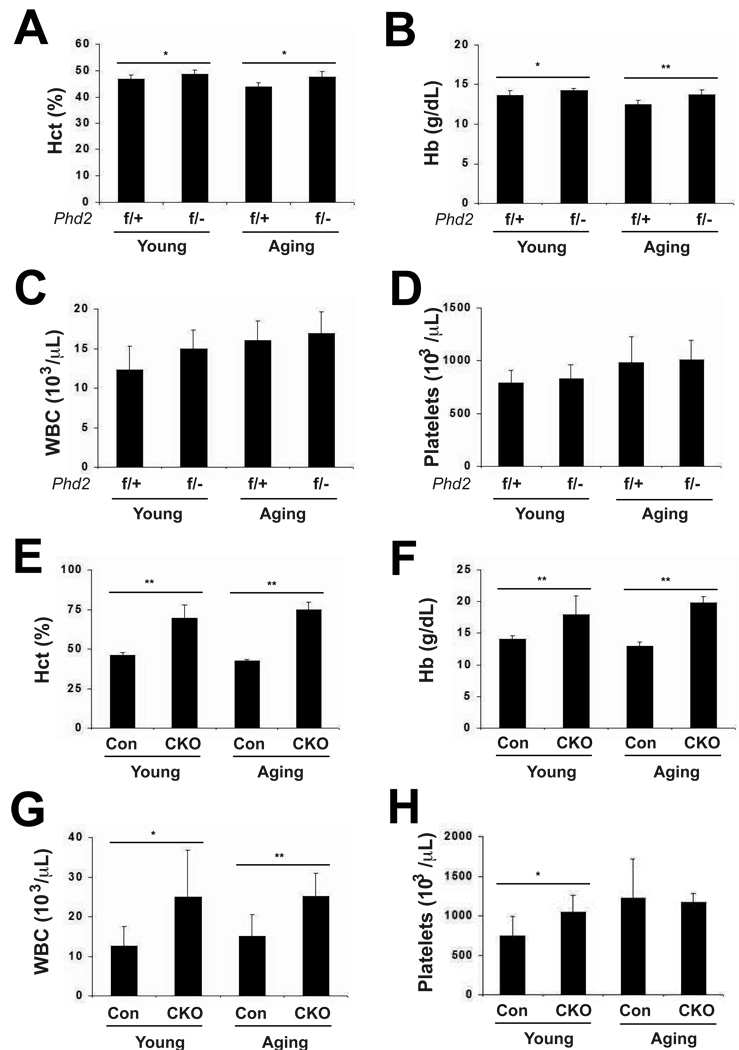
Towards this end, we examined the Phd2 conditional knockout mouse model employed previously [19; 20]. In this strain, Phd2 exon 2 is flanked by loxP sites. Exon 2 contains two of the three essential iron-chelating residues of Phd2; hence, its deletion will abolish enzymatic activity. We generated two groups of mice with the following genotypes: Phd2 f/−; Rosa26-CreERT2 (the experimental group) and Phd2 f/+ (the control group). We examined them at six to eight months (which we hereafter refer to as the young group) and at sixteen to twenty months (which we hereafter refer to as the aging group). This strategy allowed us to examine both the effect of Phd2 heterozygosity and the effect of acute global deletion of Phd2 as a function of age. We first obtained baseline hematocrit and complete blood counts. We find that the Phd2 heterozygotes display a mild erythrocytosis in both the young and aging groups (Fig. 1A and B). In the young mice, the Hct was 46.8 ± 1.8% (mean ± S.D.) in the controls, and 48.8 ± 1.4% in the heterozygotes (p < 0.05). In the aging mice the corresponding values were 43.9 ± 1.6% and 47.4 ± 2.3%, respectively (p < 0.05). Similar results were obtained with hemoglobin (Hb) measurements (Fig. 1B). No significant differences were seen in white blood cell or platelet counts in either age group (Fig. 1C and D, respectively).
Fig. 1.
Analysis of peripheral blood in young and aging mice. (A, E) hematocrit (Hct), (B, F) hemoglobin (Hb), (C, G) white blood cell count (WBC), and (D, H) Platelets were measured. For all panels, n = 6–8. For (A–D), genotypes are as follows. f/+ = Phd2 f/+; f/− = Phd2 f/−; Rosa26-CreERT2. * indicates p < 0.05 and ** indicates p < 0.01 in comparing f/+ and f/− groups at a given age. For (E–H), genotypes are as follows. Controls (Con): Phd2 f/+; CKO: Phd2 f/−; Rosa26-CreERT2. Control and Phd2 CKO mice were administered tamoxifen for five consecutive days. Four weeks after the initial tamoxifen dose, peripheral blood was collected. * indicates p < 0.05 and ** indicates p < 0.01 in comparing control and CKO groups at a given age.
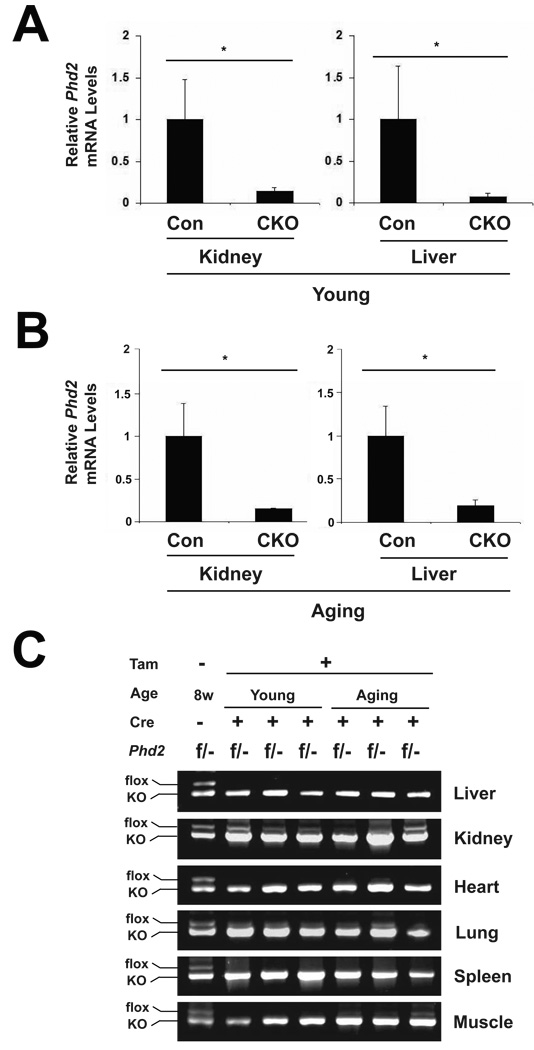
We next treated both the experimental group and the control group with tamoxifen by oral gavage (the experimental group treated with tamoxifen is hereafter referred to as CKO). RNA was isolated from kidneys and livers of animals from both the young and aging groups. We examined, by Real Time PCR, the levels of Phd2 mRNA. As shown in Fig. 2A and B, we find that tamoxifen induces a marked decrease of Phd2 message in the kidneys and livers of both the young and the aging mice, respectively, indicative of effective Cre-mediated recombination. We also employed a PCR reaction that can distinguish the floxed from knockout Phd2 allele to survey other tissues for recombination efficiency. For this purpose, we examined the conditional knockout mice at both age groups. Consistent with the Real Time PCR results, we find that tamoxifen treatment induces efficient recombination in both liver and kidney, with perhaps more variability in the kidney than in the liver (Fig. 2C, top two panels). Examination of other tissues, including heart, lung, spleen, and muscle reveals effective recombination in these tissues as well (Fig. 2C, bottom four panels), and is consistent with the known activity of the Rosa26-CreERT2 mouse line [33].
Fig. 2.
Phd2 exon 2 deletion efficiency in mice after tamoxifen induction. Both young and aging mice were treated with tamoxifen for five consecutive days. Four weeks after the initial tamoxifen dose, mice were sacrificed. RNA and DNA were then isolated from select mouse tissues. (A and B) Phd2 mRNA levels in liver and kidney were measured by RT-PCR in (A) young and (B) aging mice. n = 4. * indicates p < 0.05 in comparing control (Con, Phd2 f/+) and CKO (Phd2 f/−; Rosa26-CreERT2) groups at a given age. (C) Phd2 exon 2 recombination efficiency was surveyed in Phd2 CKO mice in both the young and aging groups. A PCR reaction employing three primers was performed on genomic DNA obtained from the indicated tissues. The floxed Phd2 allele without recombination produces a 0.95 kb band, while the knockout Phd2 allele produces a 0.8 kb band. In each panel, the first lane shows results obtained with a control DNA from a Phd2 f/− mouse.
We next examined hematologic parameters in these mice. We find, as before [20], that acute global deletion of Phd2 in young mice induces a dramatic erythrocytosis, as reflected by both an increase in hematocrit and hemoglobin (compare first two columns in Figs. 1E and 1F, respectively). The Hct was 45.9 ± 1.7% in the control group and 69.4 ± 8.5% in the Phd2 conditional knockout (Fig. 1E). Importantly, we find that it also induces a comparable increase in these parameters in the aging mice. Thus, for the aging mice, the Hct was 42.3 ± 1.3% in the control group, and 74.7 ± 4.9% in the Phd2 conditional knockout, representing a 77% increase (compare last two columns in Fig. 1E). Interestingly, we find that white blood cell counts (WBC) are increased in both young and aging mice upon Phd2 deletion (Fig. 1G). For example, in the aging group, the WBC was 15.2 ± 5.34 × 103/µL in the control group and 25.2 ± 5.85 × 103/µL in the Phd2 conditional knockout group. Phd2 deletion also induces an increase in platelet counts in the young mice but not in the aging mice (Fig. 1H).
Further analysis of white blood cells indicates that Phd2 deletion in aging mice results in increases in neutrophils, eosinophils, basophils, monocytes, and lymphocytes (Table 1). In the young mice, increases were observed in the first three of these.
Table 1.
WBC differential in control and Phd2 conditional knockout micea
| Young | Aging | |||||
|---|---|---|---|---|---|---|
| Control | Phd2 CKO | p valueb | Control | Phd2 CKO | p valueb | |
| Neutrophils | 2.7 ± 1.7 | 13.1 ± 10 | < 0.05 | 3.9 ± 1.8 | 8.5 ± 3.3 | < 0.01 |
| Monocytes | 0.96 ± 0.33 | 4.3 ± 4.8 | 1.5 ± 0.26 | 2.1 ± 0.33 | < 0.01 | |
| Eosinophils | 0.54 ± 0.4 | 2 ± 1.5 | < 0.05 | 0.78 ± 0.52 | 1.7 ± 0.45 | < 0.01 |
| Basophils | 0.17 ± 0.12 | 0.49 ± 0.3 | < 0.05 | 0.21 ± 0.19 | 0.5 ± 0.2 | < 0.01 |
| Lymphocytes | 8.1 ± 2.8 | 10.9 ± 2.5 | 8.9 ± 3.3 | 12.3 ± 2.3 | < 0.05 | |
Values for all cell types are in 106/µL. Measurements were made four weeks after the initial tamoxifen dose. For each group, n = 6–8. Young = 6 to 8 months, aging = 16 to 20 months.
p values are for the comparison between Controls and Phd2 CKO mice in a given age group
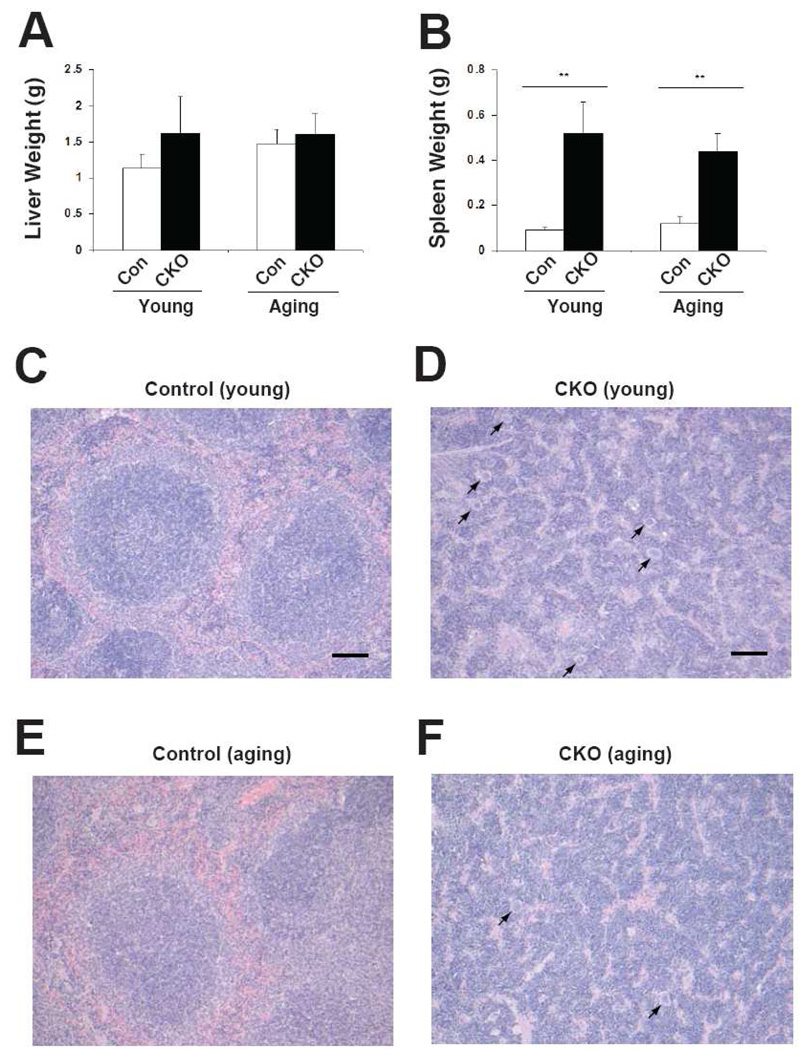
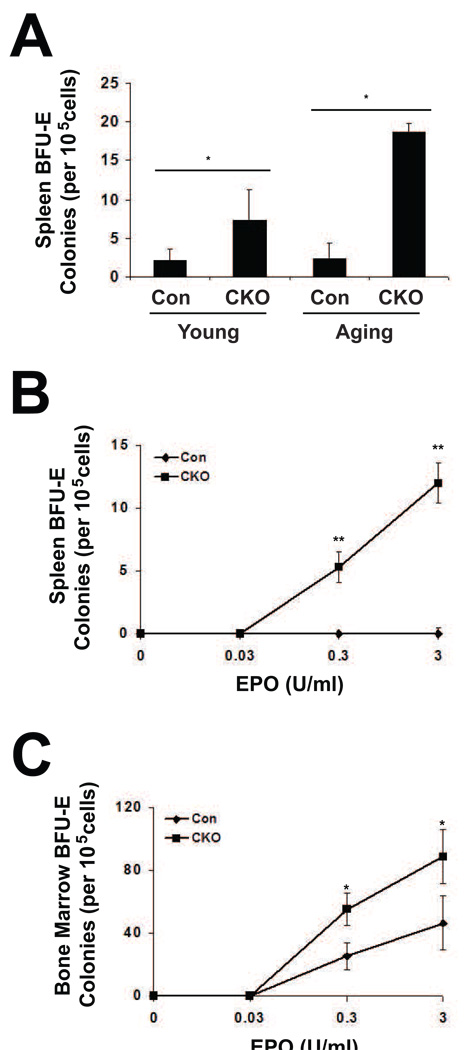
Liver weights were not increased upon Phd2 deletion in either young or aging mice (Fig. 3A). In contrast, spleens increased dramatically in size in both age groups upon Phd2 deletion (Fig. 3B). The increase was 5.8-fold in young mice, 3.7-fold in aging mice. Histologic examination of spleens revealed marked extramedullary hematopoiesis in the Phd2 conditional knockout mice in both the young and aging groups (Fig. 3C–F). Extramedullary hematopoiesis was not observed in histologic exam of the livers (data not shown). We examined erythroid progenitors from the spleens by isolating splenic cells from young and aging mice, and assessing their capacity to form BFU-E colonies. We find that Phd2 loss induces a marked increase in splenic BFU-E activity in both age groups (Fig. 4A), consistent with the histologic findings in the spleen. These BFU-E assays were performed at an EPO concentration 3U/ml. We next treated two month-old control or Phd2 CKO mice with tamoxifen, isolated splenic cells, and performed BFU-E assays at different EPO concentrations (Fig. 4B). A dose response curve for EPO indicates increased BFU-E activity not only at an EPO concentration of 3U/ml, but also at one of 0.3 U/ml.
Fig. 3.
Extramedullary hematopoiesis in Phd2 CKO mice. Both young and aging mice were treated with tamoxifen for five consecutive days. Four weeks after the initial tamoxifen dose, mice were sacrificed. Genotypes are as follows. Controls: Phd2 f/+; CKO: Phd2 f/−; Rosa26-CreERT2. (A) Liver and (B) spleen weights were measured. For (A) and (B), n=6–8. ** indicates p < 0.01. (C–F) Photomicrographs of hematoxylin and eosin stained sections of spleen from young (C,D) and aging (E,F) mice. (Leica DM2500 microscope equipped with Leica FireCam digital capture software, magnification 100×). Bars in (C) and (D) indicate 100 µm. Megakaryocytes are present in the spleens of the Phd2 CKO mice (indicated by arrows). Examination of ten high power fields from two mice in each age group reveals a higher number of megakaryocytes in the spleens of young Phd2 CKO mice (6.3 ± 2.0) as compared to aging Phd2 CKO mice (3.5 ± 1.5; p < 0.01).
Fig. 4.
Erythroid burst forming unit assays. (A) Young and aging mice were treated with tamoxifen for five consecutive days. Four weeks after the initial tamoxifen dose, cells were isolated from the spleen and BFU-E assays were performed in the presence of 3 U/ml EPO. Genotypes are as follows. Controls: Phd2 f/+; CKO: Phd2 f/−; Rosa26-CreERT2. (B and C) Two month old mice were treated with tamoxifen for five consecutive days. Three weeks after the initial treatment, cells were harvested from the (B) spleen and (C) bone marrow and BFU-E assays were performed in the absence or presence of the indicated EPO concentrations.
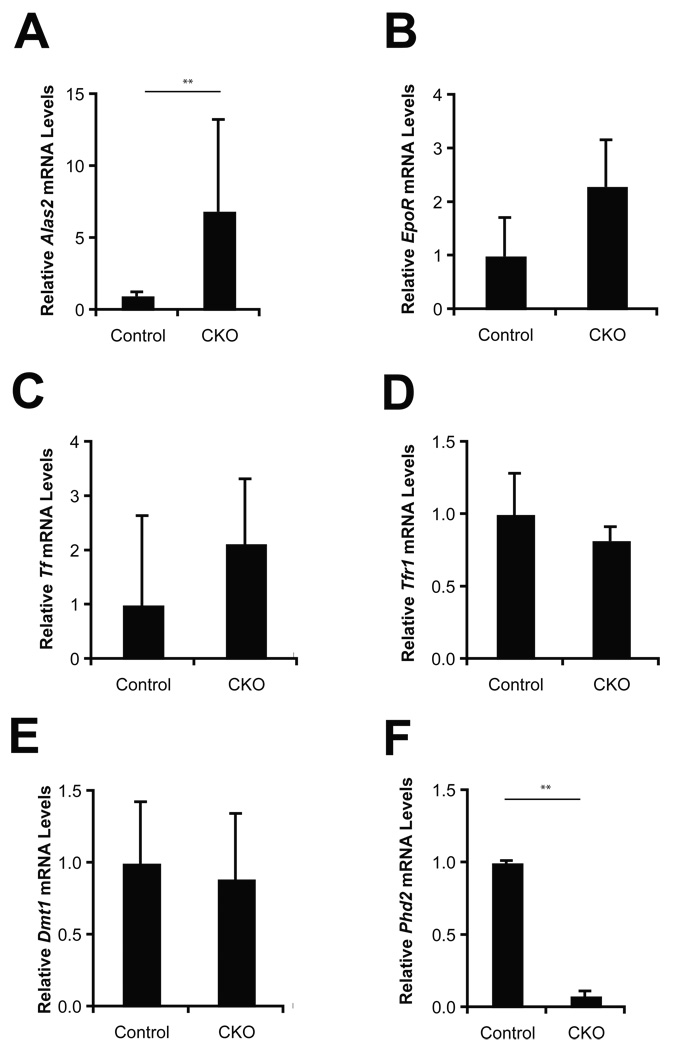
Parallel assays were performed on bone marrow cells, and these also demonstrate increased BFU-E activity at both 0.3 U/ml and 3 U/ml EPO concentrations (Fig. 4C). We examined bone marrow for the expression of genes involved in erythropoiesis and iron metabolism, including Alas2, EpoR, Tf, Tfr1, and Dmt1 (Fig. 5). Among these, we find upregulation of Alas2 mRNA in Phd2 CKO mice. While there was also a trend towards an increase in the EpoR and Tf mRNA, it was not statistically significant.
Fig. 5.
Aging mice were treated with tamoxifen for five consecutive days. Three weeks after the initial treatment, RNA was extracted from bone marrow. The mRNA levels of select genes were determined by Real Time PCR and normalized to that of β-actin. Abbreviations are as follows. Controls: Phd2 f/+ mice; CKO: Phd2 f/−; Rosa26-CreERT2. For both groups, n = 3. ** indicates p < 0.01 in comparing control and CKO groups.
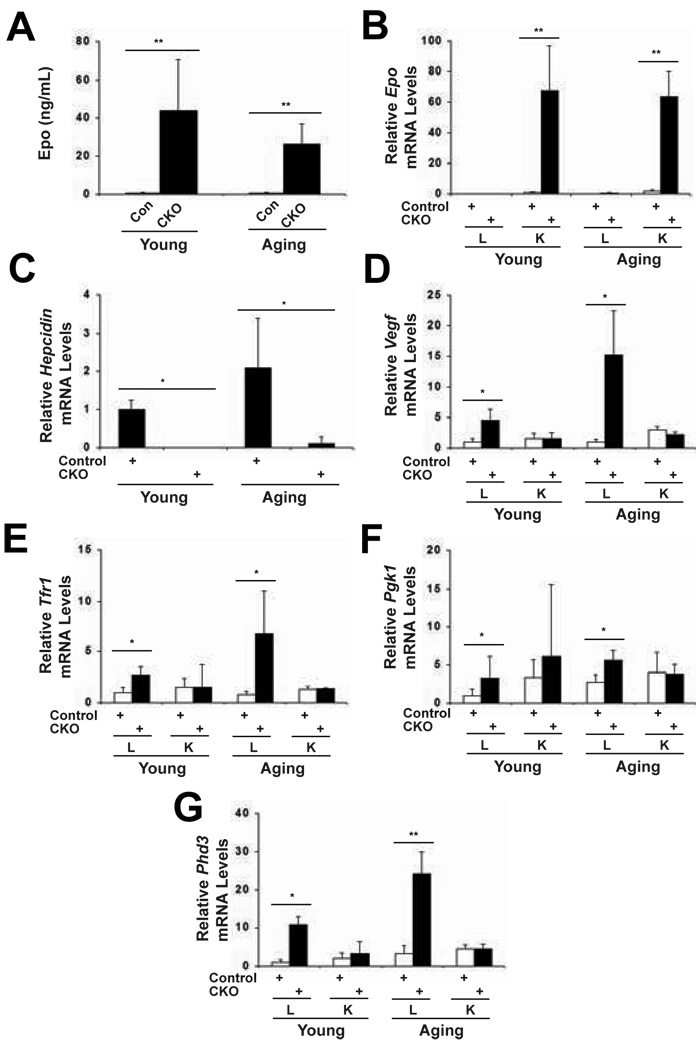
We measured plasma Epo levels and found a dramatic increase in both young and aging mice upon Phd2 deletion (Fig. 6A). It increased 62-fold in the young mice, and 46-fold in the aging mice. We did not find a statistically significant difference in Phd2 deletion-induced Epo levels between young and aging mice. To examine the tissue source of the Epo, we measured Epo mRNA levels by Real Time PCR, and observed a large increase in Epo mRNA in the kidneys of both the young and aging mice (Fig. 6B). We did not observe an increase in the Epo message from liver, thus indicating that kidney is the source of Epo upon Phd2 deletion in both the young and aging mice. Erythropoiesis is tightly coupled to iron homeostasis since approximately two-thirds of total body iron is contained within erythrocytes. Iron homeostasis, in turn, is regulated by the circulating hormone, hepcidin, which is produced by the liver [34]. Low hepcidin levels promote iron mobilization for use in cells such as erythroid progenitors, while high hepcidin concentrations inhibit this mobilization. We find that Phd2 knockout induces a marked decrease in hepcidin mRNA levels in the liver in both young and aging mice (Fig. 6C). The hepcidin message was also found to be the most highly downregulated of genes in microarray analysis of mRNA from liver upon Phd2 knockout (data not shown).
Fig. 6.
Upregulation of Hif target genes in Phd2 CKO mice. Both young and aging mice were treated with tamoxifen for five consecutive days. Four weeks after the initial dose, the mice were sacrificed. (A) Plasma Epo levels were measured by ELISA. Epo levels in young and aging CKO mice were compared by unpaired Student’s t test and the difference was not found to be statistically significant (p > 0.05). (B to G) RNA was extracted from kidney and liver. The mRNA levels of select genes were determined by Real Time PCR and normalized to that of β-actin. Abbreviations are as follows. Controls: Phd2 f/+ mice; CKO: Phd2 f/−; Rosa26-CreERT2; L: Liver; K: Kidney. For (A) n = 6–9; for (B to G) n = 4. * indicates p < 0.05 and ** indicates p < 0.01 in comparing control and CKO groups.
To examine whether other Hif target genes other than Epo are upregulated upon Phd2 knockout, we examined mRNA levels of the known Hif target genes Vegf, Pgk1, Tfr1, and Phd3. In both the young and aging groups, we find increases in all four of these genes upon Phd2 knockout in the liver, though not in the kidney. The degrees of induction varied depending on both the gene and on the age group. For example, the degree of induction of Vegf and Tfr1 was greater in the aging mice than in the young mice, whereas the degree of induction of Pgk1 was greater in the young mice than in the aging mice. Hence, global Phd2 knockout upregulates not only the Epo gene but also other Hif target genes in both young and aging mice.
Discussion
Recent studies have pointed to a central role for the PHD2 in regulating red cell mass in adult mammals [14; 16; 17; 20; 21]. In this study, we compared the role of Phd2 in regulating red cell mass in young versus aging mice. Our studies allow us to make the following observations.
First, Phd2 heterozygosity results in a mild erythrocytosis. This is in contrast to our earlier findings [20], in which no differences were observed. In the previous study, we examined mice at two to three months of age, while in the present study, we examined them at six to eight months of age. It is possible that this may be the explanation. In any case, we observe here that Phd2 heterozygosity continues to result in a mild erythrocytosis in the aging mice (Figs. 1A and B). This therefore supports Phd2 haploinsufficiency leading to erythrocytosis in the mouse. Clinically, all reported human erythrocytosis-associated PHD2 mutations are heterozygous and include missense, frameshift, and nonsense mutations [35; 36]. Based on these findings, it seems plausible that PHD2 haploinsufficiency is the underlying mechanism in at least some of these cases, such as the frameshift and nonsense mutations. However, it still leaves open the possibility that other mutations, such as the missense mutations, may operate by other mechanisms, such as dominant negative ones.
Second, the oxygen-sensitive Epo pathway is intact and robustly induced in the aging mice, since the acute global deletion of Phd2 leads to (i) the induction of Epo mRNA (Fig. 6B), (ii) an increase in circulating Epo in the plasma (Fig. 6A), (iii) extramedullary hematopoeisis and splenomegaly (Fig. 3), (iv) marked splenic BFU-E activity (Fig. 4A), and (v) dramatic increases in hematocrit and hemoglobin (Fig. 1E–F). Previous studies have shown aging-associated increases in PHD3 protein levels in tissues including heart, skeletal muscle, liver, and brain [37] [31; 38]. The present studies show that such a mechanism, if it were to occur in the Epo-producing cells of the kidney, is not sufficient to prevent robust induction of Epo upon loss of Phd2. It may also be noted that a clinical study on human patients showed that there was an appropriate increase in endogenous EPO in response to venesection in aging individuals, indicating that the EPO pathway is functional in these individuals [39].
A third observation from these studies is that the kidney continues to be the source of Epo in aging mice. Following fetal development, there is a shift in Epo production from the liver to the kidney. We do not find evidence for a shift of Epo production back to the liver in the aging mice. Indeed, the degree of induction of kidney Epo mRNA upon Phd2 deletion in aging mice is comparable to that seen in the young mice.
Fourth, Phd2 loss is associated not only with induction of the Epo pathway, but also with other effects, which include increases in Hif target genes in the liver as well as increases in white blood cell count. These changes are observed in both young and aging mice. With regard to the white blood cell count changes, it may be noted that Hif-2α has been implicated in maintaining the proper bone marrow microenvironment for hematopoeisis in the mouse [40; 41]. Hence, the PHD:HIF pathway may play a more general role in hematopoiesis beyond erythropoiesis. With regard to the induction of other Hif target genes, the upregulation of such genes in the liver is consistent with Phd2 playing a role, non-redundant with other Phd isoforms, in the control of the Hif pathway in the liver. We observed that Hepcidin mRNA is dramatically decreased upon Phd2 deletion. Further investigations will be required to determine if this decrease is due to Phd2 deletion-induced upregulation of Hif activity, which in turn has the capacity to directly affect Hepcidin gene transcription [42], or alternatively, if it is a consequence of the erythropoiesis itself—which through an unidentified factor—is known to result in potent inhibition of Hepcidin gene transcription [43].
The finding of robust induction of the Epo pathway upon Phd2 deletion would appear to contrast with a substantial body of evidence indicating impaired inducibility of the HIF pathway as a function of aging [26; 27; 28; 29; 30; 31; 32]. One possibility is that there is a shift in the dose-response curve relating HIF activation to Phd2 activity with aging such that age-dependent differences are apparent with partial loss of Phd2 activity (as with hypoxia) but not with complete loss of activity (as with genetic ablation). Arguing against this possibility is the observation that mice that are Phd2 haploinsufficient display erythrocytosis in both young and aging populations (Fig. 1A–B). Other factors that should be taken into consideration include the following. (i) Some of the other studies examined species other than mice, including rabbit and rat [27; 28; 29; 31]. (ii) The findings presented here primarily pertain to the Epo-producing interstitial cells of the renal cortex; these cells were not specifically examined in the previous studies. (iii) The present studies do not rule out the possibility that there may be impaired hypoxia-induced activation of the pathway as opposed to a defect in the integrity of the components of the pathway. For example, there may be an aging-associated defect in hypoxia-induced generation of reactive oxygen species that serve to inhibit Phd2 activity. In this scenario, the defect is not in the Phd2 protein per se, but in the signal transduction pathway that inhibits its activity under hypoxic conditions.
A clinical implication of these studies is that PHD2 continues to be a tenable target for manipulating the EPO pathway in the aging population because Phd2 loss of function in these experiments induces a robust erythropoiesis in aging mice. This, however, must be tempered by the fact that PHD2 loss of function will have systemic effects, and at least some of these effects are comparable in the young and aging. While some consequences, such as the decrease in Hepcidin message, may be desirable in the setting of augmenting erythropoiesis, others, such as the induction of Vegf mRNA, may warrant caution in other disease contexts. These studies reinforce the notion that PHD2 is not only a potent regulator of EPO, but also plays a broader role in regulating the hypoxic response.
Acknowledgments
We thank Dr. Celeste Simon and Dr. Michaela Gruber for advice on the tamoxifen treatment. This work was supported by NIH grant R01-CA090261, a Pilot Project Grant from the University of Pennsylvania Institute of Aging, and a grant from the Roche Foundation for Anemia Research (RoFAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 2.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 4.Kaelin WG. Von hippel-lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 5.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 6.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 10.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 11.Bunn HF. New agents that stimulate erythropoiesis. Blood. 2007;109:868–873. doi: 10.1182/blood-2006-08-019083. [DOI] [PubMed] [Google Scholar]
- 12.Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol Hematol. 2007;64:139–158. doi: 10.1016/j.critrevonc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008;40:160–165. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouyssegur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 16.Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110:2193–2196. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–649. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappalardi MB, Martin JD, Jiang Y, Burns MC, Zhao H, Ho T, Sweitzer S, Lor L, Schwartz B, Duffy K, Gontarek R, Tummino PJ, Copeland RA, Luo L. Biochemical characterization of human prolyl hydroxylase domain protein 2 variants associated with erythrocytosis. Biochemistry. 2008;47:11165–11167. doi: 10.1021/bi801624f. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but Not Heart Defects Are Associated with Elevated Hypoxia-Inducible Factor {alpha} Levels in Mice Lacking Prolyl Hydroxylase Domain Protein 2. Mol Cell Biol. 2006;26:8336–8846. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal N, Prchal JT. Erythropoietic agents and the elderly. Semin Hematol. 2008;45:267–275. doi: 10.1053/j.seminhematol.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenstaedt R, Penninx BW, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev. 2006;20:213–226. doi: 10.1016/j.blre.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 27.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 28.Di Giulio C, Bianchi G, Cacchio M, Artese L, Rapino C, Macri MA, Di Ilio C. Oxygen and life span: chronic hypoxia as a model for studying HIF-1alpha, VEGF and NOS during aging. Respir Physiol Neurobiol. 2005;147:31–38. doi: 10.1016/j.resp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Hwang IS, Fung ML, Liong EC, Tipoe GL, Tang F. Age-related changes in adrenomedullin expression and hypoxia-inducible factor-1 activity in the rat lung and their responses to hypoxia. J Gerontol A Biol Sci Med Sci. 2007;62:41–49. doi: 10.1093/gerona/62.1.41. [DOI] [PubMed] [Google Scholar]
- 30.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 31.Ndubuizu OI, Chavez JC, Lamanna JC. Increased Prolyl 4-Hydroxylase (PHD) Expression and Differential Regulation of Hypoxia Inducible Factors (HIFs) in the Aged Rat Brain. Am J Physiol Regul Integr Comp Physiol. 2009;297:R158–R165. doi: 10.1152/ajpregu.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 33.Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, Hennek T, Rode A, Heimann C, Faust N, Kauselmann G, Schoor M, Jaenisch R, Rajewsky K, Kuhn R, Schwenk F. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003;31:e12. doi: 10.1093/nar/gng012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [DOI] [PubMed] [Google Scholar]
- 35.Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood Rev. 2008;22:321–332. doi: 10.1016/j.blre.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Percy MJ, Lee FS. Familial erythrocytosis: molecular links to red blood cell control. Haematologica. 2008;93:963–967. doi: 10.3324/haematol.13250. [DOI] [PubMed] [Google Scholar]
- 37.Rohrbach S, Teichert S, Niemann B, Franke C, Katschinski DM. Caloric restriction counteracts age-dependent changes in prolyl-4-hydroxylase domain (PHD) 3 expression. Biogerontology. 2008;9:169–176. doi: 10.1007/s10522-008-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrbach S, Simm A, Pregla R, Franke C, Katschinski DM. Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology. 2005;6:165–171. doi: 10.1007/s10522-005-7950-9. [DOI] [PubMed] [Google Scholar]
- 39.Goodnough LT, Price TH, Parvin CA. The endogenous erythropoietin response and the erythropoietic response to blood loss anemia: the effects of age and gender. J Lab Clin Med. 1995;126:57–64. [PubMed] [Google Scholar]
- 40.Yamashita T, Ohneda O, Sakiyama A, Iwata F, Ohneda K, Fujii-Kuriyama Y. The microenvironment for erythropoiesis is regulated by HIF-2alpha through VCAM-1 in endothelial cells. Blood. 2008;112:1482–1492. doi: 10.1182/blood-2007-11-122648. [DOI] [PubMed] [Google Scholar]
- 41.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 42.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr Top Dev Biol. 2008;82:141–167. doi: 10.1016/S0070-2153(07)00006-3. [DOI] [PubMed] [Google Scholar]