Abstract
Homeless individuals (n = 187) entering contingency management (CM) for cocaine dependence were assessed for PTSD diagnosis, and a subset of 102 participants reporting traumatic exposure also periodically completed a self-report measure of PTSD symptoms. Patients with PTSD in full remission at 6 months (end of active treatment) and 12 months (end of aftercare) used substances much less frequently during aftercare than those with no PTSD diagnosis. Those whose PTSD diagnosis improved to full remission status during active treatment, and remained in full remission at 12 months, also had superior substance use outcomes. Severity of PTSD symptoms at 6 months, but not baseline or 2 months, was associated with substance use across treatment phases. Substance use during aftercare, however, was better predicted by changes in PTSD symptom severity. Patients whose PTSD symptoms improved more during active treatment fared better during aftercare than those with less improvement. Findings suggest homeless individuals with comorbid PTSD entering CM for cocaine dependence are not necessarily at increased risk for substance use compared to those without the comorbidity. However, course of PTSD does predict substance use, with the potential for CM to be unusually effective for those who respond with substantial, lasting improvements in PTSD.
Keywords: PTSD, contingency management, cocaine dependence, substance use, dual diagnosis, trauma, comorbidity
Comorbid posttraumatic stress disorder (PTSD) has been identified as a complicating factor in treatment of substance use disorders (SUDs), as researchers have proposed various functional relationships between PTSD symptoms and substance use (see Stewart & Conrod, 2003, for a review). Triggers for post-treatment relapse in these individuals differ from those with SUDs alone, as those with comorbid PTSD are more likely to relapse in response to negative emotions associated with interpersonal conflicts, but less likely to relapse due to cravings triggered by substance-related cues (e.g., Ouimette, Coolhart, Funderburk, Wade, & Brown, 2007). Trauma-related cues also trigger substance cravings in people with PTSD (Coffey et al., 2002), consistent with the self-medication hypothesis. This hypothesis describes substance misuse as a means to reduce negative affect (Khantzian, 1985) and emotional numbing, which comprise central diagnostic features of PTSD (Khantzian, 1997; APA, 2000). Additionally, avoidant coping behaviors have been linked to both SUDs (e.g., Stump & Smith, 2008) and PTSD (e.g., Stein et al., 2005), with retention of these strategies during treatment predicting poorer outcome for those with SUDs (Avants, Warburton, & Margolin, 2000). Emotional distress and avoidant coping are mediators between PTSD and increased risk of post-treatment substance use (Read, Brown, & Kahler, 2004; Ouimette, Finney, & Moos, 1999), providing support for both the self-medication and coping descriptions of the relationship between substance use and PTSD.
Despite evidence suggesting unique treatment concerns for PTSD-SUD comorbidity, as well as consumer preference for concurrent treatment of both conditions (Back, Brady, Jaanimägi, & Jackson, 2006; Brown Stout, & Gannon-Rowley, 1998), treatment guidelines favoring concurrent modalities are often abandoned in favor of referrals for SUD treatment alone (Back, Waldrop, Brady, & Hien, 2006; Ouimette, Moos, & Brown, 2003). Thus, it is pragmatically important to better understand the outcomes of individuals with PTSD pursuing SUD treatment in the absence of a PTSD-focused component (Najavits et al., 2007). It is also crucial to study such comorbidity in homeless populations. Relative to the general population, homeless people experience higher rates of SUDs and other mental disorders (see Fischer & Breakey, 1991, for a review), both of which are associated with victimization in these individuals. Trauma risk is increased by economic hardships related to homelessness, such as sleeping outdoors and obtaining income through prostitution, panhandling, and selling goods or drugs on the street (Wenzel, Koegel, & Gelberg, 2000). A set of 3 diagnostic, cross-sectional studies conducted around 1980, 1990, and 2000 suggested the proportion of homeless men and women with SUDs has increased dramatically, particularly in regard to cocaine. Further complicating effective service delivery, the prevalence of non-substance-related Axis I psychiatric disorders also increased for homeless persons during this time period (North, Eyrich, Pollio, & Spitznagel, 2004). Individuals with SUDs are also more likely to lose housing acquired after a period of homelessness (Zlotnick, Robertson, & Lahiff, 1999; Orwin, Scott, & Arieira, 2005). Together, this research suggests a vicious cycle of trauma, substance use, and homelessness, which further highlights the importance of effective treatment of SUDs for dually diagnosed homeless people.
Many studies on outcomes for individuals with PTSD in non-PTSD-focused treatments for SUDs have methodological shortcomings (Najavits et al., 2007), such as reliance on self-reported substance use, loosely defined and non-empirically supported SUD treatments, limited use of behavioral intervention, short follow-up periods, and single, brief measures of PTSD. In general, however, findings suggest individuals with comorbid PTSD have poorer outcomes (see Najavits et al., 2007; Ouimette, Brown, & Najavits, 1998). For example, Brown and colleagues (Brown, Stout, & Mueller, 1996) found that individuals with PTSD were prone to faster relapse per their self-reported substance use, although conclusions were tempered by no significant between-group differences in percentage of days of reported substance use. Additionally, Hien and colleagues (Hien, Nunes, Levin, & Frazer, 2000) used urine screenings to demonstrate that opiate-dependent individuals on methadone maintenance or detoxification protocols used more cocaine during the first 3 months of treatment if they were also diagnosed with PTSD. Finally, one large, well-controlled, multi-site outcome study drawing from a variety of treatments for cocaine-dependent individuals found mixed results: participants with PTSD did not report more drug use over 6 months of treatment than those without PTSD. However, those with a PTSD diagnosis did not mirror the decrease in alcohol use reported by those without PTSD. Diagnosis of PTSD was also associated with attenuated improvement in other addiction-related outcomes (Najavits et al., 2007). Methodological concerns notwithstanding, SUD treatment seems more challenging in the presence of PTSD.
To our knowledge, only one study (Ford, Hawke, Alessi, Ledgerwood, & Petry, 2007) has examined outcomes for individuals with PTSD symptoms in Contingency Management (CM), an empirically supported behavioral treatment for SUDs (for a review see Stitzer & Petry, 2006). Community center outpatients, who were either cocaine or heroin dependent, were randomly assigned such that one group received standard care and the other received standard care plus CM. The standard treatment involved intensive individual and group therapy, which could be adjusted according to need. The CM addition consisted of the following procedures, all provided by research assistants: (a) a needs assessment to establish treatment goals; (b) selection of goal-related activities with a research assistant; and (c) meetings for provision of vouchers or prizes for abstinence and completion of goal-related activities. Fifteen minutes of weekly educational meetings with a research assistant was provided to standard-care-only condition to provide comparable individualized attention to these CM procedures (Petry, Alessi, Marx, Austin, & Tardif, 2005).
In this study, PTSD symptoms at baseline were not associated with increased substance use during or after CM treatment. On the contrary, they were related to improved abstinence at 9 month follow-up as assessed by toxicology and self-report. Notably, for participants receiving standard care alone, PTSD symptoms were unrelated to abstinence (Ford et al., 2007). Although it is possible that participants with more severe PTSD symptoms received additional attention from the research assistants in CM and this resulted in superior outcome, there were many more opportunities for such increased attention to occur during the intensive standard treatment that both groups received. It appears more likely that an aspect of the CM component itself interacted with PTSD symptoms to produce better outcomes. The contrast between these findings and previously cited evidence of poorer outcomes for individuals with PTSD symptoms in other SUD treatment modalities suggests the possibility that PTSD may interact differently with CM. Further studies using superior measures of PTSD symptom severity are needed to replicate this unexpected finding.
Little is known regarding outcomes of homeless individuals with PTSD pursuing SUD treatment. In CM for homeless individuals with cocaine dependence, comorbid non-psychotic mental disorders in general may not reduce benefit from treatment (McNamara, Schumacher, Milby, Wallace, & Usdan, 2001). Additionally, CM targeting cocaine dependence, when combined with behavioral day treatment, reduces PTSD symptoms in homeless persons with traumatic exposure (Lester et al., 2007). Thus, baseline PTSD symptomatology may be less likely to impact outcomes in CM compared to other treatments, as PTSD symptoms may remit during CM treatment. However, it is unclear whether residual PTSD symptoms, persisting after active phases of CM, may affect distal outcomes in homeless individuals. Precedent for, and the importance of assessing PTSD at multiple time points during and after treatment, were highlighted by Read and colleagues (2004), who found that self-reported substance use 6 months after inpatient SUD treatment was associated not with baseline PTSD diagnosis but by the persistence of PTSD diagnosis during the follow-up period.
In the current study we aim to address these questions using rigorous methodology within the context of CM for homeless people with cocaine dependence. Both categorical and continuous variables from standardized self-report and interview measures were used to assess PTSD. These PTSD measures, along with urine screenings for substance use, were completed upon entry into CM and various treatment phases. Multiple assessment points, corresponding to phases of active treatment and aftercare, were used to facilitate inferences regarding the temporal relationship between PTSD and substance use in CM. Results will help to clarify when and if trauma-specific interventions should be applied in hopes of improving substance use outcomes in CM. We hypothesized that baseline PTSD diagnosis and symptoms would be unrelated to treatment outcome, while PTSD diagnosis and symptoms occurring after treatment would be associated with increased substance use during aftercare.
Method
Participants
Participants were 206 homeless individuals recruited at Birmingham Health Care (BHC), a community-based outpatient clinic serving the homeless. Case managers at BHC identified clients who may be eligible for the study and initially screened them for eligibility. Additionally, many participants originally learned about the study from other participants and then presented to case managers for the initial screening. Those who appeared eligible after this first screening were contacted by study staff, who administered a second screening to verify eligibility. Eligibility criteria included homelessness as defined by the McKinney Act (McKinney, 1987), cocaine dependence and reported cocaine use within the previous 2 weeks, psychological distress per score of 70 or greater on at least one scale of the Brief Symptom Inventory (Derogatis, 1977; Derogatis & Cleary, 1977), and intent to stay in the Birmingham area for 18 months. Participants were excluded from the study if they lacked capacity for informed consent, required immediate hospitalization, or were experiencing current psychotic symptoms. Participants meeting these admission criteria then completed a written consent form, which was at times also read aloud and explained to them.
Recruitment and study participation occurred from 2001-2004. African Americans comprised 94.2% of the sample, while the remaining 5.8% was Caucasian. This differs from the racial composition of the general homeless population in metro Birmingham metro as of 2005 (68% African American, 31% Caucasian; LaGory, Ritchey, Fitzpatrick, & Irwin, 2006), but is representative of the client base at BHC. Males comprised 72.3% of participants. Other demographic variables can be found in Table 1.
Table 1.
Demographic variables.
| Variable | Mean (SD) | Min (observed) |
Max (observed) |
|---|---|---|---|
| Age | 40.1 (7.1) | 20 | 57 |
| Years of Education | 11.9 (1.6) | 8 | 19 |
| Duration of Current Homeless Episode (months) | 30.1 (47.2) | 0 | 259 |
| Longest Full-Time Job (months) | 55.6 (50.1) | 0 | 252 |
| Years of Cocaine Use | 11.8 (6.5) | 0 | 31 |
| Years of Alcohol Use | 18.1 (10.3) | 0 | 40 |
Procedures
The University of Alabama at Birmingham Institutional Review Board approved all study procedures. Participants completed their first urine drug screen after providing consent, and were then randomly assigned such that 103 received contingency managed housing and work therapy alone (CM), while 103 received behavioral day treatment plus CM (CM+). Random draw procedures ensured equivalent group sizes and proportion of women between groups.
CM involved provision of housing contingent on urine toxicology results; if a participant tested positive for any substance or missed a urine screening without an excuse, he or she was moved to a shelter or other housing. Participants could return to the contingent housing after providing 3 consecutive, negative urine samples. Participants also received compensation for participation in vocational training activities and construction work, and their hourly wages increased or decreased contingent on abstinence and appropriate work behaviors. These wages were provided to the participants via Wal-Mart debit cards and accounts managed by the study for uses such as rent deposits. Lunches, HIV risk reduction education, and transportation to and from intervention activities, Wal-Mart, job interviews, and work were provided to all participants. Participants in the CM+ treatment group also received manualized behavioral treatment consisting of goal setting and positive reinforcement (Wal-Mart credit, monetary awards) for goal attainment, meetings with an individual counselor, group recreational outings, and group sessions on topics such as drug and alcohol education, relapse prevention, communication, and stress management. This comprised Phase I, the first 2 months of treatment.
During Phase II, lasting from months 3-6, all participants continued to receive CM, and referrals for permanent housing were made dependent upon sustained abstinence. Participants in CM+ also participated in individual counseling as needed along with weekly goal-setting and attainment group sessions. However, CM+ participants were no longer provided with tangible reinforcers for goal attainment. Phase III, lasting from months 7-12, was composed of follow-up and optional, and sustained aftercare. Aftercare consisted of weekly group therapy at BHC and low-rent, abstinence-contingent housing when available. Unexcused missing or positive urine screens resulted in permanent removal from such housing (see Milby et al., 2008, for details on methods and primary outcomes from this trial).
Measures
One week after entry into the study, participants returned for administration of the Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2001), administered by clinical psychology post-doctoral fellows and licensed psychologists on staff at University of Alabama. Responses to the PTSD module of the SCID indicated whether or not a participant met full criteria for PTSD or a diagnosis of PTSD in partial or full remission. Participants were also administered the SCID at 6- and 12-month follow-ups. Administrators of the SCID were not blind to treatment condition. They completed inter-rater reliability checks on the SCID during a training period, using patient interview recordings by First et al. (Biometrics Research Department, New York State Psychiatric Institute, New York, NY).
Nine months after the first participant was randomly assigned to a treatment group, the Posttraumatic Diagnostic Scale (PDS; Foa, Cashman, Jaycox, & Perry, 1997) was added to the measures administered in the study. The PDS symptom severity scale is comprised of summed self-reported frequency ratings for each of the 17 symptoms in the DSM-IV-TR (APA, 2000) criteria for PTSD. The symptom severity scale has demonstrated satisfactory internal consistency (alpha = .92) and test-retest reliability after a 2-3 week interval (kappa = .83). Possible scores range from 0-51, and the mean score in a sample of men and women who had experienced various forms of trauma was 33.59 (SD = 9.96) for those with a PTSD diagnosis versus 12.54 (SD = 10.54) for those not meeting criteria for PTSD (Foa et al., 1997).
The PDS was only administered to participants reporting exposure to at least one traumatic event meeting DSM-IV criterion A for PTSD. Thus, PDS data were collected for all participants diagnosed with PTSD, and also for a broader group of individuals who did not report symptoms related to the trauma they experienced or whose trauma-related symptoms did not meet full DSM-IV criteria for PTSD. The PDS was administered at baseline, 2, and 6 months, to correspond to the beginning and conclusion of active treatment Phases I and II.
Substance use was measured by urine drug screenings for cocaine, alcohol, and marijuana as assessed by OnTrack TestStik Radioimmunoassay (Varian, Lake Forest, CA). Additionally, if participants tested positive for opiates, amphetamines, or benzodiazepines at baseline, they were also tested for that substance during weekly tests in Phase I and II and then once monthly during Phase III. Urine was collected on Mondays, Wednesdays, and Fridays during Phase I and II, and randomly every week during Phase III, during which they received $20 per specimen. Unexcused absences on urine collection dates were considered substance positive (e.g., Milby et al., 2008; Higgins et al., 2007; Najavits et al., 1998), a strategy to correct for bias due to the likelihood that urine screens will be missed more frequently when an individual has used cocaine (Somoza et al., 2008, p. 133; Lavori et al., 1999). As homeless individuals may experience multiple psychosocial stressors precluding provision of all requested urine samples, absences were classified as unexcused if the participant had no legitimate excuse, and excused if staff believed the participant had been abstinent but missed the screen due to such difficulties (e.g., hospitalization). If participants missed a screening due to excused absence, this screening was removed from analysis. Three substance use variables were derived from the urine screening data: (a) number of consecutive weeks of abstinence during Phase I; (b) percentage of urine screenings positive for any substance during active treatment (Phases I and II); and (c) percentage of urine screenings positive for any substance during aftercare (Phase III).
Results
Consistent with the primary outcome study results (Milby et al., 2008), there was no difference between treatment groups (i.e., CM and CM+) in positive urine screens across active treatment Phases I and II [t(203) = 1.34, p > .18]. However, during aftercare the CM+ group tested substance-positive less often than the CM group [t(200) = 3.12, p < .01]. Table 2 details the prevalence of the PTSD diagnostic categories at each time point. General log linear analysis using multinomial distribution was run with baseline PTSD diagnostic category against treatment group. The null model was not rejected [LR(3) = .88, p > .83], indicating the diagnostic group prevalences did not differ between treatment groups at baseline. There were also no baseline differences in PDS symptom severity between treatment groups [t(100) =.27, p > .78]. See Table 3 for descriptive statistics on continuous study variables.
Table 2.
Prevalence of PTSD diagnostic categories.
| No PTSD | PTSD in Full | PTSD in Partial | PTSD Meeting Full | |
|---|---|---|---|---|
| Diagnosis | Remission | Remission | Criteria | |
| Baseline | 85.0%, n=159 | 3.7%, n=7 | 4.3%, n=8 | 7.0%, n=13 |
| 6 Months | 81.6%, n=133 | 7.4%, n=12 | 6.1%, n=10 | 4.9%, n=8 |
| 12 Months | 80.9%, n=123 | 12.5%, n=19 | 3.3%, n=5 | 3.3%, n=5 |
Table 3.
Descriptive statistics and Pearson correlations between age, PTSD symptom severity, and substance use.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Age | 1 | ||||||
| 2. PDS – Baseline | −.25* | 1 | |||||
| 3. PDS – 2 Months | −.30** | .68** | 1 | ||||
| 4. PDS – 6 Months | −.17 | .32** | .56** | 1 | |||
| 5. Consecutive Weeks Abstinent – Phase I | .25** | .01 | −.02 | −.11 | 1 | ||
| 6. % positive urines in Phases I and II | −.30** | −.02 | .05 | .22* | −.77** | 1 | |
| 7. % positive urines in Phase III | −.27** | −.01 | .01 | .33** | −.55** | .69** | 1 |
|
| |||||||
| N | 205 | 102 | 90 | 81 | 206 | 205 | 201 |
| Mean | 40.1 | 13.5 | 11.2 | 7.8 | 5.0 | 33.8 | 61.5 |
| SD | 7.1 | 14.1 | 13.4 | 12.1 | 3.0 | 35.8 | 38.4 |
| Min-Max (observed) | 20-57 | 0-51 | 0-49 | 0-47 | 0-8 | 0-100 | 0-100 |
p < .05
p < .01. PDS = Posttraumatic Diagnostic Scale, Symptom Severity Scale
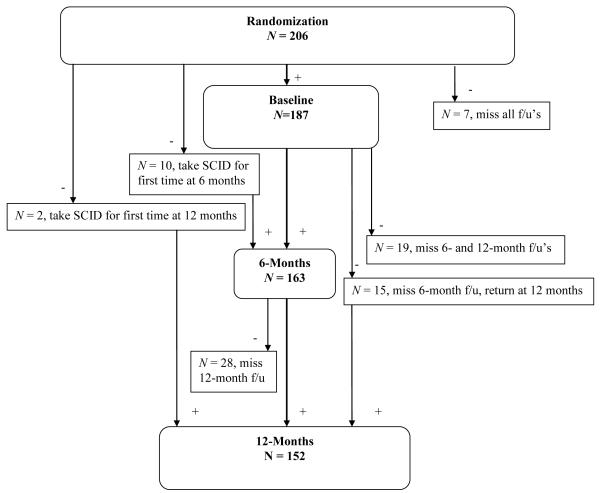
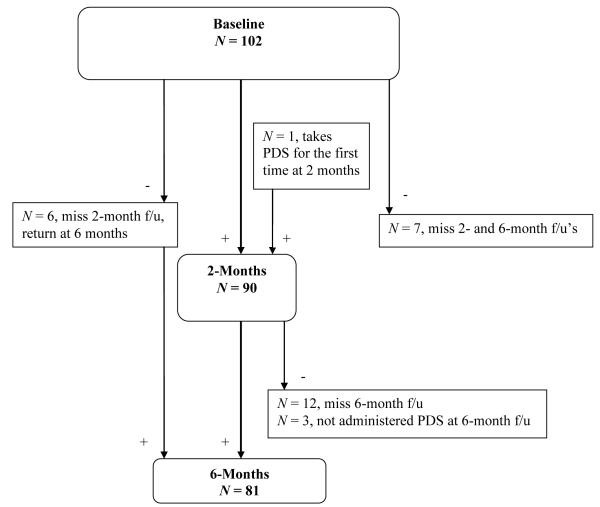
As assessed by the SCID, 11.2% of the 187 participants who returned for baseline SCID administration met criteria for PTSD or PTSD in partial remission. This is similar to the current PTSD prevalence found in a large study of non-homeless outpatients with traumatic experiences seeking treatment for cocaine dependence, with males overrepresented similarly to the current sample (10.9%, Najavits et al., 2003). Of those meeting current or lifetime criteria for PTSD at 6-months (n = 30), 6 were newly diagnosed (3 were not diagnosed with PTSD at baseline but met full criteria for PTSD at 6 months, 2 were newly diagnosed with PTSD in partial remission at 6 months, and 1 was not administered the SCID at baseline.) At 12 months, the participants with current or lifetime PTSD (n = 29) included 6 individuals diagnosed at 12 months but not at baseline or 6 months (1 met full criteria, 1 was in partial remission, and 4 were in full remission).1 At baseline, 102 participants completed the PDS symptom severity scale. At 2 months, 90 participants completed the PDS, and 81 completed the PDS at 6 months. Figures 1 and 2 outline the sources of missing data points on the primary variables.
Figure 1.
Flowchart of participants receiving the SCID throughout the study.
Figure 2.
Flowchart of participants completing the PDS throughout the study.
Relationship between PTSD Symptom Severity and Substance Use
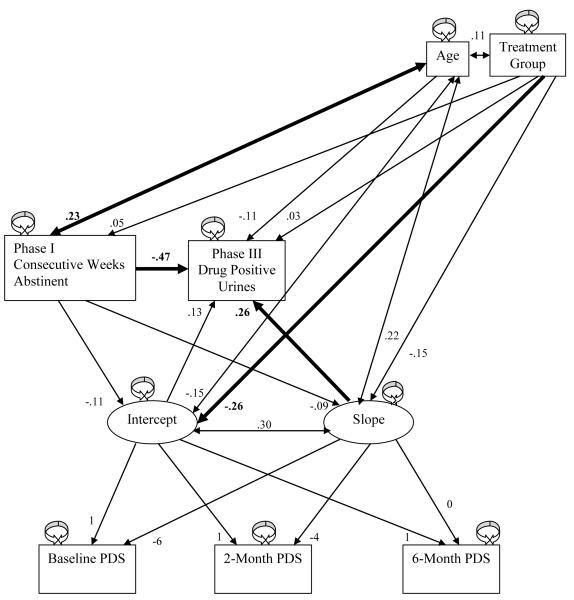
Correlations between the continuous study variables indicated the PDS symptom severity scale as assessed at 6 months was positively associated with frequency of substance use during both active treatment and aftercare (see Table 3). To further investigate the predictive value of PTSD symptom severity on substance use during aftercare, a linear growth model was constructed in which the PDS score at 6 months served as the reference point in examining both the 6 month score itself and the trajectory of PDS scores over the course of active treatment (see Figure 3). The percentage of substance positive urine screens during aftercare was transformed in this and all subsequent analyses using the 2 * arcsine(√x) formula to control for ceiling effects in percentage variables (Winer, 1971). The 6-month PDS score served as the intercept, while the slope represented change in PDS score over the 6 months of active treatment. The residual variances of the PDS scores assessed at baseline, 2 months, and 6 months were constrained to be equal, reflecting a common assumption of homoscedasticity, while path weights between the 3 PDS variables and the intercept were constrained to 1. Because the 6-month PDS score served as the reference point, the path between the 6-month PDS variable and the PDS slope variable was constrained to 0. The path weight between slope and the PDS assessed at 2 months was constrained to −4, reflecting its temporal distance from the 6 month time point; likewise, the path weight between baseline PDS and slope was constrained to −6. Treatment group was included in the model due to its aforementioned association with substance use during aftercare. Age was included as well, as older age is associated with reduced substance use (i.e., the “maturing out hypothesis”; Winick, 1962).
Figure 3.
Standardized parameter estimates in the linear growth model, with significant (p< .05) path weights in bold.
To account for the demonstrated predictive value of consecutive weeks of abstinence early in treatment on substance use at follow-up in multiple, similar samples (Vuchinich et al., 2009), the number of consecutive weeks abstinent in Phase I was assigned a path to substance use during aftercare. The consecutive-weeks-of-abstinence variable was limited to the first 2 months of treatment to maintain temporal distance and reduce excessive overlap with the outcome variable of substance use during Phase III.
The hypothesized model showed acceptable fit to the data [χ2(7) = 9.680, p = .21]; [NNFI = .93 (Bentler & Bonett, 1980)]; [CFI = .98 (Bentler, 1989)]; [RMSEA = .061 (Steiger & Lind, 1980; Browne & Cudeck, 1993)].2 Results suggested utility of using PTSD symptom trajectory to predict substance use after active treatment (β = .26, p < .05; see Table 4). The direction of this association, along with the mean slope of −.35, indicated participants whose PTSD symptoms improved less during active treatment were more likely to test positive for substances during aftercare. The intercept (6-month PDS score) of the growth curve, however, was predictive neither of substance use early in treatment nor of use during aftercare (p > .28). Consistent with previous literature, more consecutive weeks of abstinence in Phase I predicted less substance use during aftercare (β = −.47, p < .01). Age was related to consecutive weeks of abstinence in Phase I, such that older participants were able to initiate abstinence for longer periods than younger individuals (β = .23, p < .05), although there was no direct link between age and substance use during aftercare (p = .24). Finally, consistent with Lester et al. (2007), individuals in the CM+ treatment group reported fewer PTSD symptoms at 6 months than those in CM only (β = −.26, p <.05).
Table 4.
Unstandardized parameter estimates in the linear growth model.
| Variable 1 | Variable 2 | Estimate | S. E. | Est./S.E. | p | |
|---|---|---|---|---|---|---|
| Intercept | PDS - Baseline | 1 | 0 | - | - | |
| PDS – 2 months | 1 | 0 | - | - | ||
| PDS – 6 months | 1 | 0 | - | - | ||
| Slope | PDS – Baseline | −6 | 0 | - | - | |
| PDS – 2 months | −4 | 0 | - | - | ||
| PDS – 6 months | 0 | 0 | - | - | ||
| Intercept | On | Consecutive Weeks Abstinent – Phase I | −0.35 | 0.43 | −0.81 | .42 |
| Group | −4.97 | 2.52 | −1.97 | .049* | ||
| Slope | On | Consecutive Weeks Abstinent – Phase I | −0.06 | 0.09 | −0.68 | .50 |
| Group | −0.56 | 0.54 | −1.04 | .30 | ||
| Drug Positive Urines – Phase III | On | Intercept | 0.01 | 0.01 | 1.06 | .29 |
| Slope | 0.14 | 0.07 | 1.99 | .047* | ||
| Consecutive Weeks Abstinent – Phase I | −0.17 | 0.03 | −5.14 | .00** | ||
| Age | −0.02 | 0.01 | −1.18 | .24 | ||
| Group | 0.07 | 0.20 | 0.37 | .71 | ||
| Consecutive Weeks Abstinent – Phase I | On | Group | 0.29 | 0.57 | .51 | .61 |
| Intercept | With | Slope | 5.29 | 3.85 | 1.38 | .17 |
| Age | With | Intercept | −9.65 | 8.71 | −1.11 | .27 |
| Slope | 3.03 | 1.88 | 1.61 | .11 | ||
| Group | 0.39 | 0.35 | 1.11 | .27 | ||
| Consecutive Weeks Abstinent – Phase I | With | Age | 4.62 | 2.06 | 2.25 | .03* |
| PDS – Baseline, 2, and 6 months | Residual Variances (constrained equal) | 52.85 | 8.04 | 6.57 | .00** | |
| Consecutive Weeks Abstinent – Phase I | Residual Variance | 8.21 | 1.14 | 7.18 | .00** | |
| Drug Positive Urines – Phase III | Residual Variance | 0.65 | 0.12 | 5.62 | .00** | |
| Intercept | Residual Variance | 85.04 | 21.72 | 3.92 | .00** | |
| Slope | Residual Variance | 3.62 | 1.06 | 3.43 | .00** |
p < .05
p < .01
Relationship between PTSD Diagnosis and Substance Use
An analogous set of models was used to examine the predictive value of PTSD diagnosis on substance use in aftercare. A variable was constructed with 4 separate levels representing each of the PTSD diagnostic groups: (a) no PTSD diagnosis, (b) PTSD meeting full criteria, (c) PTSD in partial remission, and (d) PTSD in full remission. This diagnostic category variable was entered into an Analysis of Covariance (ANCOVA) controlling for age, consecutive weeks of abstinence in Phase I, and treatment group (see Table 5). The transformed percentage of urine screens positive for any substance during aftercare served as the dependent variable3. Three equations were constructed in this manner, separately for each PTSD diagnostic assessment point (i.e., baseline, 6 months, and 12 months).
Table 5.
ANCOVA omnibus test results with substance use during aftercare as the outcome, and PTSD diagnostic status or change as the predictor.
| Predictor Variable | |||||
|---|---|---|---|---|---|
| Diagnosis-Baseline | F(3)=.55,p>.64,ηp=.01 | ||||
| Diagnosis–6 Mos | F(3)=2.53,p=.06, ηp=.05 | ||||
| Diagnosis–12 Mos | F(3)=3.83*,ηp=.07 | ||||
| Diagnostic Change– Baseline to 6 Mos |
F(2)=5.07**, ηp=.06 | ||||
| Diagnostic Change– 6 Mos to 12 Mos |
F(3)=6.88**, ηp=.13 | ||||
| Covariates | |||||
|
| |||||
| Age | F(1)=4.35*, ηp=.02 | F(1)=2.86, p=.09, ηp=.02 | F(1)=4.10* ηp=.03 | F(1)=3.32, p=.07, ηp=.02 | F(1)=4.63*, ηp=.03 |
| Treatment Group | F(1)=4.87*, ηp=.03 | F(1)=4.59*, ηp=.03 | F(1)=4.98* ηp=.03 | F(1)=5.95*, ηp=.04 | F(1)=2.71, p=.10, ηp=.02 |
| Wks Abstinent-Phs I | F(1)=50.22**, ηp=.22 | F(1)=46.21**, ηp=.23 | F(1)=54.85**, ηp=.27 | F(1)=46.95**, ηp=.23 | F(1)=57.93**, ηp=.29 |
|
| |||||
| Corrected Model | F(6,177)=13.27** ηp=.31 | F(6,156)=13.26**, ηp=.34 | F(6,145)=15.11**, ηp=.39 | F(5,156)=16.73**, ηp=.35 | F(6,143)=7.01**, ηp=.42 |
p<.05
p<.01. Diagnosis refers to PTSD diagnosis. Wks Abstinent-Phs I refers to the number of consecutive weeks of abstinence during Phase I.
PTSD diagnostic category at baseline was not predictive of substance use in aftercare (p > .64). There was a trend (p = .06) for PTSD diagnostic group at 6 months to predict substance use during aftercare. Due to the small sample sizes of the PTSD, PTSD in partial remission, and PTSD in full remission groups, we decided to follow up on this trend despite statistical non-significance at the .05 level. Simple contrasts were used to compare the group with no PTSD diagnosis at 6 months with each of the 3 groups with a current or lifetime PTSD diagnosis at 6 months (see Table 6). Bonferroni adjustments were applied such that α = .05/3. Contrary to our hypothesis, neither the full-criteria PTSD nor the PTSD in partial remission group differed from those with no PTSD diagnoses in terms of substance use during aftercare (p’s > .39). Rather, the group with PTSD in full remission tested positive less often during aftercare than the group with no PTSD diagnosis ( p = .01), and this effect was large (d = .76; Cohen, 1988). The same pattern emerged for PTSD diagnostic category at 12 months. PTSD diagnostic group at 12 months was associated with substance use during aftercare, and simple contrasts also revealed that individuals with current or partially remitted PTSD at 12 months did not differ from the group with no 12-month PTSD diagnosis (p’s > .67). The group with PTSD in full remission at 12 months used substances much less frequently during aftercare than those with no PTSD diagnosis (p < .01; d = .84).
Table 6.
Simple contrasts between PTSD diagnostic groups in terms of percentage of substance positive urine screens during aftercare.
| Reference Group | Contrast Group | Raw Mean1(SD) | Raw Mean2(SD) | Conest | SE | p | d |
|---|---|---|---|---|---|---|---|
| 6 Months: | |||||||
| No PTSD diagnosis | PTSD meeting full criteria | .53 (.38) | .72 (.33) | .27 | .32 | .40 | .31 |
| No PTSD diagnosis | PTSD, partial remission | .53 (.38) | .66 (.32) | .10 | .29 | .74 | .11 |
| No PTSD diagnosis | PTSD, full remission | .53 (.38) | .31 (.35) | −.65 | .26 | .013* | .76 |
| No PTSD or PTSD not in full remission | Improved to full remission | .55 (.37) | .19 (.33) | −1.05 | .33 | <.01* | 1.23 |
| No PTSD or PTSD not in full remission | Remained fully remitted | .55 (.37) | .47 (.36) | −.16 | .39 | .69 | .28 |
| 12 Months: | |||||||
| No PTSD diagnosis | PTSD meeting full criteria | .55 (.39) | .73 (.37) | −.17 | .41 | .68 | .19 |
| No PTSD diagnosis | PTSD, partial remission | .55 (.39) | .75 (.33) | .06 | .41 | .88 | .07 |
| No PTSD diagnosis | PTSD, full remission | .55 (.39) | .31 (.37) | −.74 | .22 | < .01* | .84 |
| No PTSD or PTSD not in full remission | New diagnosis in full remission | .57 (.39) | .05 (.10) | −1.39 | .44 | <.01* | 1.61 |
| No PTSD or PTSD not in full remission | Improved to full remission | .57 (.39) | .58 (.34) | −.09 | .40 | .83 | .10 |
| No PTSD or PTSD not in full remission | Remained fully remitted | .57 (.39) | .19 (.29) | −1.01 | .30 | <.01* | 1.18 |
p < Bonferroni corrected α
Note. Effect sizes were derived from Rosnow, Rosenthal, & Rubin’s (2000) formula for calculating Cohen’s d (Cohen, 1988) from the t-statistic when contrasted groups are unequal in sample size.
Next, we created focused analyses on the groups diagnosed with PTSD in full remission at 6 or 12 months. The aim was to examine differing trajectories ending in PTSD in full remission, determining whether different trajectories to this same end point are differentially associated with substance use. A grouping variable was created to capture changes in PTSD diagnostic status from baseline to 6-month assessment. Individuals who were diagnosed with PTSD meeting full criteria, or in partial remission, at baseline comprised one group representing those whose diagnostic status improved to full remission by 6 months (n = 7). Individuals diagnosed with PTSD in full remission at both baseline and 6 months comprised a group with no change in full remission status (n=5), while the remaining participants comprised a reference group that was not diagnosed with PTSD in full remission at 6 months (n = 151). This change variable was entered into an ANCOVA, again with substance use during aftercare as the dependent variable and age, consecutive weeks abstinent during Phase I, and treatment group serving as covariates (see Table 5). The PTSD change variable was significant in this model, and simple contrasts with Bonferroni corrections against the reference group revealed a large group difference. Specifically, that those whose PTSD diagnostic status improved to full remission during active treatment were less likely to use substances during aftercare (p < .01; d = 1.23). The group whose diagnosis remained in full remission from baseline to 6 months did not differ from the reference group (p = .69).
The same ANCOVA model was then tested using a similar variable representing diagnostic change between 6 months and 12 months, but containing an additional group that was not diagnosed with PTSD at 6 months but met PTSD in full remission criteria at 12 months. Thus, Bonferroni correction for the simple contrasts was α = .05/4. The PTSD diagnostic change variable was significant (p < .01). In contrast to results for the 6-month time point, simple contrasts revealed that the group whose PTSD diagnosis improved to full remission status during aftercare (n = 5) did not differ from the reference group (n = 133; p > .83). However, those who maintained full remission status from 6 to 12 months (n = 9) used substances less often than the reference group (p < .01, d = 1.18), as did the group having no PTSD diagnosis at 6 months but newly diagnosed with PTSD in full remission at 12 months (n = 4; p < .01, d = 1.61). Both these group differences constituted large effects (Cohen, 1988).
Discussion
Findings indicate homeless individuals with comorbid PTSD entering CM for cocaine dependence are not necessarily at increased risk for substance use during or after treatment. However, individuals reporting trauma exposure whose PTSD-related symptoms do not sufficiently improve during active treatment used substances more often than those whose posttraumatic symptoms respond well to CM. Many individuals reported trauma exposure and related symptoms, but did not meet full criteria for a diagnosis of PTSD. Even if they are not diagnosed with PTSD, results suggest individuals with posttraumatic symptoms should be monitored during active treatment and considered for focused PTSD intervention if their symptoms are not substantially improving. Our findings also enabled some inferences about critical time periods during this form of active treatment. PTSD at baseline and 2 months did not predict substance use during any treatment phase, but PTSD symptoms at 6 months correlated with substance use during treatment and aftercare. Although the correlation with substance use during aftercare was accounted for by changes in PTSD rather than 6-month PTSD severity per se, the correlation does suggest important changes may occur specifically during the 2-6 month time period.
Analyses of PTSD diagnostic status echoed our findings on the importance of improvement in PTSD symptom severity during active treatment. Full remission of PTSD was required for superior substance use outcomes. As remission of PTSD only predicted substance abuse when remission occurred during active treatment, these findings also suggest individuals whose PTSD symptoms are not improving should receive additional intervention during active treatment. PTSD remission during aftercare did not improve outcomes, indicating active treatment may be a critical period for additional treatment efforts. Although no studies to our knowledge have compared sequential versus concurrent treatment of PTSD and substance use disorders, our findings are consistent with treatment guidelines suggesting concurrent treatment of PTSD and SUDs (Back, Waldrop, Brady, & Hien, 2006; Ouimette, Moos, & Brown, 2003). Additional services during aftercare are also suggested, as people who maintained full remission of PTSD during aftercare also had superior substance use outcomes. This is consistent with other studies reporting improved outcomes for dually diagnosed persons who, after completion of treatment for substance use, receive additional services such as PTSD-focused intervention or continued substance-related treatment (Ouimette, Moos, & Finney, 2003; Ouimette, Moos, & Finney, 2000).
Exposure therapy for individuals with PTSD and SUDs is a potential adjunct in future attempts to increase the efficacy of CM. Exposure therapy is recommended by the International Consensus Group on Depression and Anxiety as the gold standard psychological treatment for individuals with PTSD (Ballenger et al., 2000). Preliminary examination of exposure therapy in conjunction with empirically supported SUD treatment revealed that substance abuse did not increase due to the exposure component, as feared by many clinicians (Brady, Dansky, Back, Foa, & Carroll, 2001). Although drop-out rates were high, these authors described their attrition rates as lower than those of other trials of psychotherapies addressing cocaine dependence. Participants who left the study cited reasons for drop-out such as transportation and other logistical barriers, but not avoidance of exposures. The nature of our CM treatment reduces some of these psychosocial barriers, suggesting addition of exposure therapy may not necessarily increase attrition. An alternative adjunct is Seeking Safety (Najavits, 2002), a non-exposure-based cognitive behavioral treatment (CBT) targeting PTSD and SUDs concurrently. Studies with female samples showed that although Seeking Safety evidenced superior outcomes in terms of both PTSD and substance use relative to treatment as usual (Hien, Cohen, Miele, Litt, & Capstick, 2004), randomized controlled trials failed to demonstrate superiority of Seeking Safety to relapse prevention for substance use (Hien et al., 2004) or health education (Hien et al., 2009). Also, our CM+ group, which reported less severe PTSD symptoms at conclusion of active treatment, received a behavioral day treatment that already included topics germane to some of the modules in Seeking Safety. It thus seems more likely that exposure therapy could confer additional benefit to this particular treatment program. Selective serotonin reuptake inhibitors, family therapy, and self-help groups have also been suggested as potentially helpful additions to SUD treatment for individuals with comorbid PTSD (Back, Waldrop, Brady, & Hien, 2006; Ouimette, Ahrens, Moos, & Finney, 1998).
It is also important to work towards identifying individuals at baseline whose PTSD symptoms are either less likely to improve during CM, or less likely to remain in remission during aftercare. If such predictors could be identified, services could be lengthened or better tailored to their needs proactively. One potential predictor of refractory PTSD may be coping style. In another analysis using a subset of the same data used in the current study, positive distraction and negative avoidance assessed at baseline emerged as negative and positive predictors, respectively, of PTSD symptoms at the conclusion of active treatment phase. These authors also found that adding the behavioral day treatment to contingency management was associated with reduced PTSD symptoms at the conclusion of active treatment (Lester et al., 2007). Perhaps individuals with these or other risk factors for refractory PTSD could be prioritized to also receive such behavioral day treatment as part of a stepped care approach (e.g., Milby et al., 2008, p. 181). The idea that coping style may predict which patients have PTSD symptoms that will remain or even worsen during SUD treatment is also supported by a study of male veterans. Coping style and abstinence-related self-efficacy, rather than PTSD diagnosis, were associated with exacerbated psychiatric symptoms during residential SUD treatment (Ilgen & Moos, 2006). For some individuals with these risk factors, substance use may serve to mask psychiatric symptoms, describing one pathway toward failure of PTSD to fully remit in order to reduce the risk of substance use during aftercare.
Another factor that may help distinguish individuals with comorbid PTSD who will experience symptom relief during SUD treatment is order of onset. Individuals who developed PTSD prior to the onset of alcohol dependence respond better to both the addition of sertraline to CBT for SUDs (Brady et al., 2005) and CBT for SUDs alone (Back, Jackson, Sonne, & Brady, 2005), relative to those who developed PTSD after onset of alcohol dependence. The most striking of our findings was that individuals who fully recovered from PTSD by the end of active CM treatment had better substance use outcomes than those without any PTSD diagnosis, echoing the findings of Ford et al. (2007). This suggests CM may be particularly effective in targeting the difficulties of a subset of dually diagnosed persons. Considering the evidence on order of onset and treatment response, this efficacy could be due to varying motivations for cocaine use. Motivations to use cocaine range from reducing negative affect, increasing positive affect, and social cohesion (Newcomb, Chou, Bentler, & Huba, 1988), to weight control (Cochrane, Malcolm, & Brewerton, 1998) and paradoxical relaxation and attentional control for individuals with attention-deficit/hyperactivity disorder (Khantzian, 1985). CM is unlikely to target all of these motives, and individuals who use cocaine to increase positive affect in particular may have sought cocaine to replace the contingencies removed during aftercare. In fact, a recent study indicated cocaine-dependent individuals may require long-term contingencies to maintain abstinence (DeFulio, Donlin, Wong, & Silverman, 2009), and the present findings suggest this could be more or less true depending on comorbidity that may be driving the cocaine use. For participants whose drug use was preceded by PTSD and potentially a more direct result of PTSD symptoms, reduction of PTSD symptoms during CM may have reduced their need for self-medication and led to better outcomes.
Discussion of other ways that CM potentially targets the difficulties of individuals with comorbid PTSD may aid in clarifying directions for future studies. One limitation to the current study is the unknown direction of causality between PTSD symptoms and substance use. PTSD symptom trajectory during treatment temporally preceded substance use during aftercare, consistent with self-reports of most dually diagnosed individuals describing changes in PTSD symptoms as predictors of later cocaine use (Back, Brady, Jaanimägi, & Jackson, 2006; Brown et al., 1998). However, there was also a positive association between PTSD symptom severity at 6 months and the toxicology results during active treatment. A period of abstinence may help lead to reduction in PTSD symptoms, particularly given the anxiogenic properties of cocaine (APA, 1994, p. 244). Participants who reduced their substance use may have also decreased their risk of victimization during treatment, particularly since private housing was provided based on abstinence.
A third variable may also be responsible for the connection between improvements in PTSD and reduced substance use. There is evidence that individuals with comorbid PTSD derive different benefits from SUD treatment, as those with PTSD increased use of approach-oriented coping styles to a greater degree after 12-step involvement than patients with SUDs only. Such individuals also experienced greater reductions in psychological distress than patients with SUDs alone, in response to 12-step involvement, family therapy, and additional substance-related counseling sessions (Ouimette, Ahrens, Moos, & Finney, 1998). As previously discussed, both coping style and emotional distress are related to substance use in people with PTSD.
Control is also an issue salient in PTSD, SUDs, and homelessness (Lester et al., 2007, p. 573). The “control heuristic” model suggests perceived control increases when: (a) people believe that their actions are connected with an outcome; (b) the outcome is desirable; (c) the action was intended to produce the outcome; and (d) positive reinforcement is frequent (Thompson et al., 2004; Thompson, Armstrong, & Thomas, 1998). CM may thus foster a sense of control due to clearly communicated, consistent connection between participant behavior and contingencies, presumed desire and intention on the part of participants to obtain the outcomes (e.g., housing in the current study), and frequent administration of positive reinforcers (Peer, Strachan, & Spaulding, 2008). As reduced perceived control is associated with stress reactions, increased perceived control may help reduce PTSD-related anxiety (for a review, see Benight & Bandura, 2004) in addition to assisting maintenance of abstinence. Indeed, self-efficacy predicts less severe PTSD symptoms longitudinally, and substance-related self-efficacy beliefs are associated with reduced substance use in trauma survivors (Luszczynska, Benight, & Cieslak, 2009). Increased perceived control is associated with increased active coping and decreased avoidant coping (for a review, see Benight & Bandura, 2004), so a combination of CM-related changes may help to explain our findings.
Limitations
As previously discussed, future studies should attempt to clarify the mechanism by which substance use and PTSD are related during and after CM. In particular, data on trauma chronology was unavailable in the current study, limiting our understanding of the effects of previous trauma versus more recent trauma. Although removing individuals newly diagnosed with PTSD at 6 or 12 months did not substantially change the pattern of results, those with a preexisting PTSD diagnosis may have been re-traumatized during the study. It is also unclear whether the new diagnoses of PTSD at 6 or 12 months were: 1) based on previous trauma participants failed to report during previous assessments; 2) trauma experienced just prior to study participation that did not meet the duration criterion for PTSD (APA, 2000) until 6 months; or 3) trauma occurring during the study. This is important to examine, given the unexpected finding that the group newly diagnosed with PTSD in full remission at 12 months evidenced superior outcomes. If this group was diagnosed based on recent traumas, their rapid progression to full remission status may reflect effective coping or other protective factors that could also have helped them to reduce their substance use.
Methodological limitations of the current study included the fact that participants and SCID administrators were not blind to treatment condition, which may have biased the assessments. The sample sizes for the diagnostic groups with PTSD were also small, as this study was not originally powered for these analyses. Results require replication in larger groups with PTSD in various stages of remission or non-remission. Future studies should also examine the generalizability of results, in particular to general populations pursuing CM, populations with different comorbid, non-substance related psychiatric disorders, and individuals pursuing CM for dependence on other substances. Finally, incorporation of more frequent assessments of PTSD symptoms would enable greater precision in identifying temporal sequencing between PTSD and substance use.
Conclusion
To our knowledge, this is only the second study to have examined PTSD and outcomes in CM, and our use of multiple, more objective assessments of both PTSD and substance use begins to bridge this critical gap in the dual diagnosis literature. We have also extended the seminal Ford et al. (2007) findings to homeless individuals, who despite critical need of efficacious treatment for multiple psychosocial difficulties, are largely excluded from research studies. Results suggest that some individuals with posttraumatic symptoms have the potential to respond better to CM than those without, highlighting promising new directions for future research aiming to improve the efficacy of this empirically supported treatment for SUDs.
Acknowledgements
This research was supported by National Institute on Drug Abuse (NIDA) grant R01 DA11789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To verify the following results were not driven solely by newly diagnosed participants, we tried removing the 12 participants newly diagnosed at 6 or 12 months from analyses. The pattern of results for the predictive value of PTSD diagnosis and diagnostic change remained identical. In the linear growth model, the association between PTSD symptom trajectory and drug use during aftercare became a trend (p = .09) rather than a significant result. The path weight between the slope and drug use, however, was larger than in the reported model, and its statistical non-significance may have been due to a larger SE stemming from reduced sample size.
Consistent with recommendations made elsewhere (e.g., Preacher, Wichman, MacCallum, & Briggs, 2008; Widaman & Thompson, 2003), the null model used to derive fit indices differed from that typically employed in SEM applications. In this model, only (invariant) mean level of PDS and common residual variance of the PDS indicators was estimated, and no relationship was estimated among this mean level variable and other variables subsequently used in the hypothesized model. As is typical, this null model inadequately represented the data [χ2(25) = 160.55, p < .0001].
Three analogous ANCOVAs were also run with substance use during active treatment as the dependent variable, and at no time point (i.e., baseline, 6 months, 12 months) was PTSD diagnostic category significant (p’s > .12).
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- Avants S, Warburton L, Margolin A. The influence of coping and depression on abstinence from illicit drug use in methadone-maintained patients. American Journal of Drug and Alcohol Abuse. 2000;26:399–416. doi: 10.1081/ada-100100252. [DOI] [PubMed] [Google Scholar]
- Back S, Brady K, Jaanimägi U, Jackson J. Cocaine dependence and PTSD: A pilot study of symptom interplay and treatment preferences. Addictive Behaviors. 2006;31:351–354. doi: 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Back S, Dansky B, Coffey SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without Posttraumatic Stress Disorder: A comparison of substance use, trauma history, and psychiatric comorbidity. American Journal on Addictions. 2000;9:51–62. doi: 10.1080/10550490050172227. [DOI] [PubMed] [Google Scholar]
- Back S, Jackson J, Sonne S, Brady K. Alcohol dependence and posttraumatic stress disorder: Differences in clinical presentation and response to cognitive-behavioral therapy by order of onset. Journal of Substance Abuse Treatment. 2005;29:29–37. doi: 10.1016/j.jsat.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Back S, Waldrop A, Brady K, Hien D. Evidence-based time-limited treatment of co-occurring substance-use disorders and civilian-related posttraumatic stress disorder. Brief Treatment and Crisis Intervention. 2006;6:283–294. [Google Scholar]
- Ballenger JC, Davidson JRT, Lecrubier Y, Nutt DJ, Foa EB, Kessler RC, McFarlane AC. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. Journal of Clinical Psychiatry. 2000;61:60–66. [PubMed] [Google Scholar]
- Benight C, Bandura A. Social cognitive theory of posttraumatic recovery: The role of perceived self-efficacy. Behaviour Research and Therapy. 2004;42:1129–1148. doi: 10.1016/j.brat.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS structural equations program manual. BMDP Statistical Software; Los Angeles: 1989. [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Brady K, Dansky B, Back S, Foa E, Carroll K. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. Journal of Substance Abuse Treatment. 2001;21:47–54. doi: 10.1016/s0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Brown P, Stout R, Gannon-Rowley J. Substance use disorder-PTSD comobidity: Patients’ perceptions of symptom interplay and treatment issues. Journal of Substance Abuse Treatment. 1998;15:445–448. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- Brown P, Stout R, Mueller T. Posttraumatic stress disorder and substance abuse relapse among women: A pilot study. Psychology of Addictive Behaviors. 1996;10:124–128. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage; Newbury Park, CA: 1993. pp. 136–162. [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addictive Behaviors. 1998;23:201–207. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Coffey S, Saladin M, Drobes D, Brady K, Dansky B, Kilpatrick D. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug and Alcohol Dependence. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Ouimette P. Crosby, Brown P, Najavits L. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addictive Behaviors. 1998;23:785–796. doi: 10.1016/s0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Donlin W, Wong C, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: A randomized controlled trial. Addiction. 2009;104:1530–1538. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. The SC-R-90 manual: I. Scoring, administration and procedures for the SCL-90. Clinical Psychometric Research; Baltimore, MD: 1977. [Google Scholar]
- Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90-R: A study in construct validation. Journal of Clinical Psychology. 1977;33:981–989. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Fischer P, Breakey W. The epidemiology of alcohol, drug, and mental disorders among homeless persons. American Psychologist. 1991;46:1115–1128. doi: 10.1037//0003-066x.46.11.1115. [DOI] [PubMed] [Google Scholar]
- Foa E, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of PTSD: The Posttraumatic Diagnostic Scale. Psychological Assessment. 1997;9:445–451. [Google Scholar]
- Ford JD, Hawke J, Alessi S, Ledgerwood D, Petry N. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behaviour Research and Therapy. 2007;45:2417–2431. doi: 10.1016/j.brat.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hien D, Cohen L, Miele G, Litt L, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. The American Journal of Psychiatry. 2004;161:1426–1432. doi: 10.1176/appi.ajp.161.8.1426. [DOI] [PubMed] [Google Scholar]
- Hien D, Nunes E, Levin F, Frazer D. PTSD and short-term outcome in early methadone treatment. Journal of Substance Abuse Treatment. 2000;19:31–37. doi: 10.1016/s0740-5472(99)00088-4. [DOI] [PubMed] [Google Scholar]
- Hien D, Wells E, Jiang H, Suarez-Morales L, Campbell A, Cohen L, Miele GM, Kileen T, Brigham GS, Zhang Y, Hansen C, Hodgkins C, Hatch-Maillette M, Brown C, Kulaga A, Kristman-Valente A, Chu M, Sage R, Robinson JA, Liu D, Nunes EV. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. Journal of Consulting and Clinical Psychology. 2009;77:607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S, Heil S, Dantona R, Donham R, Matthews M, Badger G. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Ilgen M, Moos R. Exacerbation of psychiatric symptoms during substance use disorder treatment. Psychiatric Services. 2006;57:1758–1764. doi: 10.1176/ps.2006.57.12.1758. [DOI] [PubMed] [Google Scholar]
- Khantzian E. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. The American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Khantzian The self-medication hypothesis of substance use disorders: reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- LaGory M, Ritchey FJ, Fitzpatrick K, Irwin J. A Needs Assessment of the Homeless of Birmingham and Jefferson County. Department of Sociology, University of Alabama at Birmingham; Birmingham: 2006. 2006. [Google Scholar]
- Lavori PW, Bloch DA, Bridge PT, Leiderman DB, LoCastro JS, Somoza E. Plans, designs, and analyses for clinical trials of anti-cocaine medications: Where we are today. NIDA/VA/SU Working Group on Design and Analysis. Journal of Clinical Psychopharmacology. 1999;19:246–256. doi: 10.1097/00004714-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Lester KM, Milby JB, Schumacher JE, Vuchinich R, Person S, Clay OJ. Impact of behavioral contingency management intervention on coping behaviors and PTSD symptom reduction in cocaine-addicted homeless. Journal of Traumatic Stress. 2007;20:565–575. doi: 10.1002/jts.20239. [DOI] [PubMed] [Google Scholar]
- Luszczynska A, Benight C, Cieslak R. Self-efficacy and health-related outcomes of collective trauma: A systematic review. European Psychologist. 2009;14:51–62. [Google Scholar]
- McKinney SB. McKinney Homelessness Assistance Act. Public Law. 1987:100–177.
- McNamara C, Schumacher J, Milby J, Wallace D, Usdan S. Prevalence of nonpsychotic mental disorders does not affect treatment outcomes in a homeless dependent sample. American Journal of Drug and Alcohol Abuse. 2001;27:91–106. doi: 10.1081/ada-100103120. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, Vuchinich RE, Freedman MJ, Kertesz S, Wallace D. Toward cost-effective initial care for substance-abusing homeless. Journal of Substance Abuse Treatment. 2008;34:180–191. doi: 10.1016/j.jsat.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM. Seeking safety: A treatment manual for PTSD and substance abuse. Guilford; New York: 2002. [DOI] [PubMed] [Google Scholar]
- Najavits L, Gastfriend D, Barber J, Reif S, Muenz L, Blaine J, Frank A, Crits-Christoph P, Thase M, Weiss RD. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse. American Journal of Psychiatry. 1998;155:214. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Harned MS, Gallop RJ, Butler SF, Barber JP, Thase ME, Crits-Christoph P. Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multisite national trial. Journal of Studies on Alcohol and Drugs. 2007;68:353–361. doi: 10.15288/jsad.2007.68.353. [DOI] [PubMed] [Google Scholar]
- Najavits L, Runkel R, Neuner C, Frank A, Thase M, Crits-Christoph P, Elaine J. Rates and Symptoms of PTSD among Cocaine-Dependent Patients. Journal of Studies on Alcohol. 2003;64:601–606. doi: 10.15288/jsa.2003.64.601. [DOI] [PubMed] [Google Scholar]
- Newcomb M, Chou C, Bentler P, Huba G. Cognitive motivations for drug use among adolescents: Longitudinal tests of gender differences and predictors of change in drug use. Journal of Counseling Psychology. 1988;35:426–438. [Google Scholar]
- North C, Eyrich K, Pollio D, Spitznagel E. Are Rates of Psychiatric Disorders in the Homeless Population Changing? American Journal of Public Health. 2004;94:103–108. doi: 10.2105/ajph.94.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin RG, Scott CK, Arieira C. Transitions through homelessness and factors that predict them: three-year treatment outcomes. Journal of Substance Abuse Treatment. 2005;28(Suppl 1):S23–39. doi: 10.1016/j.jsat.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Ahrens C, Moos RH, Finney JW. During treatment changes in substance abuse patients with posttraumatic stress disorder: The influence of specific interventions and program environments. Journal of Substance Abuse Treatment. 1998;15:555–564. doi: 10.1016/s0740-5472(97)00315-2. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Coolhart D, Funderburk J, Wade M, Brown P. Precipitants of first substance use in recently abstinent substance use disorder patients with PTSD. Addictive Behaviors. 2007;32:1719–1727. doi: 10.1016/j.addbeh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Finney J, Moos R. Two-year posttreatment functioning and coping of substance abuse patients with posttraumatic stress disorder. Psychology of Addictive Behaviors. 1999;13:105–114. [Google Scholar]
- Ouimette P, Moos R, Brown P. Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. American Psychological Association; Washington, DC US: 2003. Substance use disorder-posttraumatic stress disorder comorbidity: A survey of treatments and proposed practice guidelines; pp. 91–110. [Google Scholar]
- Ouimette P, Moos R, Finney J. Two-year mental health service use and course of remission in patients with substance use and posttraumatic stress disorders. Journal of Studies on Alcohol. 2000;61:247–253. doi: 10.15288/jsa.2000.61.247. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Moos R, Finney J. PTSD treatment and 5-year remission among patients with substance use and posttraumatic stress disorders. Journal of Consulting and Clinical Psychology. 2003;71:410–414. doi: 10.1037/0022-006x.71.2.410. [DOI] [PubMed] [Google Scholar]
- Peer J, Strachan E, Spaulding W. Heterogeneity in behavioral treatment response in severe mental illness. Journal of Nervous and Mental Disease. 2008;196:198–206. doi: 10.1097/NMD.0b013e318165c7d2. [DOI] [PubMed] [Google Scholar]
- Petry N, Alessi S, Marx J, Austin M, Tardif M. Vouchers versus prizes: Contingency Management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent growth curve modeling. Sage; Los Angeles: 2008. [Google Scholar]
- Read J, Brown P, Kahler C. Substance use and posttraumatic stress disorders: Symptom interplay and effects on outcome. Addictive Behaviors. 2004;29:1665–1672. doi: 10.1016/j.addbeh.2004.02.061. [DOI] [PubMed] [Google Scholar]
- Rosnow R, Rosenthal R, Rubin D. Contrasts and correlations in effect-size estimation. Psychological Science. 2000;11:446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- Somoza E, Somoza P, Lewis D, Li S, Winhusen T, Chiang N, Vocci F, Horn P, Ahmed E. The SRPHK1 outcome measure for cocaine-dependence trials combines self-report, urine benzoylecgonine levels, and the concordance between the two to determine a cocaine-use status for each study day. Drug and Alcohol Dependence 93. 2008:132–140. doi: 10.1016/j.drugalcdep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Steiger JH, Lind JC. Statistically based tests for the number of factors. Paper presented at the annual spring meeting of the Psychometric Society; Iowa City, IA. 1980. [Google Scholar]
- Stein A, Tran G, Lund L, Haji U, Dashevsky B, Baker D. Correlates for posttraumatic stress disorder in Gulf War veterans: A retrospective study of main and moderating effects. Journal of Anxiety Disorders. 2005;19:861–876. doi: 10.1016/j.janxdis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Stewart S, Conrod P. Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. American Psychological Association; Washington, DC US: 2003. Psychosocial models of functional associations between posttraumatic stress disorder and substance use disorder; pp. 29–55. [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Stump M, Smith J. The relationship between posttraumatic growth and substance use in homeless women with histories of traumatic experience. The American Journal on Addictions. 2008;17:478–487. doi: 10.1080/10550490802409017. [DOI] [PubMed] [Google Scholar]
- Thompson SC, Armstrong W, Thomas C. Illusions of control, underestimations, and accuracy: A control heuristic explanation. Psychological Bulletin. 1998;123:143–161. doi: 10.1037/0033-2909.123.2.143. [DOI] [PubMed] [Google Scholar]
- Thompson SC, Kyle D, Osgood A, Quist RM, Phillips DJ, McClure M. Illusory control and motives for control: The role of connection and intentionality. Motivation and Emotion. 2004;28:315–330. [Google Scholar]
- Vuchinich R, Wallace D, Milby J, Schumacher J, Mennemeyer S, Kertesz S. Relations between in-treatment and follow-up abstinence among cocaine-dependent homeless persons in three clinical trials. Experimental and Clinical Psychopharmacology. 2009;17:165–172. doi: 10.1037/a0015296. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Koegel P, Gelberg L. Antecedents of physical and sexual victimization among homeless women: A comparison to homeless men. American Journal of Community Psychology. 2000;28:367–390. doi: 10.1023/A:1005157405618. [DOI] [PubMed] [Google Scholar]
- Widaman KF, Thompson JS. On specifying the null model for incremental fit indices in structural equation modeling. Psychological Methods. 2003;8:16–37. doi: 10.1037/1082-989x.8.1.16. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2nd Ed McGraw Hill; New York: 1971. [Google Scholar]
- Winick C. Maturing out of narcotic addiction. Bulletin on Narcotics. 1962;14:1–7. [Google Scholar]
- Zlotnick C, Robertson MJ, Lahiff M. Getting off the streets: economic resources and residential exits from homelessness. Journal of Community Psychology. 1999;27:209–224. [Google Scholar]