Abstract
Mucosal leishmaniasis (ML) follows localized cutaneous leishmaniasis (CL) caused by Leishmania braziliensis. Proinflammatory responses mediate CL self-healing but are exaggerated in ML. Proinflammatory monocyte chemoattractant protein 1 (MCP-1; encoded by CCL2) is associated with CL. We explore its role in CL/ML through analysis of the regulatory CCL2 -2518bp promoter polymorphism in CL/ML population samples and families from Brazil. Genotype frequencies were compared among ML/CL cases and control groups using logistic regression and the family-based association test (FBAT). MCP-1 was measured in plasma and macrophages. The GG recessive genotype at CCL2 -2518bp was more common in patients with ML (N=67) than in neighborhood control (NC; N=60) subjects (OR 1.78; 95% CI 1.01–3.14; P=0.045), than in NC combined with leishmanin skin-test positive (N=60) controls (OR 4.40; 95% CI 1.42–13.65; P=0.010), and than in controls combined with CL (N=60) patients (OR 2.78; 95% CI 1.13–6.85; P=0.045). No associations were observed for CL compared to any groups. FBAT (91 ML and 223 CL cases in families) confirmed recessive association of ML with allele G (Z=2.679; P=0.007). Higher levels of MCP-1 occurred in plasma (P=0.03) and macrophages (P<0.0001) from GG compared to AA individuals. These results suggest that high MCP-1 increases risk of ML.
Keywords: Mucosal leishmaniasis, genetic association, MCP-1, CCL2
1. Introduction
Leishmania parasites cause a spectrum of disease phenotypes which differ according to clinical manifestations and immune response. Although distinct species of Leishmania can cause different forms of the disease, a single species can also be associated with two or more distinct clinical forms of leishmaniasis (Almeida et al., 1996; Barral et al., 1991; Carvalho et al., 1985). The immune response and genetic background of the host may be important in disease pathogenesis.
Following infection with L. braziliensis, cutaneous leishmaniasis (CL) is the most common form disease involving the skin, characterized by one or more (<10) granular ulcers with elevated borders. Concomitantly, or months to years later, ~3% of individuals affected by CL develop ML (Carvalho et al., 1985). This severe form is characterized by destructive lesions which may be incapacitating and disfiguring; occasionally becoming life-threatening when lesions of the pharynx and larynx obstruct the respiratory passages and/or cause difficulty in swallowing (Marsden et al., 1998). ML is characterized by a strong cell-mediated immune response with intense infiltrate, pronounced production of inflammatory cytokines such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) (Bacellar et al., 2002). There is also reduced response to the immunomodulatory cytokine interleukin(IL)-10 and lower expression of IL-10 receptor (Faria et al., 2005).
Chemokines are produced as one of the earliest responses against Leishmania parasites, providing a signal for the initiation of the immune response such as chemotaxis, cytokine production, and activation of different cellular subsets (Antoniazi et al., 2004). The chemokine, CC Motif, Ligand 2 (CCL2), also known as Monocyte Chemotactic Protein 1 (MCP-1), is produced by lymphocyte and monocyte lineages and plays a role in both cellular immune reactions and responses to acute tissue injury (Leonard and Yoshimura, 1990). Herein we refer to the gene as CCL2 and the protein as MCP-1. Several studies have reported putative roles for MCP-1 in leishmaniasis from infection studies in vitro (Bhattacharyya et al., 2002; (Ritter and Moll, 2000) as well as by analysis of human (Ritter et al., 1996) and murine (de Moura et al., 2005) lesions, but it is unclear whether the pro-inflammatory response associated with this chemokine is potentially disease exacerbatory or protective. As for TNF-α, the balance between enough proinflammatory activity to promote parasite killing, but not to cause tissue damage, is a central feature of CL versus ML disease, whilst induction of anti-inflammatory cytokines like IL-10 plays an important modulating role.
One way to explore the role of chemokines and other pro- and anti-inflammatory molecules in humans is to determine whether polymorphisms that affect expression or function are associated with disease outcome. In Venezuela, for example, polymorphisms at both TNF and LTA, the genes encoding TNF-α and lymphotoxin-α respectively, have been associated with increased susceptibility to ML caused by L. braziliensis infection (Cabrera et al., 1995). In Brazil, the -174bp G/C single nucleotide polymorphism (SNP) in the promoter region of the gene (IL6) encoding IL-6 was also shown to be associated with ML (Castellucci et al., 2006), while the IL10 -819bp C/T promoter region SNP is associated with susceptibility to CL (Salhi et al., 2008). Recently, Flores-Villanueva et al. (Flores-Villanueva et al., 2005) demonstrated an association between the rarer G allele at the CCL2 -2518bp A/G (rs1024611) promoter SNP and susceptibility to developing active pulmonary tuberculosis in populations from Mexico and Korea. Tuberculosis patients carrying the G allele had the highest plasma levels of MCP-1 and the lowest plasma levels of IL12p40. This led the authors to conclude that individuals with the CCL2 -2518GG genotype produce high concentrations of MCP-1, which inhibits IL-12p40 production in response to Mycobacterium tuberculosis and increases the likelihood of tuberculosis infection progressing to active disease by preventing T helper 1 immunity. Since the IL-12p40 chain is shared with IL-23, this cytokine might also be affected. On the other hand, Ramasawmy and colleagues (Ramasawmy et al., 2006) showed an association between the common A allele, which is known to be associated with low transcriptional activity (Rovin et al., 1999), and chronic Chagas cardiomyopathy in Brazilians, suggesting that MCP-1 was required to prevent disease. Here we report on a small case-control study, underpinned by family-based analysis, which provides evidence for association between the CCL2 -2518bp G allele with high plasma and macrophage MCP-1 levels, and susceptibility to ML but not CL disease caused by L. braziliensis.
2. Materials and Methods
2.1 Case patients, control subjects and study design
As previously (Castellucci et al., 2006), the study was conducted in the area of Corte de Pedra, Bahia, Brazil where L. braziliensis is endemic (Cuba-Cuba et al., 1984; Rosa et al., 1988). All participants were enrolled between January 2001 and November 2004. The majority of cases were retrospective cases that had not had parasites isolated. For all prospective cases of CL and ML studied in this endemic area over the period 2001–2007, L. braziliensis has been identified as the etiological agent for CL and ML. The Corte de Pedra area comprises 20 municipalities in a total area of ~10.000 km2 around the “Corte de Pedra Health Post”, the referral center for leishmaniasis treatment. Corte de Pedra is in a rural rain forest region where agriculture underpins the local economy. Seventy five percent of study participants were farm labourers. Both case-control and family-based cohorts were collected. Index cases of ML were ascertained from medical records of the health post, and families and neighbourhoods re-visited. Informed consent was obtained from all participants or their parents/guardians. Human experimentation guidelines of the US Department of Health and Human Services and from Brazil were followed. The research was approved by the ethical committee of the Hospital Universitário Professor Edgard Santos, Salvador, Bahia and from CONEP (Conselho Nacional de Etica em Pesquisa), Brazil. Protocols were approved by the US National Institutes of Health and the University of Iowa.
Initially 67 ML index cases (48 males: 19 females; mean age±SD=40±17 years) were selected and matched by age and gender to 60 unrelated CL cases (47 males: 13 females; mean age±SD=41±17.8 years) and 60 unrelated neighbourhood controls (47 males: 13 females; mean age±SD=40±18 years). These neighborhood controls (NC) had no clinical history of disease or leishmaniasis scars, but their leishmanin skin test status was unknown. For this reason, a second control group known to be positive for the leishmanin delayed hypersensitivity skin-test response (referred to as DTH+) was included in the study comprising 60 age- and sex-matched unrelated individuals (47 males: 13 females; mean age±SD=38±18 years) also from Corte de Pedra endemic area but not living in the neighbourhood from the index case. The advantage of this group is that they have confirmed infection with no clinical symptoms. This second site was geographically and demographically equivalent to the first neighbourhood studied. Environmental risk factors were analysed previously (Castellucci et al., 2006). The only significant difference in environmental risk factors between case and control groups was that patients with ML lived significantly (P=0.04) closer to forest than did the case patients with CL, although they did not live closer than the NC or DTH-positive control subjects. The 67 ML index cases were also used to ascertain a total of 67 multi-case leishmaniasis (mixed for CL and ML) pedigrees (101 nuclear families; reference (Castellucci et al., 2006), providing a total of 91 ML cases (i.e. 24 additional cases) and 223 CL cases (exclusive of the 60 CL cases used in thecase-control study). Unaffected family members contributed genotype information to increase statistical power of the FBAT analysis, especially for families with missing parents. Full demographic and epidemiological information relating to the multicase families are presented elsewhere (Castellucci et al., 2005).
2.2 Sample collection and DNA extraction
Blood (8 ml) was taken by venipuncture and collected into dodecyl citrate acid (DCA)-containing vacutainers (Becton Dickinson). Genomic DNA was prepared using the proteinase K and salting-out method (Sambrook et al., 1989).
2.3 MCP-1 -2518 A/G genotyping
The CCL2 -2518bp A/G (rs1024611) promoter SNP was typed by PCR and restrictio fragment-length polymorphism analysis as described (Rovin, BH et al., 1999). The A allele appears as a unique band of 930 bp on ethidium stained agarose gels, whereas the G allele generates 2 fragments of 708 and 222 bp.
2.4 ELISA assays for MCP-1, IL-12, IL-23, IL-6, TNF-α and IL-10
Individuals selected for cytokine assays were a mixture of cured cases (> 3 years) and neighbourhood controls for each subgroup with different CCL2 -2518bp genotypes (GG, GA and AA). Cytokines were assayed by enzyme-linked immunosorbent assay (ELISA) using commercial kits (R&D Systems, Minneapolis, MN, USA) in plasma (n=14) and macrophage supernatants (n=10–15, according to TNF and IL6 genotypes). Macrophages were isolated from peripheral blood mononuclear cells (PBMC) by adherence to Petri dishes as described (Castellucci et al., 2006). Macrophages were incubated with medium alone or stimulated with 10 μg/ml of soluble leishmania antigen (SLA) from L. braziliensis (Carvalho et al., 2007) or 10 μg/ml LPS (Sigma) for 24 h.
2.5 Statistical analyses
A test for deviation from Hardy-Weinberg equilibrium was performed on unrelated founders of the families or individuals married into the pedigrees. Tests for Hardy-Weinberg equilibrium were carried out using STATA (version 8.2; available at: http://www.stata.com/) with the free GenAssoc package (available at: http://www-gene.cimr.cam.ac.uk/clayton/software/stata/). Logistic regression analysis was performed in STATA to determine allele-wise (1 df test) and genotype-wise (2 df test) associations at the CCL2-2518 bp SNP, comparing the ML, CL, NC, and DTH+ groups. Global test statistics were generated for both 1 df and 2 df tests, and odds ratios (ORs) with 95% confidence intervals (CIs) were computed to compare risk of ML and CL disease for the variant G allele relative to the common A allele. A likelihood ratio test comparing the 1 and 2 df tests provided a test for dominance effects. Interlocus stepwise logistic regression analysis (Cordell and Clayton, 2002) was used to determine whether associations observed at the CCL2 -2518bp SNP were independent of those observed earlier (Castellucci et al., 2006) for the IL6 -174bp polymorphism. PEDCHECK (O'Connell and Weeks, 1998) was used to determine and delete Mendelian inconsistencies within families. Family-based allelic association tests based on the transmission disequilibrium test (TDT) but generalized to allow analysis under additive and dominant models of inheritance were performed within FBAT (Horvath et al., 2001; Laird et al., 2000) under the null hypothesis of “no linkage and no association”. Unaffected members of the pedigrees contributed genotype information to increase statistical power of the FBAT analysis, especially for families with missing parents. FBAT analyses were carried out under additive and dominant models. For the functional assays, the levels of MCP-1, IL-12p40, IL-12p70, IL-23, IL-6, TNF-α and IL-10 were compared for statistical differences between the 3 genotype groups (GG, GA and AA) using an unpaired Mann Whitney U test. Tests were considered statistically significant if the probability of a type I error was less than 5%.
3. Results
3.1 Population-based analysis of MCP-1 -2518 A/G bp polymorphism
There was no evidence of deviation from Hardy-Weinberg equilibrium using unrelated individuals in these families. Table 1 presents the frequency distribution for genotypes and alleles in the different population-based patient and control groups. To determine initially whether the CCL2 -2518bp SNP was associated with susceptibility to leishmaniasis per se, we compared (Table 2) the ML and CL groups against NC and DTH+ groups. This comparison was significant in the global genotype-wise but not the allele-wise test, with a significant likelihood ratio test indicating a dominance effect. The odds for leishmaniasis for the GG genotype was 3.55 (95% CI 1.25–10.09; P=0.017). Analysis for the CL phenotype compared with all non-CL groups (Table 2) showed no significant associations with the CCL2 -2518bp SNP under either additive or dominant models, suggesting that this polymorphism is not influencing the CL phenotype. In contrast, when the ML group was compared with the three non-ML groups, a significant genotype-wise association were observed, which was most significant (odds ratio 4.40; 95% CI 1.42–13.65; P=0.010) when the ML group was compared with the combined NC and DTH+ control groups but also achieved significance under an additive model (genotype test not valid) for comparison of the ML group with the NC group (Table 2). Overall, the results of the case-control analysis pointed to a role for the CCL2 -2518bp SNP in determining susceptibility to ML but not CL disease.
Table 1.
Allele and genotype distributions for study group comparisons. Data are no. (%) of subjects. ML, mucosal leishmaniasis, CL cutaneous leishmaniasis, NC, neighbourhood control and DTH positive, positive for leishmanin delayed-hypersensivity skin-test response.
| Cases | Controls | |||
|---|---|---|---|---|
| Category | ML | CL | NC | DTH positive |
| Allele | ||||
| G | 42 (32) | 31 (26) | 24 (20) | 29 (25) |
| A | 90 (68) | 89 (74) | 96 (80) | 87 (75) |
| Genotype | ||||
| G/G | 11 (17) | 7 (12) | 0 (0) | 5 (9) |
| G/A | 20 (30) | 17 (28) | 24 (40) | 19 (33) |
| A/A | 35 (53) | 36 (60) | 36 (60) | 34 (58) |
Table 2.
Results of logistic regression analyses for study group comparisons. The 2df test represents the genotype-wise test, and the 1df test represents the allele-wise test. CI, confidence interval, OR, odds ratio, ML, mucosal leishmaniasis, CL cutaneous leishmaniasis, NC, neighbourhood control and DTH positive, positive for leishmanin delayed-hypersensivity skin-test response.
| Comparisons | Global 2df | Global 1df | MCP-1 −2518 Allele/Genotype | OR (95% CI) | P |
|---|---|---|---|---|---|
| (CL + ML) vs. (NC + DTH positive) | 0.018 | 0.124 | GG vs. AA1 | 3.55 (1.25–10.09) | 0.017 |
| CL vs. non-CL (ML + NC + DTH positive) | 0.616 | 0.997 | GG vs. AA | 1.28 (0.49–3.35) | 0.621 |
| CL vs. (NC + DTH positive) | 0.147 | 0.494 | GG vs. AA | 2.72 (0.81–9.18) | 0.106 |
| CL vs. DTH positive | 0.791 | 0.893 | GG vs. AA | 1.32 (0.38–4.57) | 0.659 |
| CL vs. NC | -2 | 0.289 | G vs. A2 | 1.38 (0.76–2.52) | 0.292 |
| ML vs. non-ML (CL + NC + DTH positive) | 0.081 | 0.089 | GG vs. AA | 2.78 (1.13–6.85) | 0.027 |
| ML vs. CL | 0.648 | 0.355 | GG vs. AA | 1.62 (0.56–4.65) | 0.373 |
| ML vs. (NC + DTH positive) | 0.019 | 0.063 | GG vs. AA1 | 4.40 (1.42–13.65) | 0.010 |
| ML vs. DTH positive | 0.401 | 0.284 | GG vs. AA | 1.32 (0.79–2.18) | 0.287 |
| ML vs. NC | -2 | 0.040 | G vs. A2 | 1.78 (1.01–3.14) | 0.045 |
| DTH positive vs. NC | -2 | 0.345 | G vs. A2 | 1.35 (0.72–2.55) | 0.348 |
Significant likelihood ratio test consistent with dominant model.
The 2 df test was invalid, because of a zero value for GG genotype in the NC control group
3.2 Confirmation of the association between the CCL2 -2518bp SNP and ML by FBAT analysis
One concern with genetic analysis using a case-control design in this Brazilian population is that ethnic admixture could lead to false positive results. Although the low prevalence of ML disease precluded sampling of a completely independent patient group for replication, these 67 index cases were used to ascertain a total of 67 multi-case families, which also included an independent sample of CL cases. We genotyped all members of these pedigrees, thus increasing the power of our analysis to evaluate transmission of A or G alleles at the CCL2 -2518bp SNP from heterozygous parents to ML or CL affected offspring using FBAT analysis. This analysis confirmed the association between the G allele and ML disease under a dominant model (Table 3), where ML disease was associated with the recessive G allele (GG genotype Z=+2.679, P=0.007) and protection from disease with the dominant A allele (GA genotype Z=−2.460; P=0.014). Association was not significant under an additive model for either ML or CL (Table 3), and no association was observed for CL disease under a dominant model, further confirming our case-control findings.
Table 3.
Results of FBAT analyses for different disease phenotypes. FBAT analysis for transmission of alleles from heterozygous parents to CL, ML and L. braziliensis per se (CL and ML) individuals in families. # families = number of families informative for the FBAT analysis. Data are only shown for the genotype tests where there were sufficient numbers of informative families (>10) contributing to the analysis. S and E(S) represent the observed and expected transmissions. A positive Z score indicates association with disease; a negative Z score indicates the non-associated or protective allele or genotype.
| Phenotype | Model | # Families | Allele/Genotype | S | E(S) | Z score | P |
|---|---|---|---|---|---|---|---|
| CL+ML | Additive | 60 | G | 146 | 143.53 | 0.363 | 0.717 |
| CL+ML | Dominant | 29 | A | 46 | 51.44 | −1.454 | 0.146 |
| CL+ML | Genotype | 29 | GG | 33 | 27.56 | 1.454 | 0.146 |
| CL+ML | Genotype | 60 | GA | 80 | 88.41 | −1.394 | 0.163 |
| CL | Additive | 58 | G | 142 | 139.028 | 0.443 | 0.658 |
| CL | Dominant | 28 | A | 45 | 49.329 | −1.175 | 0.240 |
| CL | Genotype | 28 | GG | 31 | 26.671 | 1.175 | 0.240 |
| CL | Genotype | 58 | GA | 80 | 85.685 | −0.954 | 0.340 |
| ML | Additive | 41 | G | 41 | 37.344 | 0.955 | 0.339 |
| ML | Dominant | 20 | A | 10 | 15.77 | −2.679 | 0.007 |
| ML | Genotype | 20 | GG | 13 | 7.23 | 2.679 | 0.007 |
| ML | Genotype | 41 | GA | 15 | 22.884 | −2.46 | 0.014 |
3.3 Demonstrating independent effects for IL6 and CCL2 SNPs on ML disease
We previously reported an association between the C allele of IL6 −174bp G/C SNP and susceptibility to ML disease in the same study population (Castellucci et al., 2006). Stepwise logistic regression analysis was therefore undertaken (Table 4) to determine whether these SNPs at IL6 and CCL2 contribute independent main effects. Models comparing the addition of the IL6 SNP to a model in which CCL2 alone was considered added significant independent effects.
Table 4.
Inter-locus forward stepwise regression analysis. Test to determine whether SNPs at IL6 and CCL2 contribute independent main effects. A significant Wald χ2 test comparing null and alternative models indicates that the marker added (bold) under the alternative model is contributing a separate main effect from marker considered under the null hypothesis. Robust variance estimates to control for family clustering were used throughout.
| Null Model | Alternative Model | Test statistic | ||
|---|---|---|---|---|
| χ 2 | df | P | ||
| (a) Adding SNP at CCL2 | ||||
| IL6 −174bp | IL6 −174bp + CCL2 −2518bp | 4.38 | 1 | 0.036 |
| (b) Adding SNP at IL6 | ||||
| CCL2 −2518bp | CCL2 −2528bp + IL6 −174bp | 5.91 | 1 | 0.015 |
Conversely, addition of the CCL2 SNP to a model in which the IL6 alone was considered also added significant independent effects. Genetically, the two loci therefore appear to be having independent effects on susceptibility to ML disease. Our sample size was too small to look for interaction between the loci.
3.4 Association between genotypic variation at the CCL2 -2518bp SNP and differences in MCP-1 levels and regulation of other cytokines
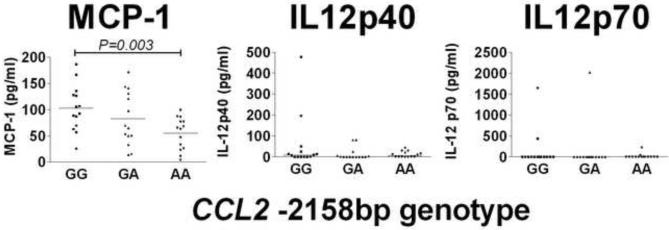
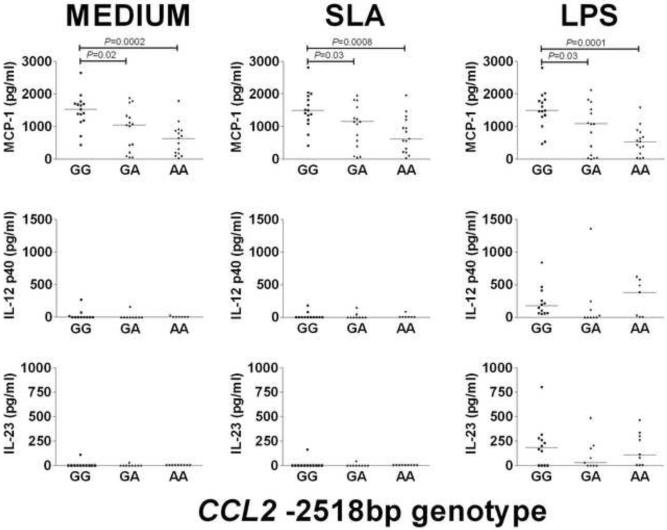
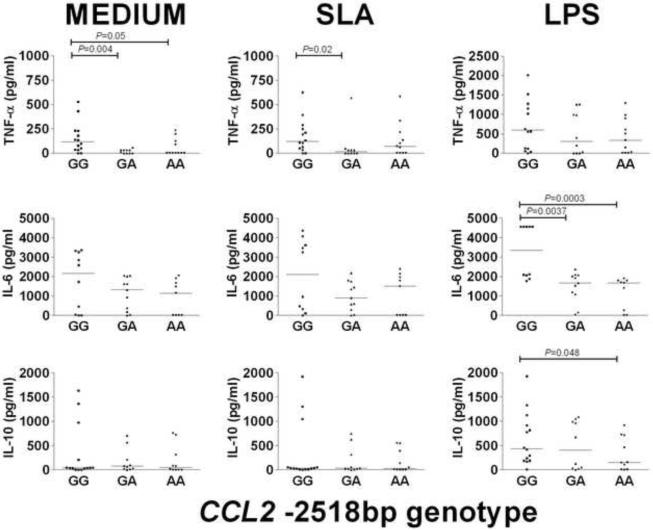
Previous studies have shown that allele G for the CCL2 -2518bp promoter SNP increases gene expression (Flores-Villanueva et al., 2005; Gonzalez et al., 2002; Rovin et al., 1999). In our study the G allele also correlated with differences in plasma levels of MCP-1, with GG individuals having significantly (P=0.003) elevated levels of MCP-1 compared to AA individuals (Figure 1). In contrast to data published for tuberculosis patients (Flores-Villanueva et al., 2005), plasma levels of IL-12p40 and IL-12p70 were low and did not differ significantly between the CCL2 -2518bp genotype groups (p >0.05). We also observed higher MCP-1 levels in the supernatants of macrophages from GG compare to AA genotypes in un-stimulated (P=0.0002) and stimulated (SLA P=0.0008; LPS P=0.0001) cultures, although the magnitude of the MCP-1 response was not significantly enhanced by either stimulus (Figure 2). Neither the plasma IL-12p40 nor the plasma IL-23 was significantly different between individuals with each of the 3 genotypes (Figure 2). To look for any functional interplay between the CCL2 -2518bp promoter SNP and the products of other genes, TNF (Cabrera et al., 1995), IL6 (Castellucci et al., 2006) and IL10 (Salhi et al., 2008), we examined these cytokine levels in supernatants from un-stimulated and stimulated macrophages. TNF-α levels were significantly higher in supernatants of non-stimulated macrophages from individuals with the CCL2 -2518bp GG genotype compared to the GA or the AA genotypes, but not following stimulation with SLA or LPS (Figure 3). In contrast, IL-6 was only statistically higher between supernatants from individuals with different genotypes after LPS stimulation, and differences in IL-10 levels were not significantly different in supernatants of macrophages from individuals with different CCL2 -2518bp genotypes (Figure 3).
Fig. 1.
Levels of MCP-1, IL-12p40 and IL-12p70 measured in plasma from individuals carrying GG, GA or AA genotypes (n=14 for each genotype) at the CCL2 −2158bp promoter region polymorphism. Bars represent the median.
Fig. 2.
MCP-1 (n=15), IL-12p40 (n=12) and IL-23 (n=12) chemoking/cytokine production in un-stimulated (MEDIUM), SLA-stimulated, and LPS-stimulated macrophages from individuals carrying GG, GA or AA genotypes at the CCL2 −2158bp promoter region polymorphism. Optimal conditions for time (24 h) and dose (10 μg/ml) of stimulation were determined after comparing different incubation times and concentrations. Bars represent the median. Differences in numbers evaluated for IL-12p40 and IL-23 was due to either shortage of supernatant. There were no underlying differences in chemokine levels according to previous disease phenotypes (data not shown).
Fig. 3.
TNF-α (n=10), IL-6 (n=10) and IL-10 (n=14) cytokine production in un-stimulated (MEDIUM); SLA-stimulated; and LPS-stimulated macrophages from individuals carrying GG, GA or AA genotypes at the CCL2 −2158bp promoter region polymorphism. Optimal conditions for time (24 h) and dose (10 μg/ml) of stimulation were determined after comparing different incubation times and concentrations. Bars represent the median. Differences in numbers evaluated occur because for TNF-α we excluded subjects with genotypes AA and AG for the −308bp TNF gene SNP known to induce high TNF-α, and only IL6 −174bp GG individuals were included in the IL-6 analysis. All groups contained a mixture of individuals with different IL10 promoter haplotypes. There were no underlying differences in cytokine levels according to previous disease phenotypes (data not shown).
4. Discussion
The data presented here provide both population-based and family-based evidence for an association between the G allele of the CCL2 -2518bp promoter SNP and susceptibility to ML disease following infection with L. braziliensis. This correlated with elevated plasma levels of MCP-1 in GG individuals, and with higher release of MCP-1 by both un-stimulated and stimulated macrophages. Previous work has demonstrated that MCP-1 enhances the cytotoxic response against L. donovani amastigotes via a NO-dependent mechanism, concomitant with up-regulation of TNF-α in infected macrophages (Bhattacharyya et al., 2002). In addition, Ritter and Moll (Ritter and Moll, 2000) showed that MCP-1 operates synergistically with IFN-γ to clear L .major from infected macrophages by induction of reactive oxygen intermediates, whereas IL-4 abrogates MCP-1 expression in infected monocytes. Moreover, in patients with self-healing CL, high levels of MCP-1 were detected in infected skin whereas in the non-healing lesions of diffuse cutaneous leishmaniasis MCP-1 expression was much lower with a predominance of another CCL chemokine, CCL3 or Macrophage Inflammatory Protein 1-α (MIP-1α) (Ritter et al., 1996). More recently, it was demonstrated that the chemokines MCP-1, MIP-1α and Chemokine, CXC Motif, Ligand 1 (CXCL1, also known as KC) were expressed in ears and draining lymph nodes of mice infected in the ear pinna with L. braziliensis. (de Moura et al., 2005). These data suggested that the leishmanicidal capacity of MCP-1 contributed to lesion healing. Our results suggest that high levels of MCP-1 appear to exacerbate ML disease. This supports the alternative view that the proinflammatory capacity of MCP-1 in recruiting host monocytes could provide both the environment for parasite replication and for tissue damage and lesion development. This could be due to a direct effect of MCP-1 in bringing fresh monocytes to the site of infection and/or to downstream events regulated by MCP-1 in macrophages and other cells.
In their study of the influence of the CCL2 -2518bp promoter SNP on tuberculosis infection, Flores-Villanueva and colleagues (Flores-Villanueva et al., 2005) found that the elevated levels of MCP-1 associated with the GG genotype correlated with low plasma levels of IL12p40, and that MCP-1 inhibited IL12p40 production in stimulated macrophages. They postulated that reduced T helper 1 immune responses in GG individuals were therefore more likely to lead to active tuberculosis. In our study we failed to replicate either the correlation between CCL2 -2518bp genotype and IL12p40 levels, or the association with IL12p40 (or IL23) responses in macrophages in vitro. It seems unlikely, therefore, that the effect of MCP-1 in ML disease is mediated through either of these T helper 1 promoting cytokines which, in any case, did not fit logically with the ML disease model in which elevated T helper 1 immune responses are observed. In contrast to the Flores-Villanueva et al (Flores-Villanueva et al., 2005) study, Thye et al. (Thye et al., 2009) found that the CCL2 -2518bp G allele was associated with resistance to pulmonary tuberculosis in a large study of 2,000 cases compared to 2,300 healthy controls, supported by 332 affected nuclear families, from Ghana, West Africa. In the same study (Thye et al., 2009) no association was found in case-control analysis of 1,400 tuberculosis patients and 1,500 controls from Russia. In the Ghanaian population, analysis of eight additional CCL2 polymorphisms led the authors to conclude that the primary association was with the CCL2 -362bp SNP, and that the effect of CCL2 -2518bp could be explained in part by linkage disequilibrium with the -362bp SNP.
The differences in tuberculosis disease associations with CCL2 promoter polymorphisms between populations could be due to the presence of different haplotypes carrying different combinations of functional promoter region variants, and/or to differences in functional interaction between variants. Further work is required to determine whether CCL2 -2518bp is the functional variant, or the only functional variant, affecting ML disease in our study population. Nevertheless, our study did show that the G allele at CCL2 -2518bp (or something in strong LD with it) was associated with elevated MCP-1 levels. Having ruled out IL-12 (or IL-23) as a possible downstream mediator of the MCP-1 effect, we examined whether other pro- or anti-inflammatory cytokines correlated with CCL2 genotype. In particular we were interested in the products of other pro- and anti-inflammatory cytokines for which genetic associations with ML or CL had been observed for L. braziliensis infection (Cabrera et al., 1995; Castellucci et al., 2006; Salhi et al., 2008). Interestingly, baseline levels of TNF-α were higher in macrophages from individuals with CCL2 -2518bp GG compared to AA genotypes. This was not due to the influence of the TNF -308bp variant (Cabrera et al., 1995), as this was controlled for in the choice of individuals studied. The combination of high MCP-1 and high TNF-α is consistent with increased risk of ML disease, and suggests that the CCL2 -2518bp GG genotype can increase TNF- release on the genetic background of TNF -308bp low responders. Results for IL-6 were more complex. In our previous study (Castellucci et al., 2006) we demonstrated an association between ML and the low IL-6 producing IL6 -174bp C allele. Here we kept the IL6 -174bp constant by only including individuals carrying the high IL-6 producing IL6 -174bp GG genotype. Nevertheless, we observed apparently higher IL-6 production associated with the high MCP-1 producing CCL2 -2518bp GG, at least after LPS stimulation of macrophages. Interestingly, however, all genotype groups for CCL2 -2518bp genotypes appeared to divide into higher and lower responders for IL-6, leading us to postulate other genetic differences that reflect underlying regulation of IL-6 production independently of IL6 -174bp and CCL2 -2518bp. Although the genetic effects of the IL6 -174bp and CCL2 -2518bp polymorphisms were shown statistically to contribute independent main effects, we did not have sufficient power in our study to adequately determine whether there was genetic interaction between the two loci. The sample size used here had limited power and the study requires replication. Our observations would be interesting to pursue in a larger study, along with a more detailed analysis of regulatory polymorphisms at both loci. No functional associations were seen between CCL2 -2518bp genotype and IL-10 responses.
In summary, the work presented here has shown an association between ML disease and the high MCP-1producing CCL2 −2518bp GG genotype, which also associates with enhanced TNF-α production. This suggests that, despite previous reports suggesting a protective role for MCP-1 in self-healing CL disease associated with L. major (Ritter and Moll, 2000) and L. amazonensis (Ritter et al., 1996) infections, too much MCP-1 can contribute to the exaggerated proinflammatory and ML disease. Our study contributes to the increasing importance that genetic regulation of pro- and anti-inflammatory cytokines have in determining underlying susceptibility to ML disease following L. braziliensis infection.
Acknowledgements
We thank Ednaldo Lima do Lago and other staff at the Health Post of Corte de Pedra for helping with patient recruitment. We thank Paulo Machado and Albert Schriefer for help in diagnosis of patients. We thank Elbe M. Silva and Lúcia Reis for secretarial assistance. This work was funded in part by NIH grants P50 AI-30639 and R03AI070909 (LC, EMC, MEW), NIH/FIC 1 D43 TW007127-01 (JO, AM), R01 AI076233 (JMB and MEW), R01AI067874 (JMB and MEW), CNPq (ARJ, RA, EMC), a VA Merit Review grant (MEW), and grants from The Wellcome Trust (JMB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida RP, Barral-Netto M, De Jesus AM, De Freitas LA, Carvalho EM, Barral A. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal, or visceral leishmaniasis in BALB/C mice. Am. J. Trop. Med. Hyg. 1996;54:178–184. doi: 10.4269/ajtmh.1996.54.178. [DOI] [PubMed] [Google Scholar]
- Antoniazi S, Price HP, Kropf P, Freudenberg MA, Galanos C, Smith DF, Muller I. Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infect. Immun. 2004;72:5168–5174. doi: 10.1128/IAI.72.9.5168-5174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral A, Pedral-Sampaio D, Grimaldi G, Junior, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, Almeida R, Badaro R, Barral-Netto M, Carvalho EM, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 1991;44:536–546. doi: 10.4269/ajtmh.1991.44.536. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J. Infect. Dis. 2002;185:1704–1708. doi: 10.1086/340820. [DOI] [PubMed] [Google Scholar]
- Cabrera M, Shaw M-A, Sharples C, Williams H, Castes M, Convit J, Blackwell JM. Polymorphism in TNF genes associated with mucocutaneous leishmaniasis. J. Exp. Med. 1995;182:1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JLM, Reed S, Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 1985;135:4144–4148. [PubMed] [Google Scholar]
- Carvalho LP, Passos S, Bacellar O, Lessa H, Almeida RP, Magalhaes A, Dutra WO, Gollob KJ, Machado P, de Jesus AR. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci L, Cheng LH, Araujo C, Guimaraes LH, Lessa H, Machado P, Almeida MF, Oliveira A, Ko A, Johnson WD, Wilson ME, Carvalho EM, AR DEJ. Familial aggregation of mucosal leishmaniasis in northeast Brazil. Am. J. Trop. Med. Hyg. 2005;73:69–73. [PubMed] [Google Scholar]
- Castellucci L, Menezes E, Oliveira J, Magalhaes A, Guimaraes LH, Lessa M, Ribeiro S, Reale J, Noronha EF, Wilson ME, Duggal P, Beaty TH, Jeronimo S, Jamieson SE, Bales A, Blackwell JM, de Jesus AR, Carvalho EM. IL6 -174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J. Infect. Dis. 2006;194:519–527. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- Cordell HJ, Clayton DG. A unified stepwise regression procedure for evaluating the relative effects of polymorphisms within a gene using case/control or family data: application to HLA in type 1 diabetes. Am. J. Hum. Genet. 2002;70:124–141. doi: 10.1086/338007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuba-Cuba C, Llanos-Cuentas EA, Barreto AC, Magalhaes AV, Lago EL, Reed S, Marsden PD. Human cutaneous leishmaniasis in Tres Bracos, Bahia, Brasil. An area of Leishmania brazilienses braziliensis transmission. I. Laboratory diagnosis. Rev. Soc. Bras. Med. Trop. 1984;17:161–167. [Google Scholar]
- de Moura TR, Novais FO, Oliveira F, Clarencio J, Noronha A, Barral A, Brodskyn C, de Oliveira CI. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 2005;73:5827–5834. doi: 10.1128/IAI.73.9.5827-5834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria DR, Gollob KJ, Barbosa JJ, Schriefer A, Machado PR, Lessa H, Carvalho LP, Romano-Silva MA, De Jesus AR, Carvalho EM, Dutra WO. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect. Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, Montano M, Barnes PF, Selman M, Granados J. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J. Exp. Med. 2005;202:1649–1658. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc. Natl Acad. Sci. U. S. A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype--phenotype associations. Eur. J. Hum. Genet. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet. Epidemiol. 2000;19(Suppl 1):S36–42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol.Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Marsden PD, Lessa HA, Oliveira MR, Romero GA, Marotti JG, Sampaio RN, Barral A, Carvalho EM, Cuba CC, Magalhaes AV, Macedo VO. Clinical observations of unresponsive mucosal leishmaniasis. Am. J. Trop. Med. Hyg. 1998;59:543–545. doi: 10.4269/ajtmh.1998.59.543. [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am. J. Hum. Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasawmy R, Cunha-Neto E, Fae KC, Martello FG, Muller NG, Cavalcanti VL, Ianni B, Mady C, Kalil J, Goldberg AC. The monocyte chemoattractant protein-1 gene polymorphism is associated with cardiomyopathy in human chagas disease. Clin. Infect. Dis. 2006;43:305–311. doi: 10.1086/505395. [DOI] [PubMed] [Google Scholar]
- Ritter U, Moll H. Monocyte chemotactic protein-1 stimulates the killing of leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur. J. Immunol. 2000;30:3111–3120. doi: 10.1002/1521-4141(200011)30:11<3111::AID-IMMU3111>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ritter U, Moll H, Laskay T, Brocker E, Velazco O, Becker I, Gillitzer R. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 1996;173:699–709. doi: 10.1093/infdis/173.3.699. [DOI] [PubMed] [Google Scholar]
- Rosa AC, Cuba CC, Vexenat A, Barreto AC, Marsden PD. Predominance of Leishmania braziliensis braziliensis in the regions of Tres Bracos and Corte de Pedra, Bahia, Brazil. Trans R. Soc. Trop. Med. Hyg. 1988;82:409–410. doi: 10.1016/0035-9203(88)90138-1. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem. Biophys. Res. Commun. 1999;259:344–348. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- Salhi A, Rodrigues V, Jr., Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, Rafati S, Chevillard C, Prata A, Alcais A, Argiro L, Dessein A. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J. Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- Thye T, Nejentsev S, Intemann CD, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, Zeitels LR, Herb F, Horstmann RD, Meyer CG. MCP-1 promoter variant −362C associated with protection from pulmonary tuberculosis in Ghana, West Africa. Hum. Mol. Genet. 2009;18:381–388. doi: 10.1093/hmg/ddn352. [DOI] [PMC free article] [PubMed] [Google Scholar]