Abstract
We have recently shown that stimulation of cultured neonatal cardiomyocytes with endothelin-1 (ET-1) first produces conformational disorder within the ryanodine receptor (RyR2) and diastolic Ca2+ leak from the sarcoplasmic reticulum (SR), then develops hypertrophy (HT) in the cardiomyocytes [Hamada et al., 2009]. The present paper addresses the following question. By what mechanism does crosstalk between defective operation of RyR2 and activation of the HT gene program occur? Here we show that the immuno-stain of calmodulin (CaM) is localized chiefly in the cytoplasmic area in the control cells; whereas, in the ET-1-treated/hypertrophied cells, major immuno-staining is localized in the nuclear region. In addition, fluorescently labeled CaM that has been introduced into the cardiomyocytes using the BioPORTER system moves from the cytoplasm to the nucleus with the development of HT. The immuno-confocal imaging of Ca2+/CaM-dependent protein kinase II (CaMKII) also shows cytoplasm-to-nucleus shift of the immuno-staining pattern in the hypertrophied cells. In an early phase of hypertrophic growth, the frequency of spontaneous Ca2+ transients increases, which accompanies with cytoplasm-to-nucleus translocation of CaM. In a later phase of hypertrophic growth, further increase in the frequency of spontaneous Ca2+ transients results in the appearance of trains of Ca2+ spikes, which accompanies with nuclear translocation of CaMKII. The cardio-protective reagent dantrolene (the reagent that corrects the de-stabilized inter-domain interaction within the RyR2 to a normal mode) ameliorates aberrant intracellular Ca2+ events and prevents nuclear translocation of both CaM and CaMKII, then prevents the development of HT. These results suggest that translocation of CaM and CaMKII from the cytoplasm to the nucleus serves as messengers to transmit the pathogenic signal elicited in the surface membrane and in the RyR2 to the nuclear transcriptional sites to activate HT program.
Keywords: cardiomyocytes, hypertrophy, protein translocation, calmodulin, CaMKII, ryanodine receptor
INTRODUCTION
Since initial demonstration of Paul Simpson that neurohormonal stimulation of cultured neonatal cardiomyocytes causes hypertrophy (HT), this system has been used extensively as a cellular model of cardiac HT to investigate characteristic changes in gene expression and protein kinase signaling [1, 2]. The strong advantage of this cellular disease model is that upon stimulation of the cell surface receptor (e.g. the Gαq-mediated receptor) by endothelin-1 (ET-1), the cells develop HT within a day, making it possible to investigate the process of development of HT in a relatively short time. Using this neonatal cell culture model, we have recently shown that the cell developed HT not only by stimulation of the cell with ET-1, but also by DPc10, a domain peptide that de-stabilizes normal inter-domain interaction within the cardiac ryanodine receptor (RyR2) and causes diastolic Ca2+ leak [3]. Furthermore, dantrolene or K201, the reagent that corrects the de-stabilized inter-domain interaction to a normal mode [4–9], prevented the development of HT that would have been induced by ET-1 or DPc10 [3]. These findings suggest that stimulation of cultured neonatal cardiomyocytes with ET-1 first produces conformational disorder within the RyR2 and diastolic Ca2+ leak from the sarcoplasmic reticulum (SR), then develops HT in the cardiomyocytes [3].
By what mechanism does crosstalk between defective operation of RyR2 and activation of the HT gene program occur? The present paper addresses this question. As is well known, RyR2-bound CaM inhibits RyR2 channels at a physiological concentration of cytoplasmic Ca2+ [10–12]. This implies that the RyR2-bound CaM stabilizes the closed state of RyR2 channels in the resting state of normal cells. Accordingly, CaM dissociation from RyR2 will likely activate the channel in an otherwise resting condition, causing a diastolic Ca2+ leak, which represents the pro-HT conditions. Consistent with this prediction, Meissner and his colleagues have shown that a mouse with 3 amino acid substitutions in the CaM binding domain of RyR2, which make the RyR2 unable to bind CaM, developed HT and early death [13]. Accumulated evidence also suggests that increased CaMKII-dependent phosphorylation of RyR2 leads to increased SR Ca2+ leak, causing elevated cytosolic Ca2+ levels, thereby providing a potential arrhythmogenic substrate that triggers cardiac disorder, such as heart failure and atrial fibrillation [14–17]. On the other hands, intensive investigations of in vitro and in vivo models of HT have revealed crosstalk among multiple parallel pro-hypertorphic signaling pathways, many of which are regulated by CaM and CaMKII. For instance, Ca2+/CaM-dependent activation of calcineurin dephosphorylates NFAT-P to NFAT; the de-phosphorylated NFAT is translocated into the nucleus to activate HT gene program [18]. It is also well established that CaM/CaMKII•-mediated phosphorylation of histone deacetylase (HDAC) exports the phosphorylated HDAC from the nucleus, and activates HT gene program as a result of removal of the transcriptional suppressor HDAC [19–21]. Thus, it seems that CaM and CaMKII are involved in the upstream pathway leading to the arrhythmogenic diastolic Ca2+ leak through RyR2 as well as in the downstream pathway leading to the activation of pro-HT gene program. The above background information suggests the hypothesis that the pathogenic proteins, CaM and CaMKII, also serve as messengers for the crosstalk between the conformational disorder of RyR2 and development of HT. Here we present the data suggesting that translocation of CaM and CaMKII from the cytoplasm to the nucleus serves as messengers to transmit the pathogenic signal elicited in the cytoplasm (i.e. defective inter-domain interaction within the RyR2 and resultant aberrant cytoplasmic Ca2+ events) to the nuclear transcriptional sites to activate HT program.
MATERIALS AND METHODS
Reagents
Dantrolene and endothelin-1 were obtained from Sigma.
Isolation of primary cardiomyocytes
Neonatal cardiac myocytes were prepared using a Percoll gradient method as described in ref. [22]. Myocytes from 1- to 2-day-old Sprague–Dawley rats were cultured in a serum-containing medium (Dulbecco’s modified Eagle’s medium, 10% horse serum, 5% fetal bovine serum, 1 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 mg/ml Amphotericin B, 0.1 mM Brdu and 2 mM L-glutamine) for 24 hrs.
Induction of hypertrophy by ET-1
After isolation, cardiomyocytes were cultured in a serum-free Dulbecco’s modified Eagle’s medium containing 0.5% Nutridoma (Roche Applied Science) for another 24 hrs. At this time point, the cells were treated with either (i) 0.1 μM ET-1 alone or (ii) 0.1 μM ET-1 supplemented with 10 μM dantrolene or (iii) none (control). Development of HT in the cardiomyocytes was followed at different time points (6–24 hrs).
Determination of cell size of the cardiomyocytes
The treated (ET-1 and ET-1 + dantrolene) and control cells were fixed with 3.7% formaldehyde at different times of incubation. For the measurements of cell size, the fixed cells were stained with anti-sarcomeric alpha-actinin antibody (Sigma) and TO-PRO-3 (Invitrogen) (for staining of nucleus). Areas of individual cells in the confocal fluorescence microscopy (BioRad) images were determined using ImageJ software (NIH).
Immunostaining of cardiomyocytes
The treated (ET-1 and ET-1 + dantrolene) and control cells fixed with 3.7% formaldehyde at 6 hrs, 12 hrs and 24 hrs of incubation in the course of development of HT were taken for co-immunostaining using anti-CaM monoclonal antibody (Epitomics) and anti CaMKII monoclonal antibodies (Santa Cruz Biotechnology) and imaged in fluorescence confocal microscope.
CaM-alexa translocation study
For the exogenously introduced CaM relocation study, mutant CaM (with Cysteine at 34 position) was conjugated with alexa546-maleimide. The purified CaM-alexa conjugate was introduced into living cardiomyocytes using the BioPORTER protein-loading reagent (Gene Therapy Systems, Inc.) just before the treatment with ET-1 and incubated for 4 hrs for the delivery of CaM-alexa across the cell membrane. The CaM-alexa loaded cells were then treated with ET-1 for 24 hrs for the development of HT, the control cells were incubated without any treatment. Live cells were imaged by following the alexa fluorescence in confocal microscope.
Studies of calcium transients
At 6, 12 and 24 hrs time points of ET-1 treatment, the cardiomyocytes were treated with 5 μM of fluo 4 AM (cell-permeable calcium indicator, Invitrogen) in an imaging buffer solution (25 mM HEPES, 6 mM glucose, 2 mM CaCl2, 150 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, pH 7.4) and incubated at 37°C for 30 min. The cells were washed with the imaging buffer three times and incubated another 30 min at 37°C. Live cells were imaged in confocal microcope for the synchronous calcium transients following the green fluorescence of fluo 4.
RESULTS AND DISCUSSION
Endothelin-1-induced development of hypertrophy in neonatal cardiomyocytes is mediated by intracellular translocation of CaM
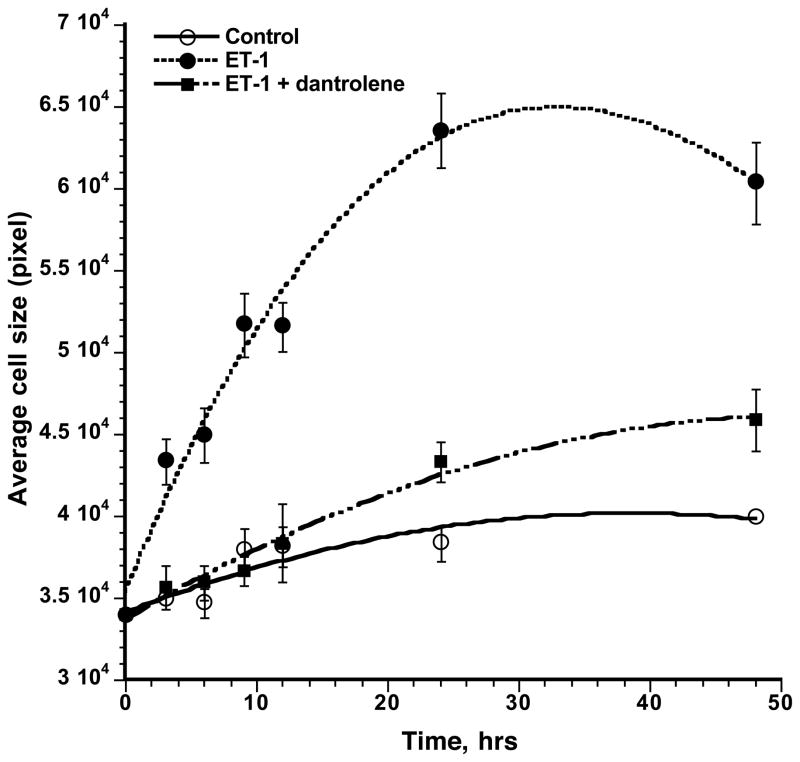
Stimulation of neonatal rat cardiomyocytes with endothelin-1 (ET-1) produces HT as evidenced by three criteria: increased cell size, increased uptake of [3H]leucine and up-regulation of fatal gene expression of ANP and BNP [22]. In the present study we treated the neonatal rat cardiomyocytes with ET-1, and the cell size was determined at different times of incubation of three groups of cells: control, ET-1 and ET-1 + dantrolene, by determining the area of n number of cells immuno-stained with anti-alpha actinin antibody (Fig. 1). In agreement with our previous report [3], ET-1 produced a significant increase in the cell size, and dantrolene (the reagent that corrects the de-stabilized inter-domain interaction within the RyR2 to a normal mode [4–6]) prevented hypertrophic cell growth, confirming the concept that HT stimulus applied to the surface membrane first produces aberrant inter-domain interaction within the RyR2, and then the conformational disorder of RyR2 is transmitted to the nucleus as a pathogenic signal to activate HT gene program. The following is the result of our attempt to identify putative messengers for the crosstalk between defective operation of RyR2 and activation of the HT gene program.
Fig. 1. Effects of ET-1 and dantrolene on the time course of cell growth of neonatal cardiomyocytes.
Cardiomyocytes fixed at 3, 6, 9, 12, 24 and 48 hrs time points of incubation with ET-1 (0.1 μM), ET-1 (0.1 μM) + dantrolene (10 μM) or none (control) were immuno-stained with anti-α-actinin antibody and the areas of individual cells (the cell size) were determined using ImageJ program. The average cell size at each time point was plotted against the time of incubation for all three groups of cardiomyocytes : control (○), ET-1 (●), ET-1 + dantrolene (■).
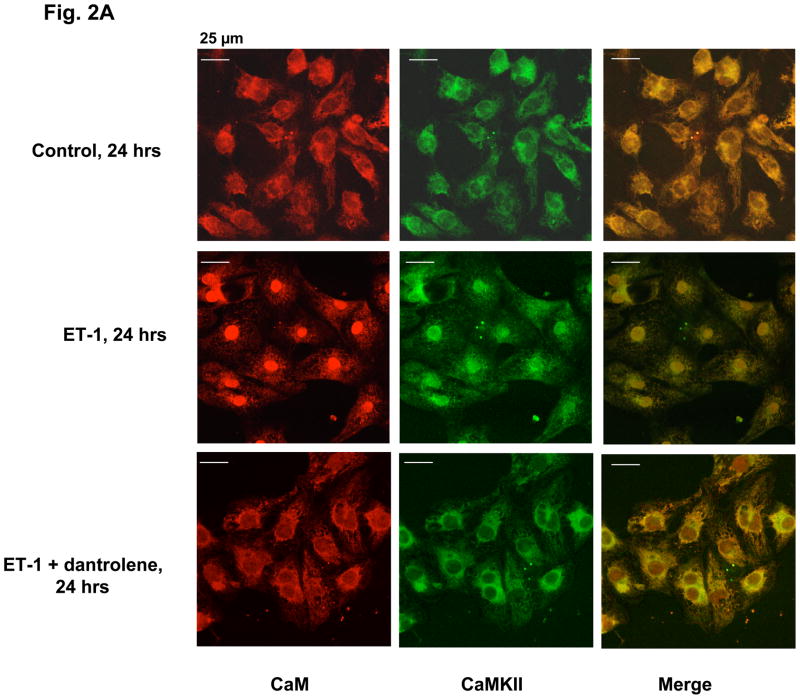
To determine the intracellular CaM distribution, the three groups of cardiomyocytes (control cells, cells treated with ET-1, and cells treated with ET-1 and dantrolene) were immuno-stained with anti-CaM antibody after 24 hrs of incubation. As shown in Fig. 2A (‘CaM’ column), in the control cells, CaM is localized chiefly in the cytoplasmic area, giving rise to a hollow appearance of the nucleus. In the ET-1-treated cells, however, CaM is chiefly localized in the nucleus. The cells treated with ET-1 in the presence of 10 μM dantrolene show basically the same staining pattern as control, indicating that dantrolene prevented the CaM re-distribution from the cytoplasm to the nucleus.
Fig. 2.
Fig. 2A. ET-1-induced hypertrophic growth of neonatal cardiomyocytes accompanies with redistribution of CaM and CaMKII from the cytoplasm to the nucleus. Cardiomyocytes were treated with ET-1, ET-1 + dantrolene or none (control) for 24 hrs as described in the legend to Fig. 1, and co-immunostained with ani-CaM (red) and ani-CaMKII (green) monoclonal antibodies.
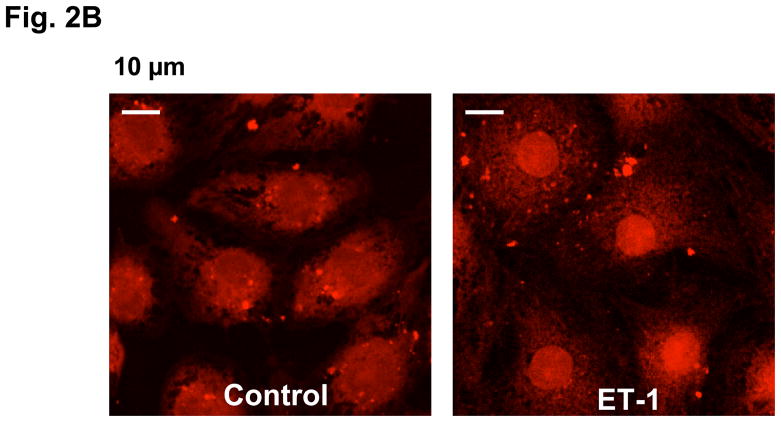
Fig. 2B. Exogenously introduced CaM-alexa translocates to the nucleus of cardiomyocytes upon development of hypertrophy. CaM-alexa546 was introduced into the live cardiomyocytes using BioPORTER protein-loading reagent and then treated with ET-1 or none (control) for 24 hrs. The cells were subjected to live cell confocal imaging following the red fluorescence of alexa546.
In order to examine whether this cytoplasm-to-nucleus re-distribution of CaM represents the actual HT-linked translocation of CaM or an increased expression of nuclear CaM relative to that of cytoplasmic CaM, we exogenously introduced fluorescently labeled CaM (CaM-alexa: conjugate of CaM T34C mutant with alexa Fluor 546) to the cardiomyocytes and followed its intracellular movement associated with the development of HT. As shown in Fig. 2B, the CaM-alexa, which was introduced into the cardiomyocytes with the aid of BioPORTER protein loading reagent in an early stage of the culture, is localized chiefly in the cytoplasm in the control cells, but the majority of the introduced CaM-alexa has moved into the nucleus after incubation of the cells with ET-1 for 24 hrs. This indicates that the observed cytoplasm-to-nucleus re-distribution of CaM in fact represents intracellular translocation of cytoplalsmic CaM to the nucleus.
Ca2+/CaM-dependent protein kinase II (CaMKII) is also translocated to the nucleus during the development of hypertrophy
CaMKII is one of the major binding partners of CaM, is expressed in a relatively large amount in the cardiomyocyte [23], and plays an important role for signaling and regulation in the heart [24–26]. Therefore, we thought of the possibility that CaMKII may also be involved in the intracellular signal transduction and pathogenesis of HT. This possibility was examined by co-immunostaining of endogenous CaM and CaMKII of the cardiomyocytes with corresponding antibodies after incubation of neonatal cardiomyocytes with ET-1 for 24 hrs. As shown in Fig. 2A (‘CaMKII’ column), in the control cells, CaM and CaMKII are localized chiefly in the cytoplasm (cf. Fig. 2A ‘merge’), but in the cells treated with ET-1, both CaM and CaMKII are chiefly co-localized in the nucleus, although some scattered stain remains in the cytoplasmic area. This suggests that both cytoplasmic CaM and CaMKII migrated into the nucleus during the development of HT in the neonatal myocytes. Dantrolene, which repairs defective inter-domain interactions within the RyR2, inhibited the intracellular translocation of both CaM and CaMKII (Fig. 2, ET-1 plus dantrolene) and prevented the development of HT (cf. Fig. 1). These results suggest that the intracellular translocation of CaM and CaMKII is a causative mechanism for the development of HT.
Cytoplasm-to-nucleus translocation of CaM and CaMKII in an early phase of the development of hypertrophy
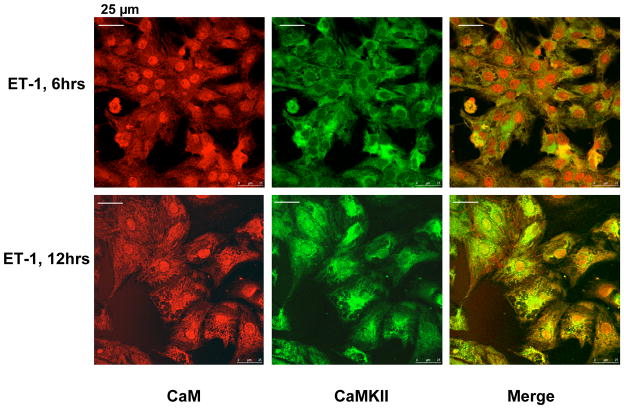
Fig. 3 shows the results of co-immunostaining of endogenous CaM and CaMKII in early stages of incubation of cardiomyocytes with ET-1, at 6 hrs and 12 hrs. As early as at 6 hrs (at an early stage of hypertrophic growth, cf. Fig. 1), CaM has already been translocated into the nucleus in most of the cells as shown. Interestingly, however, CaMKII still remains in the cytoplasmic area in most cells. After 12 hrs incubation with ET-1, some cells begin to show cytoplasm-to-nucleus translocation of CaMKII (Fig. 3). These results suggest that in an early phase of HT development, nuclear translocation of CaM precedes that of CaMKII.
Fig. 3. Immuno-cytological localization of endogenous CaM and CaMKII in early stages of hypertrophic cell growth of neonatal cardiomyocytes.
Cardiomyocytes treated with ET-1 for 6 or 12 hrs were co-immunostained with ani-CaM (red) and ani-CaMKII (green) monoclonal antibodies.
ET-1 produces characteristic intracellular Ca2+ events, which may represent upstream mechanisms of nuclear translocation of CaM and CaMKII
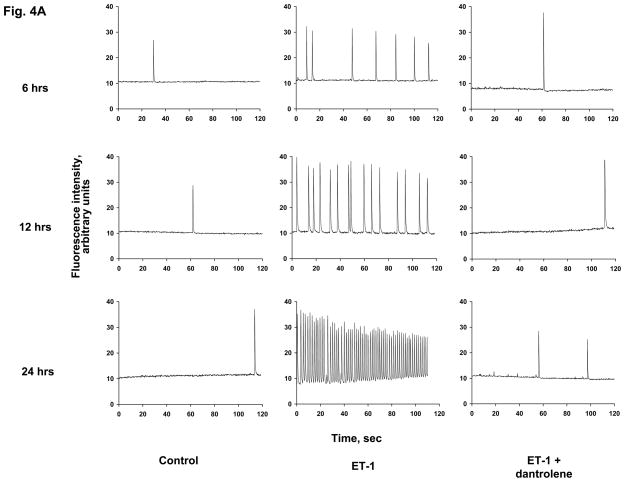
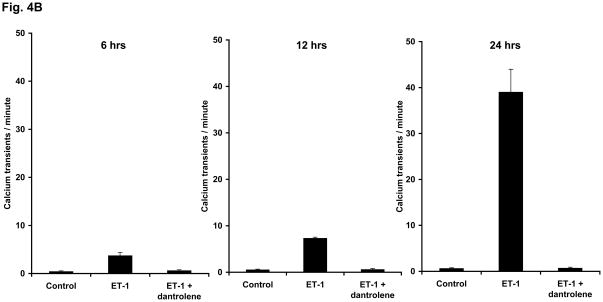
During the course of incubation of the neonatal cardiomyocyte culture, we have observed spontaneous intracellular Ca2+ transients, which were often synchronized among the cells. In the control cells, the frequency of the occurrence of Ca2+ transient was average 0.5 per minute throughout incubation from 6 hrs to 12 hrs and then to 24 hrs. The original traces of spontaneous Ca2+ transients are shown in Fig. 4A, and the frequencies of Ca2+ transients determined at these time points are shown in Fig. 4B. In the ET-1-treated cells, the frequency of spontaneous Ca2+ transients increased to an average of 3.7 per minute at 6 hrs incubation, and increased further to an average of 7.3 per minute at 12 hrs. Upon further incubation, at 24 hrs, a train of Ca2+ transients was formed, with a frequency of 39 transients per minute. It is important to note that the aforementioned nuclear translocation of CaM in early phase of ET-1 incubation well correlates with the increase of the frequency of spontaneous Ca2+ transients in early phase of hypertrophic growth, and the nuclear translocation of CaMKII in a later phase of hypertrophic growth well correlates with the appearance of the train of Ca2+ transients. Dantrolene prevented both the increase of the frequency of Ca2+ transients in an early phase of HT development and the appearance of the train of Ca2+ transients in a late phase of HT development that would have been induced by ET-1 (Figs. 4A and B).
Fig. 4. ET-1 increases the frequency of spontaneous Ca2+ transients in a time-dependent manner, and dantrolene prevents it.
A. The original trace of the Ca2+ transients. Cardiomyocytes treated with ET-1, ET-1 + dantrolene or none (control) for 6 hrs, 12 hrs and 24 hrs were used for the determination of spontaneous synchronous Ca2+ transients by adding a cell permeable Ca2+ indicator fluorescent dye fluo-4 to the live cardiomyocyte culture. The fluorescence of the dye was followed through live cell confocal imaging for 2 min; each spike indicates one spontaneous synchronous Ca2+ transient. The representative plots show the average number of spikes observed in each set of cardiomyocytes. B. Quantitation of the frequency of Ca2+ transients. Spontaneous Ca2+ transients per minute was calculated for control, ET-1 and ET-1 + dantrolene at each time point (6, 12 and 24 hrs). Five to eight experiments for each sample were averaged for each time point.
Postulated roles of CaM and CaMKII in the development of hypertrophy
In our previous study [3], we have shown that DPc10, a central domain peptide that interferes with normal inter-domain interaction within the RyR2 and produces diastolic Ca2+ leak [27], develops HT in the neonatal cardiomyocytes. We have also shown that dantrolene, the RyR2-directed cardio-protective reagent, restores normal inter-domain interaction and channel function, and prevents the development of HT that would have been induced by DPc10 as well as by ET-1. This indicates that HT stimulus by ET-1 produces defective inter-domain interaction in the RyR2, then diastolic Ca2+ leak through the de-stabilized Ca2+ channel, and in turn develops HT. One of the important findings in the present study is that ET-1 increased the frequency of spontaneous Ca2+ transients in an early phase of development of HT, and the cytoplasm-to-nucleus translocation of CaM in an early phase of HT development well correlates with the initial increase of the frequency of Ca2+ transients. We postulate that ET-1 induces defective inter-domain interaction and perhaps dissociation of RyR2-bound CaM (cf. Introduction), which result in destabilization of Ca2+ channel and diastolic Ca2+ leak, and the increased cytoplasmic Ca2+ triggers a global translocation of cytoplasmic CaM to the nucleus.
CaMKII seems to work as an intrinsic ‘Ca2+ spike frequency integrator’ [28]. According to recent studies of in vitro models [29], at a high frequency of Ca2+ stimuli, the interval between Ca2+ spikes/sparks is too short to allow full dissociation of CaM from CaMKII. This increases the probability that the successive Ca2+/CaM stimuli applied to CaMKII produce its autophosphorylation, which results in an increase in CaM affinity, coincident CaM binding and CaM trapping, whereby rendering the CaMKII to ‘memorize’ the pattern of Ca2+ transients [30, 31]. As is shown in the present study, there is a reasonable temporal correlation of the appearance of trains of Ca2+ transients with nuclear translocation of CaMKII. We propose that spontaneous Ca2+ transients with increased frequency (diastolic Ca2+ leak) eventually develop to the trains of Ca2+ transients, this event is memorized by CaMKII by virtue of CaM trapping; then, CaMKII is translocated to the nucleus to activate HT gene program as a messenger of pathogenic signal transduction (cf. Introduction). According to Ling et al. [32], attenuating CaMKII activation delimits the progression of HT to heart failure. This suggests an alternative possibility that the nuclear translocation of CaMKII described here might be involved in the progression of HT to heart failure rather than in the development of HT. If this is the case, the initial increase in the frequency of spontaneous Ca2+ transients and nuclear translocation of cytoplasmic CaM, which are observed in an early stage of ET-1 incubation, trigger HT; whereas, the CaM trapping/integration of the train of Ca2+ transients in CaMKII and its nuclear translocation, which happen in a later phase of hypertrophic growth, mediate the progression of HT to heart failure. In conclusion, the present results suggest that stimulation of neonatal cardiomyocytes with ET-1 produces first defective inter-domain interaction within the RyR2 and diastolic Ca2+ leak through the RyR2 Ca2+ channel. This triggers a global translocation of cytoplasmic CaM to the nucleus to activate some of those pro-HT pathogenic pathways. Sustained diastolic Ca2+ leak results in the appearance of trains of Ca2+ transients. This aberrant intracellular Ca2+ event is detected and memorized by the CaMKII as a pathogenic signal, which is conveyed to the pro-HT transcriptional sites as the CaMKII/CaM complex moves into the nucleus. In support of this concept, correcting the defective inter-domain interaction within the RyR2 with dantrolene prevents aberrant Ca2+ events, translocation of CaM and CaMKII, and then prevents the development of HT.
Acknowledgments
This work was supported by the National Institute of Health (NIH Grant No. RO1 HL072841).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983;72(2):732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, Chien KR. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990;265(33):20555–20562. [PubMed] [Google Scholar]
- 3.Hamada T, Gangopadhyay JP, Mandl A, Erhardt P, Ikemoto N. Defective regulation of the ryanodine receptor induces hypertrophy in cardiomyocytes. Biochem Biophys Res Commun. 2009;380(3):493–497. [Google Scholar]
- 4.Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53(21):1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Limenez LS, Morimoto H, Williams PG, Parness J. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem. 2002;277:34918–34923. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the Ryanodine receptor. J Biol Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 7.Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, Matsuzaki M. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circ. 2005;1l1(25):3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, Oda T, Kobayashi S, Ikemoto N, Matsuzaki M. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;12(117):762–772. doi: 10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, Yamamoto T, Ikeda Y, Okusa T, Ikemoto N, Matsuzaki M. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res. 2009;81(3):536–545. doi: 10.1093/cvr/cvn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodney GG, Williams BY, Strasburg GM, Beckingham K, Hamilton SL. Regulation of RyR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 11.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262:3065–3073. [PubMed] [Google Scholar]
- 12.Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol. 2000;279:C724–C733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca2+ release channel. J Clin Invest. 2007;117(5):1344–1353. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca2+ waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010 March 27; doi: 10.1016/j.yjmcc.2010.03.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neef S, Dybkove N, Sossalla S, Ort KR, Flschnik N, Neumann K, Seipelt R, Schondbe FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 16.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelu MG, Sama S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W. Old and new tools to dissect calcineurin’s role in pressure-overload cardiac hypertrophy. Cardiovasc Res. 2002;53:294–303. doi: 10.1016/s0008-6363(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 19.Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Svkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase II delta and protein kinase D overexpression reinforce the histon deacetylase 5 redistribution in heart failure. Circ Res. 2008;102:695–702. doi: 10.1161/CIRCRESAHA.107.169755. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, Mizukami M, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 21.Heineke J, Molkentin J. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nature Reviews. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 22.Toth A, Nickson P, Quin LL, Erhardt P. Differential regulation of cardiomyocyte survival and hypertrophy by MDM2, and E3 ubiquitin ligase. J Biol Chem. 2006;281:3679–3689. doi: 10.1074/jbc.M509630200. [DOI] [PubMed] [Google Scholar]
- 23.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 24.Maier LS. Role of CaMKII for signaling and regulation in the heart. Front Biosci. 2009;14:486–496. doi: 10.2741/3257. [DOI] [PubMed] [Google Scholar]
- 25.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med. 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Ikemoto N. Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem Biophys Res Comm. 2002;291:1102–1108. doi: 10.1006/bbrc.2002.6569. [DOI] [PubMed] [Google Scholar]
- 28.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364(Pt 3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279(5348):227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 30.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 31.Singla SI, Hudmon A, Goldberg JM, Smith JL, Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
- 32.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]