Abstract
PURPOSE
Radiation Therapy Oncology Group 9402 compared PCV chemotherapy plus radiation therapy (PCV+RT) versus RT alone for anaplastic oligodendroglioma. Here we report 1) longitudinal changes in cognition and quality of life, 2) effects of patient factors and treatments on cognition, quality of life and survival, and 3) prognostic implications of cognition and quality of life.
METHODS and MATERIALS
Cognition was assessed by Mini Mental Status Examination (MMSE) and quality of life by Brain-Quality of Life (B-QOL). Scores were analyzed for survivors and within five years of death. Shared parameter models evaluated MMSE/B-QOL with survival.
RESULTS
For survivors, MMSE and B-QOL scores were similar longitudinally and between treatments. For those who died, MMSE scores remained stable initially, while B-QOL slowly declined; both declined rapidly in the last year of life and similarly between arms. In the aggregate, scores decreased over time (P=0.0413 for MMSE; P=0.0016 for B-QOL) and were superior with age < 50 years (P<0.001 for MMSE; P=0.0554 for B-QOL) and KPS 80–100 (P<0.001). Younger age and higher KPS were associated with longer survival. After adjusting for patient factors and drop-out, survival was longer after PCV+RT (HR=0.66, 95% CI=0.49–0.9, P=0.0084; HR=0.74, 95% CI=0.54–1.01, P=0.0592) in models with MMSE and B-QOL. In addition, there were no differences in MMSE and B-QOL scores between arms (P=0.4752 and P=0.2767, respectively); higher scores predicted longer survival.
CONCLUSIONS
MMSE and B-QOL scores held steady in the upper range in both arms for survivors. Younger, fitter patients had better MMSE and B-QOL and longer survival.
Keywords: Cognitive function, quality of life, PCV, radiotherapy, randomized trial
INTRODUCTION
Anaplastic oligodendrogliomas (AO) respond to radiation therapy (RT) and also respond to procarbazine, lomustine and vincristine (PCV) chemotherapy (1). The RTOG designed a phase III study (9402) to test whether adding PCV to RT prolonged life in patients with newly diagnosed AO compared to RT alone. At first reporting, PCV+RT yielded longer progression-free survival times, a benefit associated with increased toxicity, and patients whose tumors lacked chromosomal arms 1p and 19q lived longer, irrespective of treatment (2). In 9402, all patients participated in a cognitive function and quality of life evaluation, completing questionnaires at baseline and subsequently, to compare clinician-administered cognitive function and patient-reported quality of life scores after PCV+RT versus RT alone. This report analyzes within-subject longitudinal changes of cognitive function and quality of life, characterizes the effects of patient specific factors on cognitive function, quality of life and survival and explores the prognostic implications of cognitive function and quality of life.
METHODS AND MATERIALS
Study participants
Eligibility criteria for RTOG 9402, characteristics of participants and early survival results have been published (2). Eligible patients were 18 years of age or older with newly diagnosed AO. Randomization to either four cycles of PCV (at intensified dosing) followed by RT (experimental arm) or RT alone (control arm) occurred within eight weeks of diagnosis. RT consisted of localized treatment fields (tumor + edema + 1–2 cm margin) to a dose of 59.4 Gy. Patients were stratified by age < 50 versus ≥ 50 years, Karnofsky Performance Score (KPS) 60–70 versus ≥ 80 and degree of anaplasia (moderate versus high) and were required to begin treatment within one week of randomization. The study was designed to accrue 146 patients per arm giving 80% power to detect a 50% increase in median survival from 3.8 years in the control arm to 5.7 [hazard ratio (HR) for death = 0.67); type I error, 0.05]. The study was approved by Institutional Review Boards and participants consented.
Cognitive function and quality of life assessment
Cognition was assessed using the Mini Mental Status Examination (MMSE) (3). The MMSE is a 30-point test for dementia; scores <27 are considered abnormal. Quality of life was assessed using the B-QOL (4–6). B-QOL measures global quality of life and emotional well-being. Each subscale contains questions, scored 1 to 4. Results are summed to yield minimum and maximum scores of 50 and 200, respectively; scores are standardized to a 100-point scale (7). MMSE and B-QOL questionnaires were given to assess the effects of treatment and disease on cognition and quality of life. Assessments were made at baseline, 9 and 12 months in the PCV + RT arm and baseline, 3, 6, 9 and 12 months in the RT alone arm; thereafter, patients in both arms were assessed every 4 months in year 2, every 6 months in years 3–5, then annually. The MMSE form was completed by the nurse, research associate, or physician; the B-QOL questionnaire was completed by the patient. If assistance was required to complete the B-QOL, the reason for assistance and the name of the assistant were recorded.
Statistical Methods
A meaningful effect size for change in cognition and quality of life is debated, without universal standardization. Cohen defined a 0.8 standard deviation (SD) as a “large” effect size and 0.5 SD as a “medium” effect (8, 9), the latter clinically meaningful by consensus. For this analysis, we had 99% power to detect a 0.5 SD effect size between PCV+RT and RT alone for the longitudinal assessment of MMSE and B-QOL score at the 0.05 level (two-sided); there was adequate power to detect differences due to treatment, allowing for drop-out and missing evaluations. All patients with baseline scores were included. Endpoints for cognition and quality of life were MMSE and B-QOL scores, respectively. For overall survival (OS), an event was death from any cause. The significance threshold was a P value of < 0.05. All analyses were performed using SAS version 9 (SAS Institute Inc, Cary, NC).
Patient features at baseline by treatment arm and patterns of completion of MMSE and B-QOL assessments were compared using chi-square tests. Analyses of cognitive function and quality of life were performed by assessment pattern on survivors and within five years of death. MMSE and B-QOL scores were compared over time; effects of patient factors and treatment on MMSE, B-QOL and survival were analyzed; and survival was assessed in relation to MMSE and B-QOL. Because assessments were often truncated due to early death or other reasons, it was necessary to consider the confounding effects of missing MMSE and B-QOL data. An analysis only of survivors would give an incomplete picture of cognitive function and quality of life among 9402 participants by failing to consider missing data from dying patients who might have had lower scores. These concerns were addressed by joint modeling of longitudinal MMSE and B-QOL measures and survival through shared parameter models (10–13). Joint modeling allowed for simultaneous consideration of the distributions of longitudinal assessments and survival and the relationship of assessments to survival. The method is well suited to estimations and comparisons of trends over time while adjusting for possible informative censoring due to patient drop out. A random-effects model for repeated assessments of MMSE and B-QOL was specified together with a Weibull model for survival.
We examined whether treatment, age, KPS, degree of anaplasia, type of surgery (resection vs. other), number of lesions (unifocal vs. multifocal) and 1p/19q co-deletion status predicted MMSE and B-QOL scores, risk of death, or both, whether patients with co-deleted tumors had higher MMSE and B-QOL scores, or whether MMSE and B-QOL scores changed over time by co-deletion status.
RESULTS
RTOG 9402 completed accrual in 2002. Seventy-six institutions and 299 patients participated. Ten patients were judged ineligible, five in each arm. The remaining 289 patients are the subject of this report which is based on data assembled for a survival analysis that took place in February 2008, when the median follow-up for surviving patients was 6.9 years, and 64% had died (14). Only patients with a baseline evaluation were included in this study; baseline MMSE and B-QOL data were missing for 17 of 289 (6%) and 22 of 289 (8%) cases, respectively. MMSE/B-QOL assessments were grouped at yearly intervals after randomization to maximize follow-up data. For MMSE: 29 patients (10%) had all evaluations; 24 (8%) had baseline, follow-up (some) and 5-year assessments; 107 (37%) missed the 5-year evaluation due to death; and 112 (39%) missed assessments for unspecified reasons. The completion rate (including those missing some evaluations) and drop out rates from death or other reasons were 18%, 37% and 39%, respectively. A similar pattern was seen for B-QOL (table 1). There was no difference in the distributions of MMSE or B-QOL assessment patterns by treatment arm.
Table 1.
MMSE and B-QOL Data Summary
| PCV+RT (n=147) | RT alone (n=142) | Total (n=289) | p-value* | ||
|---|---|---|---|---|---|
| MMSE | 0.80 | ||||
| Complete (all assessments within 5 years) | 12 (8%) | 17 (12%) | 29 (10%) | ||
| Complete (with some intermittent missing assessments) | 13 (9%) | 11 (8%) | 24 (8%) | ||
| Drop-out due to death within 5 years | 54 (37%) | 53 (37%) | 107 (37%) | ||
| Drop-out due to other reasons | 58 (39%) | 54 (38%) | 112 (39%) | ||
| Missing baseline | 10 (7%) | 7 ( 5%) | 17 (6%) | ||
| B-QOL | 0.27 | ||||
| Complete (all assessments within 5 years) | 13 (9%) | 20 (14%) | 33 (11%) | ||
| Complete (with some intermittent missing assessments) | 13 (9%) | 9 (6%) | 22 (8%) | ||
| Drop-out due to death within 5 years | 52 (35%) | 49 (35%) | 101 (35%) | ||
| Drop-out due to other reasons | 54 (37%) | 57 (40%) | 111 (38%) | ||
| Missing baseline | 15 (10%) | 7 ( | 5%) | 22 (8%) | |
Chi-square test comparing PCV+RT vs. RT alone
MMSE outcomes
The mean MMSE scores over time by treatment are shown in figure 1A for surviving patients; scores ranged from 28–29, suggesting relatively normal cognition. Those in the PCV+RT arm had increasing MMSE scores beginning two years after starting treatment, while after RT alone, scores were constant over time. Mean scores by pattern of assessment are shown in figure 1B, 1C. For both arms, those who dropped out due to death had the lowest scores. In the PCV+RT arm, those who completed assessments had slightly higher scores than those who dropped out for unspecified reasons, whereas in the RT alone arm, scores overlapped between survivors and those who survived but dropped out. Because a longitudinal analysis of cognition that captures available data will be distorted by the early loss of those with lower scores who died and had incomplete evaluations, mean MMSE scores in the five years prior to death were analyzed (figure 1D). One hundred seventy nine deceased patients completed at least one MMSE assessment. Scores were in the normal range until their last year of life and were similar in both arms in the five years prior to death.
Figure 1.
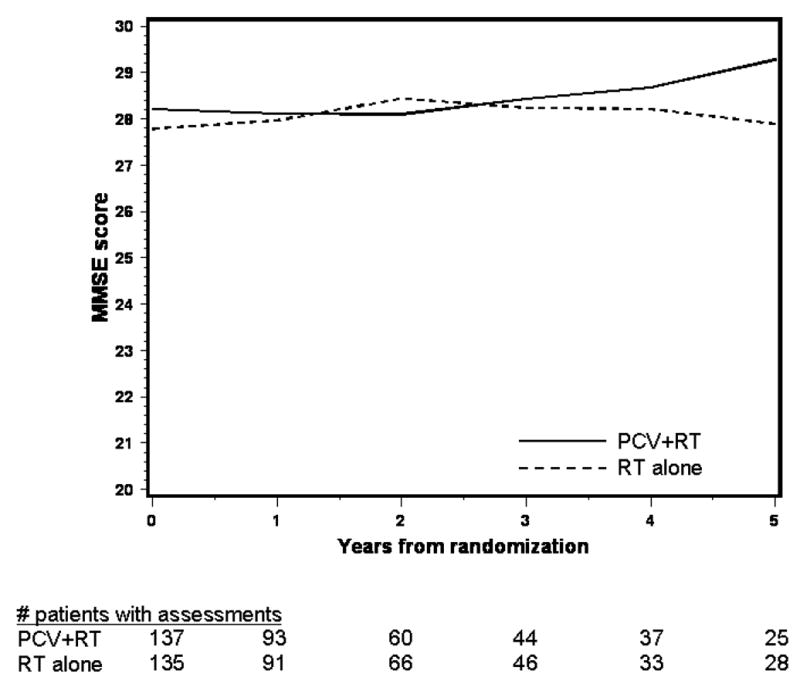
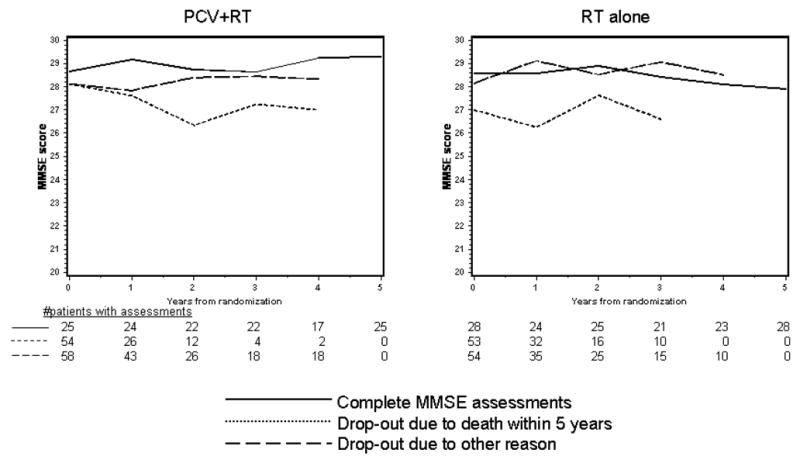
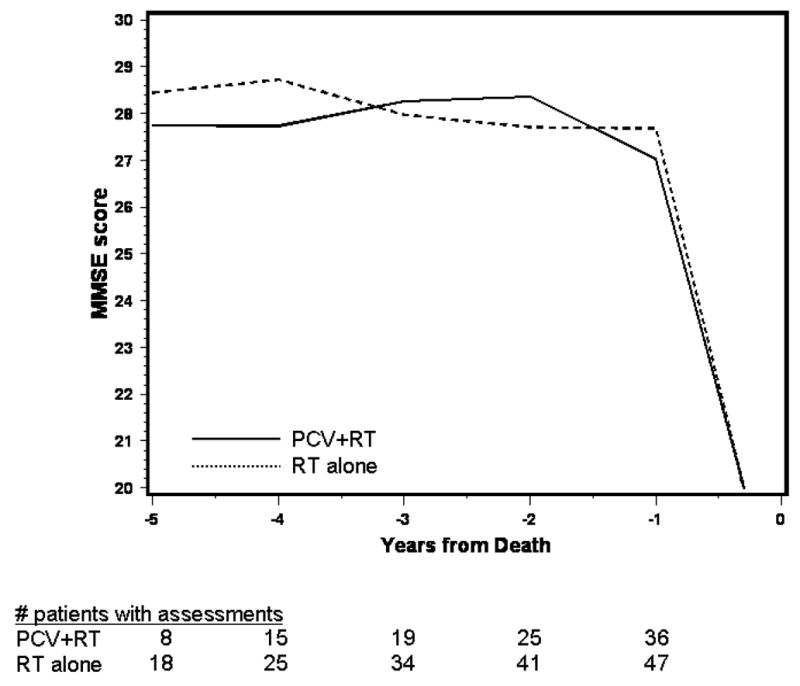
Figure 1A. Average MMSE Score over Time by Treatment
Figure 1B, 1C. Average MMSE Score over Time by Treatment and Pattern of Assessment
Figure 1D. Average MMSE Score over Time before Death
The results of joint modeling of MMSE and survival are shown in table 2. Age, type of surgery, KPS, degree of anaplasia, number of lesions, co-deletion status and treatment were analyzed in relation to MMSE and risk of death. All variables were significant in the model for survival; however, only KPS (P<0.001) and age (P<0.001) were significant in the model for MMSE in addition to the assessment time after diagnosis (P=0.0413). For cognition, positive estimates (second column in table 2) imply that scores were higher for patients with these features compared to counterparts. For survival, positive estimates (second column in table 2) imply that patients with these features had a lower risk of death. Hazard ratios (15) and 95% confidence intervals are also shown. Joint modeling results demonstrated that patients < age 50 or those with KPS 80–100 were more likely to have higher MMSE scores; moreover, lower age and higher KPS were significantly associated with increased survival. Patients with unifocal, resected, less anaplastic, co-deleted tumors, treated with PCV+RT (HR=0.66, 95%CI =0.49–0.9, P=0.0084) had a lower risk of death, but their MMSE scores did not differ from the RT group (P=0.4752). The negative value of estimate for assessment time was significant (P=0.0413) indicating that MMSE scores decreased over time. The joint modeling results also show that factors causing higher MMSE scores decrease the risk of death (P=0.0015).
Table 2.
Joint Modeling of MMSE Score and Survival Time
| Covariate | Estimate | Standard Error | p-value | |
|---|---|---|---|---|
| MMSE Score | ||||
| Assessment time (Time) | −0.0132 | 0.0064 | 0.0413 | |
| Treatment arms (RX) (PCV+RT) | 0.2398 | 0.3354 | 0.4752 | |
| Interaction between treatment and time | 0.0120 | 0.0090 | 0.1832 | |
| KPS (80–100) | 2.7240 | 0.5412 | <.0001 | |
| Age (<50) | 1.4106 | 0.3479 | <.0001 | |
| Anaplastic features (2–3) | 0.2981 | 0.3146 | 0.3441 | |
| Surgery (Resection) | 0.5322 | 0.4999 | 0.2879 | |
| Multifocal (No) | 0.2094 | 0.5909 | 0.7233 | |
| 1p/19q deletion (Both deleted) | 0.1785 | 0.3209 | 0.5786 | |
| Hazard Ratio (95% CI) | ||||
| Survival Time | ||||
| Treatment arm (PCV+RT) | 0.3556 | 0.1339 | 0.0084 | 0.66 (0.49,0.90) |
| KPS (80–100) | 0.5613 | 0.1990 | 0.0051 | 0.52 (0.33,0.82) |
| Age (<50) | 0.4642 | 0.1498 | 0.0021 | 0.58 (0.41,0.82) |
| Anaplastic features (2–3) | 0.4825 | 0.1369 | 0.0005 | 0.57 (0.42,0.78) |
| Surgery (Resection) | 0.4572 | 0.2010 | 0.0237 | 0.59 (0.37,0.93) |
| Multifocal (No) | 1.0238 | 0.2167 | <.0001 | 0.30 (0.18,0.50) |
| 1p/19q deletion (Both deleted) | 0.7370 | 0.1358 | <.0001 | 0.42 (0.31,0.58) |
Age and KPS predicted MMSE scores over time in the joint model of cognition and survival. Scores were high in patients with excellent function and did not differ over time by treatment. In the small group with poor function, scores were lower (28 patients with KPS 60–70 in both treatment arms at baseline). Those < age 50 had higher MMSE scores compared to older patients. Average MMSE scores did not differ over time after stratification for treatment and co-deletion status.
B-QOL outcomes
The mean B-QOL scores over time by treatment for survivors are depicted in figure 2A. Patients in both treatment arms had similar scores at baseline that changed little over time. B-QOL scores by pattern of assessment are shown in figure 2B, 2C. In both arms, those who dropped out due to death had the lowest scores; mean scores among those who completed assessments and those who dropped out for unspecified reasons were similar between treatments and over time. Analysis of quality of life incorporating available data from survivors will be distorted by the early loss of patients with lower scores who died and had incomplete assessments. Scores in the five years before death are shown in figure 2D. One hundred seventy seven patients who died completed at least one assessment; baseline scores were higher in the RT alone arm but declined equally in both arms prior to death.
Figure 2.
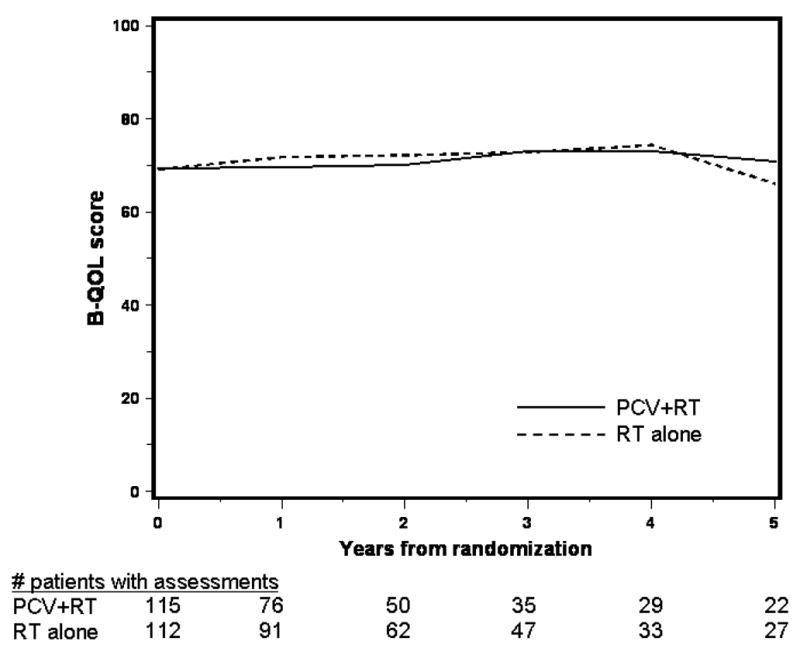
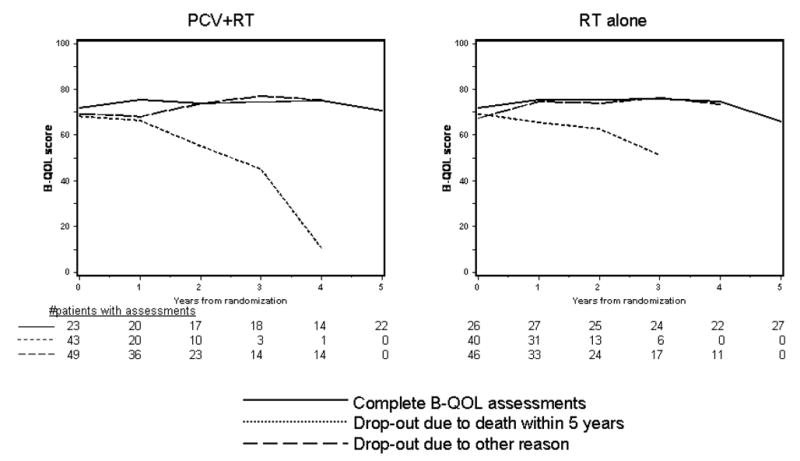
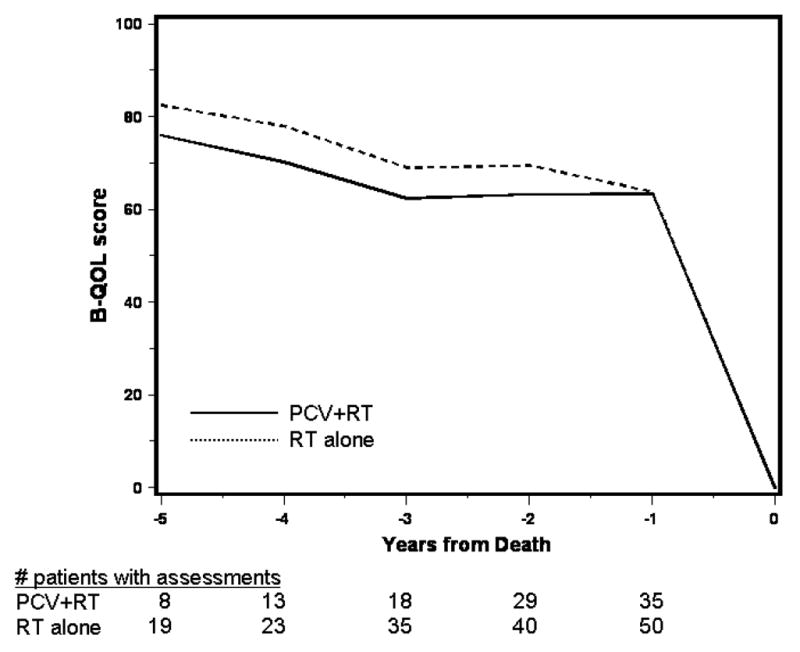
Figure 2A. Average B-QOL Score over Time by Treatment
Figure 2B, 2C. Average B-QOL Score over Time by Treatment and Pattern of Assessment
Figure 2D. Average B-QOL Score over Time before Death
Joint modeling of B-QOL and survival is summarized in table 3. Age, type of surgery, KPS, degree of anaplasia, number of lesions, and co-deletion status were significant in the joint model with survival. The p values for treatment (0.0592) did not reach the pre-specified significance level. KPS (P<0.001) was significant in the model for B-QOL in addition to the assessment time after diagnosis (P=0.0016). The p values for age (0.0554) did not reach the pre-specified significance level. Patients < age 50 or with KPS 80–100 were more likely to have higher quality of life than older or poorer function patients; younger age and higher KPS were associated with lower risk of death. Those with unifocal, resected, less anaplastic, co-deleted tumors, treated with PCV+RT (HR=0.74, 95%CI =0.54–1.01, P=0.0592) tended to have a lower risk of death, but their scores did not differ from RT alone patients (P=0.2767). The negative value of the estimate for assessment time was significant indicating that B-QOL decreased over time. Those latent factors contributed to higher quality of life also decreased the risk of death (P=0.0004).
Table 3.
Joint Modeling of B-QOL Score and Survival Time
| Covariate | Estimate | Standard Error | p-value | |
|---|---|---|---|---|
| B-QOL Score | ||||
| Assessment time (Time) | −0.1044 | 0.0326 | 0.0016 | |
| Treatment arms (RX) (PCV+RT) | −2.1620 | 1.9833 | 0.2767 | |
| Interaction between treatment and time | 0.0737 | 0.0476 | 0.1230 | |
| KPS (80–100) | 16.4225 | 3.4976 | <.0001 | |
| Age (<50) | 4.0584 | 2.1083 | 0.0554 | |
| Anaplastic features (2–3) | 3.3424 | 1.8908 | 0.0783 | |
| Surgery (Resection) | −1.9490 | 3.1640 | 0.5385 | |
| Multifocal (No) | 5.4738 | 3.6006 | 0.1297 | |
| 1p/19q deletion (Both deleted) | −0.0202 | 1.9379 | 0.9917 | |
| Hazard Ratio (95% CI) | ||||
| Survival Time | ||||
| Treatment arms (PCV+RT) | 0.2622 | 0.1383 | 0.0592 | 0.74(0.54,1.01) |
| KPS (80–100) | 0.5017 | 0.2223 | 0.0249 | 0.56 (0.33,0.93) |
| Age (<50) | 0.4591 | 0.1550 | 0.0034 | 0.58 (0.41,0.83) |
| Anaplastic features (2–3) | 0.4587 | 0.1436 | 0.0016 | 0.58 (0.42,0.81) |
| Surgery (Resection) | 0.4862 | 0.2278 | 0.0338 | 0.57 (0.34,0.95) |
| Multifocal (No) | 0.9889 | 0.2388 | <.0001 | 0.31 (0.18,0.54) |
| 1p/19q deletion (Both deleted) | 0.7820 | 0.1430 | <.0001 | 0.40 (0.29,0.56) |
Age and KPS predicted B-QOL scores in joint modeling of quality of life and survival. Scores were higher in patients with excellent function and did not differ over time by treatment. In the small group with poor function, scores were lower (20 patients with KPS 60–70 in both treatment arms at baseline). Those < age 50 had higher B-QOL scores compared to older patients. Average B-QOL scores did not differ over time after stratification for treatment and co-deletion status.
DISCUSSION
Cognitive function and quality of life are significant issues in brain tumor care (16). At first reporting of RTOG 9402, when the minimum follow-up of survivors was three years, the median survival time was 4.8 years and the median survival of co-deleted cases had not been reached (2). Further, progression-free survival was prolonged by PCV + RT, a benefit that was restricted to the co-deleted subset. In a subsequent analysis, when the minimum follow-up for survivors was 6.9 years, the adjusted overall survival was prolonged by PCV + RT (HR=0.66, 95% CI= 0.46–0.95, p=0.02) (14) and the median survival for co-deleted cases was 8.7 years. Longer follow-up is needed to ascertain whether this emerging survival difference favoring PCV+RT reflects better control of co-deleted cases or other factors (14). In the meantime, other study outcomes are gaining importance. Given the large number of long term survivors in 9402, cognitive function and quality of life, both longitudinally and between treatment arms, will be results of interest. Long survival after effective treatment is less meaningful if intellect and well being are not preserved. Data on these outcomes are also needed.
Because RTOG 9402 was a prospective trial with central pathology review and rigorous follow-up, we were able to analyze cognition and quality of life in relation to patient factors, tumor histology and survival. Because it was also a randomized trial with tissue collection, we were able to compare cognitive function and well-being after different treatments and study relationships between co-deletion status, initial therapy and survival simultaneously in a joint statistical model.
Among survivors, many of whom had co-deleted tumors, MMSE scores remained constant in the high normal range. There was no difference in scores between survivors treated with PCV+RT or RT. Interestingly, MMSE scores trended upwards over five years of follow-up for the PCV+RT group. Patients who dropped out due to death had lower baseline scores which remained lower during follow-up in both arms. Those who dropped out for other reasons had lower baseline scores than survivors, but over time in both arms, their scores more closely matched survivors than non-survivors. Similarly, B-QOL scores remained constant in the mid upper range over time for survivors and there was no difference between the treatment arms. Survivors and those who dropped out due to death had similar baseline B-QOL scores whereas the scores of those who died declined steadily in both treatment groups. Scores of those who dropped out for other reasons were similar to those of surviving patients and between treatment arms. These results are encouraging: MMSE and B-QOL scores were favorable and did not decline over time among long survivors. Further, patients who were treated aggressively with PCV+RT did not experience greater difficulties with cognition or quality of life than those treated with RT. Lack of decline in cognition and well being in the setting of long follow-up is remarkable given that large volumes of brain were irradiated to higher doses.
Among patients who died within five years of diagnosis, MMSE scores were normal and unchanged until one year prior to death and then deteriorated rapidly. There was no difference between the PCV+RT and RT alone groups. B-QOL scores, on the other hand, slowly declined from baseline, and like MMSE scores, declined rapidly in the last year of life. At baseline, B-QOL scores were somewhat lower in the PCV + RT group, but within five years of death, there was no difference in the rate of decline between treatment groups. Tumors with intact 1p or 19q alleles were over-represented among those who died within five years of diagnosis. Quality of life deteriorated relentlessly when the tumor was poorly responsive or unusually aggressive. The rapid decline in MMSE and B-QOL in the last year of life is consistent with the experience of those who care for brain tumor patients.
Using all cognitive function, quality of life and survival data, and without regard for survival or death within 5 years, there was no significant difference between PCV+RT and RT alone in either MMSE or B-QOL scores. In the aggregate, after adjusting for treatment and patient characteristics and after controlling for all missing MMSE and B-QOL assessments, cognitive function and quality of life decrease over time. Our analysis revealed that cognition and quality of life were superior in younger patients and those with good performance; age and KPS significantly predicted risk of death. Patients with unifocal, removable, less anaplastic, co-deleted tumors had a lower risk of death and there was a trend to longer survival after PCV+RT. Higher MMSE and B-QOL scores also predicted a lower risk of death. For co-deleted cases, MMSE and B-QOL scores were similar between treatment arms; there was no difference in cognition or quality of life by genotype. Lack of the co-deletion was associated with poor survival, but had no effect on cognition or quality of life.
One weakness of this study is the MMSE which may not capture aspects of cognitive decline that are subtle and important (17, 18). The test was developed as a screening tool for dementia (19–21); its sensitivity and specificity in other spheres have not been examined thoroughly (22). Therefore, although our study suggests that cognition is well-maintained in this patient group, subtle deterioration in memory, personality, or intelligence could not be assessed by this simple cognitive measurement tool. Indeed, most trials are abandoning the MMSE in favor of more refined assessment tools (18, 23–27). The RTOG now uses extended battery tools to assess cognitive decline. However, several studies have found an association between baseline MMSE score and survival, implying that MMSE, however crude, captures important prognostic information (28–31). Our results are encouraging in three respects: cognition among the more intensively treated PCV+RT group was indistinguishable from the RT group; there was no difference in quality of life between the groups, as measured by the B-QOL, a respected measure; and both MMSE and B-QOL scores were stable over time in surviving patients. Like Brown et al (32), we find that tumor growth is the main cause of decline in brain tumor patients.
Acknowledgments
We are especially indebted to the patients who agreed to participate in this study. We are also indebted to the nurses and managers at participating institutions, office staff at RTOG, SWOG, NCCTG, NCIC-CTG and ECOG, and the staff in the pathology and molecular genetics laboratories at the Mayo Clinic whose support was critical to the success of this trial. The groups, institutions and investigators who enrolled patients in RTOG 9402 were gratefully acknowledged and published previously (2).
Footnotes
Conflicts of Interest Notification
No authors of this manuscript shall derive any personal profit or gain, directly or indirectly, by reason of his or her participation with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cairncross JG, Macdonald DR, Ramsay DA. Aggressive oligodendroglioma: A chemosensitive tumor. Neurosurgery. 1992;31:78–82. doi: 10.1227/00006123-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiotherapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 3.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson NK, Bakker W, Stewart AL, et al. Multidimensional approach to the measurement of quality of life in lung cancer clinical trials. In: Aaronson NK, Beckmann J, editors. The Quality of Life of Cancer Patients. New York: Raven Press; 1987. pp. 63–82. [Google Scholar]
- 6.Mackworth N, Fobair P, Prados MD. Quality of life self-reports from 200 brain tumor patients: comparison with Karnofsky performance scores. J neuro-Oncol. 1992;14:243–253. doi: 10.1007/BF00172600. [DOI] [PubMed] [Google Scholar]
- 7.Fairclough D. Design and analysis of quality of life studies in clinical trials. Chapman & Hall; CRC: 2003. [Google Scholar]
- 8.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- 9.Sloan J, Cella D, Hays R. Clinical significance of patient-reported questionnaire data: Another step toward consensus. J Clin Epidemiol. 2005;58(12):1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat in Med. 2006;25:143–163. doi: 10.1002/sim.2249. [DOI] [PubMed] [Google Scholar]
- 11.Zeng D, Cai J. Simultaneous Modeling of Quality of Life and Survival Time. Lifetime Data Analysis. 2005;11(2):151–174. doi: 10.1007/s10985-004-0381-0. [DOI] [PubMed] [Google Scholar]
- 12.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1(4):465–80. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Carlin B. Separate and joint modeling of longitudinal and event time data using standard computer packages. The American Statistician. 2004;58(1):16–24. [Google Scholar]
- 14.Cairncross G, Wang M, Shaw E, et al. A Randomized Trial of Chemotherapy plus Radiotherapy (RT) versus RT alone for Anaplastic Oligodendroglioma (RTOG 9402): The Perspective of Longer Follow-Up [abstract] Int J Radiat Oncol Biol Phys. 2008;72(suppl 1):S206. [Google Scholar]
- 15.Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. Springer; 2003. [Google Scholar]
- 16.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 17.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4. Oxford: Oxford University Press; 2004. [Google Scholar]
- 18.Meyers C, Hess K, Yung W, Levin V. Cognitive Function as a Predictor of Survival in Patients With Recurrent Malignant Glioma. J Clin Oncol. 2000;18(3):646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 19.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: A comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 20.Salmon D, Thal L, Butters N, et al. Longitudinal evaluation of dementia of the Alzheimer type: A comparison of 3 standardized mental status examinations. NEUROLOGY. 1990;40:1225. doi: 10.1212/wnl.40.8.1225. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D, Klauber MR, Hofstetter CR, et al. The Mini-Mental State Examination in the early diagnosis of Alzheimer’s disease. Arch Neurol. 1990;47(1):49–52. doi: 10.1001/archneur.1990.00530010061020. [DOI] [PubMed] [Google Scholar]
- 22.Wade DT. Measurement in neurological rehabilitation. Oxford: Oxford University Press; 1992. [PubMed] [Google Scholar]
- 23.Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-In Phase to Randomized Trial of Motexafin Gadolinium and Whole-Brain Radiation for Patients With Brain Metastases: Centralized Assessment of Magnetic Resonance Imaging, Neurocognitive, and Neurologic End Points. J Clin Oncol. 2002;20(16):3445–3453. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 24.Mehta MP, Rodrigus P, Terhaard CHJ, et al. Survival and Neurologic Outcomes in a Randomized Trial of Motexafin Gadolinium and Whole-Brain Radiation Therapy in Brain Metastases. J Clin Oncol. 2003;21(13):2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 25.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 26.Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62. doi: 10.1215/15228517-2006-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Bentzen SM, Li J, et al. Relationship Between Neurocognitive Function and Quality of Life After Whole-Brain Radiotherapy in Patients With Brain Metastasis. Int J Radiat Oncol Biol Phys. 2008;71(1):64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 28.Taylor BV, Buckner JC, Cascino TL, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol. 1998;16:2195–2201. doi: 10.1200/JCO.1998.16.6.2195. [DOI] [PubMed] [Google Scholar]
- 29.Corn BW, Moughan J, Knisely JPS, et al. Prospective Evaluation of Quality of Life and Neurocognitive Effects in Patients With Multiple Brain Metastases Receiving Whole-Brain Radiotherapy With or Without Thalidomide on Radiation Therapy Oncology Group (RTOG) Trial 0118. Int J Radiat Oncol Biol Phys. 2008;71(1):71–78. doi: 10.1016/j.ijrobp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Murray KJ, Scott C, Zachariah B, et al. Importance of the mini-mental status examination in the treatment of patients with brain metastases: a report from the Radiation Therapy Oncology Group protocol 91-04. Int J Radiat Oncol Biol Phys. 2000;48(1):59–64. doi: 10.1016/s0360-3016(00)00600-3. [DOI] [PubMed] [Google Scholar]
- 31.Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. doi: 10.1200/JCO.2006.08.5605. [DOI] [PubMed] [Google Scholar]
- 32.Brown PD, Buckner JC, O’Fallon JR, et al. J Clin Oncol. 2003;21:2519–2524. doi: 10.1200/JCO.2003.04.172. [DOI] [PubMed] [Google Scholar]


