Four independent mechanisms for uptake of activated EGFR are identified by a combination of receptor mutagenesis and RNA interference approaches.
Abstract
Endocytosis of the epidermal growth factor receptor (EGFR) is important for the regulation of EGFR signaling. However, EGFR endocytosis mechanisms are poorly understood, which precludes development of approaches to specifically inhibit EGFR endocytosis and analyze its impact on signaling. Using a combination of receptor mutagenesis and RNA interference, we demonstrate that clathrin-dependent internalization of activated EGFR is regulated by four mechanisms, which function in a redundant and cooperative fashion. These mechanisms involve ubiquitination of the receptor kinase domain, the clathrin adaptor complex AP-2, the Grb2 adaptor protein, and three C-terminal lysine residues (K1155, K1158, and K1164), which are acetylated, a novel posttranslational modification for the EGFR. Based on these findings, the first internalization-defective EGFR mutant with functional kinase and normal tyrosine phosphorylation was generated. Analysis of the signaling kinetics of this mutant revealed that EGFR internalization is required for the sustained activation of protein kinase B/AKT but not for the activation of mitogen-activated protein kinase.
Introduction
Binding of EGF to its receptor (EGF receptor [EGFR]) at the cell surface initiates a signal transduction process that involves a dynamic network of molecular interactions and modifications ultimately leading to cell survival, proliferation, and differentiation (Schlessinger, 2000). EGF binding also results in rapid internalization of activated receptors and targeting of internalized EGF–receptor complexes to lysosomes for degradation (for review see Sorkin and Goh, 2009). Acceleration of internalization and lysosomal targeting leads to receptor down-regulation, which serves to decrease the number of activated receptors in the cell and prevent excessive signaling. For instance, activation of phosphoinositide-3-kinase and protein kinase B/AKT is thought to occur mostly from the plasma membrane and is, therefore, negatively regulated by endocytosis (for review see Sorkin and von Zastrow, 2009). However, activated EGFR can continue to emit signals from endosomes. Specifically, endocytosis has been proposed to be necessary for the normal duration and intensity of the EGFR signaling to mitogen-activated protein kinases (MAPKs; Vieira et al., 1996; Teis et al., 2006; for reviews see Fehrenbacher et al., 2009; Sorkin and von Zastrow, 2009). Therefore, endocytosis can have multiple and possibly opposing effects on signal transduction processes. The analysis of the role of EGFR endocytosis in signaling has been difficult because the molecular mechanisms of EGFR internalization remain poorly understood, and experimental approaches to specifically inhibit EGFR internalization have not been developed. In particular, mutants of EGFR that are not internalized but maintain a full signaling capacity have not been generated.
Endocytosis of EGFR has historically been and remains to be the most popular experimental system for the study of ligand-induced endocytosis. Endocytosis via clathrin-coated pits is the main pathway of activated EGFR internalization observed under most physiological conditions, although several examples of clathrin-independent EGFR endocytosis have been reported (Yamazaki et al., 2002; Sigismund et al., 2005; Orth et al., 2006). Several conventional mechanisms of cargo recruitment into clathrin-coated pits have been tested for their role in EGFR internalization. An early study demonstrated interaction of EGFR with the clathrin adaptor protein complex AP-2 (Sorkin and Carpenter, 1993). Classical tyrosine-based motif (Y974RAL) in EGFR is essential for this interaction, although an involvement of another AP-2–binding, dileucine motif (Leu1010/Leu1011 [LL1010/1011]) in EGFR has also been proposed (Sorkin et al., 1996; Huang et al., 2003). However, functional assays demonstrated that neither the YRAL motif that interacts with the μ2 subunit of AP-2 nor the LL motif is necessary for clathrin-mediated endocytosis (CME) of EGFR (Sorkin et al., 1996; Nesterov et al., 1999; Huang et al., 2003). Testing the effect of AP-2 depletion by siRNAs on EGFR internalization has yielded conflicting results, showing either an important role of AP-2 (Huang et al., 2004; Johannessen et al., 2006; Rappoport and Simon, 2009) or lack thereof (Motley et al., 2003).
Another proposed mechanism of internalization involves cargo ubiquitination and interaction of the ubiquitinated cargo with ubiquitin adaptors in coated pits, such as epsin 1 and Eps15. EGFR is ubiquitinated by the E3 ubiquitin ligase Cbl, which binds to activated EGFR directly and indirectly through the Grb2 adaptor protein (Levkowitz et al., 1999; Waterman et al., 2002). However, the ubiquitin-based mechanism has also been ruled out as essential for EGFR internalization. First, mutation of 15 Lys residues in the kinase domain of EGFR, including six major ubiquitin conjugation sites, essentially abolished receptor ubiquitination but did not affect the rate of EGFR internalization (Huang et al., 2007). Second, siRNA depletion of epsin 1 alone or together with depletion of Eps15 and Eps15R did not specifically affect the CME of EGFR (Huang et al., 2004; Sigismund et al., 2005; Vanden Broeck and De Wolf, 2006; Chen and Zhuang, 2008). Mouse embryonic fibroblasts derived from the double–epsin 1/2 knockout exhibited normal EGFR endocytosis (Chen et al., 2009). In contrast, a strong effect of epsin 1 knockdown on EGFR internalization in HeLa cells has been reported in a previous study (Kazazic et al., 2009). The latter study suggested that there might be cryptic ubiquitination of the EGFR Lys mutants, which could potentially mediate receptor internalization through interaction with epsin 1.
Controversies in the reported effects of EGFR mutagenesis and siRNA depletion of AP-2 and epsin on EGFR endocytosis prompted us to test whether (a) ubiquitin- and AP-2–based internalization mechanisms have a redundant function in EGFR endocytosis and whether (b) Lys residues in the EGFR outside of its kinase domain are involved in the ubiquitin-dependent endocytosis. Thus, we show that there are at least four either completely redundant or partially interrelated mechanisms that participate in EGFR internalization through clathrin-coated pits. These mechanisms involve ubiquitination of the receptor kinase domain, receptor interaction with AP-2, C-terminal Lys residues, and Grb2. Consecutive elimination of the molecular determinants in EGFR, which participate in multiple internalization mechanisms, led to the generation of a novel EGFR mutant that preserves normal signaling capacity, yet is very slowly internalized and not accumulated in endosomes. This mutant was used to demonstrate the impact of slow EGFR endocytosis on signaling dynamics, particularly, on the sustained activity along the EGFR–AKT signaling axis.
Results
Lys residues in the EGFR kinase and C-terminal domains play redundant roles in receptor internalization
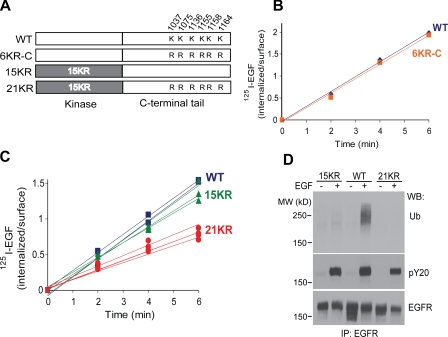
We have previously described an EGFR mutant with 15 Lys to arginine (15KR) mutations in the kinase domain (Huang et al., 2007). Despite negligible residual ubiquitination, 15KR was still internalized as efficiently as the wild-type (wt) EGFR, suggesting that receptor ubiquitination is not essential for internalization. Because there are six Lys in the C-terminal tail of EGFR (residues 1037, 1075, 1136, 1155, 1158, and 1164), we examined whether these Lys represent cryptic ubiquitination sites, which are undetectable by mass spectrometry (MS) and Western blotting, could be important for EGFR internalization. To this end, all six Lys were mutated to arginines in the wtEGFR and 15KR mutant, resulting in the 6KR-C and 21KR mutants, respectively (Fig. 1 A). We found that measurements of EGF endocytosis rates in cells transiently expressing EGFR yield low rates when compared with the typical rate of CME and the endocytic rates of endogenous as well as stably expressed heterologous EGFR (Fig. S1; Carter and Sorkin, 1998). To accurately measure the endocytic rates, EGFR mutants were stably expressed in porcine aortic endothelial (PAE) cells that do not express endogenous EGFR and detectable amounts of other ErbBs. For each EGFR mutant, several single-cell stable clones were tested to account for potential clonal variability. Finally, internalization assays were performed using low, physiological concentrations of radiolabeled 125I-EGF (1 ng/ml; plasma concentration of EGF), conditions favoring EGFR internalization through the CME pathway.
Figure 1.
Mutation of Lys residues in the C terminus decreases internalization of 125I-EGF by the 15KR mutant. (A) Schematic representation of 6KR-C and 21KR EGFR mutants in which six Lys were replaced by arginines in the C-terminal tail of wtEGFR and 15KR, respectively. (B) Time course of 1 ng/ml 125I-EGF internalization in PAE cells expressing wtEGFR (blue) or 6KR-C (orange) receptor. (C) Time course of 1 ng/ml 125I-EGF internalization in various single-cell clones of PAE cells expressing wtEGFR (blue), 15KR (green), or 21KR (red). (D) PAE cells expressing wtEGFR, 15KR, and 21KR were untreated or treated with 20 ng/ml EGF for 5 min at 37°C, and EGFR was immunoprecipitated (IP). Immunoprecipitates were probed by Western blotting with antibodies to ubiquitin (Ub), phosphotyrosine (pY20), and EGFR (1005). MW, molecular weight; WB, Western blot.
As shown in Fig. 1 B, 6KR-C mutant was internalized at a high rate. In contrast, several clones of 21KR-expressing cells internalized 125I-EGF much slower than 15KR and wtEGFR-expressing cells (Fig. 1 C). EGF-induced tyrosine phosphorylation of 21KR mutants was similar to that of wtEGFR, which indicates that the decrease in the internalization rate was not caused by altered tyrosine phosphorylation of the receptor (Fig. 1 D). Overall, these data suggest that Lys within the C-terminal tail play a role in EGFR internalization only when ubiquitination of the kinase domain is eliminated. Therefore, we hypothesized that Lys within the kinase domain and the C-terminal tail of EGFR are involved in two redundant mechanisms of EGFR internalization.
AP-2–binding motifs in the EGFR are involved in receptor internalization
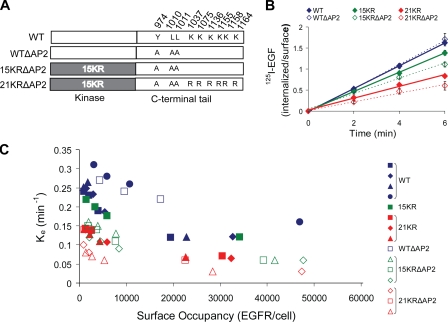
Because the 21KR mutant exhibits a 50% reduction in the rate of internalization (Fig. 1 C), additional mechanisms responsible for the residual internalization of the 21KR mutant must exist. The interaction of EGFR with the major cargo-binding protein complex in coated pits, AP-2, has been previously demonstrated, although functional experiments have shown that this interaction is not essential for EGFR internalization (Sorkin et al., 1996; Nesterov et al., 1999; Jiang et al., 2003). To investigate whether AP-2 is one of the several mechanisms involved in EGFR endocytosis, two AP-2–binding motifs in EGFR, Y974RAL and LL1010/1011, were mutated in the 15KR and 21KR constructs (15KRΔAP2 and 21KRΔAP2 mutants; Fig. 2 A). Interestingly, although mutations of these AP-2–binding motifs in wtEGFR (wtΔAP2) did not affect the internalization rate, the same mutations in conjunction with 15KR or 21KR mutations significantly reduced internalization rates of 1 ng/ml 125I-EGF (Fig. 2 B). Importantly, all mutants used in this study preserved normal EGF-induced tyrosine phosphorylation (Fig. S2). Moreover, upon EGF stimulation, 21KRΔAP2 was capable of phosphorylating two direct substrates of the EGFR kinase, c-Cbl and Shc, to the extent similar to that observed in cells expressing wtEGFR (Fig. S3). Collectively, these data suggest that the defective internalization of 21KRΔAP2 and other mutants is not caused by the impaired kinase activity.
Figure 2.
Mutation of AP-2–binding motifs (Y974RAL and LL1010/1011) decreases internalization of 125I-EGF by the 15KR and 21KR mutants. (A) Schematic representation of WTΔAP2, 15KRΔAP2, and 21KRΔAP2 EGFR mutants in which Tyr974 and LL1010/1011 were mutated to alanines in wtEGFR, 15KR, and 21KR mutants, respectively. (B) Summary of 1 ng/ml 125I-EGF internalization experiments performed in several single-cell clones of PAE cells expressing wtEGFR, WTΔAP2, 15KR, 15KRΔAP2, 21KR, or 21KRΔAP2 receptors. Bars represent SEM from three to four independent experiments. (C) Internalization saturation plots are shown. Specific internalization rate constant (ke) was measured in several clones of PAE cells expressing wtEGFR or various EGFR mutants treated with 1–20 ng/ml 125I-EGF. ke values are plotted against the number of surface EGFR occupied by 125I-EGF per cell measured as the amount of surface 125I-EGF after 5 min of continuous 125I-EGF internalization at 37°C.
The clathrin-dependent endocytosis of EGFR has limited capacity and is saturated when high concentrations of EGF-occupied receptors are present at the cell surface. In turn, the saturation leads to the decrease in the specific internalization rate of EGF, as the contribution of a slow clathrin-independent endocytosis in the overall uptake of EGF is increased (Lund et al., 1990). Because various clones of PAE cells express different levels of EGFR mutants (Fig. S2), saturation of the CME pathway can occur at different 125I-EGF concentrations. Therefore, internalization saturation experiments were performed using a range of 125I-EGF concentrations (1–20 ng/ml) to compare the maximally high internalization rates among all clones. Consistently with the results of time course internalization assays using 1 ng/ml 125I-EGF (Fig. 2 B), progressive decreases in the maximum internalization rate were observed as the redundant pathways were eliminated by mutations (Fig. 2 C). Importantly, the maximum internalization rate of the 21KRΔAP2 mutant was 20–30% to that rate of wtEGFR. Altogether, these results suggest that ubiquitination of the kinase domain, C-terminal Lys, and AP-2–binding motifs all contribute to EGFR internalization process and likely represent the redundant mechanisms of this internalization.
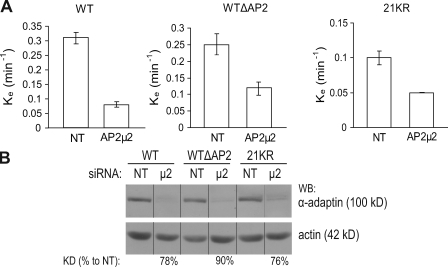
To further test whether AP-2 and Lys residues function redundantly in EGFR internalization, the effect of AP-2 depletion on the internalization of wtEGFR, 21KR, and wtΔAP2 mutant was examined using siRNA targeting µ2 subunit of AP-2. AP-2 can recruit cargo to coated pits by means of direct or indirect interaction with the cargo (Traub, 2009), and AP-2 also has a general function in the formation of coated pits and vesicles (McMahon and Mills, 2004). Therefore, it is expected that depletion of AP-2 would differentially affect endocytosis of a cargo that binds AP-2 directly (such as wtEGFR) and that does not bind AP-2 directly (such as wtΔAP2 mutant). Indeed, AP-2 depletion reduced the internalization rate of both wtEGFR and wtΔAP2 (Fig. 3 A). However, the effect of µ2 siRNA on wtΔAP2 receptor internalization was less (50% decrease) than on wtEGFR internalization (75% decrease), whereas AP-2 was similarly depleted in both cell lines (Fig. 3 B). This result is consistent with a model in which AP-2 has a smaller contribution to internalization of the wtΔAP2 mutant as compared with wtEGFR because the former mutant does not directly bind AP-2. Furthermore, AP-2 knockdown decreased the internalization rate of the 21KR mutant (Fig. 3 A), which confirmed that AP-2 interactions play an important role in endocytosis of the 21KR mutant.
Figure 3.
siRNA depletion of AP-2 decreases internalization of 125I-EGF to a various degree in cells expressing wtEGFR, WTΔAP2, and 21KR receptors. (A) Internalization rate constants (ke) were measured using 1 ng/ml 125I-EGF in wtEGFR, WTΔAP2, and 21KR-expressing cells that were transfected with nontargeting siRNA (NT) or siRNA targeting µ2 subunit of AP-2. Bars represent SEM from four experiments. (B) Lysates of cells used in internalization experiments described in A were probed by Western blotting (WB) with Ab32 to β-adaptin and actin antibody (loading control). All images are from the same Western blot. The efficiency of siRNA depletion (KD%) was calculated as a percentage of the intensity of the β-adaptin signal in depleted cells to that intensity in nontargeting siRNA–transfected cells (both signals were first normalized to the loading control). Black lines indicate that intervening lanes have been spliced out.
Internalization-defective EGFR mutants are poorly recruited into clathrin-coated pits
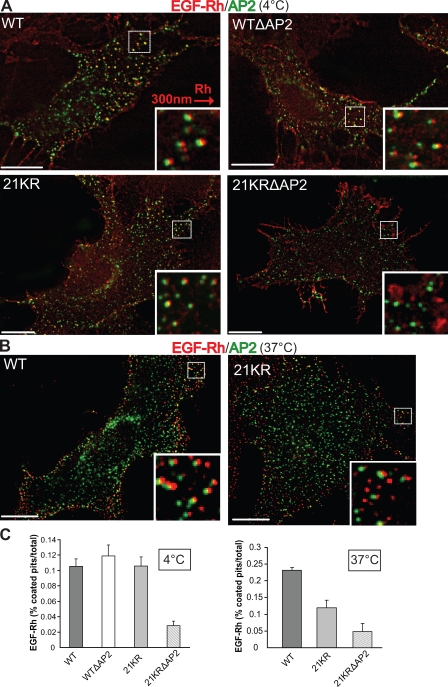
To determine whether slow internalization of 21KR and 21KRΔAP2 mutants was caused by inefficient recruitment into clathrin-coated pit, an equilibrium-coated pit localization assay was performed as previously described (Sorkina et al., 2002; Jiang et al., 2003). Cells stably expressing similar levels of wtEGFR, wtΔAP2, 21KR, or 21KRΔAP2 were transiently transfected with β2-YFP to mark plasma membrane clathrin-coated pits and vesicles. The cells were incubated with 4 ng/ml EGF-rhodamine (EGF-Rh) at 4°C for 45 min to achieve an equilibrium so that EGF-Rh binding to surface receptors and interaction of EGF-Rh–EGFR complexes with coated pit proteins could take place in the absence of endocytosis. Three-dimensional deconvolution image analysis revealed that EGF-Rh dots were often colocalized with β2-YFP dots (coated pits) in cells expressing either wtEGFR or wtΔAP2 mutant, and the extent of EGF-Rh colocalization with coated pits was 10–11% (Fig. 4, A and C). Surprisingly, 21KR mutant was also efficiently concentrated in coated pits despite its slow endocytosis (Fig. 4 A). In contrast, 21KRΔAP2 mutant was diffusely distributed in the plasma membrane and accumulated in membrane ruffles with few examples of colocalization between EGF-Rh dots and β2-YFP (Fig. 4 A). The extent of colocalization of rhodamine and YFP dots in these cells was as low as 3% (Fig. 4 C).
Figure 4.
Recruitment of EGF-Rh into clathrin-coated pits is impaired in cells expressing 21KR and 21KRΔAP2 mutants. (A) PAE cells expressing wtEGFR, WTΔAP2, 21KR, and 21KRΔAP2 were transiently transfected with pcDNA3.1–β2-YFP. After 2 d, the cells were incubated with 4 ng/ml EGF-Rh for 45 min at 4°C and fixed. A z stack of optical sections was acquired through CY3 (EGF-Rh) and FITC (β2-YFP) filter channels and deconvoluted. 21KRΔAP2 was mostly localized to the ruffles at cell periphery. (B) PAE cells expressing wtEGFR and 21KR were transiently transfected with pcDNA3.1–β2-YFP. After 2 d, the cells were incubated with 4 ng/ml EGF-Rh for 1 min at 37°C and fixed. A z stack of optical sections was acquired through CY3 (EGF-Rh) and FITC (β2-YFP) filter channels and deconvoluted. (A and B) Insets show enlarged views of boxed areas with CY3 filter channel shifted ∼300 nm to the right to better demonstrate the localization of EGF-Rh in coated pits. Bars, 10 µm. (C) At least 10 images obtained for each cell clone as described in A and B were used to quantitate the percentage of rhodamine fluorescence colocalized with the punctate β2-YFP fluorescence dots (coated pits). Error bars indicate SEM.
The discrepancy between slow endocytosis and normal recruitment of 21KR to clathrin-coated pits at 4°C could be a result of the long incubation of cells with EGF-Rh (45 min) at 4°C, allowing the process of receptor interaction with coated pits to reach the equilibrium. Under such conditions, moderate differences in the binding affinity of wtEGFR and 21KR to coated pits may not be revealed. For instance, internalization assay that included long preincubation of cells with EGF at 4°C did not reveal the effect of AP-2 siRNA depletion on EGF internalization, whereas the assay using a short time course at 37°C did demonstrate a role of AP-2 (Motley et al., 2003; Huang et al., 2004; Johannessen et al., 2006; Rappoport and Simon, 2009). Therefore, the efficiency of wtEGFR and 21KR recruitment into coated pits was compared by incubating cells with EGF-Rh for a short time (1 min) at 37°C. Based on the time course of binding and internalization of 125I-EGF, there is a substantial EGF binding to surface receptors at the 1-min time point without significant internalization. In contrast to experiments at 4°C, the extent of EGF-Rh localization in coated pits was two times lower in cells expressing 21KR mutant than wtEGFR, although EGF-Rh–occupied receptors were mainly clustered. (Fig. 4, B and C). Similar to experiments performed at 4°C, localization of the 21KRΔAP2 mutant in coated pits at 37°C was minimal (3.5%; Fig. 4 C). Collectively, these data suggest that the 21KR mutant is impaired in the ability to interact with coated pit proteins.
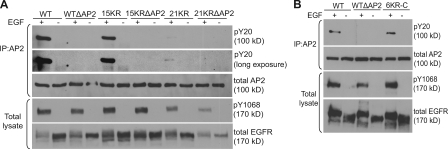
Considering the hypothesis of the redundant role of EGFR Lys residues and AP-2–binding motifs, it is unclear why the 21KR mutant, which contains AP-2–binding motifs, is internalization impaired. One possibility could be that Lys residues and their modifications regulate receptor interactions with AP-2. We have previously shown that during EGFR endocytosis, Tyr6 in the β2 subunit of AP-2 (β2-adaptin) is phosphorylated by activated EGFR and that this phosphorylation is strongly dependent on the LL1010/1011 motif of the EGFR (Huang et al., 2003). The interaction of the LL1010/1011 motif with AP-2 is likely of low affinity, as it could not be detected by coimmunoprecipitation, and the phosphorylation of AP-2 can be considered as an indirect measure of the interaction. Therefore, the ability of EGFR mutants to phosphorylate β2-adaptin was tested by immunoprecipitating AP-2 from EGF-stimulated cells and probing the immunoprecipitate with phosphotyrosine antibodies. As shown in Fig. 5 A, wtEGFR and 15KR mutant phosphorylated AP-2 to a similar extent, which indicates a similar extent of receptor interaction with AP-2. wtΔAP2 did not phosphorylate β2 despite normal recruitment to coated pits and endocytosis, supporting the hypothesis that this mutant utilizes AP-2–independent mechanisms of internalization (Fig. 5, A and B). Interestingly, β2-adaptin was only negligibly phosphorylated by the 21KR mutant (Fig. 5 A), which suggests that this mutant very weakly and/or only transiently interacts with AP-2. The weak β2 phosphorylation by the 21KR mutant was not caused by the direct effect of C-terminal Lys mutations because the 6KR-C mutant displayed normal capacity to phosphorylate AP-2 (Fig. 5 B). In summary, the decreased recruitment into coated pits at 37°C and slow internalization of the 21KR mutant correlated with its inability to phosphorylate β2-adaptin. These data support the hypothesis that EGFR Lys and possibly posttranslational modifications of these residues regulate the interaction of the receptor with AP-2.
Figure 5.
EGF-induced tyrosine phosphorylation of β2-adaptin is impaired in cells expressing 21KR and various AP-2–binding motif EGFR mutants. PAE cells expressing wtEGFR and various mutants were untreated or treated with 100 ng/ml EGF for 5 min, lysed, and AP-2 was immunoprecipitated (IP) with AP.6 antibody. Immunoprecipitates were blotted with phosphotyrosine antibody (pY20) and antibody to α-adaptin (AC.1). Overexposed blot demonstrates the presence of the weak pY20 signal in AP-2 immunoprecipitates from 21KR-expressing cells. Aliquots of lysates (bottom) were blotted with antibodies to phospho-Tyr1068 of EGFR and total EGFR (1005). Two representative experiments (A and B) are shown.
Three distal Lys in the C terminus are critical for EGFR internalization and are acetylated
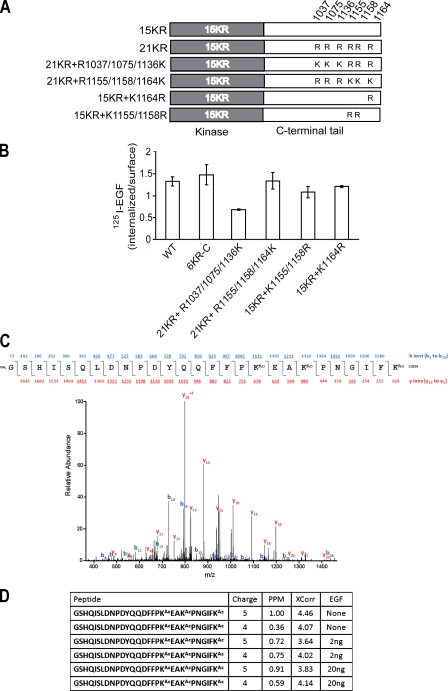
To further elucidate the mechanism by which C-terminal Lys regulate EGFR internalization, we tested which of these six Lys are responsible for the slow internalization of the 21KR mutant. Systematic arginine to Lys add-back mutations in the C-terminal tail of 21KR were made, and several monoclonal lines in PAE cells were generated (Fig. 6 A). Restoration of three kinase-proximal Lys (1037, 1075, and 1136) did not significantly rescue the internalization rate, whereas add back of three distal Lys (1155, 1158, and 1164) rescued EGFR internalization to the level of internalization observed for wtEGFR and 6KR-C mutant (Fig. 6 B). These data suggest that Lys1155, Lys1158, and Lys1164 are involved in the regulation of EGFR internalization. Individual and double mutations of these Lys did not significantly affect internalization of the 15KR mutant, indicating that the role of these three Lys is redundant (Fig. 6 B).
Figure 6.
Three distal Lys in the C terminus of EGFR (K1155, K1158, and K1164) are important for internalization and are acetylated. (A) Schematic representation of 21KR + R1037/1075/1136K, 21KR + R1155/1158/1164K, 15KR + K1164R, and 15KR + K1155/1158R EGFR mutants. (B) Internalization rates of 1 ng/ml 125I-EGF are compared in cells expressing wtEGFR, 6KR-C, and mutants depicted in A. Error bars indicate mean values of ke (SEM) obtained in two individual single-cell clones for each mutant. (C) MS analysis of wtEGFR immunoprecipitates from PAE cells treated and untreated with EGF. Representative MS/MS spectrum of EGFR with acetylation (Ac) at Lys 1155, 1158, and 1164 and the sequence of the peptide. The precursor peptide ion (m/z 837.4064) was isolated and fragmented in a mass spectrometer. Fragment ions included both the N and C termini (b and y ions, respectively) in which the detected ions are underlined. (D) The ion charge state, mass deviation, Sequest score (XCorr), and experimental condition are shown for the peptides containing Lys1155, 1158, and 1164 that were identified. In addition, a tryptic peptide containing unmodified Lys1155 was identified multiple times (not depicted).
MS analysis of wtEGFR-expressing PAE and other types of cells untreated or treated with EGF did not reveal ubiquitin conjugation of C-terminal Lys. Surprisingly, a peptide encompassing Lys1155, Lys1158, and Lys1164, in which all three Lys residues were acetylated, was identified several times (Fig. 6 C). Acetylation of these Lys was observed in wtEGFR/PAE cells independently of EGF treatment (Fig. 6 D). An identical acetylated peptide was detected in human head and neck squamous carcinoma cells expressing high levels of endogenous EGFR, although in these cells, the acetylation was EGF dependent (unpublished data). Thus, it is possible that acetylation of the distal C-terminal Lys regulates EGFR internalization.
Residual internalization of the 21KRΔAP2 mutant is clathrin and Grb2 dependent
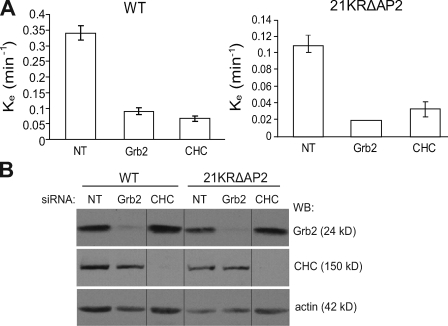
To obtain insights into the mechanisms of the residual internalization of the 21KRΔAP2 mutant (∼20–30% wtEGFR), the effects of several siRNAs on endocytosis of this mutant were analyzed. Knockdown of clathrin heavy chain (CHC) substantially inhibited internalization of 21KRΔAP2 (ke = 0.05 min−1; Fig. 7). Similarly, siRNA knockdown of the Grb2 adaptor, known to be important for ubiquitination and endocytosis of EGFR, strongly inhibited 21KRΔAP2 internalization (Fig. 7) despite a complete lack of detectable ubiquitination of this mutant (Fig. S2). siRNAs targeting several other proteins specifically implicated in EGFR endocytosis or capable of Grb2 binding, such as phospholipase D (Lee et al., 2006), CIN85 (Soubeyran et al., 2002), Tom1L1 (Puertollano, 2005), and intersectin (Martin et al., 2006), did not affect internalization of the 21KRΔAP2 mutant and wtEGFR (unpublished data). These data suggest that there is at least an additional (fourth) mechanism underlying the CME of EGFR that requires Grb2.
Figure 7.
siRNA depletion of Grb2 and CHC decreases internalization of 125I-EGF in cells expressing wtEGFR and 21KRΔAP2 mutant. (A) Internalization rate constants (ke) were measured using 1 ng/ml 125I-EGF in wtEGFR and 21KRΔAP2-expressing cells that were transfected with nontargeting siRNA (NT) or siRNA targeting Grb2 or CHC. Error bars indicate SEM from three experiments. (B) Lysates of cells used in internalization experiments described in A were probed by Western blotting with antibodies to CHC, Grb2, and actin (loading control). All images are from the same Western blot (WB). Black lines indicate that intervening lanes have been spliced out.
Altered dynamics of AKT activation by the internalization-defective 21KRΔAP2 mutant
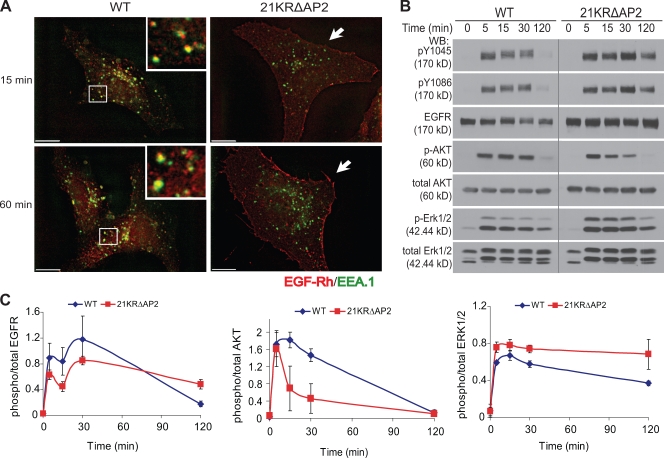
Although 21KRΔAP2 mutant could still be internalized at a slow rate, the internalization rate of this mutant approximates the rate of constitutive endocytosis. Therefore, it is expected that EGF would not cause significant accumulation of the 21KRΔAP2 mutant in endosomes. Analysis of the localization of this mutant stimulated with EGF-Rh at 37°C demonstrated that the appearance of the mutant receptor in endosomes containing EEA.1 (early endosomal antigen 1) was dramatically delayed as compared with cells expressing wtEGFR (Fig. 8 A). 21KRΔAP2 did not significantly colocalize with EEA.1 for up to 1 h after EGF stimulation. This result confirmed that in cells expressing 21KRΔAP2 receptor, most of the EGF-activated receptors remained at the cell surface, whereas in cells expressing wtEGFR, there was a large pool of EGF-occupied receptors in endosomes. Because nonubiquitinated EGFR mutants are not efficiently sorted to late endosomes and remain in early/recycling endosomes (Huang et al., 2006), enhanced recycling of the 21KRΔAP2 mutant may amplify the effect of its slow internalization, thus resulting in its predominant cell surface localization.
Figure 8.
Poor accumulation of 21KRΔAP2 mutant in endosomes and altered kinetics of ERK1/2 and AKT activation by EGF in cells expressing the internalization-defective mutant. (A) PAE cells expressing wtEGFR and 21KRΔAP2 were incubated with 5 ng/ml EGF-Rh for 15 min or 1 h. After fixation, the cells were permeabilized and stained with antibody to EEA.1. A z stack of optical sections was acquired through CY3 (EGF-Rh) and FITC (EEA.1) filter channels and deconvoluted. Insets show enlarged views of the boxed areas, showing localization of wtEGFR in EEA.1-containing early endosomes. 21KRΔAP2 mutant was accumulated in the membrane ruffles (arrows). Bars, 10 µm. (B) Serum-starved cells were treated with 5 ng/ml EGF for 0–120 min at 37°C and lysed. The lysates were probed for active EGFR (pY1045 and pY1086), total EGFR (1005), phosphorylated AKT (p-AKT), total AKT, phosphorylated ERK1/2 (p-ERK1/2), and total ERK1/2. The experiment is representative of three independent experiments. Gray line indicates that intervening lanes have been spliced out. (C) Quantification of experiments presented in B. Bars represent SEM of the amounts of activated EGFR, AKT, and ERK1/2 normalized to total amounts of EGFR, AKT, and ERK1/2, respectively, and plotted against time. The data are averaged from three experiments. WB, Western blot.
To assess whether activation of the internalization-defective 21KRΔAP2 mutant results in altered signaling outcome, cells expressing wtEGFR or 21KRΔAP2 mutant were incubated with 5 ng/ml EGF at 37°C. Western blot analysis of phospho-EGFR revealed prolonged phosphorylation of the 21KRΔAP2 mutant as compared with wtEGFR (Fig. 8 B). Both wtEGFR and the 21KRΔAP2 mutant initially activated protein kinase B/AKT to the same extent, suggesting that the mutant receptor is capable of activating upstream components of the AKT signaling cascade. However, although AKT phosphorylation was sustained in wtEGFR-expressing PAE cells, the amount of phosphorylated AKT rapidly decreased to ∼50% by the 15-min time point in cells expressing the 21KRΔAP2 mutant (Fig. 8, B and C). These data suggest that internalization is necessary for normal kinetics of AKT activation by EGFR. In contrast, phosphorylation of MAPK/extracellular stimulus–regulated kinase 1/2 (ERK1/2) in cells expressing 21KRΔAP2 mutant was relatively long lasting when compared with wtEGFR-expressing cells (Fig. 8, B and C), suggesting that EGFR endocytosis primarily down-regulates ERK1/2 signaling. Altogether, these data imply that EGFR endocytosis can have both positive and negative effects on its downstream signaling molecules.
Discussion
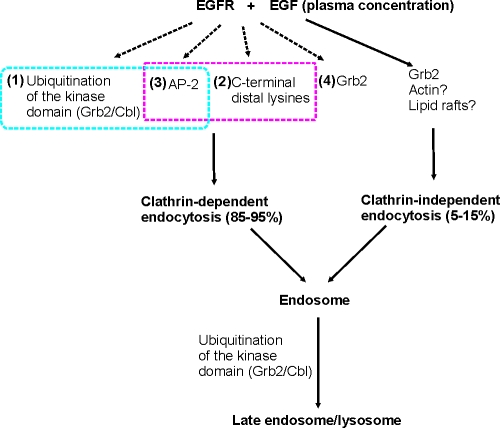
Since the first comprehensive analysis of EGF endocytosis (Carpenter and Cohen, 1976) and cloning of EGFR (Downward et al., 1984), >1,000 publications have addressed the mechanisms of EGFR endocytosis and the role of endocytosis in EGFR signaling. This study provides an explanation as to why previous studies produced many nonreconcilable models of these processes by demonstrating the extreme complexity of the clathrin-dependent internalization of EGFR, which appears to be mediated by several redundant and interdependent mechanisms. A hypothetic model of the EGFR CME is described in Fig. 9.
Figure 9.
Working model of EGFR internalization and lysosomal targeting. Grb2/Cbl-dependent ubiquitination of the kinase domain (mechanism #1) and C-terminal Lys (mechanism #2) functionally interact with AP-2 binding of the receptor (mechanism #3) in contributing to 70–80% of EGFR internalization. The CME (mechanism #4) involves other unknown Grb2-related mechanisms. Clathrin-independent mechanisms of EGFR internalization constitute ∼5–15% of the overall uptake of EGF–EGFR complexes into the cell. These mechanisms may involve Grb2, actin-dependent membrane ruffling, and cholesterol-rich membrane microdomains (rafts).
Ubiquitination of Lys within the kinase domain is one of the mechanisms that can promote EGFR internalization. Inhibition of ubiquitination by mutating ubiquitin conjugation sites or Cbl-binding site (Tyr1045) in the EGFR did not affect internalization, thus indicating that ubiquitination is not essential for receptor internalization (Waterman et al., 2002; Huang et al., 2007). Similarly, normal endocytosis of the ubiquitination-deficient mutant of the fibroblast growth factor receptor, another receptor tyrosine kinase, has also been demonstrated previously (Haugsten et al., 2008). However, add back of two major ubiquitin conjugation sites restored the internalization of the ubiquitination and kinase activity–deficient EGFR mutant, providing the first indication that ubiquitination of the EGFR kinase domain is capable of promoting receptor internalization under specific conditions (Huang et al., 2007). In this study, the involvement of ubiquitination of the kinase domain was revealed when Lys in the kinase domains and C terminus were simultaneously mutated (Figs. 1 and 2). In this case, the internalization-defective EGFR mutant maintained normal tyrosine phosphorylation and was capable of phosphorylating exogenous substrates (Figs. S2 and S3).
The precise role of the three distal C-terminal Lys, which appear to be mainly responsible for the observed slow internalization of 21KR receptor, is unknown. The simplest explanation is that these Lys are ubiquitinated in the absence of major conjugation sites in the kinase domain; however, such ubiquitination is neither detected by MS nor by Western blotting. MS analysis instead revealed a novel posttranslational modification of EGFR by acetylation (Fig. 6 C). Strikingly, Lys1155, Lys1158, and Lys1164 that are important for EGFR internalization (Fig. 6) were the only acetylated Lys in the EGFR that were identified in our analysis. It is common that acetylation occurs at clusters of Lys residues that form positive patches, as it occurs in the distal C terminus of EGFR. Lys acetylation has been shown to regulate protein function in coordination with phosphorylation and ubiquitination (Yang and Seto, 2008). Thus, Lys acetylation may have either a direct, positive effect, or an opposite, inhibitory function on EGFR endocytosis, for instance, by competing with ubiquitination of these Lys. Unfortunately, all available acetyltransferase inhibitors were highly toxic to PAE cells under various experimental conditions, precluding the analysis of the effects these inhibitors on the endocytosis of EGFR mutants (unpublished data). Finally, it is also possible that C-terminal Lys are a part of an internalization motif. For instance, the QQDFF sequence was shown to be capable of mediating CME (Chang et al., 1993). Such a sequence (residues 1149–1153) is present in the proximity to the three distal Lys of EGFR and can potentially regulate endocytosis in conjunction with these Lys.
The role of yet another internalization mechanism that involves EGFR binding to AP-2 was revealed when AP-2–binding motifs of EGFR (Y974RAL and LL1010/1011) were mutated in combination with the mutation of Lys in the kinase domain and C terminus. Although the YRAL motif is critical for the receptor interaction with AP-2 in cell lysates and in vitro, it is unlikely that this motif is involved in such interaction in the intact cell. A recent study suggested that the YRAL sequence is part of an α-helix and is not present in an extended conformation necessary for binding to the µ2 subunit of AP-2 (Jura et al., 2009). In fact, denaturing conditions and ionic detergents resulted in an increased interaction of the EGFR YRAL motif with AP-2 (Nesterov et al., 1995; Sorkin et al., 1996).
Furthermore, when YRAL motif was mutated, coimmunoprecipitation of AP-2 with EGFR was not observed (Sorkin et al., 1996). This suggests that the interaction between the LL motif of EGFR and AP-2 is very weak and transient. In fact, the LL motif in EGFR lacks a negatively charged residue in the −4 position, which is frequently present in the LL-based motifs of other proteins. However, recent structure of AP-2 complexed with the LL peptide suggested that such negative charge is not an absolute requirement (Kelly et al., 2008). Importantly, this study also predicted that phosphorylation of Tyr6 in β2-adaptin would shift the AP-2 complex to an active, open conformation capable of efficient interaction with the LL motif. Poor phosphorylation of β2-adaptin by the 21KR mutant (Fig. 5) can be the consequence of weak binding of this mutant to AP-2 and, therefore, short residence time in coated pits or vice versa, which is a basis for the insufficient LL–AP-2 interaction and coated pit recruitment of the mutant receptor. It is possible that ubiquitination of Lys in the kinase domain and/or posttranslational modifications of C-terminal Lys may cause conformation changes in the EGFR molecule, leading to better exposure of AP-2–binding motifs, as was suggested for the interferon receptor (Kumar et al., 2007). Furthermore, our model also implies that coated pit accumulation of receptors lacking AP-2 binding, such as the wtΔAP2 mutant, can be achieved by alternative, e.g., ubiquitin-based, mechanisms.
Finally, the residual internalization of the 21KRΔAP2 mutant is controlled by yet another mechanism. siRNA analysis demonstrated that internalization of 21KRΔAP2 is partially clathrin dependent and also requires Grb2. It should be emphasized that Grb2 plays a role in all ubiquitin-dependent mechanisms of EGFR endocytosis by delivering Cbl to EGFR (for review see Sorkin and Goh, 2009). In the case of the 21KRΔAP2 receptor, it is likely that Grb2 links the receptor to coated pits through ubiquitin-independent mechanisms and unknown adaptors. In addition to its role in CME, Grb2 has been shown to be important for clathrin-independent endocytosis of EGFR (Yamazaki et al., 2002). Therefore, Grb2 knockdown has the major inhibitory effect on the total uptake of EGF by wtEGFR and 21KRΔAP2 mutant (Fig. 7). Lastly, early studies in mouse fibroblasts demonstrated that EGFR mutants with deletions of the C terminus at the position 1022 (eliminating Grb2- and Cbl-binding sites) were rapidly internalized in a kinase-dependent manner (Chen et al., 1989), although the same mutants were internalized very slowly in PAE cells (Jiang et al., 2003). These data suggest that in some cells, there might be yet another mechanism of EGFR internalization that requires kinase activity but does not require Grb2 binding, receptor ubiquitination, and a large portion of the C terminus. Finally, another level of complexity is added by the recent report of the clathrin-mediated but kinase-independent internalization of EGFR (Frosi et al., 2010). In this case, internalization is mediated by the protein called receptor-associated signal transducer (RALT or MIG6) that is capable of binding to the dimerized EGFR, inhibiting its kinase activity and linking the receptor to coated pits through the interaction with AP-2 and intersectins.
In summary, our experiments reveal several redundant and, in some cases, interdependent mechanisms of EGFR internalization involving AP-2, Grb2, ubiquitination, and possibly Lys acetylation. The sheer complexity of EGFR internalization may help to explain discrepancies in the literature regarding the role of AP-2 and ubiquitin adaptors like epsin in EGFR internalization. It is possible that the relative contribution of one of the internalization mechanisms may vary in different cell types and depend on experimental conditions. Importantly, the existence of multiple internalization mechanisms does not necessarily imply that all mechanisms are simultaneously used. Instead, one mechanism may be predominantly used under physiological conditions, whereas other mechanisms are used only when the main mechanism is inhibited experimentally or under pathological conditions. It is interesting that two rate-limiting steps of EGFR down-regulation exhibit drastically different robustness (Fig. 9). Unlike the internalization step, the endosomal sorting to the degradation pathway is highly dependent on EGFR ubiquitination and, therefore, mainly controlled by a single, ubiquitin-based mechanism (Levkowitz et al., 1999; Huang et al., 2006).
The first internalization-defective EGFR mutant with normal tyrosine phosphorylation generated in this study (21KRΔAP2) is a novel and specific tool to investigate the role of EGFR endocytosis in signaling. As was observed with the EGFR mutants defective in lysosomal targeting (Levkowitz et al., 1999; Huang et al., 2006), degradation of activated 21KRΔAP2 was substantially delayed as compared with wtEGFR (Fig. 8). However, in contrast to degradation-defective mutants, 21KRΔAP2 mutant did not fully maintain AKT activity, suggesting that continuous activity of AKT requires EGFR internalization (Fig. 8). This finding contradicts the general assumption in the literature that the AKT activation pathway is mostly initiated at the plasma membrane, where phosphatidylinositol-(3,4,5)-phosphate is generated, whereas the EGFR-ERK1/2 signaling can also be triggered from endosomes (Haugh and Meyer, 2002; Sadowski et al., 2009; for reviews see Wiley, 2003; Fehrenbacher et al., 2009; Sorkin and von Zastrow, 2009). It is possible that endosomal EGFR is required for activation of a pool of AKT associated with Appl1/2 (adaptor protein, phosphotyrosine interaction, PH domain, and Leu zipper containing–1), an endosomal protein that recruits AKT and facilitates phosphorylation of its downstream substrate, glycogen synthase kinase-3β in Rab5-containing endosomes (Schenck et al., 2008). Recently, another endosomal adaptor protein, WDFY2, was found to be necessary for maintaining insulin-stimulated AKT2 phosphorylation (Walz et al., 2010). Finally, another possible explanation is that accumulation of internalization-defective EGFR at the cell surface triggers a negative feedback loop by up-regulating an AKT phosphatase, which results in down-regulation of active AKT.
The role of endocytosis in ERK1/2 activation by EGF has been previously demonstrated using general inhibitors of CME (Vieira et al., 1996), although other studies using the same inhibitors produced conflicting results with regard to the role of endocytosis in ERK activity (for reviews see Wiley, 2003; Fehrenbacher et al., 2009; Sorkin and von Zastrow, 2009). In our experiments, ERK1/2 activation was not inhibited and even slightly prolonged in cells expressing the 21KRΔAP2 receptor (Fig. 8). Thus, endocytosis of activated EGFR may primarily serve to down-regulate rather than enhance EGFR-ERK1/2 signaling. In summary, our experiments have taken the experimental approaches to specifically inhibit EGFR endocytosis and to generate valuable tools for comprehensive analysis of the role of EGFR endocytosis in signaling.
Materials and methods
Antibodies
Mouse monoclonal antibody to ubiquitin (P4D1), rabbit polyclonal antibodies to Grb2, Cbl (C-15), Shc (H-108), and EGFR (1005) were purchased from Santa Cruz Biotechnology, Inc. The monoclonal antibodies to EGFR (Ab528), α-subunit of AP-2 (AP.6), and CHC (TD.1) were obtained from American Type Culture Collection (ATCC). Monoclonal antibodies specific to phosphotyrosine (pY20), c-Cbl, Shc, and EEA.1 were obtained from BD. Monoclonal antibodies to phospho-EGFR (pY1068), phospho-AKT (Ser473), phospho-ERK1/2 (Thr202/Tyr204), and polyclonal antibodies to phospho-EGFR (pY1045), total AKT, and total ERK1/2 were obtained from Cell Signaling Technology. Rabbit polyclonal antibody to phospho-EGFR (pY1086) and monoclonal antibody to α-adaptin (AC.1) were obtained from Thermo Fisher Scientific. Polyclonal antibody to actin was obtained from Sigma-Aldrich. Rabbit polyclonal antibody specific to β-adaptins (Ab32) was described previously (Sorkin et al., 1995).
Plasmid constructs and point mutations
To generate EGFR mutant 21KR, six Lys residues in the C-terminal tail of 15KR (Huang et al., 2007) were mutated to arginines. To generate EGFR mutants 15KRΔAP2 and 21KRΔAP2, Tyr974, Leu1010, and Leu1011 were mutated to alanine on the template of 15KR and 21KR, respectively. All mutations were performed using multisite-directed mutagenesis kits according to the manufacturer’s protocols (QuickChange; Agilent Technologies). All point mutations were verified by automated dideoxynucleotide sequencing (Sequencing Core Facility, University of Colorado Denver, Aurora, CO). β2-YFP construct was made in pcDNA3.1 as previously described (Huang et al., 2003).
Cell culture and DNA transfection
PAE cells were grown in F12 medium containing 10% FBS. Transfections of DNA constructs were performed using Effectene (QIAGEN) according to the manufacturer’s protocols. PAE cells stably expressing various EGFR mutants were selected with 400 ng/ml geneticin (G418), and single-cell clones were established. Stable clones were maintained in the presence of 400 ng/ml G418. HEK293 cells were grown in DME containing 10% FBS. Transient transfection was performed using Effectene. Human head and neck squamous cell carcinoma SCC2 and intestinal adenocarcinoma HuTu 80 were grown in DME containing 5% FBS.
siRNA transfections
Synthetic siRNAs for CHC (duplex 2; Huang et al., 2004), Grb2 (duplex 3; Jiang et al., 2003), AP-2 µ2 subunit (Motley et al., 2003), ON-TARGET Cbl-interacting protein 85 (CIN 85; SMARTpool), siGENOME intersectin 1 (SMARTpool), phospholipase D1 (5′-GGUGGGACGACAAUGAGCA-3′), siGENOME TOM1L1 (SMARTpool), and siGENOME nontargeting siRNA #3 were purchased from Thermo Fisher Scientific. Duplexes were resuspended in 1× siRNA universal buffer (Thermo Fisher Scientific) to 20 µM. PAE cell lines expressing various EGFR mutants were grown in six-well plates to 50% confluence. Cells were transfected with siRNA duplexes, either singly or in combination, to the final concentration of 120 nM in 5 µl DharmaFECT1 reagent (Thermo Fisher Scientific). After 36 h, a second transfection was performed, and the cells were replated into 12-well plates on the next day for internalization experiments. To access the efficiency of knockdown, total cell lysates were resolved on 7.5% or 10% SDS-PAGE depending on the protein of interest and probed by Western blotting.
Immunoprecipitation and Western blotting
To examine the extent of ubiquitination and tyrosine phosphorylation, PAE cells stably expressing wtEGFR or EGFR mutants were grown in 60-mm dishes for 2 d and stimulated with 20 ng/ml EGF in binding medium (0.1% bovine serum albumin in F12) for 5 min at 37°C. The cells were washed twice with Ca2+Mg2+-free (CMF) PBS and solubilized by scraping with a cell lifter in TGH lysis buffer (50 mM Hepes, pH 7.3, 10% glycerol, 1% Triton X-100, 50 mM NaCl, 1 mM dithiothreitol, 1 mM EGTA, 5 mM EDTA, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 10 mM N-ethylmaleimide, and 1 mM sodium orthovanadate; Huang et al., 2004). The lysates were centrifuged for 10 min at 14,000 g. EGFR was immunoprecipitated with monoclonal antibody 528 (ATCC) for 3 h at 4°C followed by protein A–Sepharose beads (Sigma-Aldrich) for 1 h at 4°C. The precipitates were washed twice with TGH lysis buffer supplemented with 100 mM NaCl and once without NaCl. Samples were resuspended in sample buffer and heat denaturated.
For immunoprecipitation of AP-2, c-Cbl, or Shc, PAE cells expressing wt and mutant EGFR were stimulated with 100 ng/ml (for AP-2) or 20 ng/ml (for c-Cbl and Shc) EGF for 5 min at 37°C, and the cells were lysed as described previously (Huang et al., 2004). AP-2, c-Cbl, or Shc was immunoprecipitated using AP6, c-Cbl (C-15), or Shc antibodies (BD), respectively, for 3 h at 4°C followed by protein A–Sepharose beads (Sigma-Aldrich) or protein G–Sepharose beads (Invitrogen) for 1 h at 4°C. The samples were washed and heat denatured as described in the previous paragraph. To examine EGFR signaling, the cells grown in 35-mm dishes were serum starved overnight, stimulated with 5 ng/ml EGF, and lysed in TGH lysis buffer without NaCl.
The immunoprecipitates and cell lysates were resolved on 7.5% SDS-PAGE. Proteins were transferred to the nitrocellulose membrane and probed by Western blotting with various antibodies followed by species-specific secondary antibodies conjugated with horseradish peroxidase (The Jackson Laboratory). Detection of signal was performed with an enhanced chemiluminescence kit (Thermo Fisher Scientific). To determine the linear range of chemiluminescence signal, several x-ray films (Thermo Fisher Scientific) were analyzed, and the densitometric quantifications were performed using ImageJ software (National Institutes of Health).
MS
PAE cells stably expressing wtEGFR were grown in 150-mm dishes. Cells were untreated or treated with 2 or 20 ng/ml EGF for 5 min at 37°C, washed with CMF-PBS, and lysed in TGH containing 1% sodium deoxycholate. Lysate was cleared by centrifugation at 14,000 g for 10 min. EGFR was immunoprecipitated with monoclonal antibody 528 (ATCC). The precipitates were washed three times with TGH containing, sequentially, 500 mM, 100 mM, and no NaCl, and resolved by SDS-PAGE (Huang et al., 2006). Acetylation sites on hEGFR were identified by liquid chromatography MS/MS techniques described previously (Haas et al., 2006). In brief, hEGFR was digested in gel with trypsin. The peptides were separated by nanoscale reversed chromatography coupled to a mass spectrometer (LTQ-Orbitrap; Thermo Fisher Scientific). MS/MS data were collected on a data-dependent method described previously (Haas et al., 2006). Mass increment of 42.0106 (acetylation) was allowed on Lys residues for MS/MS spectra matched to hEGFR using Sequest algorithm (Eng et al., 1994).
Internalization of 125I-EGF
Mouse receptor-grade EGF (Collaborative Research, Inc.) was iodinated as previously described (Nesterov et al., 1999). In most experiments, the time course of 125I-EGF internalization was obtained using 1 ng/ml 125I-EGF, and the specific rate constant for internalization ke was calculated as the linear regression coefficient of the dependence of the ratio of internalized/surface 125I-EGF versus time as previously described (Nesterov et al., 1999). This low concentration of 125I-EGF was used to avoid saturation of the clathrin-mediated internalization pathway. In internalization saturation experiments, the ke values were determined for the range of 125I-EGF concentrations (1–20 ng/ml) and plotted against the amount of surface 125I-EGF–occupied receptors per cell. The latter value was determined at a 5-min time point of the 125I-EGF internalization assay as described previously (Huang et al., 2006).
Fluorescent microscopy
To visualize clathrin-coated pits, β2-YFP was transiently expressed in PAE cells stably expressing wt or EGFR mutants in six-well plates. 24–36 h after transfection, the cells were replated onto 25-mm glass coverslips. The cells were incubated with 4 ng/ml EGF-Rh (Invitrogen) for 45 min at 4°C or for 1 min at 37°C. The cells were washed with ice-cold CMF-PBS and fixed with freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences) for 30 min at 4°C. The coverslips were mounted on glass slides using Mowiol (Invitrogen). Detection of YFP and rhodamine fluorescence was performed as previously described (Sorkina et al., 2002; Jiang et al., 2003). A z stack of 30 images for at least 10 cells per each condition was acquired using an imaging workstation (Marianas; Intelligent Imaging Innovations, Inc.) based on an inverted microscope (300M; Carl Zeiss, Inc.) with a 63× 1.2 NA oil objective and a charge-coupled device camera (CoolSnap HQ2; Photometrics) controlled by Slidebook software (version 4.2; Intelligent Imaging Innovations, Inc.). Images were acquired in 2 × 2 binning mode. The three-dimensional images were deconvoluted using a constrained iterative algorithm (Slidebook). The percentage of total cell-associated EGF-Rh colocalized with YFP dots (coated pits) was calculated using SlideBook Mask Statistic module as described previously (Sorkina et al., 2002; Jiang et al., 2003). Images are presented as obtained in Slidebook without changing γ settings. Images were cropped to the final size using Photoshop (Adobe).
To assess colocalization of EGFR with early endosomal marker, EEA.1, the cells grown on coverslips were treated with 4 ng/ml EGF-Rh at 37°C and fixed with 4% paraformaldehyde for 12 min at room temperature. Fixed cells were mildly permeabilized with 0.02% saponin in CMF-PBS containing 0.1% bovine serum albumin for 30 min at room temperature. Permeabilized cells were incubated with antibodies to EEA.1 followed by secondary donkey anti–mouse IgG labeled with Cy5 (Jackson ImmunoResearch Laboratories, Inc.). The cells were washed and mounted on glass slides using Mowiol and imaged as described previously (Huang et al., 2006).
Online supplemental material
Fig. S1 shows the comparison of 125I-EGF internalization rates between various cell lines expressing endogenous or transfected EGFR. Fig. S2 shows that EGFR mutants retain normal phosphorylation of Tyr1068. Fig. S3 shows that 21KRΔAP2 EGFR mutant induces normal tyrosine phosphorylation of c-Cbl and Shc. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201001008/DC1.
Acknowledgments
We thank Melissa Adams for help in preparation of the manuscript and Graham Carpenter and Kalen Dionne for critical reading of the manuscript.
This work was supported by the National Institutes of Health (grants CA089151 to A. Sorkin, F. Huang, W. Kim, and L.K. Goh, CA112219 to A. Sorkin, and GM67945 to S. Gygi) and Fulbright and American Heart Association fellowships (to L.K. Goh).
Footnotes
Abbreviations used in this paper:
- CHC
- clathrin heavy chain
- CME
- clathrin-mediated endocytosis
- CMF
- Ca2+Mg2+ free
- EGFR
- EGF receptor
- EGF-Rh
- EGF-rhodamine
- ERK
- extracellular stimulus–regulated kinase
- MS
- mass spectrometry
- PAE
- porcine aortic endothelial
- wt
- wild type
References
- Carpenter G., Cohen S. 1976. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J. Cell Biol. 71:159–171 10.1083/jcb.71.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.E., Sorkin A. 1998. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 273:35000–35007 10.1074/jbc.273.52.35000 [DOI] [PubMed] [Google Scholar]
- Chang C.-P., Lazar C.S., Walsh B.J., Komuro M., Collawn J.F., Kuhn L.A., Tainer J.A., Trowbridge I.S., Farquhar M.G., Rosenfeld M.G., et al. 1993. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268:19312–19320 [PubMed] [Google Scholar]
- Chen C., Zhuang X. 2008. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc. Natl. Acad. Sci. USA. 105:11790–11795 10.1073/pnas.0803711105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.S., Lazar C.S., Lund K.A., Welsh J.B., Chang C.P., Walton G.M., Der C.J., Wiley H.S., Gill G.N., Rosenfeld M.G. 1989. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 59:33–43 10.1016/0092-8674(89)90867-2 [DOI] [PubMed] [Google Scholar]
- Chen H., Ko G., Zatti A., Di Giacomo G., Liu L., Raiteri E., Perucco E., Collesi C., Min W., Zeiss C., et al. 2009. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc. Natl. Acad. Sci. USA. 106:13838–13843 10.1073/pnas.0907008106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M.D. 1984. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 307:521–527 10.1038/307521a0 [DOI] [PubMed] [Google Scholar]
- Eng J.K., McCormack A.L., Yates J.R.I. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976–989 10.1016/1044-0305(94)80016-2 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N., Bar-Sagi D., Philips M. 2009. Ras/MAPK signaling from endomembranes. Mol. Oncol. 3:297–307 10.1016/j.molonc.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosi Y., Anastasi S., Ballarò C., Varsano G., Castellani L., Maspero E., Polo S., Alemà S., Segatto O. 2010. A two-tiered mechanism of EGFR inhibition by RALT/MIG6 via kinase suppression and receptor degradation. J. Cell Biol. 189:557–571 10.1083/jcb.201002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Faherty B.K., Gerber S.A., Elias J.E., Beausoleil S.A., Bakalarski C.E., Li X., Villén J., Gygi S.P. 2006. Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol. Cell. Proteomics. 5:1326–1337 10.1074/mcp.M500339-MCP200 [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Meyer T. 2002. Active EGF receptors have limited access to PtdIns(4,5)P(2) in endosomes: implications for phospholipase C and PI 3-kinase signaling. J. Cell Sci. 115:303–310 [DOI] [PubMed] [Google Scholar]
- Haugsten E.M., Malecki J., Bjørklund S.M., Olsnes S., Wesche J. 2008. Ubiquitination of fibroblast growth factor receptor 1 is required for its intracellular sorting but not for its endocytosis. Mol. Biol. Cell. 19:3390–3403 10.1091/mbc.E07-12-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Jiang X., Sorkin A. 2003. Tyrosine phosphorylation of the beta2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J. Biol. Chem. 278:43411–43417 10.1074/jbc.M306072200 [DOI] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279:16657–16661 10.1074/jbc.C400046200 [DOI] [PubMed] [Google Scholar]
- Huang F., Kirkpatrick D., Jiang X., Gygi S., Sorkin A. 2006. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell. 21:737–748 10.1016/j.molcel.2006.02.018 [DOI] [PubMed] [Google Scholar]
- Huang F., Goh L.K., Sorkin A. 2007. EGF receptor ubiquitination is not necessary for its internalization. Proc. Natl. Acad. Sci. USA. 104:16904–16909 10.1073/pnas.0707416104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Huang F., Marusyk A., Sorkin A. 2003. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell. 14:858–870 10.1091/mbc.E02-08-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen L.E., Pedersen N.M., Pedersen K.W., Madshus I.H., Stang E. 2006. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol. Cell. Biol. 26:389–401 10.1128/MCB.26.2.389-401.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura N., Endres N.F., Engel K., Deindl S., Das R., Lamers M.H., Wemmer D.E., Zhang X., Kuriyan J. 2009. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 137:1293–1307 10.1016/j.cell.2009.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazic M., Bertelsen V., Pedersen K.W., Vuong T.T., Grandal M.V., Rødland M.S., Traub L.M., Stang E., Madshus I.H. 2009. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 10:235–245 10.1111/j.1600-0854.2008.00858.x [DOI] [PubMed] [Google Scholar]
- Kelly B.T., McCoy A.J., Spate K., Miller S.E., Evans P.R., Honing S., Owen D.J. 2008. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 456:976–979 10.1038/nature07422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K.G., Barriere H., Carbone C.J., Liu J., Swaminathan G., Xu P., Li Y., Baker D.P., Peng J., Lukacs G.L., Fuchs S.Y. 2007. Site-specific ubiquitination exposes a linear motif to promote interferon-α receptor endocytosis. J. Cell Biol. 179:935–950 10.1083/jcb.200706034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Kim I.S., Park J.B., Lee M.N., Lee H.Y., Suh P.G., Ryu S.H. 2006. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat. Cell Biol. 8:477–484 10.1038/ncb1401 [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Waterman H., Ettenberg S.A., Katz M., Tsygankov A.Y., Alroy I., Lavi S., Iwai K., Reiss Y., Ciechanover A., et al. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 4:1029–1040 10.1016/S1097-2765(00)80231-2 [DOI] [PubMed] [Google Scholar]
- Lund K.A., Opresko L.K., Starbuck C., Walsh B.J., Wiley H.S. 1990. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J. Biol. Chem. 265:15713–15723 [PubMed] [Google Scholar]
- Martin N.P., Mohney R.P., Dunn S., Das M., Scappini E., O’Bryan J.P. 2006. Intersectin regulates epidermal growth factor receptor endocytosis, ubiquitylation, and signaling. Mol. Pharmacol. 70:1643–1653 10.1124/mol.106.028274 [DOI] [PubMed] [Google Scholar]
- McMahon H.T., Mills I.G. 2004. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr. Opin. Cell Biol. 16:379–391 10.1016/j.ceb.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Motley A., Bright N.A., Seaman M.N., Robinson M.S. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909–918 10.1083/jcb.200305145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A., Kurten R.C., Gill G.N. 1995. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J. Biol. Chem. 270:6320–6327 10.1074/jbc.270.11.6320 [DOI] [PubMed] [Google Scholar]
- Nesterov A., Carter R.E., Sorkina T., Gill G.N., Sorkin A. 1999. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 18:2489–2499 10.1093/emboj/18.9.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth J.D., Krueger E.W., Weller S.G., McNiven M.A. 2006. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 66:3603–3610 10.1158/0008-5472.CAN-05-2916 [DOI] [PubMed] [Google Scholar]
- Puertollano R. 2005. Interactions of TOM1L1 with the multivesicular body sorting machinery. J. Biol. Chem. 280:9258–9264 10.1074/jbc.M412481200 [DOI] [PubMed] [Google Scholar]
- Rappoport J.Z., Simon S.M. 2009. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J. Cell Sci. 122:1301–1305 10.1242/jcs.040030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski L., Pilecka I., Miaczynska M. 2009. Signaling from endosomes: location makes a difference. Exp. Cell Res. 315:1601–1609 10.1016/j.yexcr.2008.09.021 [DOI] [PubMed] [Google Scholar]
- Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. 2008. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 133:486–497 10.1016/j.cell.2008.02.044 [DOI] [PubMed] [Google Scholar]
- Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell. 103:211–225 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P.P., Polo S. 2005. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA. 102:2760–2765 10.1073/pnas.0409817102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Carpenter G. 1993. Interaction of activated EGF receptors with coated pit adaptins. Science. 261:612–615 10.1126/science.8342026 [DOI] [PubMed] [Google Scholar]
- Sorkin A., Goh L.K. 2009. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 315:683–696 10.1016/j.yexcr.2008.07.029 [DOI] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. 2009. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10:609–622 10.1038/nrm2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., McKinsey T., Shih W., Kirchhausen T., Carpenter G. 1995. Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associated protein complex AP-2. J. Biol. Chem. 270:619–625 10.1074/jbc.270.2.619 [DOI] [PubMed] [Google Scholar]
- Sorkin A., Mazzotti M., Sorkina T., Scotto L., Beguinot L. 1996. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J. Biol. Chem. 271:13377–13384 10.1074/jbc.271.23.13377 [DOI] [PubMed] [Google Scholar]
- Sorkina T., Huang F., Beguinot L., Sorkin A. 2002. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 277:27433–27441 10.1074/jbc.M201595200 [DOI] [PubMed] [Google Scholar]
- Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W.Y., Dikic I. 2002. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 416:183–187 10.1038/416183a [DOI] [PubMed] [Google Scholar]
- Teis D., Taub N., Kurzbauer R., Hilber D., de Araujo M.E., Erlacher M., Offterdinger M., Villunger A., Geley S., Bohn G., et al. 2006. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 175:861–868 10.1083/jcb.200607025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L.M. 2009. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10:583–596 10.1038/nrm2751 [DOI] [PubMed] [Google Scholar]
- Vanden Broeck D., De Wolf M.J. 2006. Selective blocking of clathrin-mediated endocytosis by RNA interference: epsin as target protein. Biotechniques. 41:475–484 10.2144/000112265 [DOI] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze C., Schmid S.L. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 274:2086–2089 10.1126/science.274.5295.2086 [DOI] [PubMed] [Google Scholar]
- Walz H.A., Shi X., Chouinard M., Bue C.A., Navaroli D.M., Hayakawa A., Zhou Q.L., Nadler J., Leonard D.M., Corvera S. 2010. Isoform-specific regulation of Akt signaling by the endosomal protein WDFY2. J. Biol. Chem. 285:14101–14108 10.1074/jbc.M110.110536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman H., Katz M., Rubin C., Shtiegman K., Lavi S., Elson A., Jovin T., Yarden Y. 2002. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 21:303–313 10.1093/emboj/21.3.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H.S. 2003. Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 284:78–88 10.1016/S0014-4827(03)00002-8 [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Zaal K., Hailey D., Presley J., Lippincott-Schwartz J., Samelson L.E. 2002. Role of Grb2 in EGF-stimulated EGFR internalization. J. Cell Sci. 115:1791–1802 [DOI] [PubMed] [Google Scholar]
- Yang X.J., Seto E. 2008. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol. Cell. 31:449–461 10.1016/j.molcel.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]