Abstract
Objective
To estimate which strategy is the most cost-effective for prevention of preterm birth and associated morbidity.
Study Design
We used decision-analytic and cost-effectiveness analyses to estimate which of 4 strategies was superior based on quality-adjusted life-years (QALYs), cost in US dollars ($), and number of preterm births prevented.
Results
Universal sonographic screening for cervical length and treatment with vaginal progesterone was the most cost-effective strategy and dominant over 3 alternatives: cervical length screening for women at increased risk for preterm birth and treatment with vaginal progesterone; risk-based treatment with 17 α-hydroxyprogesterone Caproate (17-OHP-C) without screening; no screening or treatment. Universal screening represented savings of $1,339 ($8,325 vs. $9,664) when compared to treatment with 17-OHP-C, and led to a reduction of 95,920 preterm births annually in the US.
Conclusion
Universal sonographic screening for short cervical length and treatment with vaginal progesterone appears cost-effective and yields the greatest reduction in preterm birth prior to 34 weeks.
Introduction
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide, and its prevention is the most important challenge to modern obstetrics1. A myriad of strategies to identify patients at risk have been investigated, and interventions have been considered2–13. Recent data have suggested the potential role of progestins in the prevention of preterm birth, such as those with mid-gestation short cervical length14, 15. Fonseca14 and colleagues reported a 44% reduction in the rate of preterm birth in women with a short cervix in the group randomly assigned to receive vaginal progesterone when compared to those without treatment. However, to identify the group of women at-risk for preterm birth based on a short cervical length, the investigators sonographically screened a population of over 24,000 women, calling the cost-effectiveness of this strategy into question. Further, there has been no direct comparison of the various evidence-based clinical strategies for reduction of preterm birth with regard to reducing the health burden of preterm birth in the population as a whole.
We sought to evaluate which comprehensive strategy for the reduction of preterm birth maximizes population pregnancy outcomes based on available published evidence. Using decision analytic and cost-effectiveness modeling, we planned to compare universal cervical length screening with intention to treat with vaginal progesterone to alternative strategies for the reduction of preterm birth and resultant neonatal morbidity and mortality.
Materials and Methods
We designed a decision analytic model in order to compare four strategies for the reduction of preterm birth in singleton pregnancies based on available published evidence to determine the optimal strategy and the cost-effectiveness of that strategy. Specifically, the model was designed to compare: 1) the strategy of universal screening of cervical length with transvaginal ultrasound at the time of routine anatomic survey and treatment with daily vaginal progesterone for women with a short cervix; 2) cervical length screening for women at increased risk for preterm birth (i.e. prior spontaneous preterm birth), and treatment with vaginal progesterone for women with a cervical length ≤ 15mm; 3) no cervical length screening; treatment with 17 α-hydroxyprogesterone Caproate (17-OHP-C) based on obstetrical history; and 4) no screening or treatment. The goal of the model was to weigh the cost of each strategy against the effectiveness of reducing health care costs associated with significant neonatal morbidity and mortality resulting from preterm birth before 34 weeks gestation. The four strategies were compared based on the probability of clinical events, their utilities, or valuation of outcome, and costs. The effectiveness of each strategy was expressed as QALYs (quality-adjusted life years).
Several definitions and assumptions were utilized: 1) the model was designed for women with singleton, non-anomalous gestations, undergoing routine standard-of-care anatomy surveys by ultrasound in the mid second trimester; 2) a short cervix was defined as ≤ 15mm; 3) all cervical length assessments in the model were measured by transvaginal ultrasound at 18–23 weeks gestation, and 4) all 4 strategies included the same measured outcomes: preterm birth before 34 weeks, neonatal death, and severe long-term neonatal morbidity.
In the universal screening arm, women found to have a sonographic cervical length ≤ 15mm received 200mg of micronized progesterone vaginally from diagnosis until 33 weeks and 6 days gestation. Baseline cost estimates were based on 14 weeks of therapy (20–34 gestational weeks). Strategies 2 and 3 identified patients at increased risk based on a history of a prior preterm birth and either screened at-risk patients with cervical sonography (strategy 2) or proceeded directly to preventative therapy (strategy 3). In strategy 2, at-risk women identified by sonographic screening to have a short cervix were given vaginal progesterone in the same fashion as strategy 1. Strategy 3, which most closely models common current practice, offered weekly intramuscular injections of 250 mg 17-OHP-C based on a history of a prior spontaneous preterm birth between 20 weeks and 36 weeks gestation without the use of sonographic screening. Baseline cost estimates were based on an average of 16 weeks of therapy (20–36 weeks gestation). The fourth strategy served as a baseline reference, employing no screening or treatment for the reduction in preterm birth.
Base-case point estimates for probabilities and utilities (or values), and their plausible ranges were derived from a quantitative review of the literature (Table 1 & Table 2). We conducted a MEDLINE and PubMed literature search using the key words: “preterm birth”, “premature birth”, “preterm labor”, “short cervix”, “progesterone”, as well as a search for pertinent references in identified bibliographies. We restricted our search to data from human subjects published in the English language in the last 14 years, and then excluded any case reports or series, meta-analyses, or review articles. Studies without control groups were only included for prevalence estimates of rare events. Probability and utility point estimates were calculated as the sample size-weighted means of estimates from the included studies, and their ranges were defined by the extreme low and high values reported in the literature. For estimates derived from a single source, a range was defined by the 95% confidence interval (CI) calculated from binomial distribution.
Table 1.
Probability Estimates for Patients with a Singleton Intrauterine Pregnancy being Screened for Risk of Preterm Birth
| Variable | Point estimate (range) |
Reference | Level of Evidence† |
|---|---|---|---|
| Pr cvx ≤ 15mm | 0.0119 (0.0100–0.0168) |
14, 23–26 | I, II-2 |
| Pr PTB if cvx ≤ 15mm | 0.2996 (0.2619–0.5952) |
14, 24, 26, 27 | I, II-2 |
| Pr PTB if cvx > 15mm | 0.0156 (0.0152–0.0227) |
14, 24 | I, II-2 |
| Pr PTB if cvx ≤ 15mm, treated with progesterone |
0.1754 (0.1106–0.2579) |
14 | I |
| Sensitivity of cvx ≤ 15mm for PTB |
0.084 (0.021 – 0.102) |
24, 27 | II-2 |
| Specificity of cvx ≤ 15mm for PTB |
0.989 (0.970 – 0.995) |
24, 27 | II-2 |
| Pr Hx of a prior PTB | 0.0730 (0.0452–0.1105) |
28, 29 | II-2, III |
| Pr cvx ≤ 15mm with Hx of a prior PTB |
0.1511 (0.1502–0.1522) |
14, 26 | I, II-2 |
| Pr PTB if cvx ≤ 15mm with Hx prior PTB, treated with progesterone |
0.2667 (0.0779–0.5510) |
14 | I |
| Pr PTB if cvx ≤ 15mm with Hx prior PTB, no treatment |
0.5625 (0.2988 – 0.8025) |
14 | I |
| Pr PTB if cvx >15mm with Hx prior PTB |
0.1960 (0.1364–0.2679) |
18 | I |
| Pr Hx prior PTB meeting criteria for 17-OHP-C |
0.0652 (0.0452–0.0805) |
28, 29 | II-2, III |
| Pr PTB if Hx prior PTB, treated with 17-OHP-C |
0.2059 (0.1620–0.2556) |
18 | I |
| Pr PTB if Hx prior PTB, not treated with 17-OHP-C |
0.3072 (0.2352–0.3868) |
18 | I |
| Pr PTB with no screening or treatment |
0.1230 (0.1227–0.1233) |
30 | II-3 |
| Pr NN death if birth < 34 weeks |
0.1420 (0.1080–0.2470) |
31, 32 | II-2 |
| Pr NN death if birth ≥ 34 weeks |
0.0004 (0.0003–0.0005) |
33 | II |
| Pr NN severe morbidity if birth < 34 weeks |
0.1720 (0.1390–0.1830) |
31, 32 | II-2 |
| Pr NN severe morbidity if birth ≥ 34 weeks |
0.0059 (0.0055–0.0063) |
33 | II |
Pr = probability, U = utility, C = cost, NN = neonatal 17-OHP-C = 17 α-hydroxyprogesterone Caproate Cvx = cervix, PTB = preterm birth, Hx = history
Local sources based on Medicaid reimbursement
Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. Preventative Task Force: A review of the process. Am J Prev Med 2001;20:(3S)
Table 2.
Utility and Cost Estimates for Patients with a Singleton Intrauterine Pregnancy being Screened and Treated to Reduce Preterm Birth Risk
| Variable | Point estimate (range) |
Reference | Level of Evidence† |
|---|---|---|---|
| U NN death | 0.01 (0.001–0.02) |
34 | III |
| U NN severe morbidity |
0.55 (0.50–0.60) |
34, 35 | III |
| C transvaginal sonogram |
$52 ($43–74) |
¶ | n/a |
| C vaginal progesterone (18–34 weeks) |
$283 ($220–344) |
¶ | n/a |
| C 17-OHP-C (18–34 weeks) |
$365 ($300–440) |
¶ | n/a |
| C NN severe morbidity |
$995,940 ($200,000–1,200,000) |
36 | III |
U = utility, C = cost, NN = neonatal, 17-OHP-C = 17 α-hydroxyprogesterone Caproate
Local sources based on Medicaid reimbursement
Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. Preventative Task Force: A review of the process. Am J Prev Med 2001;20:(3S)
Cost estimates were derived from the literature, and when unavailable, from local sources based on Medicaid reimbursement rates (Table 2). When local estimates were used, charges were multiplied by a cost-charge ratio of 0.6 as an approximation to third party reimbursements16. To account for regional variation in costs, estimates were varied widely around the point estimate. Effectiveness was expressed as quality-adjusted life years (QALYs), calculated by the product of utility value and life expectancy (in years). In accordance with standard assumptions in economic analysis, we discounted annual costs and QALYs at a rate of 3% per year17. We assumed life expectancy was 75 years.
Base-case cost-effectiveness analysis was performed, comparing strategies 1–3 to each other and to a “no screening or treating strategy” (strategy 4). A cost-effectiveness threshold of $100,000 per QALY was considered cost-effective. Sensitivity analyses were performed to determine if the optimal strategy identified in the model changed when estimates of probability, utility, and cost were varied alone or in combination across their plausible ranges. Threshold analyses were performed to determine at what hypothetical value for influential variables, such as the effectiveness of vaginal progesterone, the optimal strategy would change. Finally, Monte Carlo simulation was used as a form of multivariable sensitivity analysis, simultaneously varying all values across their plausible ranges at random over multiple iterations to estimate the frequency that the conclusion of the model (the optimal strategy) is concordant with the base-case analysis. The analytic model was constructed and analyzed using TreeAge Pro 2006 Suite (TreeAge Software, Inc, Williamstown, MA).
Since it is known that a history of a prior preterm birth is the most prevalent historical risk factor for a subsequent preterm birth, it is clinically relevant to ask if the optimal strategy differs when women are triaged by this single factor risk-assessment. We constructed two additional models for subgroup analyses based on presence or absence of a prior preterm birth history. The first model for women with a prior spontaneous preterm birth compared three strategies for the prevention of preterm birth: weekly 17-OHP-C (using the same parameters and assumptions outlined for the main model), sonographic cervical length screening and treatment with daily vaginal progesterone for patients with a cervix ≤ 15mm, and no screening or treatment. The second model for women without a prior history of a preterm birth compared two strategies: sonographic cervical length screening and treatment with daily vaginal progesterone for cervix ≤ 15mm, and no screening or treatment. As in the main model, sensitivity analyses including Monte Carlo simulation were performed to explore the stability or precision of the model’s result.
This study did not involve human subjects, making it exempt from Institutional Review Board approval.
Results
The strategy of universal sonographic screening for cervical length and daily treatment with vaginal progesterone for women with a cervical length ≤ 15mm was the most cost-effective strategy, and was dominant (lower total costs with better outcomes) over the three alternatives (Table 3). The base-case analysis also revealed all three strategies employing some form of screening and/or preventative therapy for preterm birth to be more cost-effective than no screening or treatment.
Table 3.
Base-case cost-effectiveness analysis comparing universal screening to the alternative strategies
| Cost ($) |
Incremental Cost ($) |
Effectiveness (QALY) |
Incremental Effectiveness (QALY) |
Average Cost/ Effectiveness (C/E) ($/QALY) |
Incremental C/E |
|
|---|---|---|---|---|---|---|
| Universal screening; tx vag p |
8,325 | - - | 72.3 | - - | 115 | Dominant |
| High-risk screening; tx vag p |
10,577 | 2,252 | 72.0 | −0.25 | 147 | Dominated |
| No screening; 17-OHP-C based on hx |
9,664 | 1,339 | 72.1 | −0.15 | 134 | Dominated |
| No screening or treating |
11,560 | 3,235 | 71.9 | −0.36 | 161 | Dominated |
Tx=treatment, Vag p=vaginal progesterone; 17OHp=17-hydroxyprogesterone acetate; Hx=history
We considered a hypothetical cohort of 4 million pregnant patients, estimating the annual birth rate in the Unites States (Table 4). Universal screening prevented the greatest number of preterm births before 34 weeks and cases of severe neonatal morbidity when compared to the other three strategies. Universal screening would prevent more than 95,000 preterm births and, in turn, more than 13,000 cases of severe morbidity leading to savings of almost 13 billion dollars annually. When compared to the current preventive strategy of treating with 17-OHP-C based on obstetric history, universal screening continues to yield better outcomes and greater economic savings.
Table 4.
Number of preterm births prior to 34 weeks and cases of severe morbidity prevented per dollar spent by strategy, using base-case estimates and an estimated annual delivery rate of 4 million in the United States
| Strategy | Number of Preterm Births |
Number of Cases Significa nt Morbidity |
Total Cost (100 Million $) |
Number of Preterm Births Prevented |
Number of Cases Significant Morbidity Prevented |
Total Cost Saved (100 Million $) |
|---|---|---|---|---|---|---|
| No screening or treating (ref) |
170,920 | 47,810 | 462.4 | Reference | Reference | Reference |
| Universal Screening |
75,000 | 34,220 | 333.0 | 95,920 | 13,590 | 129.4 |
| High-risk Screening |
142,160 | 43,740 | 423.1 | 28,760 | 4,070 | 39.3 |
| Standard of care; 17- OHP-C |
114,880 | 39,860 | 386.6 | 56,040 | 7,950 | 79.3 |
One-way and multi-way sensitivity analyses showed the model to be robust; when the probability, utility, and cost estimates were varied across their ranges, universal screening remained the preferred strategy. Notably, the direct comparison of the universal screening strategy and the 17-OHP-C strategy relies directly on the published efficacy from each of the positive trials for treatment14, 18. When we varied the treatment efficacy in the universal treatment arm based on the risk reduction in preterm birth and its range published by Fonseca14 and colleagues (relative risk [RR] 0.56, 95% confidence interval [CI] 0.36–0.86), and the treatment efficacy in the 17-OHP-C arm based on the risk reduction for preterm birth and its range published by Meis et al18 (RR 0.67, 95% CI 0.48–0.93), universal screening remained the preferred strategy.
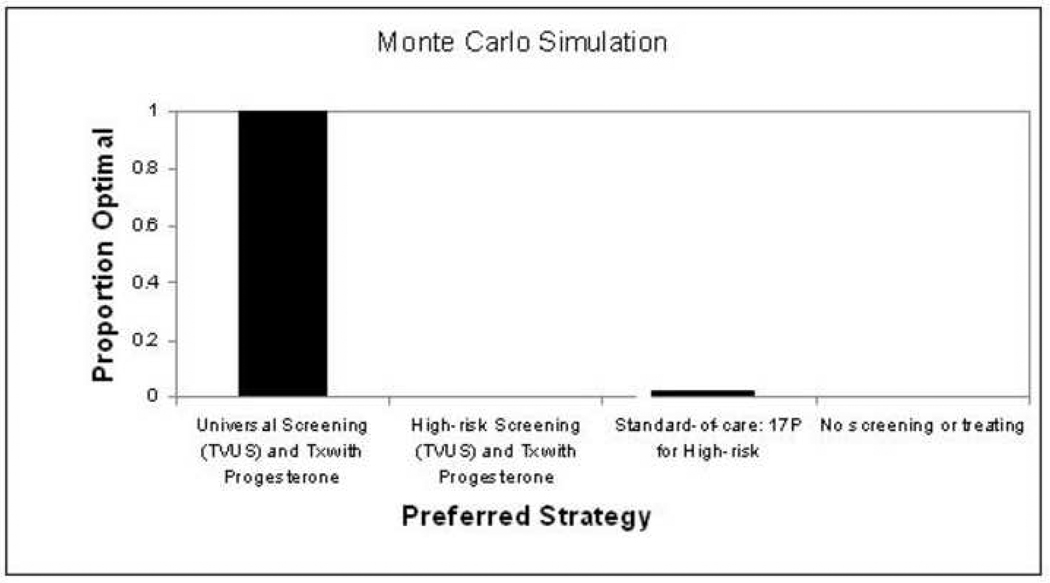
Monte Carlo simulation was used to simultaneously vary at random all variables across their plausible ranges. Using 10,000 simulations, the universal screening strategy was the dominant strategy 98.9% of the time (Figure 1). Given that the model was driven by a few specific cost estimates, it was important to consider some hypothetical extreme estimates of those values. For example, while the model was designed with a definition of preterm birth as delivery prior to 34 weeks, there is a wide range of potential outcomes, and thus costs, which could result from these births. Thus, we modeled several extreme scenarios, or values, to test the threshold of our model. Even in a ‘worst case’ scenario, modeling the cost of vaginal progesterone at 10 times the estimate in the model, minimizing the cost and maximizing the utility of neonatal morbidity, Monte Carlo simulation revealed universal screening and treating with vaginal progesterone was the dominant strategy 96.9% of the time.
Figure 1. Preferred Strategy by Monte Carlo Simulation with 10,000 iterations.
While the strategy of universal screening with the intention to treat with daily vaginal progesterone was the most cost-effective in the main analyses, we also considered the possibility that an alternative strategy may be superior when patients were stratified by risk. First we modeled two strategies for women who were non-high risk based on having no history of prior preterm birth, acknowledging this actually represents a heterogeneous group seen clinically everyday. This non-high-risk group predominantly consists of nulliparous women, with an unproven history, as well as multiparous women with prior birth(s) at term. Similar to the main analysis, we found universal screening with transvaginal ultrasound and treatment with daily vaginal progesterone to be cost-effective, resulting in cost-saving of $9,982/QALY when compared to no screening or treating, the latter being the approach most commonly used in clinical practice for such a low-risk population. Sensitivity analyses revealed the model was robust to all probability, utility, and cost estimates across their plausible ranges.
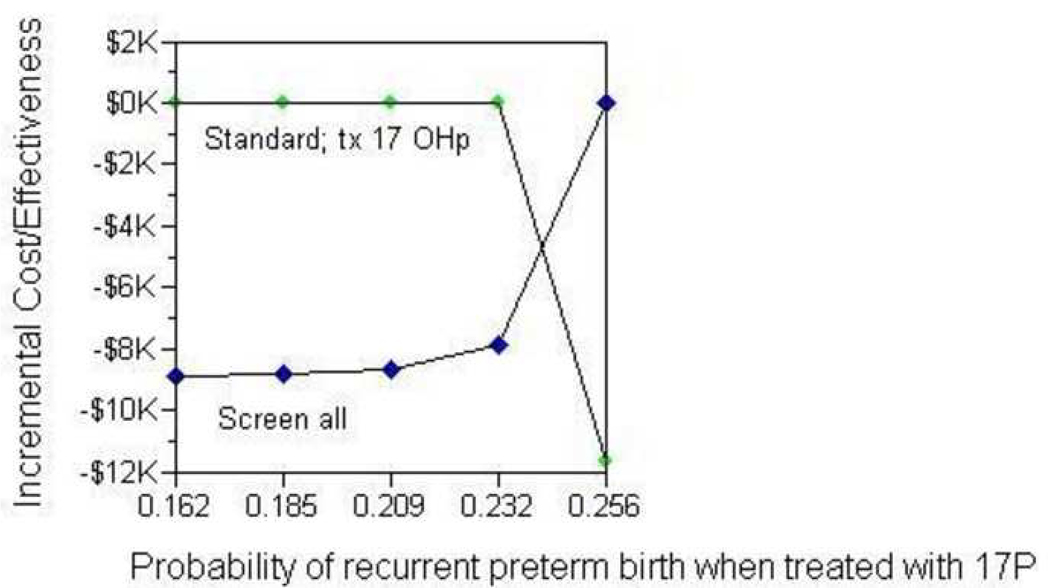
Next, we modeled three strategies for women at increased risk for preterm birth based on a history of at least one prior spontaneous preterm birth: universal screening (with treatment of cervical length ≤ 15mm with vaginal progesterone), treatment with 17-OHP-C (as described previously), and no screening or treating. In the base-case analysis, treatment with 17P and no sonographic screening was the preferred strategy. Treating a hypothetical cohort of 280,000 high-risk women with 17P would save 11,760 additional preterm births prior to 34 weeks compared to sonographic screening and treatment with vaginal progesterone for short cervical length (Table 5). In one- and two-way sensitivity analyses, the model was sensitive to the probability of recurrent preterm birth if treated with 17-OHP-C. In other words, when the estimated reduction in risk of preterm birth achieved by 17-OHP-C fell below 22% (as compared to 33% reported in the Meis18 trial), universal cervical length screening became the preferred strategy for women with a history of a prior spontaneous preterm birth (Figure 2).
Table 5.
Subgroup analysis of high-risk women; number of preterm births prior to 34 weeks prevented per dollar spent by strategy with an estimated annual cohort of 280,000*
| Strategy | Number of Preterm Births |
Total Cost (100 Million $) |
Cost per Preterm Birth Prevented† |
|---|---|---|---|
| No screening or treating (ref) |
70,280 | 112.4 | Reference |
| Sonographic screening; vag p for short cervix |
69,440 | 111.1 | $13,226,000 |
| Standard of care; 17-OHP-C |
57,680 | 95.9 | $761,400 |
Estimated number of women annually with a history of at least one prior preterm birth (“high-risk”)
Compared to no screening or treating
Figure 2. Subgroup Analysis of High-risk Women: Sensitivity Analysis on Probability of Recurrent Preterm Birth with 17-OHP-C Treatment.
Comment
It is imperative that efforts are made to optimally integrate and apply the available high-level evidence to identify the clinical strategy that maximizes and best allocates preterm birth prevention methods. This model suggests that a strategy of universal cervical length screening at the time of routine fetal anatomy sonogram to identify women with a cervical length of ≤ 15mm, and subsequent treatment with vaginal progesterone, is the most cost-effective strategy with the greatest reduction in preterm birth before 34 weeks and resultant neonatal morbidity and mortality. However, the logistical challenges of the practical application of this strategy may vary significantly based on regionally available resources. Our secondary analyses demonstrated that if women are triaged by history of preterm birth, weekly treatment of those with a positive history with 17-OHP-C results in the greatest reduction in recurrent preterm births when compared to alternatives but is dependent on the published estimated effect of 17-OHP-C.
While progestins appear to be a promising treatment for the prevention of preterm birth, only two randomized, placebo-controlled studies have provided evidence of positive effect. In 2003, Meis18 and colleagues conducted a multi-center study of women with at least one prior spontaneous preterm birth and reported a significant risk-reduction of recurrent preterm birth in the group of women who received weekly intramuscular 17-OHP-C when compared to placebo. In 2007, Fonseca14 et al reported results from a multi-center trial in which they sonographically screened a population of over 24,000 to identify women with a cervical length less than or equal to 15mm, and then randomly assigned women with a short cervix to nightly vaginal micronized progesterone or placebo. The authors reported a significant reduction in preterm birth in women who received progesterone as compared to placebo. Other studies using different formulations of progesterone19, or enrolling various patient populations at risk for preterm birth such as multiple gestations20, have not found a significant reduction in preterm birth. Thus, based on available evidence, the trials by Meis18 and Fonseca14 represent the only Level I data that suggest that progesterone reduces the risk for preterm birth. Given the significant health burden preterm birth represents, it is imperative that clinical management strategies maximize the application of preventative therapies supported by these data.
A recent Committee Opinion from the American College of Obstetricians and Gynecologists21 addressed the use of progesterone to reduce the rate of preterm birth. The Committee did not recommend routine cervical length screening, but noted that women with an incidentally diagnosed short cervix may be offered progesterone. Based on our model, this latter recommendation to avoid cervical length screening does not appear to be cost-effective. Because of the savings associated with avoiding preterm birth, the universal screening approach which has been recommended by experts in the field22, may actually be cost saving.
Decision and economic models, such as the one presented here, allow for formal comparison of clinical strategies based on available data. Decision analytic models are particularly useful for clinical decision making under conditions of uncertainty when there are multiple, complex competing risks and benefits. This is an inherent strength of this study. Initially it may seem that universal sonographic cervical length screening would be cost-prohibitive. However, when formally weighed against the long-term effects of preterm birth, the use of sonographic screening for cervical length is the most cost-effective strategy. Our model is the first to systematically weigh the economic implications of such a policy.
Our study is not without limitations. First, there are assumptions required in the construction of such models that are worth noting. The primary outcome of preterm birth in the model was defined as spontaneous birth at or before 34 weeks gestation. This was the primary outcome used in some but not all of the source trials. For example, this was the primary outcome for Fonseca14, but not for Meis18 since their primary outcome was birth prior to 37 weeks. In the case of the latter study, we modeled the data for the sub-analysis of birth at or before 35 weeks. We then varied the point estimates widely to account for discrepancies such as this one in the sensitivity analyses, but the model remained robust.
It is also important to note that there has been no trial that has directly compared or combined the strategies for progestin use. It remains unknown how much overlap exists between the groups at-risk for preterm birth that have been studied and if there exists another distinct population of patients who would benefit from multiple treatments. Our model assumed patients all received standard-of-care sonography for fetal anomaly survey, and thus the addition of cervical length measurement represented an adjunct cost and not a full additional exam and encounter. If routine fetal anomaly screening by sonogram were not routinely utilized, universal screening would have higher up-front costs. The model defined short cervix as ≤ 15mm for the purposes of treatment with vaginal progesterone as that is what had been the definition in the source trial14. We did not consider the possibility that vaginal progesterone could have an effect on the reduction of preterm birth at other cervical lengths. In addition, we did not include multiple cervical length assessments in the model, as the application of this strategy would become onerous. More importantly, while both of these clinical questions are important and relevant, published data to support them does not exist, making these questions impossible to assess accurately using decision analysis.
It is also critically important to consider the possible logistical complexities of universal screening. Our model assumed that all patients undergo a standard-of-care, mid-second trimester anomaly survey at a facility that has the equipment and trained staff capable of adding the additional study to the routine exam. Variation in regional practices, facilities, and training may dictate the need to train additional personnel to acquire and read the images, as well as the need for appropriate equipment to perform the additional exam. The potential for these additional costs were not modeled, as they would be impossible to estimate without specific regional data. But given the results of our model, regional analyses are much needed.
Finally, there are countless questions remaining regarding preterm birth prevention, such as the application of proven therapies to additional at-risk groups and alternative therapies for the prevention of preterm birth. Specifically, many hypothesize that there may be efficacy of progestins given to women with a cervical length ≤ 25mm. Others hypothesize that cerclage might reduce the risk of preterm birth in women with a short cervix. Similarly, some propose the use of combination therapy with progestin and cerclage placement in some at-risk populations. However, at the current time, there is no evidence for benefit or efficacy of these treatments in the published literature, precluding us from including them in our model. We sought to explore the optimal strategy for preterm birth prevention with the data currently available.
The present study suggests that universal transvaginal sonographic screening for short cervical length and treatment with vaginal progesterone may be cost-effective in reducing the risk of preterm birth. But further study that considers regional assets is necessary to evaluate the practical application of this approach, particularly in regions of limited health care resources.
Acknowledgments
Funding: Funded in part by the Perinatology Research Branch, Division of Intramural Research, of Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures:
We wish to disclose to the Editors that after the submission of the manuscript, Dr. Macones now serves on an Advisory Board for Ther-Rx, the company that has the rights to 17-OHP-C if it is FDA approved.
Reprints: Not available
References
- 1.Mathews TJ, Curtin SC, Macdorman MF. Infant mortality statistics from the 1998 period linked birth/infant death data set. Natl Vital Stat Rep. 2000;48:1–25. [PubMed] [Google Scholar]
- 2.Carey JC, Klebanoff MA, Hauth JC, et al. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 3.Dyson DC, Crites YM, Ray DA, Armstrong MA. Prevention of preterm birth in high-risk patients: the role of education and provider contact versus home uterine monitoring. Am J Obstet Gynecol. 1991;164:756–762. doi: 10.1016/0002-9378(91)90510-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Iams JD, Das A, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2000;182:636–643. doi: 10.1067/mob.2000.104212. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Iams JD, Mercer BM, et al. NICHD MFMU Network. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. Am J Public Health. 1998;88:233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Iams JD, Miodovnik M, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: risk factors in twin gestations. Am J Obstet Gynecol. 1996;175:1047–1053. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, Mcnellis D NICHD Maternal Fetal Medicine Units Network. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. Obstet Gynecol. 1996;87:643–648. doi: 10.1016/0029-7844(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 8.Hauth JC, Goldenberg RL, Andrews WW, Dubard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–1736. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Meis PJ, et al. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 10.Iams JD, Goldenberg RL, Mercer BM, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035–1040. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 11.Iams JD, Johnson FF, O'Shaughnessy RW. A prospective random trial of home uterine activity monitoring in pregnancies at increased risk of preterm labor. Part II. Am J Obstet Gynecol. 1988;159:595–603. doi: 10.1016/s0002-9378(88)80016-4. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669–674. doi: 10.1056/NEJM199109053251001. [DOI] [PubMed] [Google Scholar]
- 13.Mercer BM, Goldenberg RL, Das A, et al. The preterm prediction study: a clinical risk assessment system. Am J Obstet Gynecol. 1996;174:1885–1893. doi: 10.1016/s0002-9378(96)70225-9. discussion 1893-5. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 15.Defranco EA, O'Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 16.Washington, DC: US Department of Health and Human Services, National Limitations-Clinical Lab; Medicare. 1995
- 17.Drummond MFSM, Torrance GW, Stoddart GL. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2005. [Google Scholar]
- 18.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 20.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Committee Opinion number 419 October 2008 (replaces no. 291, November 2003) Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112:963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 22.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 23.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 24.Heath VC, Souka AP, Erasmus I, Gibb DM, Nicolaides KH. Cervical length at 23 weeks of gestation: the value of Shirodkar suture for the short cervix. Ultrasound Obstet Gynecol. 1998;12:318–322. doi: 10.1046/j.1469-0705.1998.12050318.x. [DOI] [PubMed] [Google Scholar]
- 25.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks' gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–978. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 26.To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 27.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 28.Mercer BM, Goldenberg RL, Moawad AH, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol. 1999;181:1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 29.Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 31.Stoelhorst GM, Rijken M, Martens SE, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project On Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow-Up Project on Prematurity 1996–1997. Pediatrics. 2005;115:396–405. doi: 10.1542/peds.2004-1497. [DOI] [PubMed] [Google Scholar]
- 32.Zeitlin J, Draper ES, Kollee L, et al. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics. 2008;121:e936–e944. doi: 10.1542/peds.2007-1620. [DOI] [PubMed] [Google Scholar]
- 33.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbussche FP, De Jong-Potjer LC, Stiggelbout AM, Le Cessie S, Keirse MJ. Differences in the valuation of birth outcomes among pregnant women, mothers, and obstetricians. Birth. 1999;26:178–183. doi: 10.1046/j.1523-536x.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 35.Pham CT, Crowther CA. Birth outcomes: utility values that postnatal women, midwives and medical staff express. BJOG. 2003;110:121–127. [PubMed] [Google Scholar]
- 36.Waitzman NJ, Romano PS, Scheffler RM. Estimates of the economic costs of birth defects. Inquiry. 1994;31:188–205. [PubMed] [Google Scholar]